STRUCTURED SUMMARY
Background
Fibrosis in Ulcerative colitis has remained largely unexplored despite its clinical implications.
Aims
This cross-sectional study was aimed at characterizing the presence, anatomical location and degree of Ulcerative colitis-associated fibrosis and its possible link to clinical parameters.
Methods
Seven hundred and six individual tissue cross-sections derived every 10 cm along the length of 89 consecutive Ulcerative colitis colectomy specimens were examined and compared to Crohn’s disease colitis, diverticular disease and uninvolved areas from colorectal cancer patients. Degree of inflammation, fibrosis and morphometric measurements of all layers of the intestinal wall were evaluated. Three gastrointestinal pathologists independently assessed colon sections stained with hematoxylin and eosin, Masson trichrome and Sirius red. Clinical data was collected prospectively.
Results
Submucosal fibrosis was detected in 100% of Ulcerative colitis colectomy specimens, but only in areas affected by inflammation. Submucosal fibrosis was associated with the severity of intestinal inflammation (Spearman correlations rho (95% confidence interval): 0.58 (p<0.001) and histopathologic changes of chronic mucosal injury, but not active inflammation. Colectomy for refractory disease rather than presence of dysplasia was associated with increased fibrosis and a thicker muscularis mucosae, whereas a thinner muscularis mucosae was associated with anti-tumor necrosis factor therapy. No feature on endoscopic mucosal biopsies could predict the underlying amount of fibrosis or the thickness of the muscularis mucosae.
Conclusions
A significant degree of fibrosis and muscularis mucosae thickening should be considered as common complications of chronic progressive Ulcerative colitis. These features may have clinical consequences such as motility abnormalities and increased wall stiffness.
Keywords: Tissue repair, stricture, progressive disease
INTRODUCTION
Fibrosis is a common complication of chronic inflammation and can affect all organs and tissues 1. In the intestine, fibrosis is a well-recognized complication of Crohn’s disease, leading to clinically relevant intestinal strictures in at least one-third of patients throughout their disease course 2, 3. In Crohn’s disease, strictures are predominantly located in the terminal ileum, but are far less common in the colon (around 8%) 4. Very little attention has been devoted to fibrosis in other forms of chronic intestinal inflammation such as Ulcerative colitis. In this form of inflammatory bowel disease, colonic stricture formation also occurs, with strictures ranging from 1% to 11.2%, the large majority of which are benign 5–8.
Fibrosis in the Ulcerative colitis colorectal wall may have significant clinical implications, such as colonic motility abnormalities 9, 10, possibly leading to important symptoms such as loose stools, diarrhea, abdominal discomfort, pain and anorectal dysfunction, or may present as rectal urgency and incontinence associated with a decreased compliance of the rectal wall 11–13. These clinical symptoms can occur in the absence of obvious macroscopic or microscopic inflammation and an apparently normal mucosa, and may be frequently misclassified as irritable bowel syndrome 14, 15.
Fibrosis is defined as an excessive accumulation of extracellular matrix (ECM) and an expansion of the mesenchymal cell pool, and both components are likely to be present in chronic intestinal inflammation-associated strictures, including in Ulcerative colitis. The muscularis mucosae in the area of Ulcerative colitis strictures is markedly thickened, while the other intestinal layers are not different compared to non-strictured areas of Ulcerative colitis or normal colon 16. Some studies have described excessive ECM deposition, mainly in the submucosa, as a significant component of stricturing Ulcerative colitis 17. Surprisingly, few reports of Ulcerative colitis-associated fibrosis outside of the area of strictures are available 18–21. These reports, however, are based on the evaluation of single tissue sections per patient, often comparing distinct colonic locations in different patients, which limit their value in regard to learning about the real frequency and degree of severity of localized fibrosis. Until today, data on global assessment of the extent of fibrosis along the colon length, the overall fibrotic burden, and its correlation with clinical features are missing. Nevertheless, these data may be critical to establish an association with clinical phenotypes, and this study was designed to fill this knowledge gap.
MATERIALS AND METHODS
Patient population
Eighty-nine consecutive Ulcerative colitis patients who underwent total colectomy or proctocolectomy at the Cleveland Clinic in 2007 were included. The diagnosis of Ulcerative colitis was based on clinical, radiographic, endoscopic and histopathologic criteria, and confirmed according to the European Crohn’s and Colitis Organization (ECCO) clinical consensus guidelines 22. Patient demographics and clinical characteristics are shown in Table 1. Exclusion criteria were age below 18 years, the presence of indeterminate colitis or the absence of Masson trichrome stain.
Table 1.
Demographic and Clinical Characteristics
| Factor | Total (N=89) | |
|---|---|---|
|
| ||
| n | Summary | |
| Gender | 89 | |
| Female | 43 (48.3) | |
| Race | 89 | |
| Caucasian | 86 (96.6) | |
| Asian | 1 (1.1) | |
| Other | 2 (2.2) | |
| Age at diagnosis | 86 | 33.8 ± 14.9 |
| Family history (non-exclusive) | ||
| Family History of IBD | 88 | 19 (21.6) |
| Family History of CD | 88 | 10 (11.4) |
| Family History of UC | 88 | 9 (10.2) |
| Smoking | 88 | |
| Never | 57 (64.8) | |
| Remote (Quit >1 month ago) | 28 (31.8) | |
| Current | 3 (3.4) | |
| EIM (non-exclusive) | ||
| Any EIM | 89 | 8 (9.0) |
| Skin | 89 | 3 (3.4) |
| Joint | 89 | 6 (6.7) |
|
| ||
| Disease duration at colectomy (months) | 86 | 62.8 [24.7,123.8] |
| Reason for Colectomy | 89 | |
| Dysplasia / Cancer | 6 (6.7) | |
| Refractory Disease | 81 (91.0) | |
| Other | 2 (2.2) | |
|
| ||
| Disease Extent of Colitis | 89 | |
| Extensive colitis | 84 (94.4) | |
| Left sided colitis | 5 (5.6) | |
| Backwash Ileitis present at time of colectomy | 87 | 16 (18.4) |
|
| ||
| Medication prior to colectomy | ||
| Biologics (at any time) | 85 | 39 (45.9) |
| Immunomodulators (at any time) | 87 | 48 (55.2) |
| 6-MP (at any time) | 87 | 20 (23.0) |
| Duration of 6-MP use (months) | 12 | 8.1 [3.9,15.8] |
| AZA (at any time) | 87 | 26 (29.9) |
| Duration of AZA use (months) | 13 | 23.6 [7.2,38.5] |
| Anti-TNF (at any time) | 87 | 40 (46.0) |
| Duration of anti-TNF use (months) | 23 | 4.7 [1.3,9.0] |
| Medication at time of colectomy | ||
| 6-MP | 86 | 10 (11.6) |
| AZA | 86 | 15 (17.4) |
| Anti-TNF | 86 | 9 (10.5) |
|
| ||
| Length of colectomy specimen (cm) | 89 | 74.5 [60.0,84.0] |
| Rectum included | 87 | 36 (41.4) |
| Post-colectomy follow-up (months) | 89 | 37.1 [10.2,68.5] |
Values presented as Mean ± SD, Median [P25, P75] or N (column %).
Abbreviations: CD-Crohn’s disease, UC-Ulcerative colitis, IBD-Inflammatory bowel disease, EIM-Extraintestinal manifestations, MP-mercaptopurine, AZA-azathioprine, TNF-tumor necrosis factor
The comparison groups consisted of a single representative well-oriented section from each of 30 subjects: 10 patients who underwent colectomy for Crohn’s colitis, 10 patients who underwent sigmoid resection for diverticular disease, and 10 patients who underwent colectomy for colorectal cancer. In the latter patient group sections were obtained at least 2 cm from the neoplastic area in macroscopically and histologically normal colon tissue. The diagnosis of Crohn’s disease was made based on clinical, radiographic, endoscopic and histopathologic criteria as summarized in the ECCO clinical consensus guidelines 23. The diagnosis of diverticular disease was based on pathological assessment, and clinical and radiographic data.
Clinical data including age at diagnosis, disease duration at colectomy, gender, race, indication of colectomy, disease extent including presence of backwash ileitis, duration of pre-operative biologics or immunomodulators, administration of immunomodulators or biologics at the time of colectomy, family history of inflammatory bowel disease, smoking status, presence of extraintestinal manifestations, length of colectomy specimen and post-colectomy follow-up. All data were obtained from a prospectively collected database, and then transferred and stored in a secure coded anonymized database for analysis. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Histopathologic staining procedure
A total of 706 sections from 89 Ulcerative colitis specimens and 30 sections from the comparison groups were stained for hematoxylin & eosin and Masson trichrome using the Gomori One-Step Trichrome kit (Newcomer Supply, Middleton, WI). A set of well oriented control sections from 50 Ulcerative colitis specimens with different degrees of fibrosis and the 30 sections from the comparison groups were additionally stained for Sirius red. The surface area of Sirius red was examined under polarized light to determine the amount of collagen in the tissue, as previously described 24.
Grading of inflammation
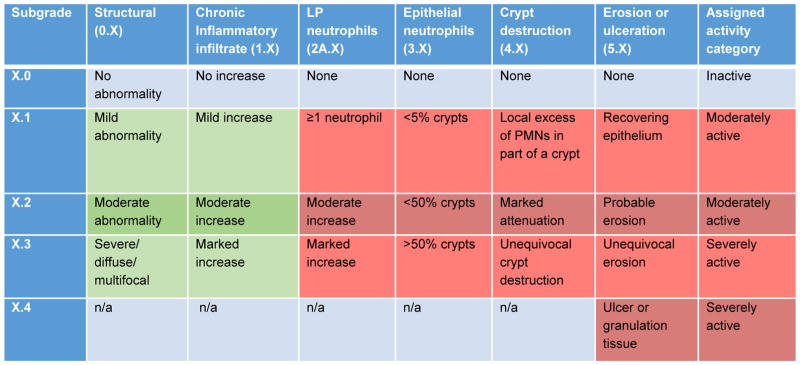
Inflammation was graded based on the Geboes score 25 and we separated histologic features of chronic mucosal injury and active inflammation. This is consistent with current standard pathology terminology for ulcerative colitis 26 and is also reflected in the latest ECCO clinical consensus on Ulcerative colitis 22. The numerical Geboes score was determined as previously described 27, 28 using a continuous scale from 0 to 22 by adding up the numerical values of the seven subscores. We also separated histologic features of chronic mucosal injury and active inflammation were determined as follows: Chronic mucosal injury: a. Yes: Geboes of 0.1, 0.2, 0.3, 1.1, 1.2, or 1.3; b. No: Geboes of 0.0 and 1.0; Active inflammation: a. Inactive: Geboes of 2B.0, 3.0, 4.0 and 5.0; b. Moderately active: 2B.1, 2B.2, 3.1, 3.2, 4.1, 4.2, 5.1 or 5.2; c. Severely active: 2B.3, 3.3, 4.3, 5.3 or 5.4. Involved segments were classified as having active inflammation and/or chronic mucosal injury. This is consistent with current standard pathology terminology for ulcerative colitis 26 and is also reflected in the latest ECCO clinical consensus on Ulcerative colitis 22. The classification is depicted in Figure 1.
