Abstract
Conflicting evidence has suggested that low mean nocturnal hemoglobin oxygen saturation (SpO2) predicts future hospital days for acute severe pain in children with sickle cell anemia (SCA). In an unselected multi-center prospective cohort study, we tested the hypothesis that either low mean nocturnal SpO2 or high obstructive apnea-hypopnea index (OAHI; the number of obstructive apneas and hypopneas with ≥ 3% desaturation or arousal per hour of sleep) or high oxygen desaturation index (ODI; number of ≥ 3% desaturation from baseline saturation per hour of sleep) is associated with increased incidence rates of pain. A total of 140 children with SCA with a median age of 10.8 years (interquartile range 7.2) were followed for a median 4.9 years (interquartile range 1.8). Overnight polysomnography evaluations at baseline health were measured and adjudicated centrally. Multivariable models created in two steps were included. First all plausible covariates were included in a screening model. Subsequently, covariates meeting significance criteria of p < 0.20 were included in the final model. Contrary to our hypothesis, higher (but not lower) mean nocturnal SpO2 was associated with higher rates of pain episodes (IRR 1.10, 95% CI [1.03-1.18], p = 0.004). Higher log OAHI did not pass screening criteria. Higher log ODI was not significantly associated with higher rates of pain episodes (IRR 0.93, 95% CI [0.82-1.06], p = 0.28). Neither low nocturnal SpO2, higher OAHI, nor higher ODI were associated with clinically relevant increased incidence rates of acute severe pain episodes.
Introduction
Sickle cell anemia (SCA) is the most common inherited blood disorder in the United States, and the burden is continuing to increase worldwide.1 The two most common complications of SCA requiring hospitalization are acute vaso-occlusive pain (referred to as pain) and acute chest syndrome (ACS) episodes. One potential risk factor for sickle cell disease (SCD) related morbidity is hemoglobin oxygen desaturation, which leads to hemoglobin polymerization, sickling of erythrocytes, and increased red blood cell adherence to the endothelium, ultimately resulting in vaso-occlusion and tissue hypoxia.2,3
Previous studies have investigated the relationship between hemoglobin oxygen saturation, as measured by pulse oximetry (SpO2), and SCA-related morbidity with conflicting results. In a prospective cohort of 130 children with SCA, there was no association between lower mean daytime SpO2 obtained at baseline health exam and incidence rates of pain or ACS.4 Similar findings were seen in a retrospective cohort of 183 children with SCA from the Dallas Newborn Cohort for incidence rates of ACS episodes.5 In a third study, a prospective single-center cohort of 39 consecutive children with homozygous SS or S/β0 thalassemia, neither mean daytime nor mean nocturnal SpO2 was associated with increased incidence rates of pain or ACS episodes.6
The prior three studies measuring mean SpO2 levels (three daytime studies and one nighttime study) have not demonstrated any association between lower mean SpO2 and increased incidence of pain or ACS in children with SCA. In contrast, only one study has demonstrated a positive association. A single site prospective study by Hargrave et al. demonstrated that low mean nocturnal SpO2, based on overnight pulse oximetry measured either at home or in-patient, was associated with an increased number of hospital days for pain episodes.7 Conflicting results describing the relationship between low mean nocturnal SpO2 measurements and SCA morbidity (pain and ACS episodes) has resulted in ambiguity as to whether children with low mean nocturnal SpO2 should be prescribed supplemental oxygen at night to prevent SCA related morbidity.
The National Heart Lung and Blood Institute funded our multi-disciplinary research team of pediatric hematologists, pulmonologists, and sleep experts to address whether mean nocturnal SpO2 predicted future pain episodes requiring hospitalization. We created a multi-center prospective cohort of unselected children with SCA that received a full 12-channel polysomnography (PSG). In this cohort, we tested the primary hypothesis that low mean nocturnal SpO2 is associated with increased incidence rates of pain episodes in children with SCA. We also tested the hypothesis that obstructive sleep apnea syndrome (OSAS), as defined by high obstructive apnea-hypopnea index (OAHI) or by high oxygen desaturation index (ODI), is associated with an increased incidence of pain episodes in children with SCA. Finally, we explored whether there is any relationship between mean nocturnal SpO2, OAHI, and ODI and increased incidence of ACS episodes in children with SCA.
