Abstract
Host-derived matrix metalloproteinases (MMPs) and bacterial proteases mediate destruction of extracellular matrices and supporting alveolar bone in periodontitis. The Treponema denticola dentilisin protease induces MMP-2 expression and activation in periodontal ligament (PDL) cells, and dentilisin-mediated activation of pro-MMP-2 is required for cellular fibronectin degradation. Here we report that T. denticola regulates MMP-2 expression through epigenetic modifications in the periodontium. PDL cells were treated with epigenetic enzyme inhibitors before or after T. denticola challenge. Fibronectin fragmentation, MMP-2 expression and activation were assessed by immunoblot, zymography and qRT-PCR, respectively. Chromatin modification enzyme expression in T. denticola-challenged PDL cells and periodontal tissues were evaluated using gene arrays. Several classes of epigenetic enzymes showed significant alterations in transcription in diseased tissue and T. denticola-challenged PDL cells. T. denticola-mediated MMP-2 expression and activation were significantly reduced in PDL cells treated with inhibitors of aurora kinases and histone deacetylases. In contrast, DNA methyltransferase inhibitors had little effect, and inhibitors of histone acetyltransferases, methyltransferases and demethylases exacerbated T. denticola-mediated MMP-2 expression and activation. Chronic epigenetic changes in periodontal tissues mediated by T. denticola or other oral microbes may contribute to the limited success of conventional treatment of chronic periodontitis and may be amenable to therapeutic reversal.
Introduction
Periodontitis, a bacterially-mediated chronic inflammatory disease of the tissues supporting the tooth is one of the most common inflammatory diseases in humans and it can adversely affect systemic health (Armitage, 2004, Armitage, 2008). National surveys show that the majority of adults suffer from mild-to-moderate periodontitis, with up to 15% of the population being affected by severe forms at some stage in their lives (Pihlstrom et al., 2005). Periodontitis causes largely irreversible destruction of the periodontal tissues, and in advanced stages, tooth loss, speech and masticatory problems, and an overall reduced quality of life. Moreover, the systemic burden of periodontitis on different organs has been investigated. It has been reported that periodontal infections can adversely predispose to coronary heart diseases (Stewart et al., 2016), ischemic strokes (Leira et al., 2016), poor glycemic control (Garcia et al., 2015), preterm labor, low-birth-weight delivery (Sitholimela et al., 2013), and pulmonary diseases (Prasanna, 2011). These systemic effects have been attributed to either direct bacterial invasion or modulation of specific host inflammatory and tissue destructive mediators (Williams et al., 2008).
Destruction of the periodontal extracellular matrices (ECM) and detrimental changes in the cellular elements of the periodontal ligament occur as a result of disruption of normal tissue homeostatic processes. One of these disrupted processes is regulation of host-derived matrix metalloproteinases (MMP) that are both directly and indirectly involved in periodontal tissue breakdown. Destruction of the ECM in periodontitis results in the release of fibronectin (FN) fragments, which are considered markers of periodontal disease status (Huynh et al., 2002). Evidence from in vitro studies further indicate that these FN fragments, induce several detrimental effects, including induction of apoptosis and suppression of osteoblast differentiation of periodontal ligament cells (Kapila et al., 1996, Kapila et al., 1998, Kapila et al., 1999, Jee et al., 2004, Dai et al., 2005, Ghosh et al., 2008, Joseph et al., 2010), thereby further potentiating disease progression.
MMPs are synthesized in latent form, then activated through proteolytic cleavage to expose the catalytic site of the MMP enzyme. MMP activation is primarily extracellular, though intracellular activation is reported in certain cases (Nagase, 1997, Murphy et al., 1999). MMPs are synthesized at low basal levels for maintenance of homeostatic processes; however their levels and activation are typically increased during disease (Mittal et al., 2016). In addition to their role in remodeling the ECM and basement membrane during various physiologic processes, MMPs are implicated in a wide range of pathologic processes, including cardiovascular (Azevedo et al., 2014), pulmonary (Navratilova et al., 2016, Pardo et al., 2016), renal (Aresu et al., 2011, Charitaki et al., 2016), and gastrointestinal diseases (Medina et al., 2004), cancer (Endres et al., 2016, Ligi et al., 2016, Liu et al., 2016, Lukaszewicz-Zajac et al., 2016, Pietruszewska et al., 2016) and inflammatory diseases, such as periodontitis (Shinkarenko et al., 2013, Nissinen et al., 2014).
MMP-2, one of several MMPs involved in tissue homeostasis and remodeling, is constitutively expressed by periodontal ligament cells as pro-MMP-2 (Kapila et al., 1996). Thus, control of the process or rate of activation of MMP- 2 has been proposed as a key regulatory step in periodontal tissue homeostasis (Madsen et al., 2013, Mosig et al., 2013, Borkham-Kamphorst et al., 2015). Activation of pro-MMP-2 is promoted by proteolytic cleavage by the membrane type-1-MMP (MT1-MMP/MMP-14) (Nagase, 1997, Murphy et al., 1999, Zucker et al., 2003). Extracellular MMP activities are controlled by the blockage of autolytic MMP activation or by endogenous proteinase inhibitors, such as tissue inhibitors of MMPs (TIMPs) (Nagase et al., 2006, Brew et al., 2010). Regulation of the proteolytic activity of MMP-2 is dependent on the balance between MT1-MMP/TIMP-2 (Shofuda et al., 1998, Hernandez-Barrantes et al., 2001, Oyarzun et al., 2010). This mechanism of MMP-2 activation by an MT1-MMP–TIMP-2 complex has been well recognized in other systems (Strongin et al., 1995, Butler et al., 1998, Kinoshita et al., 1998). Disturbances in that balance may result in excessive tissue degradation associated with inflammatory diseases (Ejeil et al., 2003).
T. denticola along with Porphyromonas gingivalis and Tannerella forsythia become prevalent in late stages of subgingival biofilm formation and comprise the bacterial “red complex” that is considered pathogenic in the etiology of periodontal disease (Socransky et al., 1998). While our understanding of the periodontal disease microbiome has greatly increased, the “red complex” bacteria continue to be recognized as important pathogens in the disease process. Oral spirochetes including T. denticola often predominate in periodontal disease, though they are typically below detectable levels in healthy gingival plaque (Choi et al., 1994, Ellen et al., 2005). The levels of T. denticola increase with the severity of periodontitis, underscoring its major role in the disease (Simonson et al., 1988, Yoshida et al., 2004).
Recognized virulence factors of T. denticola include the acylated serine protease complex (dentilisin; PrtP complex; CTLP/chymotrypsin-like protease) that degrades gelatin, laminin and various serum components and bioactive peptides (Uitto et al., 1988, Grenier et al., 1990, Makinen et al., 1995). The dentilisin complex contributes to T. denticola adherence and cytotoxic effects on epithelial cells and fibroblasts (Ellen et al., 1994, Mathers et al., 1996, Fenno et al., 1998), penetration of epithelial tissue (Chi et al., 2003), and it may play a role in complement-mediated bactericidal activity (McDowell et al., 2009) and complement evasion (McDowell et al., 2011). Of particular relevance to the current study, we previously demonstrated that dentilisin proteolytic activity induces activation of pro-MMP-2 in cultured PDL cells, and that activated MMP-2 is required for cleavage of cellular FN into fragments similar to those observed in gingival crevicular fluid from periodontal lesions (Miao et al., 2011). Furthermore, transcription and expression of MT1/MMP and TIMP-2 increased in response to T. denticola challenge (Miao et al., 2014). Taken together, these properties suggest important links between the T. denticola protease activity and regulation of the cellular and tissue processes that result in periodontal tissue destruction.
Epigenetics is defined as heritable and potentially reversible changes in gene expression without alterations in the DNA sequence (Goldberg et al., 2007, Waddington, 2012). Such modifications are not only associated with diseases but are also essential for the incorporation and integration of endogenous and environmental signals in cells. Epigenetic status can be affected by environmental factors, such as, nutrients, toxins, infections, and hypoxia with subsequent up- or down-regulation of specific gene expression patterns (Barros et al., 2009, Safronova et al., 2010, Bayarsaihan, 2011, Yin et al., 2011). Emerging evidence suggests that epigenetic modifications play a major role in inflammatory diseases, including periodontal disease (Barros et al., 2014). Several factors that mediate periodontal disease pathogenesis, including bacteria and their byproducts, smoking, and diabetes, induce marked epigenetic changes in tissue components (Offenbacher et al., 2008, Khansari et al., 2009, Medzhitov et al., 2009, Yin et al., 2011, Razzouk et al., 2013, Martinez et al., 2014, Pasquier et al., 2015). For example, chronically inflamed periodontal tissues demonstrated an increased methylation of CpG-rich regions of the PTGS2/COX2 promoter compared to healthy periodontal tissues (Zhang et al., 2010). Also, there is marked hypomethylation of the IL8 promoter in oral epithelial cells of subjects with Generalized Aggressive Periodontitis compared to control subjects (Andia et al., 2010). Emerging studies suggest that microbial pathogens, including oral species such as Porphyromonas gingivalis, induce epigenetic modifications in host cells (reviewed in (Niller et al., 2017)). We recently identified potential epigenetic links between T. denticola and genes in PDL cells involved in activation of MMP-2 (Miao et al., 2014). Thus, T. denticola may mediate epigenetic modifications that regulate MMP-2 activation and subsequent ECM degradation in the periodontium.
Epigenetic modifications are potentially reversible, and, therefore, a thorough understanding of these changes may identify new therapeutic targets for disease management. The aim of this study was to investigate T. denticola’s ability to chronically activate MMP expression through epigenetic modifications in periodontal ligament cells/tissues, and to examine potential therapeutic approaches for reversal/modification of these changes.
Results
T. denticola chronically upregulates expression of MMP-2, MT1-MMP and TIMP-2, with concomitant fibronectin fragmentation
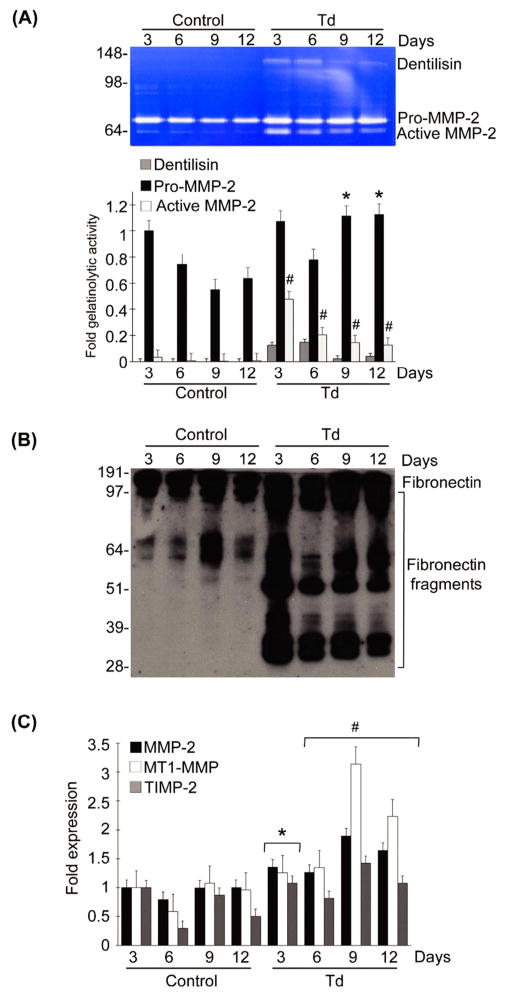
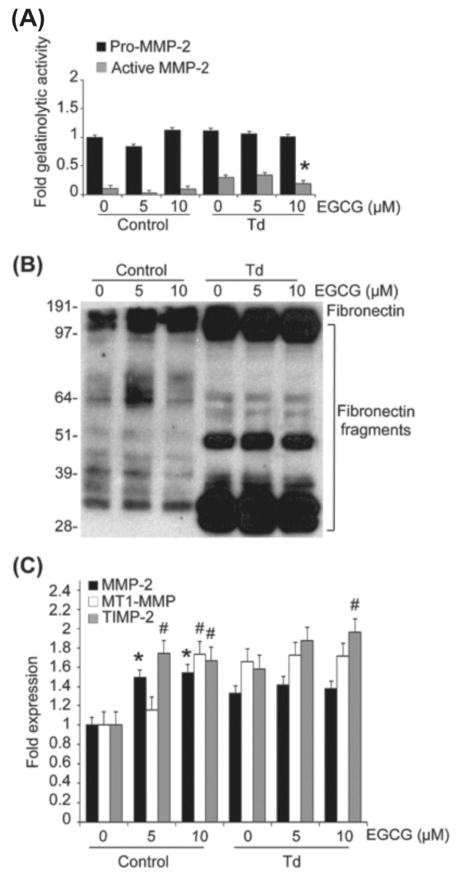
To determine the long-term effects of a brief exposure to T. denticola on MMP-2 expression in host cells, PDL cells were briefly challenged with T. denticola, then MMP-2 expression and MMP-2 activation in long-term cultures with daily medium changes were assessed by gelatin zymography and qRT-PCR. As shown in Fig. 1A, PDL cells constitutively expressed basal levels of pro-MMP-2 with minimal activation for maintenance of homeostatic functions. However, challenge with T. denticola triggered both chronic increased MMP-2 expression (pro-MMP2) and activation (active MMP-2) in PDL cells. Following a 2h exposure to T. denticola, dentilisin activity (visible as a ~100kDa band on the zymogram, Fig. 1A) persisted within these cultures throughout the experiment, though it was detected at greatly reduced levels at days 9 and 12. Expression and activation levels of MMP-2 were chronically sustained for up to 12 days, with minor reductions in the levels of activated MMP-2 on days 9 and 12 (Fig. 1A). T. denticola-mediated increases in MMP-2 expression and activation were mirrored by concomitant fibronectin fragmentation throughout the experiment (Fig. 1B).