Figure 1. Grading of inflammation.
Inflammation was graded based on the Geboes score 25, and separated into active inflammation and chronic mucosal injury. Green indicates chronic mucosal injury and red indicates active inflammation.
Assessment of fibrosis
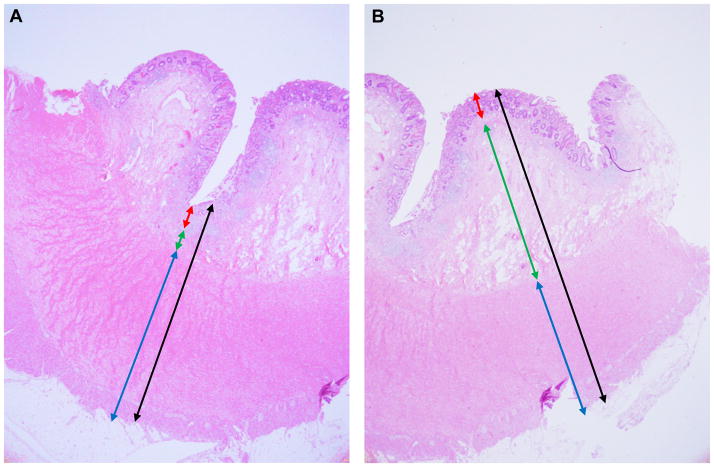
Fibrosis was assessed qualitatively and quantitatively in each section. Quantitative assessment was performed using calibrated imaging software (CellSens, Olympus Corporation, Tokyo, Japan). Specific measurements of the intestinal layers were determined on hematoxylin and eosin-stained sections and included a full-thickness measurement, as well as a measurement of each histologic bowel wall layer (mucosa, muscularis mucosae, submucosa, muscularis propria interna, muscularis propria externa, and complete muscularis propria). These measurements were performed in two planes per slide, corresponding to the most and least submucosa present (maximum and minimum submucosal thickness) (Figure 2). Qualitative assessment was performed by an experienced gastrointestinal pathologist (IOG) taking into account all of the tissue on the slide. Lamina propria fibrosis was categorized as present or absent, regardless of extent. Submucosal fibrosis was categorized as none, 1–25%, 26–50%, 51–75%, or 76–100% of the submucosa affected, corresponding to a fibrosis score of 0, 1, 2, 3, and 4, respectively. The ‘fibrosis burden score’ was defined as the average fibrosis score across all the involved segments. The fibrosis burden score was calculated per patient. As control the same fibrosis burden score was calculated for the non-involved segments. To illustrate the method we included Supplemental Table 1.
Figure 2. Measurement method for whole thickness intestinal cross sections.
Specific measurements were determined on hematoxylin and eosin stained sections and included a full-thickness measurement, as well as a measurement of each histologic intestinal wall layer (mucosa, muscularis mucosae, submucosa, muscularis propria). These measurements were performed in two areas per slide, corresponding to the highest (A) and lowest (B) thickness of submucosa present. Arrow colors: black-full thickness; blue-muscularis propria; green-submucosa; red-mucosa
Interobserver variability
Three gastrointestinal pathologists reviewed all well-oriented hematoxylin and eosin- and Masson trichrome-stained sections of colonic resections of Ulcerative colitis (n=50) with varying degrees of fibrosis and from the comparison groups including Crohn’s disease (n=10), diverticular disease (n=10), and non-neoplastic segments from colon carcinoma (n=10) resections. After review of a learning set with examples from different cases, sections for the study were assessed for presence or absence of lamina propria fibrosis and qualitative extent of submucosal fibrosis, using methods as described in ‘Assessment of fibrosis’ above.
Comparison of Trichrome and Sirius red staining
To assign the pathologist’s assessment of fibrosis to an objective measurement, the above described 80 (50+10+10+10) sections stained for Sirius red were scanned, and the percent surface area positive for Sirius red under polarized light was automatically quantified by ImagePro software (Media Cybernetics, Rockville, MD). The obtained percentages of submucosa covered by matrix were rescaled to match the pathologists’ assessment range from 0 to 100%, as described in the section ‘Assessment of fibrosis’, using the formula: 100* ((Value-Minimum)/(Maximum-Minimum)).
Association of histopathological biopsy findings with degree of fibrosis
To assess whether fibrosis in Ulcerative colitis is associated with histologic findings on mucosal biopsies obtained during endoscopies we included 21 Ulcerative colitis patients that underwent colonoscopy and endoscopic mucosal biopsies < 4 weeks prior to colectomy. In these 21 Ulcerative colitis patients, biopsies were taken from a total of 39 intestinal segments and these 39 segments were matched with the corresponding histologic cross section at time of resection. Demographics can be found in Table 2. Biopsies were stained for hematoxylin and eosin and Masson trichrome and assessed for presence of lamina propria fibrosis, thickening of the muscularis mucosae, and morphologic assessment of uniformly thickened, split or splayed muscularis mucosae. If sufficient submucosa was present (n=19 biopsies), extent of submucosal fibrosis was scored as described above. Biopsies were graded according to the Geboes score as described above.
Table 2.
Demographic and Clinical Characteristics in Patients with Endoscopic Mucosal Biopsies
| Factor | Total (N=21) | |
|---|---|---|
|
| ||
| n | Statistics | |
| Gender | 21 | |
| Female | 11 (52.4) | |
| Race | 21 | |
| Caucasian | 20 (95.2) | |
| Other | 1 (4.8) | |
| Age at diagnosis | 20 | 33.1 ± 13.9 |
| Family history (non-exclusive) | ||
| Family History of IBD | 21 | 2 (9.5) |
| Family History of CD | 21 | 2 (9.5) |
| Family History of UC | 21 | 0 (0.0) |
| Smoking | 21 | |
| Never | 12 (57.1) | |
| Remote (Quit >1 month ago) | 8 (38.1) | |
| Current | 1 (4.8) | |
| EIM (non-exclusive) | ||
| Any EIM | 21 | 1 (4.8) |
| Joint | 21 | 1 (4.8) |
|
| ||
| Disease duration at colectomy (months) | 20 | 56.7 [15.4,135.1] |
| Reason for Colectomy | 21 | |
| Dysplasia / Cancer | 4 (19.0) | |
| Refractory Disease | 17 (81.0) | |
|
| ||
| Disease Extent of Colitis | 21 | |
| Extensive colitis | 20 (95.2) | |
| Left sided colitis | 1 (4.8) | |
| Backwash Ileitis present at time of colectomy | 20 | 5 (25.0) |
|
| ||
| Medications prior to colectomy | ||
| Pre-OP Biologics (at any time) | 20 | 7 (35.0) |
| Pre-OP Immunomodulators (at any time) | 21 | 11 (52.4) |
| 6-MP | 21 | 6 (28.6) |
| Duration of 6-MP use (months) | 5 | 7.9 [1.3,8.2] |
| AZA | 21 | 4 (19.0) |
| Duration of AZA use (months) | 2 | 19.9 [4.3,35.6] |
| Anti-TNF | 21 | 7 (33.3) |
| Duration of anti-TNF use (months) | 5 | 2.0 [0.46,10.8] |
| Medications at time of colectomy | ||
| 6-MP | 21 | 3 (14.3) |
| AZA | 21 | 2 (9.5) |
| Anti-TNF | 21 | 1 (4.8) |
|
| ||
| Length of colectomy specimen (cm) | 21 | 74.5 [66.0,83.0] |
| Rectum included | 21 | 10 (47.6) |
| Post-colectomy follow-up (months) | 21 | 46.6 [12.2,74.3] |
Statistics presented as Mean ± SD, Median [P25, P75] or N (column %).
Abbreviations: CD-Crohn’s disease, UC-Ulcerative colitis, IBD-Inflammatory bowel disease, EIM-Extraintestinal manifestations, MP-mercaptopurine, AZA-azathioprine, TNF-tumor necrosis factor, OP-operation
Statistical analysis
Data are presented as mean ± standard deviation, median [25th, 75th percentiles] or N (%). Inflammation & Fibrosis Measures: Each segment of the colon was classified according to the grade of inflammation (active inflammation yes/no, chronic mucosal injury yes/no and active inflammation and/or chronic mucosal injury). Generalized linear mixed models were used to compare segments with and without inflammation while accounting for multiple measures per patient. Fibrosis Burden: The fibrosis burden score for each patient was calculated by averaging the fibrosis score in involved and non-involved colon segments, separately. The fibrosis burden scores for involved and non-involved were compared using a Wilcoxon signed rank test. In addition, a univariable analysis was performed to assess factors associated with fibrosis burden in involved areas; Kruskal-Wallis tests were used to assess association between burden score and several categorical factors of interest. Spearman’s correlation coefficients were used to assess association between fibrosis burden and disease duration and length of colectomy specimen. The same was done using average muscularis mucosae thickness as a measure of fibrosis burden. Variation of fibrosis measures across the intestine was also assessed. Area of fibrosis and thickness measures were plotted from distal to proximal colon and linear mixed models were used to assess trend while accounting for multiple segments per patient. Geboes Score & Fibrosis: The Geboes score was calculated for each segment and linear mixed models were used to assess relationship of Geboes score and area of fibrosis as well as muscularis mucosae thickness while accounting for multiple slides per patient (per segment analysis). To assess whether the association varied according to the presence of inflammation (active inflammation and/or chronic mucosal injury), the interaction between fibrosis burden and Geboes score was tested; the same was done for muscularis mucosae thickness. Comparison of Masson trichrome and Sirius red: Bowker’s test of symmetry was used to compare submucosal area of fibrosis categories between Trichrome and Sirius Red. In addition, we compared submucosal fibrosis percent by using Jonckheere-Terpstra test. Presence of any fibrosis in the mucosa was compared using McNemar’s test. Area of fibrosis was determined using Wilcoxon rank sum test. Biopsy results versus degree of fibrosis in the cross sections: Generalized estimating equations (GEE) were used to assess associations between the various features measured from biopsy samples and area of fibrosis modeled as the outcome using a cumulative logit link function. In addition, linear mixed models were used to assess relationship between the various features measured from biopsy samples and muscularis mucosae thickness measured from resection samples. To assess agreement between biopsy and resection findings we assumed independence between segments and used McNemar’s test to assess agreement for binary findings and Wilcoxon signed rank test. A p < 0.05 was considered statistically significant. All analyses were performed using SAS (version 9.4, The SAS Institute, Cary, NC).
