Abstract
Objective
The significance of polyhydramnios of one twin in the absence of oligohydramnios of the cotwin in monochorionic diamniotic (MCDA) twin pregnancies (polyhydramnios affecting a recipient-like twin [PART]) is unknown. Our aim is to assess the risk of progression to twin–twin transfusion syndrome (TTTS) with PART, progression to ≥ stage II TTTS, and neonatal survival.
Study Design
This study was a retrospective cohort study of MCDA twin pregnancies with PART evaluated at a referral center from 2008 to 2015.
Results
Sixty-four MCDA twin pregnancies with PART were identified. Fifteen (23.4%) progressed to TTTS, including 10 (15.6%) who progressed to ≥ stage II TTTS. Three pregnancies were terminated and one underwent selective reduction by radiofrequency ablation. Overall survival was 113 out of 128 (88.3%). Of those who remained stable, 91.8% (N = 45) had survival of both neonates. In multivariate analysis, the presence of arterioarterial (A-A) anastomosis by in utero Doppler ultrasound was associated with decreased risk of progression to TTTS (odds ratio: 0.12, p = 0.03, 95% confidence interval: 0.02–0.78).
Conclusion
Most MCDA twin pregnancies with PART do not progress to TTTS and have a favorable prognosis. Progression rates are higher than observed in uncomplicated MCDA twins; however, so close surveillance is warranted. The presence of an A-A anastomosis appears to confer decreased risk of progression to TTTS.
Keywords: arterioarterial anastomosis, isolated polyhydramnios, monochorionic diamniotic, PART, twin–twin transfusion syndrome
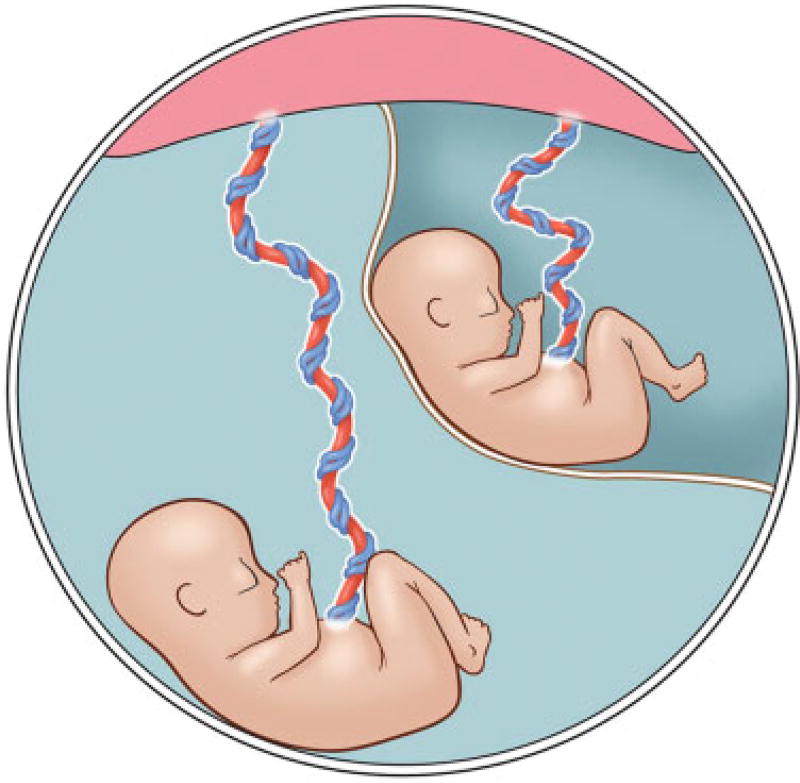
Monochorionic diamniotic (MCDA) twin pregnancies are at risk for unique, potentially severe complications, including twin–twin transfusion syndrome (TTTS). TTTS affects 8 to 10% of all monochorionic pregnancies in the United States1,2 and accounts for up to half of the total perinatal mortality in MCDA twins.2,3 Stage I TTTS is diagnosed by findings of polyhydramnios (deepest vertical pocket [DVP] >8 cm) of one twin with oligohydramnios (DVP < 2 cm)of the cotwin.4 However, little is known about the significance of isolated polyhydramnios in one twin of an MCDA twin pair in the absence of oligohydramnios in the cotwin (polyhydramnios affecting a recipient-like twin [PART]) (Fig. 1). In this condition, we refer to the twin with polyhydramnios—presumed to be due to polyuria—as “recipient-like” because the sonographic appearance mimics the excess amniotic fluid seen in the sac of the recipient in a twin pair affected by TTTS. In PART, however, there is no true “donor” as the cotwin has normal amniotic fluid.
Fig. 1.
Drawing of monochorionic diamniotic twin pair with polyhydramnios affecting a recipient-like twin. One twin with polyhydramnios and cotwin with normal amniotic fluid volume. (Image courtesy of Christine Gralapp, UCSF Fetal Treatment Center.)