Figure 1. T. denticola mediates chronic expression and activation of MMP-2, MT1-MMP, and TIMP-2 in PDL cells, with subsequent fragmentation of cellular fibronectin.
Cultured PDL cells were challenged with T. denticola (Td) at MOI = 100 or media control for two hours, then incubated for 3, 6, 9, and 12 days with daily medium changes. The experiments were repeated three times in triplicate. Data were analyzed using one-way ANOVA. (*) represents p ≤ 0.05 compared to the same time point in the control group. (#) represents p ≤ 0.001 compared to the same time point in the control group.
Panel A: A representative gelatin zymogram of PDL cell conditioned medium showing the gelatinolytic activity of pro-MMP-2 (72-kDa), active MMP-2 (64-kDa), and Td dentilisin (100-kDa). The left 4 lanes represent the control unchallenged PDL cells and the right 4 lanes represent the Td-challenged PDL cells. The bar chart below represents the densitometric analysis of gelatinolytic activity in the zymograms using ImageJ™-NIH software. The Y-axis represents fold-gelatinolytic activity of the pro-MMP-2, active MMP-2, and dentilisin relative to unchallenged control at day 3. The X-axis represents different time points.
Panel B: A representative immunoblot of PDL cell culture supernatants probed with a polyclonal anti-fibronectin antibody showing FN fragmentation in conditioned medium from Td-challenged PDL cells.
Panel C: Transcript levels of MMP-2, MT1-MMP, and TIMP-2 in PDL cells at different time points after challenge with Td or media control assayed by qRT-PCR. The Y-axis represents fold-expression level of each gene relative to unchallenged control at day 3. The X-axis represents different time points.
The chronic effects of T. denticola on MMP-2 expression in PDL cells were regulated at the transcriptional level. MMP-2 mRNA levels were upregulated for up to 12 days as assessed by qRT-PCR (Fig. 1C). Given that the MT1-MMP/TIMP-2 complex is a well-known regulator of MMP-2 activation, MT1-MMP and TIMP-2 expression were examined in T. denticola challenged periodontal ligament cells in long-term cultures. Expression of the MT1-MMP/TIMP-2 complex was also chronically upregulated by the T. denticola challenge, mirroring the changes induced in MMP-2 transcriptional expression (Fig. 1C).
T. denticola levels and MMP-2 transcription are elevated in periodontal disease
Examination of human tissues from periodontally diseased and healthy sites confirmed the association between T. denticola and elevated MMP-2 expression in diseased tissues. Human tissue specimens from periodontally diseased sites exhibited elevated levels of T. denticola concomitant with elevated levels of MMP-2 mRNA expression compared with healthy sites (Fig. 2). Tissue specimens from healthy sites exhibited negligible levels of T. denticola and low levels of MMP-2 expression. Low levels of MMP-2 expression in healthy tissues are consistent with the low basal levels of MMP-2 expression necessary for homeostatic functions within the periodontium, while increased MMP-2 expression is consistent with dysbiotic alterations of periodontal homeostasis in disease.
Figure 2. Elevated MMP-2 mRNA and T. denticola in diseased periodontal tissue.
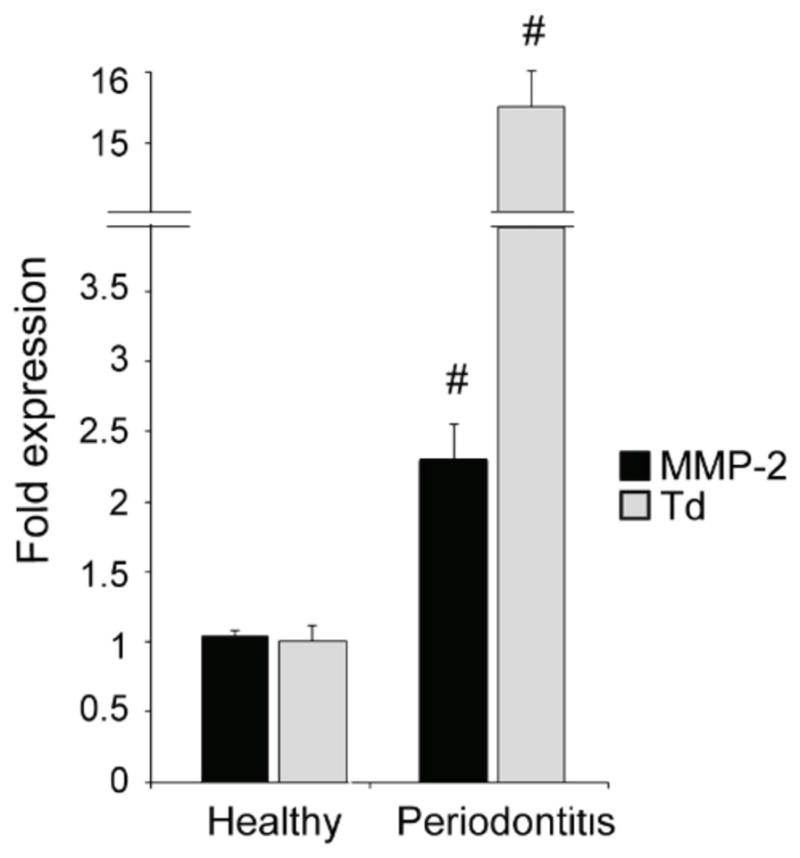
Levels of T. denticola 16S rRNA and MMP-2 mRNA in tissue specimens from periodontally diseased or healthy sites were assayed by qRT-PCR. The Y-axis represents levels of each RNA in periodontitis specimens relative to healthy specimens. The X-axis represents the source of tissue specimens. Data were analyzed using Student’s t-test. (#) represents p ≤ 0.001 compared to healthy tissue.
Expression of genes encoding chromatin modification enzymes are significantly altered in periodontal disease
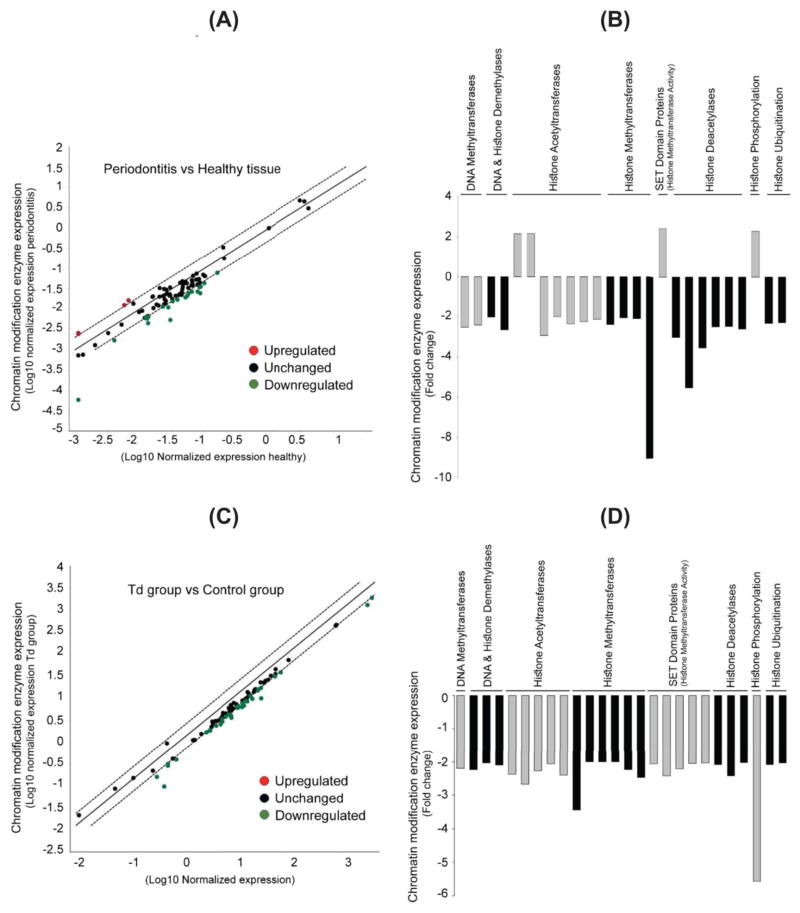
To further examine the role of epigenetics in periodontal disease, the major chromatin modification enzymes that might be involved in the disease process (Table S1) were assessed via a focused gene array. Applying a “2-fold change” as a threshold value, we found that several chromatin modification enzymes were significantly altered in diseased periodontal tissue specimens compared to healthy control tissues (Fig. 3A, B and Table S2). The most significantly altered enzymes were the histone methyltransferases (HMTs) and histone deacetylases (HDACs), including PRMT8 and HDAC11, which exhibited a significant down-regulation. Other enzymes, especially those related to SET Domain proteins, histone acetyltransferases (HATs), and histone phosphorylation kinases showed significant up-regulation, such as SETD4, ESCO1, ESCO2, and AURKB. Other significantly altered enzymes included HATs, HDACs, histone ubiquitinases, DNA demethylases (DDMs) and histone demethylases (HDMs), and DNA methyltransferases (DNMTs).
Figure 3. Transcription of multiple chromatin modification enzymes is altered in both periodontally diseased tissue and in T. denticola-challenged PDL cells.
Transcription levels of 87 chromatin modification enzymes were examined by qRT-PCR using RNA extracted from healthy and diseased periodontal tissues (Panels A and B, respectively) and from PDL cell lysates collected 9 days after Td challenge as described in Fig. 1 (Panels C and D, respectively).
Panels A and C: Scatter blots showing the transcriptional level of chromatin modification enzymes in periodontally diseased tissues relative to healthy tissue controls (Panel A) and in Td-challenged PDL cells relative to unchallenged control cells (Panel C). The Y-axis represents expression level of different enzymes relative to healthy tissue (Panel A) or unchallenged control cells (Panel C). The X-axis represents normalized expression of the respective control group.
Panels B and D: Bar-charts showing the significantly downregulated chromatin modification enzymes in periodontally diseased tissues relative to healthy tissue controls (Panel B) and in Td-challenged PDL cells relative to unchallenged control cells (Panel D). The Y-axis represents the fold-expression/downregulation level of enzymes relative to unchallenged controls. The X-axis represents different chromatin modification enzymes, with enzyme type grouped by color.
T. denticola significantly alters expression of genes encoding chromatin modification enzymes in PDL cells
To examine the potential role of epigenetics in the T. denticola-mediated upregulation of MMP-2 expression, a focused gene array was employed to study all major epigenetic chromatin modification enzymes that might be involved in this process (Table S1). Applying a “2-fold change” as a threshold value, we found that T. denticola-challenged PDL cells exhibited significantly decreased levels of all major chromatin modification enzymes (and Fig. 3C, D and Table S3). No chromatin modifying enzymes assayed showed increased expression (data not shown). The most significantly down-regulated enzymes included aurora kinases, which mediate histone phosphorylation, and HMTs. Specifically, within these classes of enzymes, the most downregulated enzymes included Aurora Kinase B and EHMT2. Other significantly downregulated enzymes included HATs, histone deacetylases (HDAC), histone ubiquitinases, DNA (DDM) and histone demethylases (HDM), and DNMTs.
Inhibitors of histone kinase/aurora kinase, DNMT and HDAC block T. denticola-mediated increases in MMP-2 activity and expression in PDL cells
Given the broad landscape of epigenetic changes mediated by T. denticola on PDL cells and the changes exhibited in diseased tissues, targeted approaches were employed to examine the role of representative members of each major class of epigenetic enzymes (Table 1) in the process of MMP-2 modulation and FN fragmentation in T. denticola-challenged PDL cells.