Methods
A prospective cohort study of children and adolescents from the Sleep and Asthma Cohort (SAC) was assembled. Children with SCA, defined as homozygous for sickle cell hemoglobin [HbSS (N = 240)] or compound heterozygous for sickle β zero thalassemia [HbSβ0 (N = 12)], ranging from 4 to 18 years of age were enrolled at three clinical SCA centers: Washington University School of Medicine in St. Louis, Missouri; Case Western Reserve University in Cleveland, Ohio; and University College London in London, UK (which recruited from three London hospitals) between 2005 and 2011. Institutional review boards for each US site approved the study protocol. Ethical approval was provided by the National Research Ethics Service which allowed for recruitment from all 3 UK sites. All study participants were unselected for respiratory disease and prospectively observed for at least 3 years. Children were not eligible for participation in the study if they received long-term blood transfusion therapy due to the known impact of transfusion therapy on pain episodes.8 Participants were also not eligible if they received long-term continuous positive airway pressure support, had chronic lung disease other than asthma, smoked cigarettes, or were known to be human immune deficiency virus (HIV)-positive due to the known association between HIV-positivity and chronic lung disease in children and adolescents.9 If patients were initiated on regular blood transfusion therapy during the study period, they were retained in the cohort and a separate analysis was performed including these participants. Parents provided written informed consent for all participants and patients provided assent when appropriate. Laboratory testing, daytime SpO2, and polysomnography were performed at study entry when patients were at baseline health. Study visits were in coordination with routine clinical care and participants were followed every three to six months.
Definition of vaso-occlusive episodes: acute pain and acute chest syndrome
An acute pain episode was defined as a SCA-associated pain episode requiring admission to the hospital and treatment with opioids. Emergency department visits were not included in this definition due to the variation in outpatient pain management practices and to capture only acute severe pain events. Headaches treated with opioids were also not included in this definition due to the difference in proposed pathophysiology of headaches as opposed to acute vaso-occlusive pain.10 To replicate the Hargrave et al. analysis, hospital days for pain rate (defined as hospital days of pain per years of follow-up) was also examined. ACS was defined as at least one new radiodensity on radiograph of the chest in addition to at least one of the following: temperature greater than 38.5°C, decreased SpO2 from baseline, or increased respiratory rate.11,12 A new diagnosis of pneumonia was considered an ACS episode. The principal investigator (MRD) visited each site and reviewed all pain and ACS episodes during the first three years of the cohort to ensure a uniform definition of pain and ACS at each site in this multi-center study.
Definition of Obstructive Sleep Apnea Syndrome (OSAS), Obstructive Apnea-Hypopnea Index (OAHI), and Oxygen Desaturation Index (ODI)
OAHI and ODI were calculated based on the American Academy of Sleep Medicine pediatric criteria.13 Respiratory event types (obstructive, mixed, hypopneas, central) were summarized as indices (the number of respiratory events divided by the hours of total sleep time). The OAHI was then defined as the sum of all obstructive and mixed apneas, plus hypopneas associated with a 50% reduction in airflow and either at least 3% desaturation or electroencephalographic arousal, divided by hours of total sleep time. The ODI was defined as the number of at least 3% desaturation from baseline saturation per hour of sleep.
Polysomnography
At each study site, SAC study-certified technicians performed overnight full 12-channel, in-laboratory research PSG including the following: monitoring of electroencephalography (EEG), eye movements (electro-oculogram; EOG), chin and limb electromyogram (EMG), thoracic and abdominal respiratory effort, airflow (thermistor and nasal pressure transducer), snore sensor, electrocardiography (ECG), and continuous measurements of nocturnal SpO2 (using Masimo SET (v2), Irvine, CA). All data collection utilized standardized protocols according to the American Academy of Sleep Medicine guidelines for data acquisition and pediatric scoring.13 All PSGs were read centrally at Vanderbilt University School of Medicine.
Statistical analysis
Negative binomial regression was used to test the association between nocturnal SpO2 and the incidence rates of pain and ACS episodes. Covariates in the primary models for pain and ACS episodes included: age at the time of the sleep study, gender, hemoglobin, white blood cell count (WBC), OAHI, and mean nocturnal SpO2. The log value of OAHI and ODI was used for a continuous measure because the distributions were positively skewed. Multivariable models were constructed in two steps. First, all covariates were included in a screening model. Subsequently, those covariates meeting a significance criterion of p < 0.20 were included in the final model for pain or ACS episodes. The variance inflation factor (VIF) was used to assess multicollinearity using a threshold of 3.0. The highest VIF value was 1.6, indicating that collinearity is not a concern in these data. All tests for significance were two-tailed. P-values of < 0.05 were considered significant in the final models. Analyses were conducted using IBM SPSS Statistics (Version 23, Armonk, NY, IBM).