RESULTS
Clinical phenotypes of the population
A total of 89 Ulcerative colitis subjects (48.3% female and 96.6% Caucasian) were included in the final analysis (Table 1). The mean age at diagnosis was 33.8 years, 21.6 % had a family history of inflammatory bowel disease, 64.8% never smoked, and 9% had an extraintestinal manifestation, primarily joint manifestations. Prior to colectomy 55.2% received at least one immunomodulatory therapy and 45.9% had anti-tumor necrosis factor therapy. The median disease duration at time of colectomy was 62.8 months; 91% underwent colectomy for refractory disease, which was extensive in 94.4% of cases. To ensure that the diagnosis of Ulcerative colitis was correct, patients were followed for a median of 37.1 months and none of them developed Crohn’s disease or other forms of inflammation during this period of time.
Establishing the fibrosis score and interobserver agreement
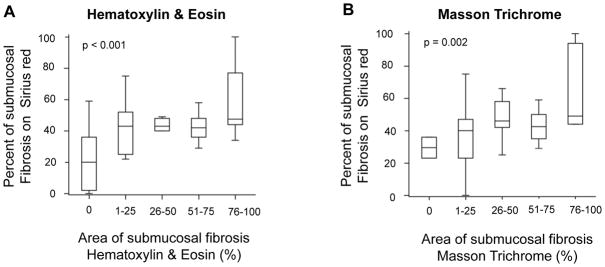
Prior to assessing the presence and quantity of intestinal fibrosis, an interobserver agreement of the fibrosis score was established among three gastrointestinal pathologists. For this purpose, a set of 80 slides (50 Ulcerative colitis with different degrees of fibrosis, 10 Crohn’s colitis, 10 diverticular disease, and 10 non-neoplastic sections from carcinoma resections) was selected. Submucosal fibrosis scores were grouped as low (score 0–1), moderate (score 2–3), and high (score 4). These criteria were chosen based on agreement among the pathologists. There was moderate interobserver agreement for Ulcerative colitis submucosal fibrosis based on hematoxylin and eosin (kappa = 0.63) and Masson trichrome (kappa = 0.57) staining. There was also moderate interobserver agreement for lamina propria fibrosis based on hematoxylin and eosin (kappa = 0.67) and Masson trichrome (kappa = 0.46) staining. These results suggest that submucosal and lamina propria fibrosis can be adequately assessed using hematoxylin and eosin, and that a “fibrosis-specific” stain, such as Masson trichrome, does not appreciably improve the detection and quantification of fibrosis in Ulcerative colitis. Our finding of moderate interobserver agreement for assessing fibrosis in the bowel wall is similar to what has been reported for pathologist assessment of fibrosis in other organs, including liver 29, kidney 30, and lung 31 (Supplemental Table 2). To investigate whether the pathologists’ scoring based on hematoxylin and eosin or Masson trichrome correlates with objective quantitative measures of fibrosis, we additionally stained the above Ulcerative colitis specimens for Sirius red and automatically quantified areas of fibrosis as stated in Material and Methods. The percentage of area for submucosal fibrosis measured by Sirius red increased in parallel with increasing fibrosis assessed by either hematoxylin and eosin (p<0.001) or Masson trichrome (p=0.002) (Figure 3). This indicates a strong positive correlation between the pathologists’ subjective assessment of fibrosis with an automated machine-based quantification method.
Figure 3. Degree of submucosal fibrosis in Ulcerative colitis.
Correlation between Sirius red vs. Hematoxylin & Eosin (A) and Masson Trichrome (B)
Determination of extent of inflammation and fibrosis
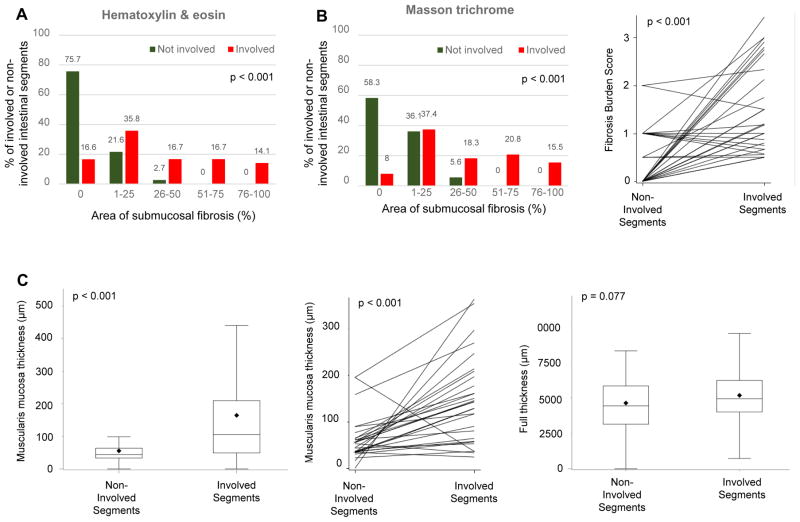
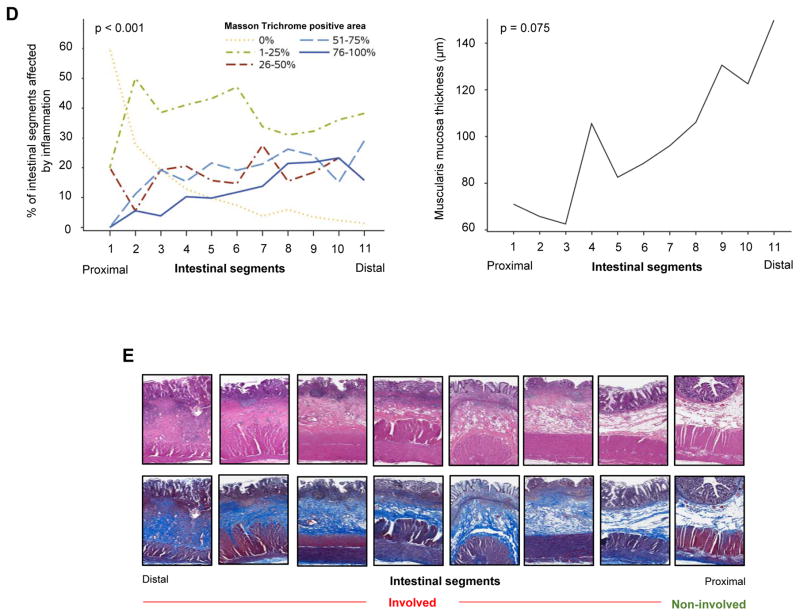
After establishing the quantification systems for inflammation and fibrosis, we first evaluated their overall magnitude. For each of the included 89 patients, between 3 and 20 colon segments were studied, with an average of 8±3 segments (median [%tiles]: 8 [6–10]) per patient leading to a total of 706 segments. We detected features of active inflammation or chronic mucosal injury in at least one segment from every subject. 60.7% and 53.9% had features of active inflammation and chronic mucosal injury, respectively (non-exclusive), in all segments of the colon. The median Geboes score was 8, confirming that inflammation of various degrees was present diffusely in the majority of Ulcerative colitis segments. In contrast, the presence of fibrosis was more restricted, being detected almost exclusively in the involved segments (median 1.7 involved versus 0 in non-involved on hematoxylin and eosin stain or median 2 involved versus 0.5 in non-involved on Masson trichrome stain (Figure 4A, B & E). Fibrosis burden was compared in 24 patients who had both involved and non-involved colonic segments, and found that fibrosis burden was significantly higher in the involved areas (p<0.001), and this was true for hematoxylin and eosin and Masson trichrome stain (Table 3; Figure 4B). In addition, the thickness of the muscularis mucosae was markedly higher in the involved compared to the non-involved segments (142.8 versus 48.6 μm; p<0.001). In the 24 patients who had both involved and non-involved segments, muscularis mucosae thickness was also significantly higher in involved areas (p<0.001) (Table 3; Figure 4C & E). The same was true for the thickness of the lamina propria (p<0.001, data not shown), but not the other layers of the intestinal wall, leading to an overall increase in wall thickness. We additionally constructed longitudinal plots to show variation in the degree of fibrosis assessing only the inflamed areas along the colon (Figure 4D & E). The degree of fibrosis increased from the proximal to distal segments (p<0.001), and a comparable pattern was found for the thickness of the muscularis mucosae (p = 0.075) (Figure 4D & E).
Figure 4. Fibrosis burden in involved and non-involved areas in Ulcerative colitis.
(A) Comparison of the degree of fibrosis on hematoxylin & eosin stain between involved and non-involved segments of Ulcerative colitis colon (n=706). (B) Comparison of the degree of fibrosis on Masson trichrome stain between involved and non-involved segments of the Ulcerative colitis colon (left panel: entire cohort (n=706); right panel: within the same patient (n=24)). (C) Comparison of the degree of muscularis mucosae thickening between involved and non-involved segments of Ulcerative colitis colon (entire cohort n=706 in bar graph on the left, involved and non-involved segments within the same patient n=24 in middle). The thickness of the entire intestinal wall remained unchanged (n=706 in bar graph on right). (D) Longitudinal plots depicting the variation for the degree of fibrosis (as evaluated by Masson trichrome, left side) and degree of muscularis mucosae thickening (right side) only in the areas affected by inflammation. The percent of segments with a particular degree of fibrosis (0–100%) is depicted. (E) Representative examples of hematoxylin & eosin as well as Masson trichrome stained colon sections showing involved and non-involved segments.