Table 1.
Chemical agents used to target chromatin modification enzymes.
| Compound | Target enzyme | Concentrations(μM) | Treatment period | Source | References | |
|---|---|---|---|---|---|---|
| Low | High | |||||
| Inhibition of DNA methyltransferases (DNMT) | ||||||
| 5-Azacytidine |
|
1 | 5 | 4 days | * | (Chernov et al., 2009, Zhang et al., 2011, Hassler et al., 2012) |
| (−)-Epigallocatechin gallate (EGCG) |
|
5 | 10 | 2 days | * | (Achour et al., 2013, Saldanha et al., 2014) |
| Inhibition of Histone Deacetylases (HDACs) | ||||||
| Trichostatin A |
|
0.05 | 0.1 | 4 days | * | (Mogal et al., 2006, Chang et al., 2012) |
| Apicidin |
|
0.5 | 1 | 1 day | * | (Ahn et al., 2012, Bauden et al., 2015) |
| Inhibition of Histone phosphorylation | ||||||
| PF-03814735 |
|
0.25 | 0.5 | 2 days | * | (Hook et al., 2012) |
| Inhibition of Histone Acetyltransferases (HATs) | ||||||
| Curcumin |
|
25 | 50 | 2 hours | * | (Balasubr amanyam et al., 2004, Ahn et al., 2012) |
| Inhibition of Histone Methyltransferases (HMTs) | ||||||
| BIX 01294 |
|
0.5 | 1 | 2 days | * | (Kubicek et al., 2007) |
| Inhibition of Histone Demethylases (HDMs) | ||||||
| Tranylcypromine, HCl (TCP) |
|
2 | 5 | 4 days | # | (Nebbioso et al., 2012) |
Sigma-Aldrich, St. Louis, MO, USA
EMD Millipore, Temecula, CA, USA
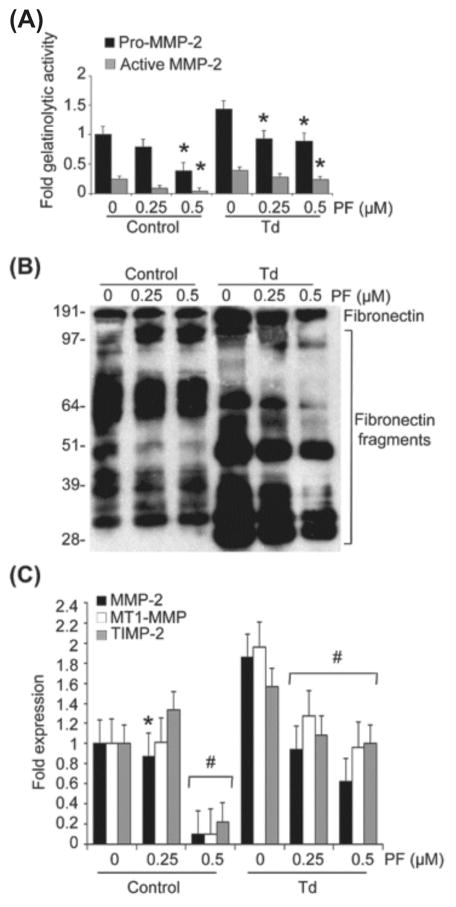
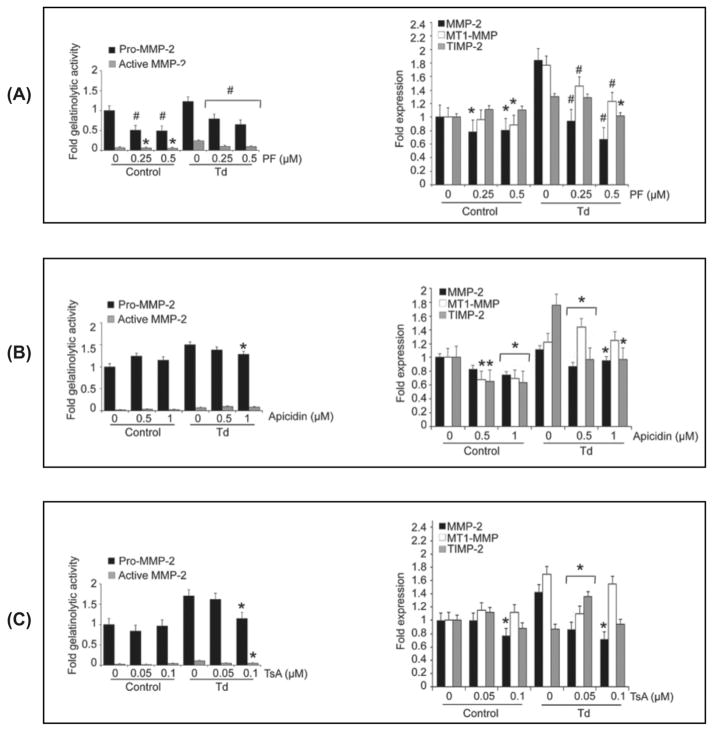
Pretreatment of PDL cells with the histone kinase/phosphorylation inhibitor, PF-03814735, inhibited the potential increase in MMP-2 activation and expression mediated by T. denticola (Fig. 4A to C). Pretreatment with PF-03814735 also prevented the transcriptional increase in the MMP-2 activator complex, MT1-MMP/TIMP-2, and FN fragmentation mediated by the T. denticola challenge. This aurora kinase inhibitor exhibited a dose-response effect in terms of preventing the changes in MMP-2 expression and activation. At the highest dose tested, PF-03814735 also suppressed MMP-2 expression and activation in control cells. All enzyme inhibitor concentrations were selected in ranges that did not alter proliferation or induce cytotoxicity in PDL cells (data not shown).
Figure 4. Inhibition of histone phosphorylation using PF-03814735 results in decreased expression and activation of MMP-2, MT1-MMP and TIMP-2, and decreased fibronectin fragmentation in T. denticola-challenged and control PDL cells.
Cultured PDL cells were pre-treated with 0.25 or 0.5 μM PF-03814735 (PF) for two days. The cells were then challenged with T. denticola (Td) at MOI = 100 or media control for two hours then incubated for three days. The conditioned medium and cell lysates were collected for zymography, western blotting, and qRT-PCR. The experiments were repeated three times in triplicate. Data were analyzed using one-way ANOVA. (*) represents p ≤ 0.05 compared to the “0” concentration in the same group. (#) represents p ≤ 0.001 compared to the “0” concentration in the same group.
Panel A: Densitometric analysis of pro-MMP-2 (72kDa) and active MMP-2 (64kDa) detected by gelatin zymography. The X-axis represents different concentrations of histone kinase/phosphorylation inhibitor, PF-03814735 (PF) in the control and Td groups. The Y-axis represents fold-gelatinolytic activity of the pro-MMP-2 and active MMP-2 relative to unchallenged and untreated controls.
Panel B: A representative immunoblot of PDL cell culture supernatants probed with a polyclonal anti-fibronectin antibody showing FN fragmentation in conditioned medium from Td-challenged PDL cells, control and PF-treated.
Panel C: Bar chart showing transcript levels of MMP-2, MT1-MMP and TIMP-2 in the control and PF-treated PDL cells as determined by qRT-PCR. The Y-axis represents fold-expression level of each gene relative to unchallenged and untreated controls. The X-axis represents different concentrations of PF in the control and Td groups.
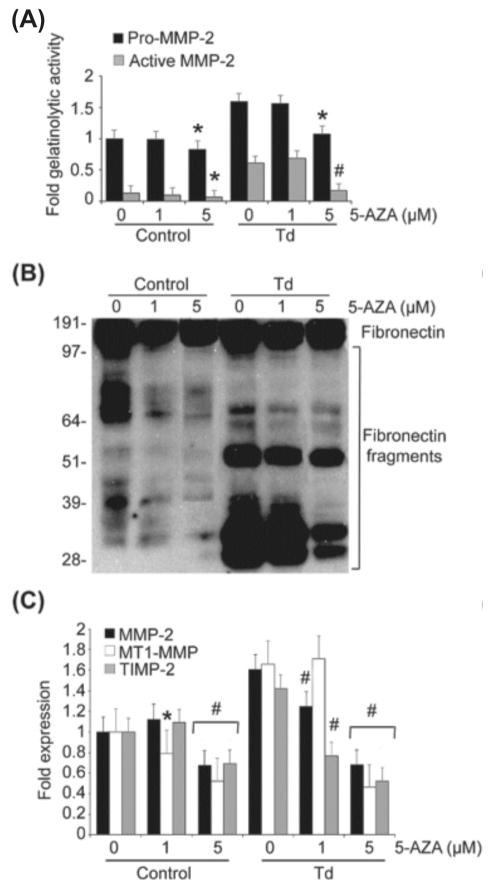
Prevention of the potential increase in MMP-2 activation and expression mediated by T. denticola was also achieved by pretreatment of PDL cells with Azacytidine (AZA), a DNMT inhibitor (Fig. 5A to C). AZA had a dose-response effect, inhibiting increases in MMP-2 expression and activation, MT1-MMP/TIMP-2 expression, and fibronectin fragmentation mediated by the T. denticola challenge. In contrast, epigallocatechin gallate (EGCG), a different DNMT inhibitor with a broad spectrum of activity that includes inhibition of HATs, had considerably less effect than AZA. At the highest concentration tested, EGCG had only minor effects on T. denticola-mediated MMP-2 activation and TIMP-2 expression, but this did not result in modulation of FN fragmentation (Fig. 6A to C).
Figure 5. Inhibition of DNA methyltransferases using AZAcytidine (AZA) result in decreased expression and activation of MMP-2, MT1-MMP and TIMP-2, and decreased fibronectin fragmentation in T. denticola-challenged and control PDL cells.
Cultured PDL cells were pre-treated with 1 or 5 μM AZA for four days. The cells were then challenged with T. denticola (Td) at MOI = 100 or media control for two hours then incubated for three days. The conditioned medium and cell lysates were collected for zymography, western blotting, and qRT-PCR. The experiments were repeated three times in triplicate.
Panel A: Densitometric analysis of pro-MMP-2 (72kDa) and active MMP-2 (64kDa) detected by gelatin zymography. The X-axis represents different concentrations of DNA methyltransferases inhibitor Azacytidine (AZA) in the control and Td groups. The Y-axis represents fold-gelatinolytic activity of the pro-MMP-2 and active MMP-2 relative to unchallenged and untreated controls. Data were analyzed using one-way ANOVA. (*) represents p ≤ 0.05 compared to the “0” concentration in the same group. (#) represents p ≤ 0.001 compared to the “0” concentration in the same group.
Panel B: A representative immunoblot of PDL cell culture supernatants probed a polyclonal anti-fibronectin antibody showing FN fragmentation in conditioned medium from Td-challenged PDL cells, control and AZA-treated.
Panel C: Bar chart showing transcript levels of MMP-2, MT1-MMP and TIMP-2 in the control and AZA-treated PDL cells as determined by qRT-PCR. The Y-axis represents fold-expression level of each gene relative to unchallenged and untreated controls. The X-axis represents different concentrations of AZA in the control and Td groups.
Figure 6. Inhibition of DNA methyltransferases using epigallocatechin gallate (EGCG) mediate a decrease in MMP-2 activation, an increase in TIMP-2 transcription and minimal decrease in fibronectin fragmentation in Td-challenged PDL cells.
Cultured PDL cells were pre-treated with 5 or 10 μM EGCG for two days before being challenged with T. denticola (Td) at MOI = 100 or media control for two hours, then incubated for three days. The conditioned medium and cell lysates were collected for zymography, western blotting, and qRT-PCR. The experiments were repeated three times in triplicate. Data were analyzed using one-way ANOVA. (*) represents p ≤ 0.05 compared to the “0” concentration in the same group. (#) represents p ≤ 0.001 compared to the “0” concentration in the same group.
Panel A: Densitometric analysis of pro-MMP-2 (72kDa) and active MMP-2 (64kDa) detected by gelatin zymography. The left 3 lanes represent the control group (unchallenged PDL cells) and the right 3 lanes represent the Td group (Td-challenged PDL cells). The cells in both groups were treated with indicated concentrations of EGCG. The X-axis represents different concentrations of EGCG in the control and Td groups. The Y-axis represents fold-gelatinolytic activity of pro-MMP-2 and active MMP-2 relative to unchallenged and untreated controls.
Panel B: A representative immunoblot of PDL cell culture supernatants probed with a polyclonal anti-fibronectin antibody showing FN fragmentation in conditioned medium from Td-challenged PDL cells, control and EGCG-treated.