Results
Recruitment and baseline characteristics
Of the 252 participants with SCA who underwent PSG, 243 had complete data that were able to be scored according to the American Academy of Sleep Medicine guidelines.13 Of these, 223 participants had values for pain and ACS after PSG; missing data were due to lack of follow-up. Among the 223 participants, 18 participants were on hydroxyurea therapy at the time of PSG and one had a missing hydroxyurea status. These 19 participants were excluded from the primary analyses due to the known association between hydroxyurea and increased baseline SpO2 measurements (hydroxyurea results in an increase in the percentage of fetal hemoglobin [HbF] and shifting of the oxygen dissociation curve to the left).14 After PSG was completed, 59 patients were started on hydroxyurea therapy, 6 were started on chronic blood transfusion therapy, and 1 participant was started on both therapies. These individuals were not included in the primary analysis (Figure 1). We did include the participants that started hydroxyurea, regular blood transfusion therapy or both after polysomnography in a secondary analysis, and there was no appreciable difference in the results (See Online Supplement).
Figure 1.
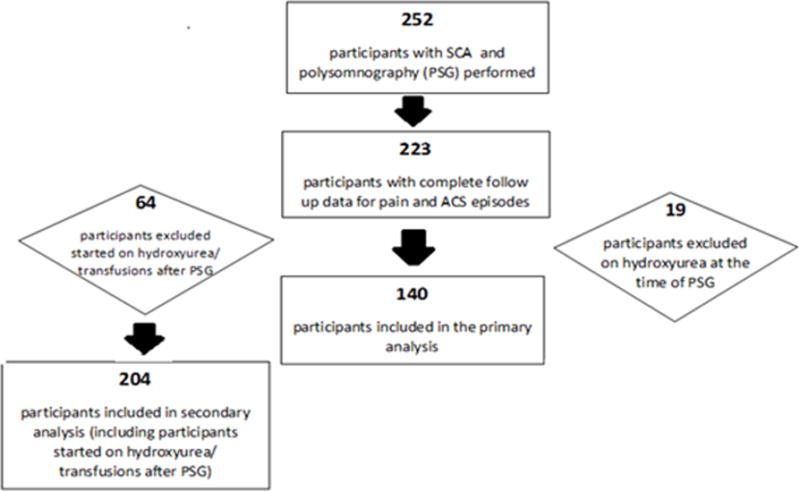
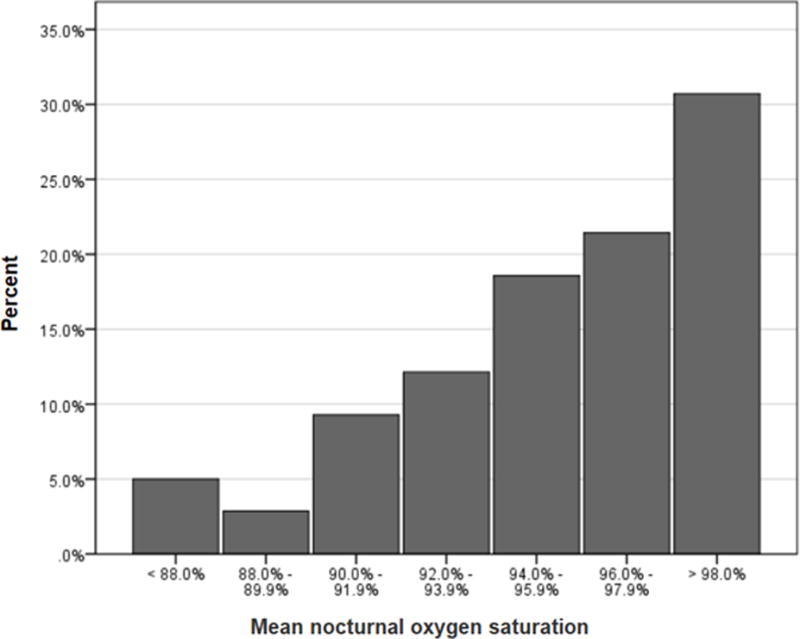
The final sample included 140 participants with a median age of 10.8 years (IQR 7.2). Participants were followed for a median of 4.9 years (IQR 1.8) after PSG, Table 1. The median (IQR) values were 96.4% (4.6) for mean nocturnal SpO2 and 97.0% (4.9) for mean daytime SpO2. Approximately 17% of our cohort (N=24) had a mean nocturnal SpO2 less than 92% (Figure 2). Participants prescribed hydroxyurea, compared to those who were not prescribed hydroxyurea, were noted to have higher rates, not lower rates, of acute severe pain (mean 1.34 episodes/patient year versus 0.58 episodes/patient year; p < 0.001) and ACS (mean 0.33 versus 0.19; p < 0.001) episodes during the follow-up period.