Table 3.
Geboes Score & Categorical Extent of Fibrosis
| Factor | Total (N=89) | |
|---|---|---|
|
| ||
| n | Summary | |
| Geboes Score | ||
| Any chronic inflammation | 89 | 89 (100.0) |
| Chronic inflammation in all segments | 89 | 48 (53.9) |
| Any active inflammation | 89 | 89 (100.0) |
| Active inflammation in all segments | 89 | 54 (60.7) |
| Percent of colon segements involved (chronic and/or active) | 89 | 100.0 [90.0,100.0] |
| Total Geboes Score | 89 | 8.0 [5.4,9.7] |
| Categorical Extent of Fibrosis | ||
| Fibrosis Burden in involved segments (H&E) | 89 | 1.7 [0.89,2.6] |
| Fibrosis Burden in non-involved segments (H&E), if any | 24 | 0.00 [0.00,0.75] |
| Fibrosis Burden in involved segments (Masson trichrome) | 89 | 2.0 [1.1,2.9] |
| Fibrosis Burden in non-involved segments (Masson trichrome), if any | 23 | 0.50 [0.00,1.00] |
| Numerical Measurement of Fibrosis | ||
| Muscularis mucosa thickness in involved areas | 89 | 142.8 [80.3,213.5] |
| Muscularis mucosa thickness in non-ivolved areas | 24 | 48.6 [34.6,62.5] |
Values presented as Median [P25, P75] or N (column %).
Abbreviations: H&E: Hematoxylin & eosin
A so called ‘lead pipe colon’ has been described in chronic Ulcerative colitis, indicating a shortened and stiff colon by radiological or endoscopic findings 32 that could be related to the amount of fibrosis or thickness of the muscularis mucosae in the organ. To account for anatomic and surgical variation, we evaluated the proctocolectomy specimens separately. There was no significant correlation between the specimen length and the fibrosis burden measured by hematoxylin and eosin in involved (rho=−011; p=0.53) or non-involved areas (rho=0.02; p=0.98). The fibrosis burden measured by Masson trichrome was higher but still did not significantly correlate with colon length. A significant correlation was also absent between length of colectomy specimen and average muscularis mucosae thickness in involved or non-involved areas.
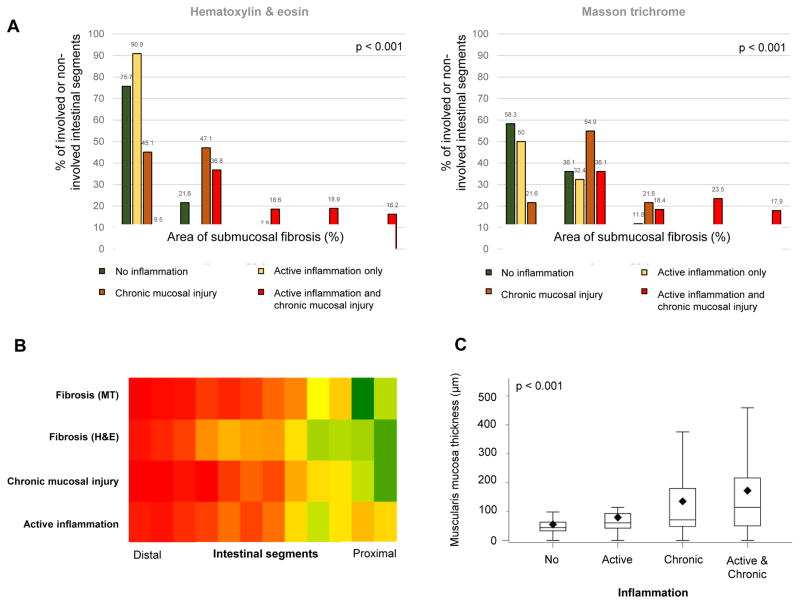
Chronic mucosal injury, but not active inflammation is linked to fibrosis in Ulcerative colitis
To evaluate if active inflammation or chronic mucosal injury, defined as stated in Material and Methods, influence the degree of fibrosis we separated each segment into no inflammation, active inflammation only, chronic mucosal injury only and active inflammation plus chronic mucosal injury at the same time (Figure 5). There was a clear association (p < 0.001), with no inflammation having the lowest and chronic mucosal injury plus active inflammation having the highest degree of fibrosis. Comparable results were obtained when investigating the muscularis mucosae with the greatest thickness in patients with active inflammation and chronic mucosal injury at the same time (Figure 5). As a control, we additionally measured the thickness of all other layers of the intestinal wall. The overall wall thickness was highest in the segments with active inflammation plus chronic mucosal injury and this increase was mainly driven by an increased thickness of the lamina propria and muscularis mucosae (data not shown).
Figure 5. Fibrosis and muscularis mucosae thickness in Ulcerative colitis colonic segments grouped according to active inflammation and chronic mucosal injury.
(A) Bar graphs show segments with no inflammation, active inflammation only, chronic mucosal injury only or both active inflammation and chronic mucosal injury linked to degree of fibrosis (0–100%) as measured by hematoxylin & eosin (left) and Masson trichrome (right). (B) Heat map for distribution of grades of active inflammation, chronic mucosal injury and degrees of fibrosis along the colon. Segments were ordered from distal to proximal. The grades of active inflammation, chronic mucosal injury and degree of fibrosis were plotted as the signal with red indicating a high and green indicating a low severity. The orange colored segments in the proximal area for active inflammation represent increased active inflammation, but no chronic mucosal changes due to cecal patches. (C) Box plots showing segments with no inflammation, active inflammation only (active), chronic mucosal injury only (chronic) or both active inflammation and chronic mucosal injury (active and chronic) linked to the thickness of the muscularis mucosae.
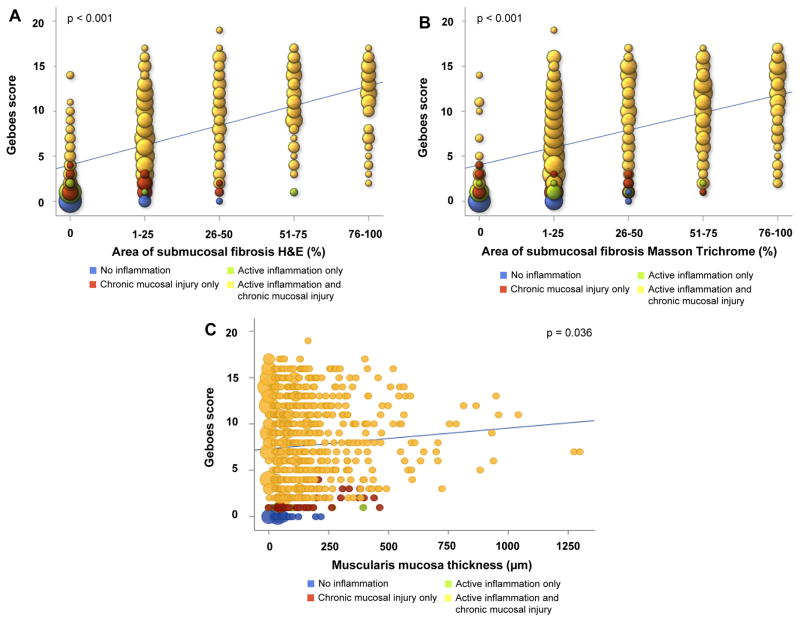
After accounting for multiple segments per patient, fibrosis burden and muscularis mucosae thickness were both found to be associated with the numerical total Geboes score. For every unit increase in the hematoxylin and eosin fibrosis score, the Geboes score increased an average of 2.2 points (95% CI: 1.9, 2.4; p<0.001). Similarly, for every unit increase in Masson trichrome fibrosis score the Geboes score increased an average of 1.9 points (95% CI: 1.7, 2.2; p<0.001). For every 100 μm increase in the muscularis mucosae thickness, the Geboes score increased an average of 0.22 points (95% CI: 0.015. 0.43; p=0.034) (Figure 6). Among all immune cells present in the inflamed mucosa, in particular eosinophils have been reported to be linked to fibrosis of several organs 33. Eosinophils in the lamina propria, analyzed separately using Geboes Grade 2A criteria, were not linked to the degree of fibrosis in Ulcerative colitis (data not shown).
Figure 6. Correlation between degree of intestinal inflammation and submucosal fibrosis or muscularis mucosae thickness.
(A) Hematoxylin & eosin stain and numerical Geboes score; (B) Masson trichrome and numerical Geboes score; (C) Thickness of the muscularis mucosae and numerical Geboes score. The different histologic findings are color coded. The size of the circles represents the number of patients per condition.
Overall fibrosis burden and clinical characteristics
Clinical characteristics could influence the degree of fibrosis in Ulcerative colitis. We investigated an association between clinical characteristics, medication intake and the overall fibrosis burden (Table 4). Refractory disease, as opposed to dysplasia, as the indication for colectomy was the only clinical feature linked to the overall burden of fibrosis in Ulcerative colitis based on both hematoxylin and eosin (p=0.023) and Masson trichrome (p=0.009) staining. Interestingly, none of the other clinical features, including disease duration, age at colectomy or location/extent of disease were associated with fibrosis. There was a strong agreement between assessment of fibrosis burden on hematoxylin and eosin compared to Masson trichrome, with male gender being the only additional parameter associated with fibrosis burden in Ulcerative colitis. All medications (intake or duration of administration either prior to or at time of colectomy) also failed to have an association with the degree of fibrosis (Table 4). We also performed a subgroup analysis in patients with refractory disease only. In this subgroup, none of the examined clinical parameters were associated with the fibrotic burden on Masson trichrome and only male gender conferred a higher risk for fibrosis on hematoxylin and eosin (Table 5).
Table 4.