Panel C: Bar chart showing transcript levels of MMP-2, MT1-MMP and TIMP-2 in the control and ECGC-treated PDL cells as determined by qRT-PCR. The Y-axis represents fold-expression level of each gene relative to unchallenged and untreated controls. The X-axis represents different concentrations of ECGC in the control and Td groups.
Examination of HDAC inhibitors Apicidin and Trichostatin revealed that pretreatment with these two inhibitors at the highest doses tested (1 μM and 0.1 μM, respectively) also inhibited the T. denticola-mediated increase in MMP-2 expression and activation as well as that of its activator complex, MT1-MMP/TIMP-2. However, as with EGCG, neither of these inhibitors affected FN fragmentation (data not shown).
HMT, HDM and HAT inhibitors exacerbate T. denticola-mediated increases in MMP-2 expression and activity in PDL cells
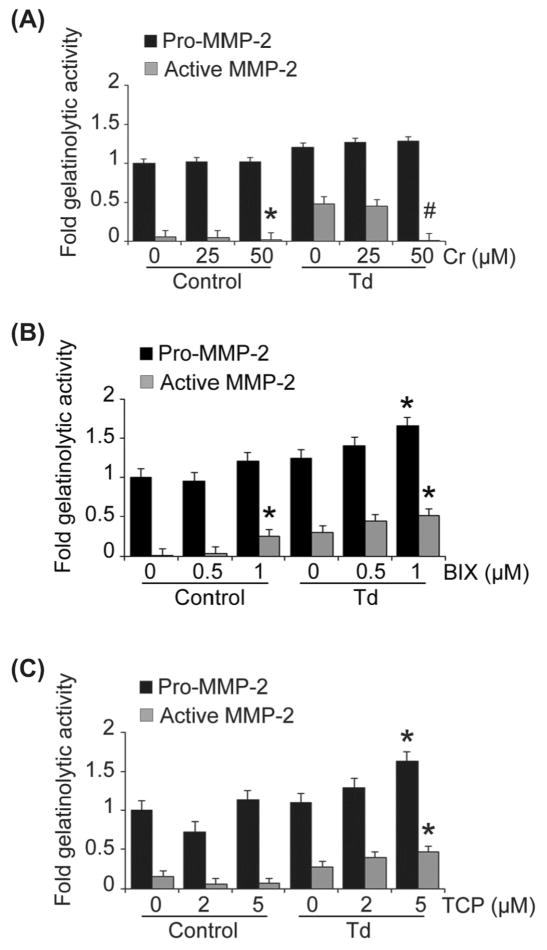
Inhibitors of histone demethylases (HDM), histone methyltransferases (HMT), and histone acetyltransferases (HAT) were not effective in preventing the T. denticola-mediated increases in pro-MMP-2 expression and activation. Treatment with Curcumin, a HAT inhibitor, resulted in moderately increased expression of pro-MMP-2, but significantly decreased its MMP-2 activation in a dose-dependent manner (Fig. 7A). In contrast, the HDM inhibitor, Tranylcypromine, HCl (TCP) and the HMT inhibitor, BIX 01295/trihydrochloride hydrate further augmented the T. denticola-mediated increases in MMP-2 expression and activation in PDL cells (Fig. 7B, C).
Figure 7. Inhibition of different chromatin modification enzymes mediate alterations in levels of expression and activation of MMP-2 in Td-challenged and control PDL cell cultures.
PDL cells were pre-treated with enzyme inhibitors at the indicated concentrations as follows: curcumin (histone acetyltransferase inhibitor) for two hours (A), 0.5 μM and 1 μM BIX-01294 (histone methyltransferase inhibitor) for two days (B), and 2 μM and 5 μM tranylcypromine/TCP (histone demethylase inhibitor) for four days (C). The cells were then challenged with T. denticola (Td) at MOI = 100 or media control for two hours, then incubated for three days. The conditioned medium and cell lysates were collected for zymography. Shown are densitometric analyses of pro-MMP-2 (72kDa) and active MMP-2 (64kDa) detected by gelatin zymography. The X-axis represents different concentrations of enzyme inhibitors in the control and Td groups. The Y-axis represents fold-gelatinolytic activity of the pro-MMP-2 and active MMP-2 relative to unchallenged and untreated controls. Panels: A, curcurmin (Cr); B, BIX-01294 (BIX); C, tranylcypromine (TCP). The experiments were repeated three times in triplicate. Data were analyzed using one-way ANOVA. (*) represents p ≤ 0.05 compared to the “0” concentration in the same group. (#) represents p ≤ 0.001 compared to the “0” concentration in the same group.
Inhibitors of histone phosphorylation and histone deacetylase reverse T. denticola-mediated increases in MMP-2 activity and expression in PDL cells
An important remaining question was whether existing epigenetic modifications on periodontal ligament cells mediated by T. denticola could be reversed in a post-treatment scenario. To this end, periodontal ligament cells were first challenged with T. denticola then the same enzyme inhibitors tested above were employed to address this question. In agreement with the pretreatment results, post-treatment of T. denticola challenged PDL cells with three different epigenetic enzyme inhibitors reversed the effects on MMP-2 expression and activation: the histone kinase inhibitor, PF-03814735 (Fig. 8A) and the HDAC inhibitors apicidin (Fig. 8B) and trichostatin (Fig. 8C).
Figure 8. Inhibitors of histone phosphorylases and deacetylases reverse T. denticola-mediated increases in MMP-2 activity and expression in PDL cells.
Cultured PDL cells were challenged with T. denticola (Td) at MOI = 100 or media control for two hours, incubated for three days in serum- and antibiotic-free MEM-α with daily culture medium refreshment, then treated with the following enzyme inhibitors: (Panel A) 0.25 or 0.5 μM PF-03814735 (PF) for two days; (Panel B) 0.5 or 1 μM apicidin for one day; (Panel C) 0.05 or 0.1 μM trichostatin A (TsA) for four days. The conditioned medium and cell lysates were collected for zymography, western blotting, and qRT-PCR. The left portion of each panel shows densitometric analysis of pro-MMP-2 (72kDa) and active MMP-2 (64kDa) detected by gelatin zymography. The X-axis represents different concentrations of the indicated inhibitor in the control and Td-challenged cultures. The Y-axis represents fold-gelatinolytic activity of the pro-MMP-2 and active MMP-2 relative to unchallenged and untreated controls. The right panel shows transcript levels of MMP-2, MT1-MMP and TIMP-2 in the control and inhibitor-treated PDL cells as determined by qRT-PCR. The Y-axis represents fold-expression level of each gene relative to unchallenged and untreated controls. The X-axis represents different concentrations of PF in the control and Td groups. The experiments were repeated three times in triplicate. Data were analyzed using one-way ANOVA. (*) represents p ≤ 0.05 compared to the “0” concentration in the same group. (#) represents p ≤ 0.001 compared to the “0” concentration in the same group.
It is important to note that the results of the pre- and post-challenge inhibition experiments should not be compared in parallel because of different sample collection times used. In the pre-treatment experiments, samples were collected after 3 days of a T. denticola (2-hour exposure) challenge, whereas in the post-challenge treatment experiments, the samples were collected 2 hours to 4 days after a T. denticola (2 hour exposure) challenge, depending on the inhibitor used. Similarly, in contrast to the pre-challenge enzyme inhibition experiments, no decrease in FN fragmentation was observed in post-challenge inhibition experiments (data not shown), presumably because the MMP-2-mediated fragmentation process had begun before addition of enzyme inhibitors.
Discussion
ECM destruction, a key event in the pathogenesis of periodontitis, is mediated by host-derived enzymes such as MMP-2 that are involved in ECM homeostasis and remodeling. Strong evidence has accumulated over the past two decades that bacterial components, including proteases and lipopolysaccharides, are key factors contributing to dysregulation of ECM homeostasis. It is of particular interest that activation of pro-MMP-2, which is constitutively expressed in PDL cells, is required for the fragmentation of cellular FN that is typically seen in periodontal disease (Miao et al., 2011). Thus, identifying factors that control MMP-2 expression or activation can help us better understand and modulate periodontal disease pathogenesis.
T. denticola is a member of a very complex microbiota involved in the pathogenesis of periodontal diseases. MMP-2 production and activation is a major event in the pathogenesis of these diseases via its role in extracellular matrix destruction. The T. denticola dentilisin protease plays an important role in the up-regulation and activation of MMP-2 in PDL cells (Miao et al., 2011, Miao et al., 2014), thereby promoting further ECM destruction and release of fibronectin fragments, which have deleterious effects on the periodontal environment (Kapila et al., 1996, Kapila et al., 1999, Kapila et al., 2002, Jee et al., 2004, Dai et al., 2005, Tafolla et al., 2005, Ghosh et al., 2008, Joo et al., 2008, Joseph et al., 2010, Miao et al., 2011, Miao et al., 2014, Pereira et al., 2014). The oral spirochete T. lecithinolyticum is also reported to activate MMP-2, as are P. gingivalis and A. actinomycetemcomitans (Choi et al., 2001, Chang et al., 2002, Song et al., 2003). The molecular mechanism(s) involved remain unstudied or unresolved for these species.
T. denticola dentilisin activity was present for several days in PDL cell conditioned medium, long after the brief (2 hour) challenge, washing and multiple changes of growth medium. This is consistent with our prior reports (Miao et al., 2011, Miao et al., 2014) and is likely due to persistent adherence of T. denticola to PDL cells, though we cannot yet rule out potential downstream effects of previously documented uptake of T. denticola by PDL cells (Miao et al., 2014) and gingival epithelial cells (Shin et al., 2012, Jo et al., 2014, Inagaki et al., 2016). Importantly, while dentilisin activity levels decreased steadily over time, MMP-2 and its activating complex, MT1-MMP and TIMP-2, were clearly expressed and activated through the 12th day of culture. These data confirm our previous results that dentilisin is an important factor in the activation of pro-MMP-2 (Miao et al., 2011). Additionally, in agreement with our earlier findings (Miao et al., 2014), the current data show that T. denticola chronically activates MMP-2, in concert with MT1-MMP and TIMP-2 expression up to 12 days. Importantly, degradation of cellular FN in PDL cell cultures is dependent on MMP-2 activation, which is the result of T. denticola dentilisin activity (Miao et al., 2011). The persistent fibronectin fragmentation in these samples may help explain the chronicity of tissue destruction mediated by bacterial proteases during periodontal disease pathogenesis.
Previous studies showed that T. denticola has the ability to adhere to and be internalized by several different host cells, including gingival epithelial cells, PDL cells, and polymorphonulear leukocytes (Ding et al., 1997, Konermann et al., 2012, Shin et al., 2012, Miao et al., 2014). It should be noted that, unlike other oral pathogens, such as Porphyromonas gingivalis which exhibits a cellular-invasive phenotype (Lamont et al., 1995), there is no reported evidence that T. denticola survives in the intracellular environment. Taken in aggregate, these results and the findings from this study, support the concept that the alteration of the PDL cells towards a destructive phenotype is a consequence of exposure to T. denticola. These data are consistent with evidence from other inflammatory diseases pointing toward an epigenetic role in the pathogenic process. We hypothesized that epigenetic mechanisms may imprint the periodontium and set in motion the process of chronic periodontal tissue destruction.
Mechanisms of epigenetic modifications include, histone acetylation, histone methylation, DNA methylation, positioning of histone variants, and gene regulation by non-coding micro RNAs (miRNAs) (Bayarsaihan, 2016, Herceg, 2016, Perkins et al., 2016). Several enzymes are involved in these mechanisms including; histone acetyltransferases (HATs), histone deacetylases (HDACs), histone methyltransferases (HMTs), histone demethylases (HDMs), histone phosphorylases, and DNA methyltransferases (DNMTs).
Perturbing the balance between acetylation/deacetylation or methylation/demethylation is profoundly associated with numerous diseases including developmental abnormalities, cancer and chronic inflammatory conditions (Bird, 2002, Barros et al., 2009). Epigenetic modifications are potentially reversible; therefore, a thorough understanding of these changes may identify new therapeutic targets for disease management. Using gene arrays that target chromatin modification enzymes, our data provides the first evidence that T. denticola mediates downregulation of many of these enzymes in PDL cells. In similar studies, P. gingivalis lipopolysaccharides were shown to downregulate many chromatin modification enzymes in cultured oral keratinocytes (de Camargo Pereira et al., 2013) and change the expression of multiple miRNAs in PDL cells (Du et al., 2016). However, since T. denticola-dependent MMP-2 activation requires dentilisin proteolytic activity (Miao et al., 2011), it is likely that the molecular mechanisms responsible for this downregulation differ from those driven by P. gingivalis lipopolysaccharides. Specific mechanisms by which T. denticola or its dentilisin protease may regulate epigenetic enzyme expression are under study in our laboratories.