Table 1.
Characteristics of the Sleep and Asthma Cohort (SAC) study population (N = 140) that completed both baseline polysomnography and were followed prospectively to assess acute vaso-occlusive pain events requiring hospitalization and acute chest syndrome events for a median of 5.0 years.
| Characteristic | Summary statistics in cohort (n=140)# |
|---|---|
| Age at polysomnography, years | 10.8 (7.2) |
| Male (%) | 47.9 |
| Total follow-up time after polysomnography (years) | 4.9 (1.8) |
| Hemoglobin (g/dl) | 8.1 (1.9) |
| White blood cell count (× 109/L) | 11.7 (3.9) |
| Reticulocytes (%), n=139 | 10.2 (6.9) |
| Daytime hemoglobin oxygen saturation (%), n=135 | 97.0 (4.9) |
| Obstructive apnea hypopnea index | 0.6 (2.0) |
| Nocturnal hemoglobin oxygen saturation (%) | 96.4 (4.6) |
| Oxygen desaturation index, 3% | 2.2 (5.7) |
| Prospective rate of pain episodes per year, mean (std. dev.) | 0.57 (0.76) |
| Prospective rate of ACS episodes per year, mean (std. dev.) | 0.17 (0.29) |
Median and IQR are presented for variables, unless otherwise indicated.
Figure 2.
Low nocturnal oxygen saturation is not associated with increased incidence rates of acute severe pain episodes or acute severe pain days
In the opposite direction of our hypothesis, after adjusting for age in the final model, higher, not lower, nocturnal SpO2 was associated with increased incidence of pain episodes, such that each increase of 1% in SpO2 was associated with a 10% increased rate of future pain episodes (incidence rate ratio (IRR) 1.10; 95% CI [1.03-1.18]; p =0.004), Table 2. Higher nocturnal SpO2 was not associated with more pain days per year (IRR 1.09; 95% CI [1.00, 1.19]; p =0.058), again in the opposite direction of our hypothesis. The slope of the bivariate relationship between nocturnal SpO2 and incidence of pain was positive (i.e., the higher the SpO2, the higher the incidence of pain events), Figure 3. The positive slope means there is no clear-cut threshold (90%, 92%, 95%, etc.) below which mean nocturnal SpO2 values will be associated with higher rates of pain events when compared to individuals above the threshold. No significant difference was seen in including or excluding participants receiving disease modifying therapy such as hydroxyurea or chronic blood transfusions. The analysis was repeated including the 59 participants started on hydroxyurea and the 6 participants started on chronic blood transfusion therapy (N=204). Higher nocturnal SpO2 was again associated with an increased incidence rate of future pain episodes (IRR 1.11; 95% CI [1.06-1.17]; p < 0.001) (see Online Supplement).
Table 2.
Final negative binomial regression models for prospective rates of vaso-occlusive pain or pain days per year** with nocturnal oxygen saturation and obstructive apnea hyponea index (OAHI) in children with sickle cell anemia (N = 140) from the Sleep and Asthma Cohort (SAC) study. The initial model included age at the time of the sleep study, gender, hemoglobin, white blood cell count (WBC), OAHI, and mean nocturnal SpO2. The log value of OAHI was used for a continuous measure because the distribution was positively skewed
| IRR | 95% CI | P-Value* | |
|---|---|---|---|
| 1. Prospective rates of pain events, final model | |||
| Age at polysomnography | 1.10 | 1.04-1.16 | <0.001 |
| Nocturnal oxygen saturation | 1.10 | 1.03-1.18 | 0.004 |
| 2. Pain days per year, final model | |||
| Age at polysomnography | 1.12 | 1.05-1.21 | 0.001 |
| Sex (male) | 0.61 | 0.34-1.12 | 0.112 |
| Nocturnal oxygen saturation | 1.09 | 1.00-1.19 | 0.058 |
Two-tailed
Acute chest syndrome is not shown since no variable passed screening
Figure 3.