Fibrosis Burden and Clinical Characteristics
| Factor | n | Hematoxylin & Eosin | Masson trichrome | ||
|---|---|---|---|---|---|
|
|
|
||||
| Fibrosis Burden | p-value | Fibrosis Burden | p-value | ||
| Gender | 0.019 | 0.18 | |||
| Male | 46 | 2.1 [1.00, 2.8] | 2.4 [1.2, 3.0] | ||
| Female | 43 | 1.2 [0.75, 2.5] | 1.8 [1.1, 2.7] | ||
| Family History of IBD | 0.56 | 0.72 | |||
| No | 69 | 1.8 [0.88, 2.6] | 2.0 [1.1, 2.8] | ||
| Yes | 19 | 1.2 [1.00, 2.5] | 1.9 [1.1, 3.0] | ||
| Family History of CD | 0.58 | 0.81 | |||
| No | 78 | 1.8 [0.88, 2.7] | 1.9 [1.1, 2.9] | ||
| Yes | 10 | 1.3 [1.00, 2.0] | 2.3 [1.00, 3.0] | ||
| Family History of UC | 0.83 | 0.81 | |||
| No | 79 | 1.8 [0.88, 2.6] | 2.0 [1.1, 2.9] | ||
| Yes | 9 | 1.1 [1.00, 2.7] | 1.5 [1.1, 3.0] | ||
| Smoking | 0.47 | 0.84 | |||
| No | 57 | 1.5 [0.86, 2.5] | 1.8 [1.1, 2.9] | ||
| Yes | 31 | 1.8 [1.00, 2.7] | 2.0 [1.00, 2.9] | ||
| EIM | 0.35 | 0.53 | |||
| No | 81 | 1.8 [1.00, 2.6] | 2.0 [1.1, 2.9] | ||
| Yes | 8 | 0.94 [0.65, 2.4] | 1.2 [1.07, 2.8] | ||
| EIM: Joint | 0.26 | 0.29 | |||
| No | 83 | 1.8 [1.00, 2.7] | 2.0 [1.1, 3.0] | ||
| Yes | 6 | 0.94 [0.80, 1.8] | 1.2 [1.00, 2.7] | ||
|
| |||||
| Disease duration at colectomy (months) | 86 | −0.13 (−0.33, 0.09) | 0.25 | −0.16 (−0.36, 0.06) | 0.15 |
| Reason for colectomy | 0.023 | 0.009 | |||
| Dysplasia/Cancer or other | 8 | 0.66 [0.49, 1.7] | 0.92 [0.60, 1.7] | ||
| Refractory disease | 81 | 1.8 [1.00, 2.7] | 2.0 [1.2, 3.0] | ||
| Backwash Ileitis | 0.06 | 0.14 | |||
| No | 71 | 1.5 [0.88, 2.6] | 1.9 [1.00, 2.9] | ||
| Yes | 16 | 2.5 [1.3, 2.8] | 2.4 [1.5, 3.1] | ||
|
| |||||
| Disease Extent of Colitis | 0.59 | 0.99 | |||
| Extensive colitis | 84 | 1.8 [0.94, 2.6] | 2.0 [1.1, 2.9] | ||
| Left sided colitis | 5 | 1.2 [0.88, 1.5] | 1.6 [1.00, 3.0] | ||
|
| |||||
| Pre-OP Biologics (at any time) | 0.97 | 0.38 | |||
| No | 46 | 1.6 [0.80, 2.7] | 1.8 [1.00, 2.9] | ||
| Yes | 39 | 1.5 [1.00, 2.5] | 2.3 [1.2, 3.0] | ||
| Pre-OP Immunomodulators (at any time) | 0.55 | 0.56 | |||
| No | 39 | 1.5 [0.71, 2.7] | 1.8 [1.00, 3.0] | ||
| Yes | 48 | 1.8 [1.00, 2.5] | 2.0 [1.2, 2.7] | ||
| Pre-OP 6-MP | 0.86 | 0.26 | |||
| No | 67 | 1.5 [0.88, 2.7] | 2.3 [1.1, 3.0] | ||
| Yes | 20 | 1.8 [1.3, 2.4] | 1.9 [1.07, 2.5] | ||
| Duration of 6-MP use (mo) | 23 | −0.21 (−0.57, 0.22) | 0.34 | −0.29 (−0.63, 0.13) | 0.17 |
| Pre-OP AZA | 0.58 | 0.65 | |||
| No | 61 | 1.8 [0.88, 2.7] | 2.0 [1.1, 3.0] | ||
| Yes | 26 | 1.4 [1.00, 2.0] | 1.8 [1.1, 2.8] | ||
| Duration of AZA use (mo) | 12 | −0.21 (−0.70, 0.42) | 0.53 | −0.34 (−0.76, 0.29) | 0.29 |
| Pre-OP Anti-TNF | 0.73 | 0.5 | |||
| No | 47 | 1.8 [0.83, 2.8] | 1.9 [1.00, 3.0] | ||
| Yes | 40 | 1.5 [1.00, 2.5] | 2.3 [1.2, 2.9] | ||
| Duration of anti-TNF use (mo) | 13 | −0.12 (−0.63, 0.46) | 0.71 | −0.08 (−0.60, 0.50) | 0.81 |
|
| |||||
| Meds at colectomy: 6-MP | 0.62 | 0.47 | |||
| No | 76 | 1.6 [0.88, 2.6] | 2.2 [1.1, 3.0] | ||
| Yes | 10 | 1.8 [1.3, 2.7] | 1.9 [1.3, 2.5] | ||
| Meds at colectomy: AZA | 0.49 | 0.95 | |||
| No | 71 | 1.8 [0.89, 2.7] | 2.0 [1.1, 3.0] | ||
| Yes | 15 | 1.2 [0.80, 2.4] | 1.8 [1.1, 2.9] | ||
| Meds at colectomy: Anti-TNF | 0.31 | 0.087 | |||
| No | 77 | 1.5 [0.88, 2.7] | 1.8 [1.1, 2.9] | ||
| Yes | 9 | 2.2 [1.8, 2.6] | 2.7 [2.4, 3.0] | ||
Values presented as median [25th, 75th percentile] with Kruskal-Wallis test or Spearman’s rho (95% CI).
Abbreviations: CD-Crohn’s disease, UC-Ulcerative colitis, IBD-Inflammatory bowel disease, EIM-Extraintestinal manifestations, MP-mercaptopurine, AZA-azathioprine, TNF-tumor necrosis factor, OP-operation
Table 5.
Fibrosis Burden and Clinical Characteristics - Patients with Refractory Disease
| Factor | n | Hematoxylin & Eosin | Masson trichrome | ||
|---|---|---|---|---|---|
|
|
|
||||
| Fibrosis Burden | p-value | Fibrosis Burden | p-value | ||
| Gender | 0.039 | 0.29 | |||
| Male | 42 | 2.1 [1.2, 2.8] | 2.4 [1.2, 3.0] | ||
| Female | 39 | 1.3 [0.83, 2.5] | 1.9 [1.2, 2.7] | ||
| Family History of IBD | 0.29 | 0.95 | |||
| No | 61 | 1.8 [1.00, 2.7] | 2.0 [1.3, 2.9] | ||
| Yes | 19 | 1.2 [1.00, 2.5] | 1.9 [1.1, 3.0] | ||
| Family History of CD | 0.38 | 0.98 | |||
| No | 70 | 1.8 [1.00, 2.7] | 2.0 [1.3, 2.9] | ||
| Yes | 10 | 1.3 [1.00, 2.0] | 2.3 [1.00, 3.0] | ||
| Family History of UC | 0.62 | 0.95 | |||
| No | 71 | 1.8 [1.00, 2.6] | 2.0 [1.2, 2.9] | ||
| Yes | 9 | 1.1 [1.00, 2.7] | 1.5 [1.1, 3.0] | ||
| Smoking | 0.28 | 0.87 | |||
| No | 53 | 1.5 [0.89, 2.5] | 1.9 [1.2, 3.0] | ||
| Yes | 27 | 2.0 [1.00, 2.7] | 2.3 [1.3, 2.9] | ||
| EIM | 0.56 | 0.56 | |||
| No | 74 | 1.8 [1.00, 2.7] | 2.1 [1.3, 3.0] | ||
| Yes | 7 | 1.00 [0.80, 3.0] | 1.2 [1.00, 3.0] | ||
| EIM: Joint | 0.47 | 0.33 | |||
| No | 76 | 1.8 [1.00, 2.7] | 2.1 [1.3, 3.0] | ||
| Yes | 5 | 1.00 [0.89, 1.8] | 1.2 [1.00, 2.7] | ||
|
| |||||
| Disease duration at colectomy (months) | 78 | −0.03 (−0.25, 0.20) | 0.81 | −0.09 (−0.31, 0.13) | 0.43 |
| Backwash Ileitis | 0.098 | 0.24 | |||
| No | 64 | 1.6 [0.94, 2.6] | 2.0 [1.2, 2.9] | ||
| Yes | 16 | 2.5 [1.3, 2.8] | 2.4 [1.5, 3.1] | ||
|
| |||||
| Disease Extent of Colitis | 0.84 | 0.68 | |||
| Extensive colitis | 77 | 1.8 [1.00, 2.7] | 2.0 [1.2, 2.9] | ||
| Left sided colitis | 4 | 1.4 [1.04, 2.3] | 2.3 [1.3, 3.3] | ||
|
| |||||
| Pre-OP Biologics (at any time) | 0.49 | 0.91 | |||
| No | 38 | 1.8 [1.00, 2.7] | 2.0 [1.1, 3.0] | ||
| Yes | 39 | 1.5 [1.00, 2.5] | 2.3 [1.2, 3.0] | ||
| Pre-OP Immunomodulators (at any time) | 0.77 | 0.1 | |||
| No | 31 | 2.1 [0.86, 2.9] | 2.4 [1.3, 3.2] | ||
| Yes | 48 | 1.8 [1.00, 2.5] | 2.0 [1.2, 2.7] | ||
| Pre-OP 6-MP | 0.88 | 0.13 | |||
| No | 60 | 1.8 [0.94, 2.8] | 2.4 [1.2, 3.0] | ||
| Yes | 20 | 1.8 [1.3, 2.4] | 1.9 [1.07, 2.5] | ||
| Duration of 6-MP use (mo) | 23 | −0.21 (−0.57, 0.22) | 0.34 | −0.29 (−0.63, 0.13) | 0.17 |
| Pre-OP AZA | 0.34 | 0.35 | |||
| No | 54 | 2.1 [1.00, 2.7] | 2.3 [1.3, 3.0] | ||
| Yes | 26 | 1.4 [1.00, 2.0] | 1.8 [1.1, 2.8] | ||
| Duration of AZA use (mo) | 12 | −0.21 (−0.70, 0.42) | 0.53 | −0.34 (−0.76, 0.29) | 0.29 |
| Pre-OP Anti-TNF | 0.36 | 0.94 | |||
| No | 40 | 1.9 [1.00, 2.8] | 2.0 [1.2, 3.0] | ||
| Yes | 40 | 1.5 [1.00, 2.5] | 2.3 [1.2, 2.9] | ||
| Duration of anti-TNF use (mo) | 13 | −0.12 (−0.63, 0.46) | 0.71 | −0.08 (−0.60, 0.50) | 0.81 |
|
| |||||
| Meds at colectomy: 6-MP | 0.83 | 0.29 | |||
| No | 68 | 1.8 [1.00, 2.7] | 2.3 [1.2, 3.0] | ||
| Yes | 10 | 1.8 [1.3, 2.7] | 1.9 [1.3, 2.5] | ||
| Meds at colectomy: AZA | 0.28 | 0.63 | |||
| No | 63 | 1.8 [1.00, 2.7] | 2.3 [1.3, 3.0] | ||
| Yes | 15 | 1.2 [0.80, 2.4] | 1.8 [1.1, 2.9] | ||
| Meds at colectomy: Anti-TNF | 0.42 | 0.14 | |||
| No | 69 | 1.7 [1.00, 2.7] | 1.9 [1.2, 3.0] | ||
| Yes | 9 | 2.2 [1.8, 2.6] | 2.7 [2.4, 3.0] | ||
Values presented as median [25th, 75th percentile] with Kruskal-Wallis test or Spearman’s rho (95% CI).