The association between T. denticola and periodontitis has been reported in numerous studies (Simonson et al., 1988, Sakamoto et al., 2001, Asai et al., 2002, Yoshida et al., 2004). Consistent with the existing literature, we found that T. denticola levels were significantly elevated in human tissue specimens from periodontally diseased sites compared with healthy sites, as measured by qPCR normalized to human GAPDH (data not shown). To date, studies of the role of epigenetic modifications in periodontal disease have focused on DNA methylation patterns in genes involved in inflammatory responses (Barros et al., 2014). The present study used gene array approaches to examine healthy and diseased periodontal tissues, revealing for the first time significant alterations in the expression of several chromatin modification enzymes in diseased tissues. Although expression levels of some enzymes (such as Aurora Kinase B) differed in the gene array of the PDL cells compared to the tissues, this likely reflects the fact that tissues exhibit the net expression of diverse cell types that comprise the periodontium, as well as the net effects of the diverse oral microbiome on the periodontium, and net effects of other epigenetic effectors, including smoking and medications.
Different inhibitors show promise in reversing epigenetic changes in the context of our study and in other reports. Chronic T. denticola-mediated MMP-2 expression and activation were decreased in T. denticola challenged PDL cells either pre- or post-treated with inhibitors of histone phosphorylases, histone deacetylases (HDACs), and DNA methyltransferases (DNMTs). This indicates that T. denticola induces epigenetic changes mediated by histone phosphorylation, histone deacetylation, or DNA methylation pathways. Specifically, pre- or post-treatment inhibition of histone phosphorylation in PDL cells using PF-03814735 significantly prevented or reversed the T. denticola-mediated increase in MMP-2 and the MT1-MMP/TIMP-2 complex. This study is the first to show the effect of inhibition of histone phosphorylation on MMP-2 expression in PDL cells. In a broader context, PF has been used in phase I clinical trials for the treatment of advanced solid tumors (Jani et al., 2010, Schoffski et al., 2011).
Inhibition of HDACs using Apicidin and/or Trichostatin was also effective in preventing or reversing the T. denticola-mediated effects on the expression of MMP-2 in PDL cells. Similar data were obtained by other studies investigating the effect of Trichostatin on MMP-2 expression in murine fibroblasts (Ailenberg et al., 2002). Trichostatin was further shown to decrease MMP-2 and MMP-9 expression in murine heart cells (Mani et al., 2015) and in human esophageal squamous cell carcinoma cells (Wang et al., 2013). Trichostatin, in combination with SAHA, was reported to inhibit the respiratory syncytial virus (RSV)-mediated increase in HDAC2 expression with resultant decrease in airway inflammation and oxidative stress in vivo (Feng et al., 2016). Apicidin was also reported to inhibit the expression of MMP-2 in different cancer cells (Kim et al., 2000, Park et al., 2011, Ahn et al., 2012). On the other hand, inhibition of DNMTs using AZA showed a reduction in the expression of MMP-2 and its activating complex, while the use EGCG was not effective in this process.
Our results are consistent with those in other studies reporting that AZA causes a down-regulation of MMP-2 and MMP-9 expression in cultured esophageal squamous cell carcinoma cells (Liu et al., 2014) and breast cancer cells (Chang et al., 2014b) respectively. AZA was approved by the U. S. Food and Drug Administration (FDA) for treatment of myelodysplastic syndromes (Nebbioso et al., 2012). It was also investigated in many clinical trials for treatment of several disorders, including hematological and neoplastic disorders (Liu et al., 2005, Mirza et al., 2010, Chen et al., 2012). Regarding the effect of EGCG on MMP-2 expression in our study, the data are inconsistent with other studies, which reported the ability of EGCG to inhibit MMP-2 in different cancer cells (Chang et al., 2014a, Nowakowska et al., 2016). This inconsistency may be due to the use of different cell types, different concentrations of EGCG, or both. Concentrations higher than 10 μM were toxic to PDL cells as examined by cell proliferation and cytotoxicity assays (data not shown). Due to the weak effect of EGCG on the expression of MMP-2, we did not test its effects in a post-challenge scenario.
Negative consequences of using epigenetic enzyme inhibitors are possible, as seen in the current study. Specifically, some inhibitors, namely those inhibiting HDM with TCP and those inhibiting HMT with BIX 01295, further increased MMP-2 expression and activation. Although TCP and BIX are thought to mediate opposite actions, namely BIX inhibits histone methylation and TCP promotes histone methylation, both increased MMP-2 expression and activation. Thus, due to these undesirable effects, BIX and TCP were not evaluated further in post-challenge scenarios. There are no previous studies investigating their effect on expression of MMPs. However, Pereira et al. showed that increases in MMP-2 expression were stimulated by decreases in histone methylation in the context of the MMP-2 promoter (Pereira et al., 2014). Additionally, in another study, the MMP-1, 3, 9, and 13 genes were shown to be actively transcribed in rheumatoid arthritis-derived synovial fibroblasts, and this transcription correlated with an elevation in H3K4me3 and suppression of H3K27me3 in the MMP promoter genes (Araki et al., 2016). These studies, which both showed increases in MMP-2 expression despite different degrees of histone methylation, can be explained by the fact that activation or repression of gene expression by histone modifications/methylations depends on the type of lysine being modified and the degree of its methylation. For example, H3K4me, H3K36me, or H3K79me are associated with transcriptional activation (Jenuwein et al., 2001, Zhang et al., 2001, Barski et al., 2007, Guenther et al., 2007, Koch et al., 2007, Kouzarides, 2007), whereas H3K9me, H3K27me, or H4K20me are implicated in gene repression (Jenuwein et al., 2001, Nakayama et al., 2001, Talbert et al., 2006, Barski et al., 2007, Kouzarides, 2007).
The HAT inhibitor curcumin also increased the expression of MMP-2 but significantly inhibited its activation. The effect of curcumin in decreasing MMP-2 and MMP-9 expression levels was reported in cancer cells, such as squamous cell carcinoma and osteoclastoma (Cao et al., 2015, Lee et al., 2015). Additionally, in vivo studies in humans and animals demonstrated curcumin’s effectiveness in decreasing the severity of periodontal diseases (Elburki et al., 2014, Nagasri et al., 2015, Bakir et al., 2016, Elburki et al., 2016). Curcumin has been investigated in many clinical trials for treatment of several disorders, such as ulcerative colitis (Baliga et al., 2012), breast cancer (Nagaraju et al., 2012), pancreatic cancer (Veeraraghavan et al., 2011), and diabetes (Abdel Aziz et al., 2012). Additional investigation into the role of curcumin in MMP activation and regulation in periodontal diseases is further warranted, given these reports and the current study findings.
In summary, T. denticola plays a key role in the transcriptional regulation of MMP-2 and its activating complex MT1-MMP/TIMP-2 in PDL cells. T. denticola also mediates alterations of chromatin modification enzyme expresssion in PDL cells, and an array of epigenetic modifications are associated with periodontally diseased tissues. Furthermore, inhibition of enzymes that mediate epigenetic modifications can prevent T. denticola-mediated increases in MMP-2, MT1-MMP, and TIMP-2 in PDL cells. These inhibitors can also reverse the T. denticola-mediated effects on MMP-2 and its activating complex in PDL cells. These data indicate that T. denticola mediates its effects on MMP-2 activation through epigenetic modifications in these cells. This knowledge can be useful as a first step toward the development of novel targeted therapeutics for the treatment of periodontal diseases.
Materials and Methods
Periodontal ligament (PDL) cell culture
As described previously, the primary culture of PDL cells was obtained via the direct cell outgrowth method by isolating cells from the PDL tissue around the middle third of extracted healthy human teeth (Scanlon et al., 2011, Tanaka et al., 2011). Cells were maintained in minimal essential medium-α (MEM-α) augmented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S), and 1% amphotericin B (Gibco, Grand Island, NY, USA) in a humid atmosphere with 95% air and 5% CO2 at 37°C. Cell outgrowths were passaged when they reached confluency. Cells passaged three to six times were used for experimentation. The cell counting kit-8 (Dojindo, Rockville, MD, USA) was used for assaying PDL cell proliferation and cytotoxicity at different time intervals in response to different concentrations of the inhibitors used in the study (data not shown). Protocols involving the collection and use of human teeth and PDL cells/tissue were approved by the Health Sciences Institutional Review Board of the University of Michigan.
Human periodontal tissues
Periodontal tissues were obtained by collecting the tissues around the coronal third of the roots of extracted periodontally-involved teeth and healthy teeth, both from the posterior mandibular region. Periodontal status was diagnosed clinically by testing for bleeding on probing of the gingival sulcus and periodontal pocket depth measurements. The samples were collected from six different periodontitis patients and six healthy subjects. The collection and use of human teeth and PDL tissues was approved by the Health Sciences Institutional Review Board of the University of Michigan.
Culture of Treponema denticola
Treponema denticola ATCC 35405 was grown as described previously under anaerobic conditions at 37°C in New Oral Spirochete (NOS) broth medium (Haapasalo et al., 1991, Fenno, 2005). Purity of spirochete cultures was confirmed by darkfield microscopy prior to use in experiments.
Challenge of PDL cells with Treponema denticola
PDL cells were prepared in MEM-α free of serum and antibiotics. The bacteria in broth culture were collected by centrifugation, then re-suspended in serum- and antibiotic-free MEM-α to an optical density of 0.1 at 600 nm, such that the cellular density was approximately 2.4×108 cells/ml.
T. denticola in serum-and antibiotic-free MEM-α was added to the test group of PDL cells (T. denticola group) at a multiplicity of infection (MOI) = 100, whereas only MEM-α was added to the control group. Both groups were then incubated for two hours at 37°C in 5% CO2-containing air. After the two-hour challenge, PDL cells were washed three times with PBS and incubated for the planned time periods in serum-and antibiotic-free MEM-α with daily medium changes as described previously (Miao et al., 2011). Subsequently, the culture conditioned medium and cell lysates were collected, RNA was extracted from the cell lysates, and all samples were stored at −80°C for further investigations.
Pre-challenge inhibition of epigenetic chromatin modification enzymes in PDL cells
Different inhibitors of epigenetic chromatin modification enzymes (listed in Table 1) were obtained and prepared according to the manufacturer’s instructions, then brought to the desired concentrations via dilution in serum- and antibiotic-free MEM-α. With the exception of tranylcypromine (EMD Millipore, Temecula, CA, USA), all enzyme inhibitors were purchased from Sigma Aldrich (St. Louis, MO, USA).
PDL cells were treated with the indicated concentrations of the enzyme inhibitors either as single agents or in combinations, and incubated for the indicated times with daily culture medium replacement. The cells were then challenged with T. denticola at an MOI of 1:100 for 2 hours, washed, and incubated for three days in serum- and antibiotic-free MEM-α with daily culture medium refreshment. At the end of this incubation period, conditioned culture medium and cell lysates were harvested and stored at −80°C for further investigations.
Post-challenge inhibition of epigenetic chromatin modification enzymes in PDL cells
For the post-challenge experiments, the PDL cells were first challenged with T. denticola as described before, then treated with the indicated concentrations of the inhibitors either as single agents or in combinations for certain periods. At the end of the treatment period, the conditioned culture medium and cell lysates were harvested and stored at −80°C for further investigations. All experiments were repeated at least three times and each experiment was performed in triplicate.
Gelatin zymography
Culture supernatants were concentrated approximately 10-fold in Amicon centrifugal concentrators (10,000-molecular-weight cutoff; Millipore) and total protein concentration was measured using the BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) normalized to an albumin standard. Equivalent protein concentrations from each sample were mixed with non-reducing sample buffer (0.25 M tris-base, 40% glycerol, 0.8% SDS, and 0.05% bromophenol blue stain in distilled deionized water/ddH2O at pH 6.8) and loaded into 8% polyacrylamide gels co-polymerized with 0.4% SDS and 0.2% gelatin. Samples were electrophoretically resolved on gelatin-containing gels at 125 V for 110 minutes at 4°C. Gels were then washed twice for 15 minutes under continuous agitation using renaturation/washing buffer (2.5% v/v Triton-X100 and 0.05 M Tris-base in ddH2O at pH 7.5) to eliminate SDS and promote the renaturation of MMP enzymes. Subsequently, gels were incubated in developing/incubation buffer (0.05 M Tris-base, 0.15 M sodium chloride, 0.01 M calcium chloride, and 0.02% sodium azide in ddH2O at pH 7.5) for 30 minutes under agitation, then the buffer was replaced and gels incubated for 16–20 hours at 37°C. After that, gels were stained using filtered Coomassie Brilliant blue stain for one or two hours under agitation. Destaining of the gels was performed using a methanol/acetic acid destaining buffer (40% methanol and 10% acetic acid in ddH2O) until the bands on the gel appeared clear. Zymograms were scanned and the densitometry of the gelatinolytic activity represented by the clear bands was analyzed using ImageJ software (NIH, USA). Brightness and contrast levels of zymogram images were slightly adjusted for publication only.