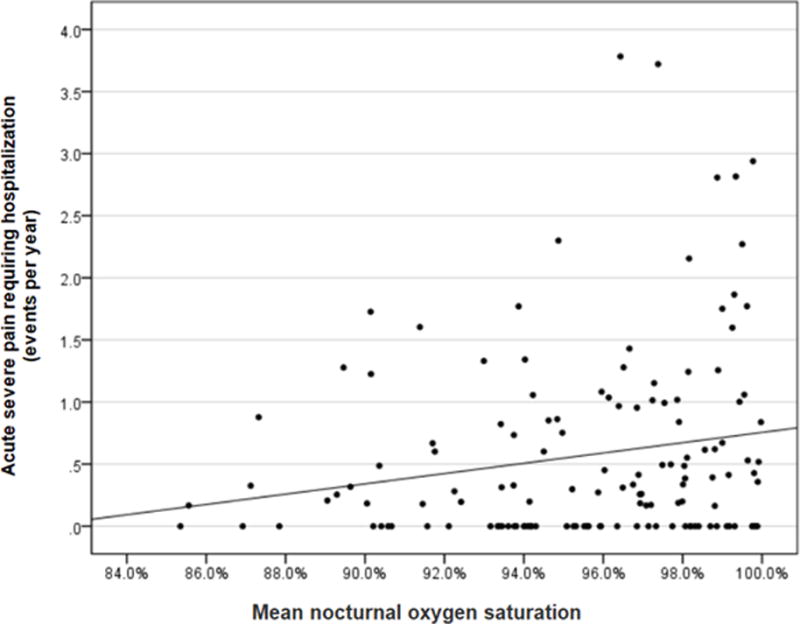
Neither low nocturnal oxygen saturation, high OAHI, nor high ODI are associated with increased incidence rates of pain or acute chest syndrome
In the screening model used to evaluate the potential effect of OAHI on future rates of pain episodes, after adjusting for age, sex, WBC, hemoglobin, and mean nocturnal SpO2, log OAHI did not pass the screening threshold (p = 0.31). In the final model evaluating the effect of ODI on future rates of pain episodes, ODI was not significant after adjusting for age (p = 0.28).
Mean nocturnal SpO2 did not pass screening in the model to evaluate the effect of mean nocturnal SpO2 on future ACS rates, after adjusting for age, sex, WBC, hemoglobin, and log OAHI (IRR 1.05; 95% CI [0.95-1.16]; p = 0.37). In the final model evaluating for the effect of log ODI on future ACS episode, ODI was not significant with the same set of covariates (p = 0.74).
Similar to our previous findings, no significant difference in the effect of SpO2 on ACS rate was seen by including or excluding participants receiving disease modifying therapy such as hydroxyurea or chronic blood transfusions. The analysis was repeated including the 59 participants started on hydroxyurea and the 6 participants started on chronic blood transfusion therapy (N=204). No relationship was noted between mean nocturnal SpO2 and incidence of ACS episodes (IRR 1.13; 95% CI [0.99-1.28]; p = 0.06) (see Online Supplement).
Low daytime oxygen saturation is not associated with increased incidence rates of acute severe pain or ACS
Higher mean daytime SpO2 was also associated with increased incidence of pain episodes after adjusting for age in the final multivariable model (IRR 1.11; 95% CI [1.04-1.19]; p = 0.001). Higher mean daytime SpO2 was not associated with ACS after adjusting for sex, age, WBC, hemoglobin, and log OAHI (IRR 1.09; 95% CI [0.98-1.20; p = 0.11).
Discussion
When a child with SCA has a low nocturnal SpO2 and a history of increased acute pain episodes, clinicians are without clear evidence that the use of supplemental oxygen will attenuate the incidence of future acute pain episodes. We conducted a prospective cohort study of children with SCA receiving rigorously performed sleep studies to determine if mean nocturnal SpO2 was associated with either future pain or ACS episodes. Our results did not provide any evidence that low mean nocturnal SpO2, high OAHI, or high ODI measurements were associated with increased incidence rates of pain or ACS episodes.