Abbreviations: CD-Crohn’s disease, UC-Ulcerative colitis, IBD-Inflammatory bowel disease, EIM-Extraintestinal manifestations, MP-mercaptopurine, AZA-azathioprine, TNF-tumor necrosis factor, OP-operation
Based on the above data, thickening of the muscularis mucosae seems to be a hallmark of fibrosis in Ulcerative colitis. We therefore linked the prior tested clinical characteristics to the average thickness of the muscularis mucosae in the involved areas. Considering all patients, the longer the disease duration, the thicker was the muscularis mucosae (Table 6), and preoperative biologic use was associated with a reduced thickness of the muscularis mucosae. In the subgroup of patients with refractory disease, only duration of anti-tumor necrosis factor use was linked to a thinner muscularis mucosae (Table 6).
Table 6.
Muscularis Mucosa Thickness and Clinical Characteristics
| Factor | All Patients | Patients with Refractory Disease | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | Muscularis Mucosa Thickness (μm) | p-value | n | Muscularis Mucosa Thickness (μm) | p-value | |
| Gender | 0.76 | 0.89 | ||||
| Male | 46 | 150.1 [80.3, 212.5] | 42 | 138.3 [80.3, 212.5] | ||
| Female | 43 | 139.0 [77.7, 216.6] | 39 | 117.1 [74.9, 203.4] | ||
| Family History of IBD | 0.25 | 0.15 | ||||
| No | 69 | 123.7 [74.9, 212.5] | 61 | 115.5 [73.4, 191.2] | ||
| Yes | 19 | 177.0 [111.8, 219.8] | 19 | 177.0 [111.8, 219.8] | ||
| Family History of CD | 0.53 | 0.39 | ||||
| No | 78 | 140.9 [74.9, 214.2] | 70 | 120.4 [73.4, 212.5] | ||
| Yes | 10 | 177.9 [111.8, 203.4] | 10 | 177.9 [111.8, 203.4] | ||
| Family History of UC | 0.36 | 0.3 | ||||
| No | 79 | 131.5 [77.7, 212.5] | 71 | 117.1 [74.9, 196.7] | ||
| Yes | 9 | 175.8 [155.1, 219.8] | 9 | 175.8 [155.1, 219.8] | ||
| Smoking | 0.45 | 0.36 | ||||
| No | 57 | 145.2 [80.3, 220.9] | 53 | 142.8 [80.3, 216.6] | ||
| Yes | 31 | 139.0 [66.6, 203.4] | 27 | 116.6 [64.6, 196.6] | ||
| EIM | 0.8 | 0.8 | ||||
| No | 81 | 139.0 [80.3, 213.5] | 74 | 126.1 [80.3, 212.5] | ||
| Yes | 8 | 164.4 [65.8, 283.1] | 7 | 149.9 [56.7, 203.4] | ||
| EIM: Joint | 0.21 | 0.47 | ||||
| No | 83 | 131.5 [77.7, 213.5] | 76 | 120.4 [76.3, 204.6] | ||
| Yes | 6 | 191.1 [149.9, 362.8] | 5 | 178.8 [149.9, 203.4] | ||
|
| ||||||
| Disease duration at colectomy (months) | 86 | 0.24 (0.03, 0.43) | 0.03 | 78 | 0.18 (−0.04, 0.39) | 0.11 |
| Reason for colectomy | 0.12 | |||||
| Dysplasia/Cancer or other | 8 | 210.9 [148.7, 285.8] | -- | |||
| Refractory disease | 81 | 128.5 [77.7, 203.4] | -- | |||
| Backwash Ileitis | 0.35 | 0.27 | ||||
| No | 71 | 131.5 [73.4, 212.5] | 64 | 120.4 [70.0, 200.0] | ||
| Yes | 16 | 165.6 [83.1, 257.1] | 16 | 165.6 [83.1, 257.1] | ||
|
| ||||||
| Disease Extent of Colitis | 0.99 | 0.54 | ||||
| Extensive colitis | 84 | 144.0 [79.0, 213.9] | 77 | 131.5 [77.7, 212.5] | ||
| Left sided colitis | 5 | 128.5 [117.1, 177.0] | 4 | 122.8 [75.1, 152.8] | ||
|
| ||||||
| Pre-OP Biologics (at any time) | 0.05 | 0.12 | ||||
| No | 46 | 172.3 [85.9, 265.2] | 38 | 167.7 [83.9, 265.2] | ||
| Yes | 39 | 116.6 [73.4, 169.0] | 39 | 116.6 [73.4, 169.0] | ||
| Pre-OP Immunomodulators (at any time) | 0.24 | 0.53 | ||||
| No | 39 | 158.5 [83.9, 238.0] | 31 | 142.8 [80.3, 238.0] | ||
| Yes | 48 | 120.2 [74.1, 184.7] | 48 | 120.2 [74.1, 184.7] | ||
| Pre-OP 6-MP | 0.27 | 0.16 | ||||
| No | 67 | 131.5 [66.6, 213.5] | 60 | 116.3 [65.6, 200.1] | ||
| Yes | 20 | 162.0 [102.0, 219.9] | 20 | 162.0 [102.0, 219.9] | ||
| Duration of 6-MP use (mo) | 23 | −0.12 (−0.51, 0.31) | 0.59 | 23 | −0.12 (−0.51, 0.31) | 0.59 |
| Pre-OP AZA | 0.73 | 0.93 | ||||
| No | 61 | 139.0 [80.3, 216.6] | 54 | 120.4 [80.3, 212.5] | ||
| Yes | 26 | 138.3 [64.6, 196.7] | 26 | 138.3 [64.6, 196.7] | ||
| Duration of AZA use (mo) | 12 | 0.20 (−0.42, 0.69) | 0.54 | 12 | 0.20 (−0.42, 0.69) | 0.54 |
| Pre-OP: Anti-TNF | 0.05 | 0.11 | ||||
| No | 47 | 168.8 [83.9, 265.2] | 40 | 167.7 [82.1, 251.6] | ||
| Yes | 40 | 116.0 [74.1, 166.8] | 40 | 116.0 [74.1, 166.8] | ||
| Duration of anti-TNF use (mo) | 13 | −0.55 (−0.85, −0.01) | 0.05 | 13 | −0.55 (−0.85, −0.01) | 0.048 |
|
| ||||||
| Meds at colectomy: 6-MP | 0.65 | 0.47 | ||||
| No | 76 | 140.9 [74.1, 213.0] | 68 | 126.1 [70.0, 200.1] | ||
| Yes | 10 | 140.0 [92.2, 196.6] | 10 | 140.0 [92.2, 196.6] | ||
| Meds at colectomy: AZA | 0.25 | 0.35 | ||||
| No | 71 | 145.2 [80.3, 216.6] | 63 | 128.5 [77.7, 212.5] | ||
| Yes | 15 | 115.5 [59.9, 177.0] | 15 | 115.5 [59.9, 177.0] | ||
| Meds at colectomy: Anti-TNF | 0.07 | 0.09 | ||||
| No | 77 | 149.9 [83.9, 213.5] | 69 | 142.8 [80.3, 203.4] | ||
| Yes | 9 | 111.8 [37.9, 131.5] | 9 | 111.8 [37.9, 131.5] | ||
Values presented as median [25th, 75th percentile] with Kruskal-Wallis test or Spearman’s rho (95% CI).
Abbreviations: CD-Crohn’s disease, UC-Ulcerative colitis, IBD-Inflammatory bowel disease, EIM-Extraintestinal manifestations, MP-mercaptopurine, AZA-azathioprine, TNF-tumor necrosis factor, OP-operation
Comparative assessment of fibrosis in endoscopic mucosal biopsies and resected specimens
We next assessed whether findings in endoscopic mucosal biopsies reflect the presence and degree of submucosal fibrosis detected using surgically resected specimens. For this purpose Ulcerative colitis subjects undergoing colonoscopy with endoscopic mucosal biopsies taken < 4 weeks prior to colectomy were selected. The location of the biopsies was recorded and the same exact location was used to assess fibrosis in the corresponding resected specimen. A total of 39 segments from 21 patients (demographics described in Table 2) were included in the analysis (average of 2±1 segments per patient). Neither the presence of lamina propria fibrosis nor morphology of the muscularis mucosae (uniformly thickened, split or splayed) on biopsy findings corresponded with submucosal fibrosis. Accordingly, the thickness of the muscularis mucosae in the biopsies was not linked to the amount of submucosal fibrosis in the corresponding resection specimen (data not shown). Next, biopsies were graded for the Geboes scores. There was no association between the Geboes scores in the biopsy and the underlying degree of fibrosis (p=0.14) or the thickness of the muscularis mucosae (p=0.29). Taken together, these data indicate that there were no features on the endoscopic biopsies corresponding with features of fibrosis in Ulcerative colitis resections.