Immunoblotting
Equivalent protein samples consisting of 10 fold concentrates of PDL cell culture conditioned media were standardized as described above, subjected to standard SDS-PAGE (4–12% polyacrylamide gels; Invitrogen, Carlsbad, CA, USA) and transferred to PVDF membranes using standard techniques. The membranes were exposed to a rabbit polyclonal anti-fibronectin IgG (Santa Cruz, Dallas, TX, USA) primary antibody diluted 1:2000 in TBST solution for 2 hours at room temperature followed by a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz, Dallas, TX, USA) diluted 1:10,000 in TBST for 1 hour at room temperature with agitation. Western blots using anti-GAPDH antibodies were used to further confirm equal protein loading (data not shown). Blots were developed using the SuperSignal® West Pico kit (ThermoFischer Scientific, Pittsburgh, PA, USA) and scanned for digitization.
Quantitative reverse transcriptase PCR (qRT-PCR)
qRT-PCR was performed to assess the transcriptional levels of MMP-2, MT1-MMP, and TIMP-2 in PDL cells. Cell lysates were collected and the RNA was extracted and purified using the RNeasy® mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Reverse transcription of the RNA into cDNA was then performed using the SuperScript™ II RT kit (Invitrogen, Carlsbad, CA, USA). The cDNA was then amplified by qPCR using the TaqMan® Universal PCR Master Mix (Invitrogen, Carlsbad, CA, USA)” on a ViiA™ 7 Applied Biosystems® PCR system. The following TaqMan® human probes were used; MMP-2 (Hs01548727_m1), MT1-MMP (Hs01037003_g1), TIMP-2 (Hs00234278_m1), and GAPDH (Hs03929097_g1) (Invitrogen, Carlsbad, CA, USA). The cycle threshold (Ct) values were obtained, analyzed and the quantitative expression of target genes in challenged PDL cells was normalized to GAPDH and compared to the control cells using the 2−ΔΔCT method (Livak et al., 2001), applying a minimum 2 fold change in expression as the cut off.
Similar methodology was utilized to assess the levels of MMP-2 transcription and the levels of T. denticola in human periodontal tissue samples. Periodontal tissue specimens were collected by scraping the most coronal portion of the tissue around the roots of extracted healthy and periodontally-diseased teeth. Total RNA was extracted from healthy and diseased periodontal tissue specimens and cDNA was generated. Target genes were amplified using TaqMan® Universal PCR Master Mix (Invitrogen, Carlsbad, CA, USA). Tissue sample human genome content was normalized using a custom TaqMan® primer/probe set for GAPDH (Hs03929097_g1; Invitrogen, Carlsbad, CA, USA). T. denticola 16S rRNA was amplified in parallel using the SYBR® Green PCR Master Mix (Invitrogen, Carlsbad, CA, USA) with the following primer set: 16SrRNA-987F AGGGATATGGCAGCGTAGCA and 16SrRNA-1077R TTGCGGGACTTAACCCAACA.
Quantitative reverse transcription (qRT-PCR) gene micro-array
qRT-PCR arrays were used to assess the transcriptional levels of the main epigenetic chromatin modification enzymes in cultured PDL cells and in periodontal tissues using the RT2 Profiler PCR Array kit (Qiagen, Valencia, CA, USA) to assay transcription of the genes listed in Table S1. Periodontal tissue specimens were collected by scrapping the most coronal portion of the tissue around the roots of extracted healthy and periodontally-diseased teeth. RNA was extracted and purified from PDL cell cultures using the RNeasy® Mini kit, while extraction and purification of RNA from periodontal tissues was achieved using the RNeasy® Protect Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Reverse transcription of RNA into cDNA was performed using the RT2 First Strand Kit (Qiagen, Valencia, CA, USA). The target genes were then amplified using the RT2 Profiler PCR Array kit and RT2 SYBR® Green Master mix (Qiagen, Valencia, CA, USA) on a ViiA™ 7 Applied Biosystems® PCR system. Applying a minimum 2 fold change in expression as the cut off, Ct values were analyzed and the quantitative expression of the genes of interest in T. denticola-challenged PDL cells was normalized to supplied housekeeping genes; β-actin, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-2-microglobulin, Hypoxanthine phosphoribosyltransferase-1, and large ribosomal protein-P0 and then compared to expression levels in unchallenged PDL cells and the healthy tissues (Livak et al., 2001).
Statistical analysis
The data were analyzed using the statistical software SPSS® v.22 (IBM, Armonk, NY, USA). Results were evaluated by a one-way ANOVA when comparing more than two groups, whereas the student’s t-test was used when comparing two groups. p ≤ 0.05 was considered significant, whereas p ≤ 0.001 was considered highly significant.
Supplementary Material
Acknowledgments
These studies were supported by an NIH grant (RO1 DE025225) to Yvonne L. Kapila and J. Christopher Fenno and a Fellowship from the Egyptian Ministry of Higher Education to Islam Ateia. We thank Hongriu Liu for technical assistance. The authors declare no conflict of interest.
References
- Abdel Aziz MT, El-Asmar MF, El-Ibrashy IN, Rezq AM, Al-Malki AL, Wassef MA, et al. Effect of novel water soluble curcumin derivative on experimental type- 1 diabetes mellitus (short term study) Diabetol Metab Syndr. 2012;4:30. doi: 10.1186/1758-5996-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achour M, Mousli M, Alhosin M, Ibrahim A, Peluso J, Muller CD, et al. Epigallocatechin-3-gallate up-regulates tumor suppressor gene expression via a reactive oxygen species-dependent down-regulation of UHRF1. Biochem Biophys Res Commun. 2013;430:208–212. doi: 10.1016/j.bbrc.2012.11.087. [DOI] [PubMed] [Google Scholar]
- Ahn MY, Kang DO, Na YJ, Yoon S, Choi WS, Kang KW, et al. Histone deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell migration via class II histone deacetylase 4 silencing. Cancer Lett. 2012;325:189–199. doi: 10.1016/j.canlet.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Ailenberg M, Silverman M. Trichostatin A-histone deacetylase inhibitor with clinical therapeutic potential-is also a selective and potent inhibitor of gelatinase A expression. Biochem Biophys Res Commun. 2002;298:110–115. doi: 10.1016/s0006-291x(02)02420-8. [DOI] [PubMed] [Google Scholar]
- Andia DC, de Oliveira NF, Casarin RC, Casati MZ, Line SR, de Souza AP. DNA methylation status of the IL8 gene promoter in aggressive periodontitis. J Periodontol. 2010;81:1336–1341. doi: 10.1902/jop.2010.100082. [DOI] [PubMed] [Google Scholar]
- Araki Y, Tsuzuki Wada T, Aizaki Y, Sato K, Yokota K, Fujimoto K, et al. Histone Methylation and STAT-3 Differentially Regulate Interleukin-6-Induced Matrix Metalloproteinase Gene Activation in Rheumatoid Arthritis Synovial Fibroblasts. Arthritis Rheumatol. 2016;68:1111–1123. doi: 10.1002/art.39563. [DOI] [PubMed] [Google Scholar]
- Aresu L, Benali S, Garbisa S, Gallo E, Castagnaro M. Matrix metalloproteinases and their role in the renal epithelial mesenchymal transition. Histol Histopathol. 2011;26:307–313. doi: 10.14670/HH-26.307. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Challenges in diagnosis and classification of periodontal diseases and conditions. Zhonghua Kou Qiang Yi Xue Za Zhi. 2008;43:260–263. [PubMed] [Google Scholar]
- Asai Y, Jinno T, Igarashi H, Ohyama Y, Ogawa T. Detection and quantification of oral treponemes in subgingival plaque by real-time PCR. J Clin Microbiol. 2002;40:3334–3340. doi: 10.1128/JCM.40.9.3334-3340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo A, Prado AF, Antonio RC, Issa JP, Gerlach RF. Matrix metalloproteinases are involved in cardiovascular diseases. Basic Clin Pharmacol Toxicol. 2014;115:301–314. doi: 10.1111/bcpt.12282. [DOI] [PubMed] [Google Scholar]
- Bakir B, Yetkin Ay Z, Buyukbayram HI, Kumbul Doguc D, Bayram D, Candan IA, Uskun E. The effect of curcumin treatment on systemic Th17 response; gingival expression of IL-17 and retinoic acid receptor-related orphan receptor γt; and alveolar bone loss in experimental periodontitis. J Periodontol. 2016:1–17. doi: 10.1902/jop.2016.150722. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemone V, Fayad R. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food Funct. 2012;3:1109–1117. doi: 10.1039/c2fo30097d. [DOI] [PubMed] [Google Scholar]
- Barros SP, Offenbacher S. Epigenetics: connecting environment and genotype to phenotype and disease. J Dent Res. 2009;88:400–408. doi: 10.1177/0022034509335868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros SP, Offenbacher S. Modifiable risk factors in periodontal disease: epigenetic regulation of gene expression in the inflammatory response. Periodontol 2000. 2014;64:95–110. doi: 10.1111/prd.12000. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bauden M, Tassidis H, Ansari D. In vitro cytotoxicity evaluation of HDAC inhibitor Apicidin in pancreatic carcinoma cells subsequent time and dose dependent treatment. Toxicol Lett. 2015;236:8–15. doi: 10.1016/j.toxlet.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res. 2011;90:9–17. doi: 10.1177/0022034510378683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarsaihan D. Epigenetic mechanisms involved in modulation of inflammatory diseases. Curr Opin Clin Nutr Metab Care. 2016;19:263–269. doi: 10.1097/MCO.0000000000000281. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Borkham-Kamphorst E, Alexi P, Tihaa L, Haas U, Weiskirchen R. Platelet-derived growth factor-D modulates extracellular matrix homeostasis and remodeling through TIMP-1 induction and attenuation of MMP-2 and MMP-9 gelatinase activities. Biochem Biophys Res Commun. 2015;457:307–313. doi: 10.1016/j.bbrc.2014.12.106. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, et al. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- Cao F, Liu T, Xu Y, Xu D, Feng S. Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-kappaB and JNK signaling pathways. Int J Clin Exp Pathol. 2015;8:6037–6045. [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Hsieh YH, Yang WE, Yang SF, Chen Y, Hu DN. Epigallocatechingallate inhibits migration of human uveal melanoma cells via downregulation of matrix metalloproteinase-2 activity and ERK1/2 pathway. Biomed Res Int. 2014a;2014:141582. doi: 10.1155/2014/141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Wang HC, Chen CY, Hung TW, Hou MF, Yuan SS, et al. 5-azacytidine induces anoikis, inhibits mammosphere formation and reduces metalloproteinase 9 activity in MCF-7 human breast cancer cells. Molecules. 2014b;19:3149–3159. doi: 10.3390/molecules19033149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Varghese DS, Gillam MC, Peyton M, Modi B, Schiltz RL, et al. Differential response of cancer cells to HDAC inhibitors trichostatin A and depsipeptide. Br J Cancer. 2012;106:116–125. doi: 10.1038/bjc.2011.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yang SF, Lai CC, Liu JY, Hsieh YS. Regulation of matrix metalloproteinase production by cytokines, pharmacological agents and periodontal pathogens in human periodontal ligament fibroblast cultures. J Periodontal Res. 2002;37:196–203. doi: 10.1034/j.1600-0765.2002.00663.x. [DOI] [PubMed] [Google Scholar]
- Charitaki E, Kastritis E, Petraki C, Liapis K, Adamidis K, Apostolou T, et al. Glomerular expression of matrix metalloproteinases in AL-amyloidosis and association with renal function at the time of kidney biopsy. Clin Nephrol. 2016;85:44–54. doi: 10.5414/CN108670. [DOI] [PubMed] [Google Scholar]
- Chen WF, Huang F, Zha J. The comparison of short-term efficacies between decitabine and HA regimen for MDS/AML patients. Zhonghua Xue Ye Xue Za Zhi. 2012;33:143–144. [PubMed] [Google Scholar]
- Chernov AV, Sounni NE, Remacle AG, Strongin AY. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J Biol Chem. 2009;284:12727–12734. doi: 10.1074/jbc.M900273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi B, Qi M, Kuramitsu HK. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res Microbiol. 2003;154:637–643. doi: 10.1016/j.resmic.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Choi BK, Jung JH, Suh HY, Yoo YJ, Cho KS, Chai JK, Kim CK. Activation of matrix metalloproteinase-2 by a novel oral spirochetal species Treponema lecithinolyticum. J Periodontol. 2001;72:1594–1600. doi: 10.1902/jop.2001.72.11.1594. [DOI] [PubMed] [Google Scholar]