Despite having results contrary to our hypothesis, we believe our results are credible for the following reasons: 1) consistency of our findings with the two other prospective cohort studies demonstrating that mean daytime SpO2 was not associated with increased incidence rates of pain4,6; 2) the incidence rates of acute pain episodes requiring hospitalization of 0.80 events per patient year (including patients receiving disease modifying therapy, N= 204) was similar to two large cohorts of children with SCA, the SIT Trial (n=934) and the Cooperative Study of Sickle Cell Disease (n=349), 0.61 and 0.58 per patient year, respectively15; 3) rigorously obtained PSG evaluations with central adjudication in 140 children followed for 5 years coupled with the prevalence of sleep disordered breathing in this cohort being similar to the range of sleep characteristics in African-American children without SCA.16
Undoubtedly after completion of any study that did not confirm the primary hypothesis (and found a reverse association), there is some concern about spurious results and power of the study to test the hypothesis. However, these concerns are reduced for our study because we did find statistically significant results, with a moderate-sized coefficient, albeit in the opposite direction of the hypothesis (higher, not lower, SpO2 measurements are associated with increased acute pain episodes). Second, in our cohort, similar to previous studies that did not find a relationship between low mean daytime SpO2 and SCA related morbidity.4, 5, 6 In our study, for the primary hypothesis, the 95% confidence interval is narrow, describing the relationship between mean nocturnal SpO2 oxygen measurements and pain episode incidence rate (IRR 1.10; 95% CI [1.03-1.18]; p = 0.004). Additionally, OAHI and ODI are more refined approaches to determine sleep-disordered breathing than mean nocturnal SpO2. We did not find any evidence that OAHI or ODI were associated with increased rates of pain. Our results demonstrate no clinically significant relationship between lower mean nocturnal SpO2, OAHI or ODI, and future pain events in an unselected cohort of children with SCA, but instead a relationship between higher SpO2 and increased incidence of pain events. We are unaware of the biological basis explaining why an increase in daytime or nocturnal SpO2 would be associated with increased incidence rates of pain. Additional studies are needed to assess the biological basis, if any, of these findings.
Our prospective cohort study differed significantly from the only other prospective cohort of children with SCA to assess the influence of mean nocturnal SpO2 oxygen measurements and its relationship to acute pain. Some of the key characteristics in the Hargrave et al. study which distinguish their study design from our study, include, but are not limited to: a single-center study which typically overestimates the effect size;17 the use of a pulse oximeter reading without the full benefit of polysomnography evaluation, which is considered the gold standard for assessment of nocturnal desaturations; and the primary analysis which did not correct for multicollinearity. Additionally, in our study we determined mean nocturnal SpO2 measurement as part of an overnight PSG study using a Masimo pulse oximeter. An inherent challenge in using pulse oximetry measurements is the ability to differentiate true desaturation from motion artifact. A study of 20 pulse oximetry machines demonstrated that the Masimo SET R machine used in our cohort exhibited the best overall performance, with a sensitivity and specificity of 98% and 93%, respectively.18 In contrast, the Hargrave et al. analysis used Ohmeda Biox 3700, a machine with relatively poor test characteristics.19 Another major difference between our study and Hargrave et al. was the primary outcome measure. Our primary measure for severity was discrete events of acute vaso-occlusive pain, the standard strategy for assessment of pain in SCA therapeutic trials;20, 21 in comparison, Hargrave et al. elected to use the number of acute pain days in the hospital, an outcome that may be more variable based on family dynamics and physician preference. Table 3 summarizes the similarities and differences between the current study and Hargraves et al.
Table 3.