Comparison of fibrosis in Ulcerative colitis, Crohn’s disease, diverticular disease and controls
We compared the degree of submucosal fibrosis in Ulcerative colitis with control colonic segments derived from Crohn’s colitis, diverticular disease and non-neoplastic areas of resections for colorectal cancer. All specimens were selected from corresponding anatomical colonic segments (Table 7). The Geboes score was highest in Ulcerative colitis and Crohn’s disease compared to diverticular disease and normal colon, and there was no significant difference in regard to the presence or grade of lamina propria or submucosal fibrosis between Ulcerative colitis, Crohn’s disease and diverticular disease as measured by the percent of submucosa affected by fibrosis. There was also no significant difference in the thickness of the lamina propria, muscularis mucosae or submucosa among all four groups, with a slight increase in Ulcerative colitis and Crohn’s disease compared to diverticular disease and normal colon, but this increase did not reach statistical significance.
Table 7.
Comparison of Inflammation and Fibrosis Measurements between Groups
| Factor | UC† (N=52) | CD (N=10) | Diverticular disease (N=10) | Normal (N=10) | p-value |
|---|---|---|---|---|---|
| Submucosa Fibrosis‡ | <0.001 b | ||||
| 0% | 0(0.0) 4 | 0(0.0) 4 | 0(0.0) 4 | 5(50.0) 123 | |
| 1–25% | 15(30.0) | 1(10.0) | 7(70.0) | 5(50.0) | |
| 26–50% | 7(14.0) | 3(30.0) | 1(10.0) | 0(0.0) | |
| 51–75% | 16(32.0) | 3(30.0) | 2(20.0) | 0(0.0) | |
| 76–100% | 12(24.0) | 3(30.0) | 0(0.0) | 0(0.0) | |
| Lamina Propria Fibrosis†† | 24(47.1) 4 | 6(60.0) 4 | 3(30.0) | 0(0.0) 12 | 0.021 c |
|
| |||||
| Active Inflammation Grade* | <0.001 b | ||||
| Inactive | 1(1.9) 4 | 0(0.0) 4 | 2(20.0) | 6(66.7) 12 | |
| Moderately active | 36(69.2) | 9(90.0) | 7(70.0) | 3(33.3) | |
| Severely active | 15(28.8) | 1(10.0) | 1(10.0) | 0(0.0) | |
| Any Active Inflammation* | 51(98.1) 4 | 10(100.0) 4 | 8(80.0) | 3(33.3) 12 | <0.001 c |
| Any Chronic Inflammation* | 51(98.1) 4 | 9(90.0) 4 | 8(80.0) | 2(22.2) 12 | <0.001 c |
| Inflammation* | <0.001 d | ||||
| No inflammation | 0(0.0) 4 | 0(0.0) 4 | 0(0.0) 4 | 6(66.7) 123 | |
| Active inflammation only | 1(1.9) | 1(10.0) | 2(20.0) | 1(11.1) | |
| Chronic inflammation only | 1(1.9) | 0(0.0) | 2(20.0) | 0(0.0) | |
| Active and Chronic inflammation | 50(96.2) | 9(90.0) | 6(60.0) | 2(22.2) | |
| Geboes score* | 10.1±3.6 34 | 8.8±4.3 4 | 5.2±4.2 14 | 0.56±0.88 123 | <0.001 a |
|
| |||||
| Lamina Propia (um) | 698.9±285.1 | 676.7±135.7 | 618.7±177.8 | 539.4±89.4 | 0.27a |
| Muscularis Mucosa (um) | 155.4±168.9 | 171.2±281.7 | 62.3±14.3 | 50.9±17.2 | 0.14a |
| Submucosa (um) | 1870.0±797.5 | 2315.0±877.7 | 1860.5±862.7 | 2273.2±614.6 | 0.24a |
Data not available for 1 UC patient
Data not available for 2 UC patients
Data not available for 1 subject in normal group.
Values presented as Mean ± SD or N (column %).
p-values:
ANOVA,
Kruskal-Wallis test,
Pearson’s chi-square test,
Fisher’s Exact test.
Significantly different from UC
Significantly different from CD
Significantly different from Diverticular disease
Significantly different from Normal
A significance level of 0.008 was used for pairwise ad-hoc comparisons.
Abbreviations: CD-Crohn’s disease, UC-Ulcerative colitis, um-micrometer
DISCUSSION
This manuscript characterizes a long-neglected problem of fibrosis complicating Ulcerative colitis. Multiple lines of evidence indicate that colonic strictures occur in Ulcerative colitis with frequencies that range from 1 to 11.2% 5–8. Using strict criteria (i.e. difficulty in passing the endoscope or a >50% reduction of the colonic lumen) strictures were found in 1.5% of patients 34. While there is a justified concern about the risk of malignancy, most colonic strictures in Ulcerative colitis are benign 4, 6, 17, strongly suggesting that fibrosis is the leading cause of these strictures. An increase in the thickness of the muscularis mucosae, but not the submucosa, and increased fibrosis in the submucosa was noted when looking at single cross sections per patient 8. A recent report by de Bruyn and colleagues assessed matrix deposition in a small cohort of Ulcerative colitis patients and found increased collagens and a thicker muscularis mucosae 20. In this study, a single section per patient taken from different segments of the colon was used and compared with each other 20, introducing a possible bias. Little effort has been devoted to better understand colonic fibrosis in Ulcerative colitis, regardless of stricture formation, and this absence of information is stunning, since fibrosis in the colorectal wall may have significant implications for the clinical features of Ulcerative colitis. Colonic motility abnormalities 9, 10, 35, possibly leading to symptoms such as loose stools or diarrhea, abdominal discomfort, pain and anorectal dysfunction as well as rectal urgency and incontinence 11–13, can occur in the absence of macroscopic or microscopic inflammation. In fact, intestinal fibrosis may be a major underlying factor of Ulcerative colitis symptoms that are commonly classified as irritable bowel syndrome 14, 15.
This report represents the first comprehensive evaluation of fibrosis in Ulcerative colitis. Some degree of submucosal fibrosis was detected in 100% of the involved colonic segments, it was linked to chronic mucosal injury, but not active inflammation, and the severity of inflammation correlated with the degree of fibrosis. The same was found in regard to the thickness of the muscularis mucosae. A gradient was detected in involved segments that increased from the proximal to the distal colon, and reached the highest degree of fibrosis and muscularis mucosae thickness in the distal portions of the colon. Refractory disease as the indication for colectomy was also associated with more fibrosis and a thicker muscularis mucosae, while a longer duration of anti-tumor necrosis factor medication was associated with a thinner muscularis mucosae. No feature on endoscopic mucosal biopsies taken within 4 weeks prior to colectomy could predict the underlying amount of fibrosis or the thickness of the muscularis mucosae in subsequent resection specimens.
What does the present investigation add to current knowledge? This is the first comprehensive evaluation of fibrosis in Ulcerative colitis, based on a careful and detailed examination of multiple cross sections per patient longitudinally along the colon and the use of established histological and clinical parameters. We developed a fibrosis score that is robust in terms of interobserver reliability, concordance between hematoxylin and eosin and Masson trichrome stain, and agreement with objective measures of fibrosis, such as the area stained by Sirius red. The moderate interobserver agreement is considered acceptable among experienced pathologists in multiple other conditions and scoring systems with interobserver variability reported as moderate are rountinely used in clinical practice (Supplemental Table 2). By including consecutive segments of colon for each Ulcerative colitis patient, we were able to identify involved and non-involved segments within each subject. Fibrosis and muscularis mucosae thickening were restricted to involved areas of the colon when compared with the non-involved areas from the same patients, and we clearly link the degree, severity and histologic features of active inflammation and chronic mucosal injury with the grade of fibrosis. Interestingly, when considering refractory Ulcerative colitis patients only, no clinical parameter was linked to the degree of fibrosis, but a longer duration of anti-tumor necrosis factor was associated with a thinner muscularis mucosae. We purposefully excluded Ulcerative colitis patients with strictures to show that fibrosis is present even when not immediately apparent. In fact, the same phenomenon can be found in Crohn’s disease, where significant amount of fibrosis are present in the absence of stenosis (as shown by our own data in this manuscript).
The results of this study allow us speculate on various important pathogenic aspects of fibrosis in Ulcerative colitis. Inflammation appears essential for the initiation of fibrosis given the lack of ECM accumulation in the non-inflamed segments. Refractory disease, compared to dysplasia as a cause for colectomy, was the only clinical feature linked to the overall burden of fibrosis in Ulcerative colitis, supporting the concept of inflammation-driven fibrogenesis, which is well established in Crohn’s disease. Interestingly, none of the other clinical features, such as disease duration or location, were linked to fibrosis. This is in contrast to Crohn’s disease where both phenotypes show clear associations with the development of clinically apparent fibrostenosis 2. None of the administered medications (intake and duration of administration) showed an association with fibrosis. Surprisingly, we detected fibrosis even in the colon of Ulcerative colitis patients with short disease duration, an observation suggesting that fibrosis may accumulate rapidly once inflammation is established. The amount of fibrosis was not linked to the time since first diagnosis, but to the severity of inflammation. This could be explained by the location of Ulcerative colitis being restricted to the mucosa and submucosa. Based on our data the submucosa does not expand in diameter in Ulcerative colitis associated fibrosis, which may facilitate a faster ECM accumulation until 100% of the submucosa is affected.
It should be mentioned that our results showing no link between the amount of fibrosis and disease duration stand in contrast to several series of Ulcerative colitis patients with colonic fibrosis with and without strictures, in which fibrosis severity was linked to longer disease duration 8, 18, 19, 34, although as described above, there was no comprehensive evaluation of samples longitudinally along the colon in these studies, but rather single slides per patient. The use of biologics and immunomodulators was not linked to less fibrosis, but prolonged anti-tumor necrosis factor use was associated with a thinner muscularis mucosae. This may suggest that inflammation control has an effect on remodeling of the intestinal wall. However, these findings may be confounded by the fact that patients with more severe disease are more likely to receive immunomodulators or biologics.