- Choi BK, Paster BJ, Dewhirst FE, Gobel UB. Diversity of cultivable and uncultivable oral spirochetes from a patient with severe destructive periodontitis. Infect Immun. 1994;62:1889–1895. doi: 10.1128/iai.62.5.1889-1895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R, Iwama A, Wang S, Kapila YL. Disease-associated fibronectin matrix fragments trigger anoikis of human primary ligament cells: p53 and c-myc are suppressed. Apoptosis. 2005;10:503–512. doi: 10.1007/s10495-005-1880-5. [DOI] [PubMed] [Google Scholar]
- de Camargo Pereira G, Guimaraes GN, Planello AC, Santamaria MP, de Souza AP, Line SR, Marques MR. Porphyromonas gingivalis LPS stimulation downregulates DNMT1, DNMT3a, and JMJD3 gene expression levels in human HaCaT keratinocytes. Clin Oral Investig. 2013;17:1279–1285. doi: 10.1007/s00784-012-0816-z. [DOI] [PubMed] [Google Scholar]
- Ding Y, Haapasalo M, Kerosuo E, Lounatmaa K, Kotiranta A, Sorsa T. Release and activation of human neutrophil matrix metallo- and serine proteinases during phagocytosis of Fusobacterium nucleatum, Porphyromonas gingivalis and Treponema denticola. J Clin Periodontol. 1997;24:237–248. doi: 10.1111/j.1600-051x.1997.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Du A, Zhao S, Wan L, Liu T, Peng Z, Zhou Z, et al. MicroRNA expression profile of human periodontal ligament cells under the influence of Porphyromonas gingivalis LPS. J Cell Mol Med. 2016;20:1329–1338. doi: 10.1111/jcmm.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, Gogly B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol. 2003;74:188–195. doi: 10.1902/jop.2003.74.2.188. [DOI] [PubMed] [Google Scholar]
- Elburki MS, Moore DD, Terezakis NG, Zhang Y, Lee HM, Johnson F, Golub LM. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J Periodontal Res. 2016 doi: 10.1111/jre.12381. [DOI] [PubMed] [Google Scholar]
- Elburki MS, Rossa C, Guimaraes MR, Goodenough M, Lee HM, Curylofo FA, et al. A novel chemically modified curcumin reduces severity of experimental periodontal disease in rats: initial observations. Mediators Inflamm. 2014;2014:959471. doi: 10.1155/2014/959471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen RP, Dawson JR, Yang PF. Treponema denticola as a model for polar adhesion and cytopathogenicity of spirochetes. Trends Microbiol. 1994;2:114–119. doi: 10.1016/0966-842x(94)90597-5. [DOI] [PubMed] [Google Scholar]
- Ellen RP, Galimanas VB. Spirochetes at the forefront of periodontal infections. Periodontol 2000. 2005;38:13–32. doi: 10.1111/j.1600-0757.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- Endres M, Kneitz S, Orth MF, Perera RK, Zernecke A, Butt E. Regulation of matrix metalloproteinases (MMPs) expression and secretion in MDA-MB-231 breast cancer cells by LIM and SH3 protein 1 (LASP1) Oncotarget. 2016 doi: 10.18632/oncotarget.11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Su Z, Song S, Chiu H, Zhang B, Yi L, et al. Histone deacetylase inhibitors suppress RSV infection and alleviate virus-induced airway inflammation. Int J Mol Med. 2016 doi: 10.3892/ijmm.2016.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno JC. Laboratory maintenance of Treponema denticola. Curr Protoc Microbiol. 2005;Chapter 12(Unit 12B):11. doi: 10.1002/9780471729259.mc12b01s00. [DOI] [PubMed] [Google Scholar]
- Fenno JC, Hannam PM, Leung WK, Tamura M, Uitto VJ, McBride BC. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect Immun. 1998;66:1869–1877. doi: 10.1128/iai.66.5.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Tarima S, Okunseri C. Periodontitis and glycemic control in diabetes: NHANES 2009 to 2012. J Periodontol. 2015;86:499–506. doi: 10.1902/jop.2014.140364. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Park JY, Fenno C, Kapila YL. Porphyromonas gingivalis, gamma interferon, and a proapoptotic fibronectin matrix form a synergistic trio that induces c-Jun N-terminal kinase 1-mediated nitric oxide generation and cell death. Infect Immun. 2008;76:5514–5523. doi: 10.1128/IAI.00625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Grenier D, Uitto VJ, McBride BC. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M, Singh U, McBride BC, Uitto VJ. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991;59:4230–4237. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler MR, Klisaroska A, Kollmann K, Steiner I, Bilban M, Schiefer AI, et al. Antineoplastic activity of the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine in anaplastic large cell lymphoma. Biochimie. 2012;94:2297–2307. doi: 10.1016/j.biochi.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z. Epigenetic Mechanisms as an Interface Between the Environment and Genome. Adv Exp Med Biol. 2016;903:3–15. doi: 10.1007/978-1-4899-7678-9_1. [DOI] [PubMed] [Google Scholar]
- Hernandez-Barrantes S, Shimura Y, Soloway PD, Sang QA, Fridman R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem Biophys Res Commun. 2001;281:126–130. doi: 10.1006/bbrc.2001.4323. [DOI] [PubMed] [Google Scholar]
- Hook KE, Garza SJ, Lira ME, Ching KA, Lee NV, Cao J, et al. An integrated genomic approach to identify predictive biomarkers of response to the aurora kinase inhibitor PF-03814735. Mol Cancer Ther. 2012;11:710–719. doi: 10.1158/1535-7163.MCT-11-0184. [DOI] [PubMed] [Google Scholar]
- Huynh QN, Wang S, Tafolla E, Gansky SA, Kapila S, Armitage GC, Kapila YL. Specific fibronectin fragments as markers of periodontal disease status. J Periodontol. 2002;73:1101–1110. doi: 10.1902/jop.2002.73.10.1101. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Kimizuka R, Kokubu E, Saito A, Ishihara K. Treponema denticola invasion into human gingival epithelial cells. Microb Pathog. 2016;94:104–111. doi: 10.1016/j.micpath.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Jani JP, Arcari J, Bernardo V, Bhattacharya SK, Briere D, Cohen BD, et al. PF-03814735, an orally bioavailable small molecule aurora kinase inhibitor for cancer therapy. Mol Cancer Ther. 2010;9:883–894. doi: 10.1158/1535-7163.MCT-09-0915. [DOI] [PubMed] [Google Scholar]
- Jee SW, Wang S, Kapila YL. Specific pro-apoptotic fibronectin fragments modulate proteinase expression in periodontal ligament cells. J Periodontol. 2004;75:523–530. doi: 10.1902/jop.2004.75.4.523. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jo AR, Baek KJ, Shin JE, Choi Y. Mechanisms of IL-8 suppression by Treponema denticola in gingival epithelial cells. Immunol Cell Biol. 2014;92:139–147. doi: 10.1038/icb.2013.80. [DOI] [PubMed] [Google Scholar]
- Joo NE, Watanabe T, Chen C, Chekenya M, Stallcup WB, Kapila YL. NG2, a novel proapoptotic receptor, opposes integrin alpha4 to mediate anoikis through PKCalpha-dependent suppression of FAK phosphorylation. Cell Death Differ. 2008;15:899–907. doi: 10.1038/cdd.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Kapila YL, Hayami T, Kapila S. Disease-associated extracellular matrix suppresses osteoblastic differentiation of human periodontal ligament cells via MMP-1. Calcif Tissue Int. 2010;86:154–162. doi: 10.1007/s00223-009-9321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapila YL, Kapila S, Johnson PW. Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol. 1996;15:251–261. doi: 10.1016/s0945-053x(96)90116-x. [DOI] [PubMed] [Google Scholar]
- Kapila YL, Lancero H, Johnson PW. The response of periodontal ligament cells to fibronectin. J Periodontol. 1998;69:1008–1019. doi: 10.1902/jop.1998.69.9.1008. [DOI] [PubMed] [Google Scholar]
- Kapila YL, Wang S, Dazin P, Tafolla E, Mass MJ. The heparin-binding domain and V region of fibronectin regulate apoptosis by suppression of p53 and c-myc in human primary cells. J Biol Chem. 2002;277:8482–8491. doi: 10.1074/jbc.M108932200. [DOI] [PubMed] [Google Scholar]
- Kapila YL, Wang S, Johnson PW. Mutations in the heparin binding domain of fibronectin in cooperation with the V region induce decreases in pp125(FAK) levels plus proteoglycan-mediated apoptosis via caspases. J Biol Chem. 1999;274:30906–30913. doi: 10.1074/jbc.274.43.30906. [DOI] [PubMed] [Google Scholar]
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- Kim MS, Son MW, Kim WB, In Park Y, Moon A. Apicidin, an inhibitor of histone deacetylase, prevents H-ras-induced invasive phenotype. Cancer Lett. 2000;157:23–30. doi: 10.1016/s0304-3835(00)00465-1. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem. 1998;273:16098–16103. doi: 10.1074/jbc.273.26.16098. [DOI] [PubMed] [Google Scholar]
- Koch CM, Andrews RM, Flicek P, Dillon SC, Karaoz U, Clelland GK, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17:691–707. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konermann A, Deschner J, Allam JP, Novak N, Winter J, Baader SL, et al. Antigen-presenting cell marker expression and phagocytotic activity in periodontal ligament cells. J Oral Pathol Med. 2012;41:340–347. doi: 10.1111/j.1600-0714.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Fan CC, Chen YA, Cheng CW, Sung YJ, Hsu CP, Kao TY. Curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-E-cadherin pathway. Integr Cancer Ther. 2015;14:484–490. doi: 10.1177/1534735415588930. [DOI] [PubMed] [Google Scholar]
- Leira Y, Seoane J, Blanco M, Rodriguez-Yanez M, Takkouche B, Blanco J, Castillo J. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur J Epidemiol. 2016 doi: 10.1007/s10654-016-0170-6. [DOI] [PubMed] [Google Scholar]
- Ligi D, Mannello F. Do matrix metalloproteinases represent reliable circulating biomarkers in colorectal cancer? Br J Cancer. 2016;115:633–634. doi: 10.1038/bjc.2016.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Chang TC, Lin YT, Yu YL, Ko BS, Sung LY, Liou JY. Paracrine regulation of matrix metalloproteinases contributes to cancer cell invasion by hepatocellular carcinoma-secreted 14-3-3sigma. Oncotarget. 2016 doi: 10.18632/oncotarget.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Sang MX, Hou SY, Zhang C, Shan BE. Low-dose decitabine induces MAGE-A expression and inhibits invasion via suppression of NF-kappaB2 and MMP2 in Eca109 cells. Biomed Pharmacother. 2014;68:745–750. doi: 10.1016/j.biopha.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang L, Ding F, Li J, Guo M, Li W, et al. 5-Aza-2′-deoxycytidine induces retinoic acid receptor-beta(2) demethylation and growth inhibition in esophageal squamous carcinoma cells. Cancer Lett. 2005;230:271–283. doi: 10.1016/j.canlet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz-Zajac M, Szmitkowski M, Litman-Zawadzka A, Mroczko B. Matrix Metalloproteinases and Their Tissue Inhibitors in Comparison to Other Inflammatory Proteins in Gastric Cancer (GC) Cancer Invest. 2016;34:305–312. doi: 10.1080/07357907.2016.1197237. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Jurgensen HJ, Ingvarsen S, Melander MC, Albrechtsen R, Hald A, et al. Differential actions of the endocytic collagen receptor uPARAP/Endo180 and the collagenase MMP-2 in bone homeostasis. PLoS One. 2013;8:e71261. doi: 10.1371/journal.pone.0071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen PL, Makinen KK, Syed SA. Role of the chymotrypsin-like membrane-associated proteinase from Treponema denticola ATCC 35405 in inactivation of bioactive peptides. Infect Immun. 1995;63:3567–3575. doi: 10.1128/iai.63.9.3567-3575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Kern CB, Kimbrough D, Addy B, Kasiganesan H, Rivers WT, et al. Inhibition of class I histone deacetylase activity represses matrix metalloproteinase-2 and -9 expression and preserves LV function postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2015;308:H1391–1401. doi: 10.1152/ajpheart.00390.