Similarities and differences between prior prospective cohort of children with mean nocturnal oxygen saturation demonstrating increased incidence of severe pain days7 and the current multi-Sleep and Asthma Cohort (SAC) study, demonstrating no increase incidence rates of acute pain episodes requiring hospitalization.
| Hargrave et al. | Sleep and Asthma Cohort (SAC) | |
|---|---|---|
| Sample size | N = 95 | N = 140 |
| Number of institutions involved in the study | Single institution | Multi-institution (3 large pediatric centers) |
| Management of hydroxyurea | Excluded from all analyses | Excluded from all analyses |
| Definition of pain | Hospital treatment (emergency department visit or hospital admission) | Hospital admission only |
| Outcome measurement used | Hospital days for pain per years of follow-up | Incidence rate of hospitalization for acute pain per patient year |
| Measurement of oxygen saturation | Pulse oximetry at home (N = 63) or in laboratory (N = 27) | Full 12-channel, in-laboratory research polysomnography with central adjudication (N = 140) |
| Pulse oximetry machine used | Ohmeda Biox 3700 | Masimo SET (v2) |
| Measurement modality | Pulse oximetry (SpO2) | Pulse oximetry (SpO2), oxygen desaturation index (ODI), and obstructive apnea hypopnea index (OAHI) |
| Statistical analysis | Multiple linear regression without adjustment for collinearity | Multiple negative binomial regression with tests for collinearity |
Despite the observation that an increase in OAHI was not associated with an increase in the incidence of ACS in our study, the direction of the association (higher OAHI is associated with higher incidence rate of ACS) is in agreement with our understanding of the pathophysiology of SCA. When including the cohort receiving disease modifying therapy, our results are just barely above the level of statistical significance, and do not exclude the possibility that higher OAHI correlates with higher risk for ACS. In this cohort, the median OAHI of 0.6 is in the range of normal,22 suggesting our population is similar to the general population of children without SCA. Thus, in a child with SCA that has recurrent ACS episodes and evidence of severe OSAS, specific interventions may be considered given the benefits in the general pediatric population.23 However, a prospective cohort study would need to validate the potential benefits and risks of this intervention for decreasing SCA related morbidities or other morbidities.
Our study had several limitations. In our study, hydroxyurea was not associated with a decrease in the rates of pain and ACS during the study period, thus we elected to exclude participants on hydroxyurea and started on chronic blood transfusion therapy after initial PSG. However, our results are generalizable to most children with SCA because despite a clear benefit of hydroxyurea therapy, almost a third of children with severe disease are not on hydroxyurea therapy.24 Additionally, we performed a secondary analysis including those started on hydroxyurea or chronic blood transufion therapy after their first sleep study and found no significant difference in our results. Completion of a sleep study provides numerous parameters to consider to evaluate the relationship between sleep disordered breathing and other characteristics. However, we focused our analysis on only three well established measurements of sleep disordered breathing (mean nocturnal SpO2, ODI, and OAHI). Increasing the number of sleep parameters that may predict acute severe pain or ACS episodes beyond our primary and secondary outcomes would undoubtedly increase the likihood of false positive results and would be considered exploratory.
Our 5-year multi-center prospective cohort study demonstrates that in an unselected group of children with SCA, neither low mean nocturnal SpO2 nor elevated ODI or OAHI measures predict future pain or ACS events. Prior to starting nocturnal oxygen supplementation in children with decreased low mean nocturnal SpO2 or elevated ODI or OAHI measures with the intent of decreasing future SCD related morbidity, careful consideration should be undertaken to weigh the individual risks and benefits.
Supplementary Material
Acknowledgments
Supported in part by the National Heart, Lung, and Blood Institute: NIH 1R01HL079937 (DeBaun), UL1 RR024989 (CWRU CRU) and by Research and Development in the National Health Service (UK)
Footnotes
Authorship Contributions: Drs. Willen and DeBaun had full access to all of the data in the study and take responsibility for the integrity of the data, the accuracy of the data analysis, and the work as a whole. Dr. Willen interpreted the data, created the initial draft, and finalized the manuscript for submission; Dr. Rodeghier developed the statistical approach, performed those analyses, reviewed the integrity of those analyses, and reviewed and revised the manuscript; Dr. Rosen contributed to the development of the SAC project, study concepts and procedures, and reviewed and helped revise the manuscript; Dr. DeBaun served as the principal investigator for the SAC project, design the concepts for SAC and this manuscript, interpreted the results, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The authors have no conflicts of interest to disclose.