To our surprise, neither the degree of fibrosis nor the degree of muscularis mucosae thickening were linked to shortening of the colon. This stands against the intuitive belief that fibrosis may induce a reduced colonic length despite an absence of objective data. Finally, the thickness of the lamina propria, muscularis mucosae and submucosa, as well as the percentage of submucosa affected by fibrosis, were not different between Ulcerative colitis, Crohn’s disease and diverticular disease, suggesting the possibility that different intestinal conditions share common pathways of fibrogenesis.
This study has limitations. It is a cross sectional study, the results are descriptive and a selection bias may apply, as only surgical specimens were evaluated. No validated fibrosis score has been established for intestinal fibrosis in general and in Ulcerative colitis in particular. It remains unknown whether ECM composition is different in quality, quantity and function in Ulcerative colitis, Crohn’s disease or other forms of intestinal inflammation associated with fibrosis, and ECM differences could have relevant pathophysiological implications. Lamina propria fibrosis was categorized as present or absent, regardless of extent. This simplified approach was chosen to avoid too high of a variation as lamina propria fibrosis was minimal in extend and highly heterogenous. The histopathologic findings could not be linked to clinical symptoms at time of colectomy. Future studies need to address non-invasive means to quantify and monitor fibrosis in Ulcerative colitis to be able to connect histopathologic findings to clinical presentation and to allow using Ulcerative colitis as a model to test novel anti-fibrotics in the future. It has to be mentioned that at this time no method is available that allows the non-invasive determination of bowel fibrosis with high accuracy. A prospective study is underway linking clinical symptoms, wall compliance and histopathology at time of colectomy in Ulcerative colitis.
In summary, the results of this study suggest that Ulcerative colitis is a condition associated with progressive fibrosis and muscularis mucosae thickening. The degree of fibrosis and muscularis mucosae thickening is linked to the severity and chronicity of inflammation. Data from Crohn’s disease stricturoplasties indicate reversibility of fibrosis 36 and a case report of reversal of a benign rectal stricture suggests this could also be the case in Ulcerative colitis 37, underscoring the dynamic nature of fibrosis in inflammatory bowel disease. Given the serious clinical implications, this field is in critical need of further studies to characterize, diagnose, and treat fibrosis in Ulcerative colitis.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the National Institutes of Health [T32DK083251, P30DK097948 Pilot Feasibility Study, K08DK110415] and the European Crohn’s and Colitis Foundation to F.R. and Cleveland Clinic Intramural Funding to I.O.G.
Guarantor of the article: Florian Rieder
Specific author contributions:
IOG, NA, EW, RL, CF, FR performed the research, IOG, NA, EW, JRG, RL, DA, XL, DTP, LY, FEK, CF, FR collected and analyzed the data, IOG, FR designed the research study and all authors together wrote the paper, and IOG and FR contributed to the design of the study
All authors approved the final version of the manuscript.
Footnotes
Conflicts of interest: F.R. Consulting: UCB, Celgene, Samsung, Roche, Pliant, Thetis, Boehringer-Ingelheim, Helmsley; AdBoards: AbbVie, UCB, Receptos, RedX, Celgene; Speakers Bureau: AbbVie; Research Funding: UCB. The other authors have no conflicts to disclose.
References
- 1.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature medicine. 2012;18(7):1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152(2):340–350. e6. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49(6):777–82. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg HI, Caruthers SB, Jr, Nelson JA, Singleton JW. Radiographic findings of the National Cooperative Crohn’s Disease Study. Gastroenterology. 1979;77(4 Pt 2):925–37. [PubMed] [Google Scholar]
- 5.Marshak RH, Bloch C, Wolf BS. The Roentgen Findings in Strictures of the Colon Associated with Ulcerative and Granulomatous Colitis. The American journal of roentgenology, radium therapy, and nuclear medicine. 1963;90:709–16. [PubMed] [Google Scholar]
- 6.Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33(7):938–41. doi: 10.1136/gut.33.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt RH, Teague RH, Swarbrick ET, Williams CB. Colonoscopy in management of colonic strictures. Br Med J. 1975;3(5979):360–1. doi: 10.1136/bmj.3.5979.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Dombal FT, Watts JM, Watkinson G, Goligher JC. Local complications of ulcerative colitis: stricture, pseudopolyposis, and carcinoma of colon and rectum. Br Med J. 1966;1(5501):1442–7. doi: 10.1136/bmj.1.5501.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassotti G, Villanacci V, Mazzocchi A, et al. Colonic propulsive and postprandial motor activity in patients with ulcerative colitis in remission. Eur J Gastroenterol Hepatol. 2006;18(5):507–10. doi: 10.1097/00042737-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Snape WJ, Jr, Matarazzo SA, Cohen S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut. 1980;21(5):392–6. doi: 10.1136/gut.21.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alp MH, Sage MR, Grant AK. The significance of widening of the presacral space at contrast radiography in inflammatory bowel disease. Aust N Z J Surg. 1978;48(2):175–7. doi: 10.1111/j.1445-2197.1978.tb07298.x. [DOI] [PubMed] [Google Scholar]
- 12.Chrispin AR, Fry IK. The presacral space shown by barium enema. Br J Radiol. 1963;36:319–22. doi: 10.1259/0007-1285-36-425-319. [DOI] [PubMed] [Google Scholar]
- 13.Loening-Baucke V, Metcalf AM, Shirazi S. Anorectal manometry in active and quiescent ulcerative colitis. Am J Gastroenterol. 1989;84(8):892–7. [PubMed] [Google Scholar]
- 14.Gracie DJ, Williams CJ, Sood R, et al. Negative Effects on Psychological Health and Quality of Life of Genuine Irritable Bowel Syndrome-type Symptoms in Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2017;15(3):376–384. e5. doi: 10.1016/j.cgh.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Hirten R, Colombel JF. Need for Caution in Diagnosis of Irritable Bowel Syndrome in Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2017;15(8):1315. doi: 10.1016/j.cgh.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Goulston SJ, McGovern VJ. Strictures in ulcerative colitis. N Engl J Med. 1969;281(14):800. doi: 10.1056/NEJM196910022811422. [DOI] [PubMed] [Google Scholar]
- 17.Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitomi H, Okayasu I, Bronner MP, et al. Comparative histologic assessment of proctocolectomy specimens from Japanese and American patients with ulcerative colitis with or without dysplasia. Int J Surg Pathol. 2005;13(3):259–65. doi: 10.1177/106689690501300305. [DOI] [PubMed] [Google Scholar]
- 19.Warren S, Sommers SC. Pathogenesis of ulcerative colitis. The American journal of pathology. 1949;25(4):657–79. [PMC free article] [PubMed] [Google Scholar]
- 20.de Bruyn JR, Meijer SL, Wildenberg ME, Bemelman WA, van den Brink GR, D’Haens GR. Development of Fibrosis in Acute and Longstanding Ulcerative Colitis. Journal of Crohn’s & colitis. 2015;9(11):966–72. doi: 10.1093/ecco-jcc/jjv133. [DOI] [PubMed] [Google Scholar]
- 21.Ippolito C, Colucci R, Segnani C, et al. Fibrotic and Vascular Remodelling of Colonic Wall in Patients with Active Ulcerative Colitis. Journal of Crohn’s & colitis. 2016;10(10):1194–204. doi: 10.1093/ecco-jcc/jjw076. [DOI] [PubMed] [Google Scholar]
- 22.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. Journal of Crohn’s & colitis. 2017;11(6):649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 23.Gomollon F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. Journal of Crohn’s & colitis. 2017;11(1):3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 24.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 25.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–9. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odze RD, Goldblum JR. Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. 3. Philadelphia: Elsevier; 2015. [Google Scholar]
- 27.Zenlea T, Yee EU, Rosenberg L, et al. Histology Grade Is Independently Associated With Relapse Risk in Patients With Ulcerative Colitis in Clinical Remission: A Prospective Study. Am J Gastroenterol. 2016;111(5):685–90. doi: 10.1038/ajg.2016.50. [DOI] [PubMed] [Google Scholar]
- 28.Shi HY, Chan FKL, Chan AWH, et al. Accuracy of Faecal Immunochemical Test to Predict Endoscopic and Histological Healing in Ulcerative Colitis: A Prospective Study Based on Validated Histological Scores. Journal of Crohn’s & colitis. 2017;11(9):1071–1077. doi: 10.1093/ecco-jcc/jjx088. [DOI] [PubMed] [Google Scholar]
- 29.Pavlides M, Birks J, Fryer E, et al. Interobserver Variability in Histologic Evaluation of Liver Fibrosis Using Categorical and Quantitative Scores. Am J Clin Pathol. 2017;147(4):364–369. doi: 10.1093/ajcp/aqx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liapis H, Gaut JP, Klein C, et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transplant. 2017;17(1):140–150. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashisako M, Tanaka T, Terasaki Y, et al. Interobserver Agreement of Usual Interstitial Pneumonia Diagnosis Correlated With Patient Outcome. Arch Pathol Lab Med. 2016;140(12):1375–1382. doi: 10.5858/arpa.2016-0012-OA. [DOI] [PubMed] [Google Scholar]
- 32.Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20(11):2198–206. doi: 10.1097/MIB.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi H, Kephart GM, Colby TV, Gleich GJ. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. The American journal of pathology. 1992;140(2):521–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Yamagata M, Mikami T, Tsuruta T, et al. Submucosal fibrosis and basic-fibroblast growth factor-positive neutrophils correlate with colonic stenosis in cases of ulcerative colitis. Digestion. 2011;84(1):12–21. doi: 10.1159/000320773. [DOI] [PubMed] [Google Scholar]
- 35.Coulie B, Camilleri M, Bharucha AE, Sandborn WJ, Burton D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment Pharmacol Ther. 2001;15(5):653–63. doi: 10.1046/j.1365-2036.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn’s disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50(11):1968–86. doi: 10.1007/s10350-007-0279-5. [DOI] [PubMed] [Google Scholar]
- 37.Kirsner JB, Palmer WL, Klotz A. Reversibility in ulcerative colitis; clinical and roentgenologic observations. Radiology. 1951;57(1):1–14. doi: 10.1148/57.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.