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JA, Milagro FI, Claycombe KJ, Schalinske KL. Epigenetics in adipose tissue, obesity, weight loss, and diabetes. Adv Nutr. 2014;5:71–81. doi: 10.3945/an.113.004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers DA, Leung WK, Fenno JC, Hong Y, McBride BC. The major surface protein complex of Treponema denticola depolarizes and induces ion channels in HeLa cell membranes. Infect Immun. 1996;64:2904–2910. doi: 10.1128/iai.64.8.2904-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Frederick J, Miller DP, Goetting-Minesky MP, Goodman H, Fenno JC, Marconi RT. Identification of the primary mechanism of complement evasion by the periodontal pathogen, Treponema denticola. Mol Oral Microbiol. 2011;26:140–149. doi: 10.1111/j.2041-1014.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Huang B, Fenno JC, Marconi RT. Analysis of a unique interaction between the complement regulatory protein factor H and the periodontal pathogen Treponema denticola. Infect Immun. 2009;77:1417–1425. doi: 10.1128/IAI.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina C, Santana A, Quintero E, Radomski MW, Guarner F. Matrix metalloproteinases in diseases of the gastrointestinal tract. Gastroenterol Hepatol. 2004;27:491–497. doi: 10.1016/s0210-5705(03)70509-3. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Miao D, Fenno JC, Timm JC, Joo NE, Kapila YL. The Treponema denticola chymotrypsin-like protease dentilisin induces matrix metalloproteinase-2-dependent fibronectin fragmentation in periodontal ligament cells. Infect Immun. 2011;79:806–811. doi: 10.1128/IAI.01001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao D, Godovikova V, Qian X, Seshadrinathan S, Kapila YL, Fenno JC. Treponema denticola upregulates MMP-2 activation in periodontal ligament cells: interplay between epigenetics and periodontal infection. Arch Oral Biol. 2014;59:1056–1064. doi: 10.1016/j.archoralbio.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza S, Sharma G, Pandya P, Ralhan R. Demethylating agent 5-aza-2-deoxycytidine enhances susceptibility of breast cancer cells to anticancer agents. Mol Cell Biochem. 2010;342:101–109. doi: 10.1007/s11010-010-0473-y. [DOI] [PubMed] [Google Scholar]
- Mittal R, Patel AP, Debs LH, Nguyen D, Patel K, Grati M, et al. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J Cell Physiol. 2016;231:2599–2621. doi: 10.1002/jcp.25430. [DOI] [PubMed] [Google Scholar]
- Mogal A, Abdulkadir SA. Effects of Histone Deacetylase Inhibitor (HDACi); Trichostatin-A (TSA) on the expression of housekeeping genes. Mol Cell Probes. 2006;20:81–86. doi: 10.1016/j.mcp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Mosig RA, Martignetti JA. Loss of MMP-2 in murine osteoblasts upregulates osteopontin and bone sialoprotein expression in a circuit regulating bone homeostasis. Dis Model Mech. 2013;6:397–403. doi: 10.1242/dmm.007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Nagaraju GP, Aliya S, Zafar SF, Basha R, Diaz R, El-Rayes BF. The impact of curcumin on breast cancer. Integr Biol (Camb) 2012;4:996–1007. doi: 10.1039/c2ib20088k. [DOI] [PubMed] [Google Scholar]
- Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nagasri M, Madhulatha M, Musalaiah SV, Kumar PA, Krishna CH, Kumar PM. Efficacy of curcumin as an adjunct to scaling and root planning in chronic periodontitis patients: A clinical and microbiological study. J Pharm Bioallied Sci. 2015;7:S554–558. doi: 10.4103/0975-7406.163537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- Navratilova Z, Kolek V, Petrek M. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch Immunol Ther Exp (Warsz) 2016;64:177–193. doi: 10.1007/s00005-015-0375-5. [DOI] [PubMed] [Google Scholar]
- Nebbioso A, Carafa V, Benedetti R, Altucci L. Trials with ‘epigenetic’ drugs: an update. Mol Oncol. 2012;6:657–682. doi: 10.1016/j.molonc.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niller HH, Masa R, Venkei A, Meszaros S, Minarovits J. Pathogenic mechanisms of intracellular bacteria. Curr Opin Infect Dis. 2017;30:309–315. doi: 10.1097/QCO.0000000000000363. [DOI] [PubMed] [Google Scholar]
- Nissinen L, Kahari VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840:2571–2580. doi: 10.1016/j.bbagen.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Nowakowska A, Tarasiuk J. Comparative effects of selected plant polyphenols, gallic acid and epigallocatechin gallate, on matrix metalloproteinases activity in multidrug resistant MCF7/DOX breast cancer cells. Acta Biochim Pol. 2016 doi: 10.18388/abp.2016_1256. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008;79:1577–1584. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]
- Oyarzun A, Arancibia R, Hidalgo R, Penafiel C, Caceres M, Gonzalez MJ, et al. Involvement of MT1-MMP and TIMP-2 in human periodontal disease. Oral Dis. 2010;16:388–395. doi: 10.1111/j.1601-0825.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Jun JA, Jeong KJ, Heo HJ, Sohn JS, Lee HY, et al. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol Rep. 2011;25:1677–1681. doi: 10.3892/or.2011.1236. [DOI] [PubMed] [Google Scholar]
- Pasquier J, Hoarau-Vechot J, Fakhro K, Rafii A, Abi Khalil C. Epigenetics and Cardiovascular Disease in Diabetes. Curr Diab Rep. 2015;15:108. doi: 10.1007/s11892-015-0677-3. [DOI] [PubMed] [Google Scholar]
- Pereira IT, Ramos EA, Costa ET, Camargo AA, Manica GC, Klassen LM, et al. Fibronectin affects transient MMP2 gene expression through DNA demethylation changes in non-invasive breast cancer cell lines. PLoS One. 2014;9:e105806. doi: 10.1371/journal.pone.0105806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DJ, Patel MC, Blanco JC, Vogel SN. Epigenetic mechanisms governing innate inflammatory responses. J Interferon Cytokine Res. 2016;36:454–461. doi: 10.1089/jir.2016.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietruszewska W, Bojanowska-Pozniak K, Kobos J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: an immunohistochemical study. Otolaryngol Pol. 2016;70:32–43. doi: 10.5604/00306657.1202546. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. The Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Prasanna SJ. Causal relationship between periodontitis and chronic obstructive pulmonary disease. J Indian Soc Periodontol. 2011;15:359–365. doi: 10.4103/0972-124X.92570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzouk S, Sarkis R. Smoking and diabetes. Epigenetics involvement in osseointegration. N Y State Dent J. 2013;79:27–30. [PubMed] [Google Scholar]
- Safronova O, Morita I. Transcriptome remodeling in hypoxic inflammation. J Dent Res. 2010;89:430–444. doi: 10.1177/0022034510366813. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Takeuchi Y, Umeda M, Ishikawa I, Benno Y. Rapid detection and quantification of five periodontopathic bacteria by real-time PCR. Microbiol Immunol. 2001;45:39–44. doi: 10.1111/j.1348-0421.2001.tb01272.x. [DOI] [PubMed] [Google Scholar]
- Saldanha SN, Kala R, Tollefsbol TO. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp Cell Res. 2014;324:40–53. doi: 10.1016/j.yexcr.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon C, Marchesan J, Soehren S, Matsuo M, Kapila Y. Capturing the regenerative potential of periodontal ligament fibroblasts. J Stem Cells Regen Med. 2011;7:54–56. doi: 10.46582/jsrm.0701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffski P, Jones SF, Dumez H, Infante JR, Van Mieghem E, Fowst C, et al. Phase I, open-label, multicentre, dose-escalation, pharmacokinetic and pharmacodynamic trial of the oral aurora kinase inhibitor PF-03814735 in advanced solid tumours. Eur J Cancer. 2011;47:2256–2264. doi: 10.1016/j.ejca.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Shin J, Choi Y. The fate of Treponema denticola within human gingival epithelial cells. Mol Oral Microbiol. 2012;27:471–482. doi: 10.1111/j.2041-1014.2012.00660.x. [DOI] [PubMed] [Google Scholar]
- Shinkarenko TV, Rumiantsev VA, Egorova EN, Eliseeva TI. Matrix metalloproteinases in periodontitis. Stomatologiia (Mosk) 2013;92:77–80. [PubMed] [Google Scholar]
- Shofuda K, Moriyama K, Nishihashi A, Higashi S, Mizushima H, Yasumitsu H, et al. Role of tissue inhibitor of metalloproteinases-2 (TIMP-2) in regulation of pro-gelatinase A activation catalyzed by membrane-type matrix metalloproteinase-1 (MT1-MMP) in human cancer cells. J Biochem. 1998;124:462–470. doi: 10.1093/oxfordjournals.jbchem.a022136. [DOI] [PubMed] [Google Scholar]
- Simonson LG, Goodman CH, Bial JJ, Morton HE. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988;56:726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitholimela CS, Shangase LS. The association between periodontitis and pre-term birth and/or low birth weight: a literature review. SADJ. 2013;68:162–166. [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Song SE, Choi BK, Kim SN, Yoo YJ, Kim MM, Park SK, et al. Inhibitory effect of procyanidin oligomer from elm cortex on the matrix metalloproteinases and proteases of periodontopathogens. J Periodontal Res. 2003;38:282–289. doi: 10.1034/j.1600-0765.2003.02604.x. [DOI] [PubMed] [Google Scholar]
- Stewart R, West M. Increasing evidence for an association between periodontitis and cardiovascular disease. Circulation. 2016;133:549–551. doi: 10.1161/CIRCULATIONAHA.115.020869. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Tafolla E, Wang S, Wong B, Leong J, Kapila YL. JNK1 and JNK2 oppositely regulate p53 in signaling linked to apoptosis triggered by an altered fibronectin matrix: JNK links FAK and p53. J Biol Chem. 2005;280:19992–19999. doi: 10.1074/jbc.M500331200. [DOI] [PubMed] [Google Scholar]
- Talbert PB, Henikoff S. Spreading of silent chromatin: inaction at a distance. Nat Rev Genet. 2006;7:793–803. doi: 10.1038/nrg1920. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Iwasaki K, Feghali KE, Komaki M, Ishikawa I, Izumi Y. Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Arch Oral Biol. 2011;56:380–388. doi: 10.1016/j.archoralbio.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Uitto VJ, Grenier D, Chan EC, McBride BC. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan J, Natarajan M, Lagisetty P, Awasthi V, Herman TS, Aravindan N. Impact of curcumin, raspberry extract, and neem leaf extract on rel protein-regulated cell death/radiosensitization in pancreatic cancer cells. Pancreas. 2011;40:1107–1119. doi: 10.1097/MPA.0b013e31821f677d. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The epigenotype. 1942. Int J Epidemiol. 2012;41:10–13. doi: 10.1093/ije/dyr184. [DOI] [PubMed] [Google Scholar]
- Wang F, Qi Y, Li X, He W, Fan QX, Zong H. HDAC inhibitor trichostatin A suppresses esophageal squamous cell carcinoma metastasis through HADC2 reduced MMP-2/9. Clin Invest Med. 2013;36:E87–94. doi: 10.25011/cim.v36i2.19571. [DOI] [PubMed] [Google Scholar]
- Williams RC, Barnett AH, Claffey N, Davis M, Gadsby R, Kellett M, et al. The potential impact of periodontal disease on general health: a consensus view. Curr Med Res Opin. 2008;24:1635–1643. doi: 10.1185/03007990802131215. [DOI] [PubMed] [Google Scholar]
- Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4:409–419. doi: 10.1038/mi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Kawada M, Suzuki N, Nakano Y, Oho T, Saito T, Yamashita Y. TaqMan real-time polymerase chain reaction assay for the correlation of Treponema denticola numbers with the severity of periodontal disease. Oral Microbiol Immunol. 2004;19:196–200. doi: 10.1111/j.0902-0055.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xiao XQ, Jiang YF, Liang YS, Peng MY, Xu Y, Gong GZ. DNA demethylation in PD-1 gene promoter induced by 5-azacytidine activates PD-1 expression on Molt-4 cells. Cell Immunol. 2011;271:450–454. doi: 10.1016/j.cellimm.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Zhang S, Barros SP, Niculescu MD, Moretti AJ, Preisser JS, Offenbacher S. Alteration of PTGS2 promoter methylation in chronic periodontitis. J Dent Res. 2010;89:133–137. doi: 10.1177/0022034509356512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP) Curr Top Dev Biol. 2003;54:1–74. doi: 10.1016/s0070-2153(03)54004-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.