References
- 1.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of Sickle haemoglobin in neonates: A contemporary geostatistical model based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaul DK, Suzuka SM, Fabry M. Endothelial activation by sickle mouse red cells and their endothelial adhesion in vivo is abolished by catalase treatment of sickle cells, but not quiescent endothelium, implying a role of heme-mediated peroxide generation in sickle cell adhesion. Blood. 2009;114(22):902. [Google Scholar]
- 3.Hebbel RP, Boogaerts MAB, Eaton JW, Steinberg MH. Erythrocyte Adherence to Endothelium in Sickle-Cell Anemia. N Engl J Med. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 4.Uong EC, Boyd JH, DeBaun MR. Daytime pulse oximeter measurements do not predict incidence of pain and acute chest syndrome episodes in sickle cell anemia. J Pediatr. 2006;149(5):707–709. doi: 10.1016/j.jpeds.2006.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131(1):129–34. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halphen I, Elie C, Brousse V, et al. Severe nocturnal and postexercise hypoxia in children and adolescents with sickle cell disease. PLoS One. 2014;9(5):e97462. doi: 10.1371/journal.pone.0097462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargrave DR, Wade A, Evans JPM, Hewes DKM, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101(3):846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 8.Styles LA, Vichinsky E. Effects of a long-term transfusion regimen on sickle cell-related illnesses. J Pediatr. 1994;125(6 Pt 1):909–11. doi: 10.1016/s0022-3476(05)82006-2. [DOI] [PubMed] [Google Scholar]
- 9.Weber HC, Gie RP, Cotton MF. The challenge of chronic lung disease in HIV-infected children and adolescents. J Int AIDS Soc. 2013;16(1):18633. doi: 10.7448/IAS.16.1.18633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowling MM, Noetzel MJ, Rodeghier MJ, et al. Headache and Migraine in Children with Sickle Cell Disease Are Associated with Lower Hemoglobin and Higher Pain Event Rates But Not Silent Cerebral Infarction. J Pediatr. 2014;164(5):1175–1180 e1. doi: 10.1016/j.jpeds.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vichinsky EP, Styles LA, Colangelo LH, et al. Acute Chest Syndrome in Sickle Cell Disease: Clinical Presentation and Course. Blood. 1997;89(5):1787–1792. [PubMed] [Google Scholar]
- 12.Howard J, Hart N, Roberts-Harewood M, et al. Guideline on the management of acute chest syndrome in sickle cell disease. Br J Haematol. 2015;169(4):492–505. doi: 10.1111/bjh.13348. [DOI] [PubMed] [Google Scholar]
- 13.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashankar FD, Manwani D, Lee MT, Green NS. Hydroxyurea Improves Oxygen Saturation in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2015;37(3):242–3. doi: 10.1097/MPH.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi S, Bhatnagar P, Bean CJ, et al. Genome-wide association study to identify variants associated with vaso-occlusive pain in sickle cell anemia. Blood. 2017 doi: 10.1182/blood-2017-02-769661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen CL, Debaun MR, Strunk RC, et al. Obstructive Sleep Apnea and Sickle Cell Anemia. Pediatrics. 2014;134(2):273–81. doi: 10.1542/peds.2013-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;37(12):3114–3119. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 18.Barker SJ. “Motion-resistant” pulse oximetry: a comparison of new and old models. Anesth Analg. 2002;95(4):967–972. doi: 10.1097/00000539-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Clayton DG, Webb RK, Ralston AC, Dutchie D, Runciman WB. A comparison of the performance of 20 pulse oximeters under conditions of poor perfusion. Anaesthesia. 1991;46(1):3–10. doi: 10.1111/j.1365-2044.1991.tb09303.x. [DOI] [PubMed] [Google Scholar]
- 20.Heeney MM, Hoppe CC, Abboud MR, et al. A Multinational Trial of Prasugrel for Sickle Cell Vaso-Occlusive Events. N Engl J Med. 2016;374(7):625–35. doi: 10.1056/NEJMoa1512021. [DOI] [PubMed] [Google Scholar]
- 21.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR, Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. New England Journal of Medicine. 1995 May 18;332(20):1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 22.Bixler EO, Vgontzas AN, Lin H-M, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venekamp RP, Hearne BJ, Chandrasekharan D, et al. Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children. Cochrane Database Syst Rev. 2015;(10):CD011165. doi: 10.1002/14651858.CD011165.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creary SE, Chisolm DJ, Koch TL, et al. Hydroxyurea use in Children with Sickle Cell Disease: Do Severely Affected Patients Use It and Does It Impact Hospitalization Outcomes? Pediatr Blood Cancer. 2016;63(5):844–847. doi: 10.1002/pbc.25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