Few prior studies have looked at PART and the risk of progression to TTTS. In one retrospective abstract, Swiatkowska-Freund et al5 found a 26% rate of progression to TTTS in 65 monochorionic pregnancies with PART. Similarly, in a series of 74 pregnancies with PART, Chon et al6 reported 29.7% progression to TTTS including 18.9% (N = 14) progression to stage II or above. Early diagnosis (<20 weeks gestational age [GA]) and fetal growth restriction (FGR) were reported as risk factors for progression to TTTS. These findings have yet to be confirmed in other studies, and little is known regarding additional risk factors for progression such as placental vascular anastomoses.7
TTTS is thought to develop as a result of unbalanced, unidirectional arteriovenous vascular connections within the shared placenta.8,9 Bidirectional arterioarterial (A-A) anastomoses may help compensate for this unbalanced flow and have been found to be protective against the development of TTTS in uncomplicated MCDA twins.10–13 To our knowledge, the impact of A-A anastomoses on the risk of progression to TTTS has not been described for pregnancies with PART.
The main objective of this study was to assess the risk of progression to TTTS among pregnancies with PART as well as progression to ≥ stage II TTTS and neonatal survival for these pregnancies. We additionally aimed to evaluate the impact of A-A anastomoses on these outcomes. Our hypothesis was that PART carries a relatively low risk of progression to TTTS and that the presence of an identifiable A-A anastomosis is protective against progression to TTTS.
Study Design
This was a retrospective cohort study of all MCDA twin pregnancies referred for evaluation between 2008 and 2015 at the University of California, San Francisco (UCSF) Fetal Treatment Center (FTC). The FTC database was searched to identify MCDA twin pregnancies that were referred for evaluation of possible TTTS during this time frame. Upon referral, each pregnancy underwent a detailed sonographic evaluation including complete anatomic survey, assessment of amniotic fluid based on measurement of DVP, Doppler evaluation of umbilical artery (UA) and ductus venosus blood flow, Doppler interrogation for A-A anastomosis, and identification of placental cord insertion site for each fetus.
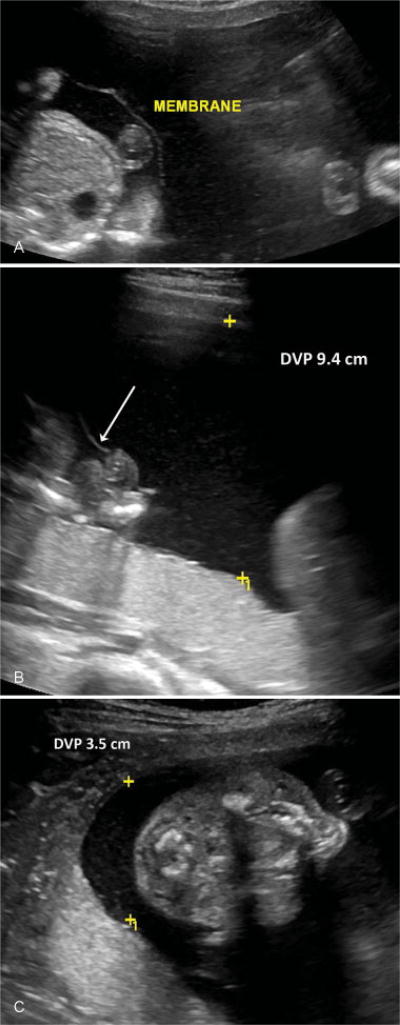
A diagnosis of PART was made if there was polyhydramnios (DVP >8 cm) of one twin (the “polytwin”) with normal amniotic fluid volume (DVP between 2 and 8 cm) in the other twin (the “cotwin”) (Fig. 2). TTTS was diagnosed and staged according to the criteria first described by Quintero et al in 1999.4 Stage I TTTS was diagnosed if there was polyhydramnios (DVP >8 cm) of the recipient twin with oligohydramnios (DVP < 2 cm) of the donor twin. These cutoffs represent the 5th and 95th percentiles of amniotic fluid measurements, respectively, and are consistent with the Society for Maternal-Fetal Medicine clinical guidelines for the diagnosis of stage I TTTS.12 Severe TTTS was characterized by additional ultrasound findings as described by Quintero et al: nonvisualization of the donor twin bladder (stage II), critically abnormal Doppler studies (stage III), hydrops fetalis (stage IV), and demise of one or both twins (stage V).
Fig. 2.
Ultrasound images from monochorionic diamniotic twin pregnancy with polyhydramnios affecting a recipient-like twin. (A) Thin intertwin membrane. (B) One twin with polyhydramnios. Deepest vertical pocket (DVP) of amniotic fluid measures 9.4 cm (membrane, arrow). (C) Cotwin with normal amniotic fluid volume, DVP 3.5 cm.
Inclusion criteria were all MCDA twin pregnancies with PART that did not previously meet criteria for TTTS based on thorough review of ultrasound reports by E.E.W. Exclusion criteria included unclear twin chorionicity, major fetal anomalies such as critical congenital cardiac anomalies, and insufficient follow-up to determine the study outcomes. The primary outcome of interest was development of TTTS. All other outcomes—including progression to ≥ stage II TTTS and neonatal survival to hospital discharge—were considered secondary. Pregnancies that ended in termination or dilation and curettage were included in the baseline characteristics and survival outcomes, but were excluded from calculations of GA at delivery.
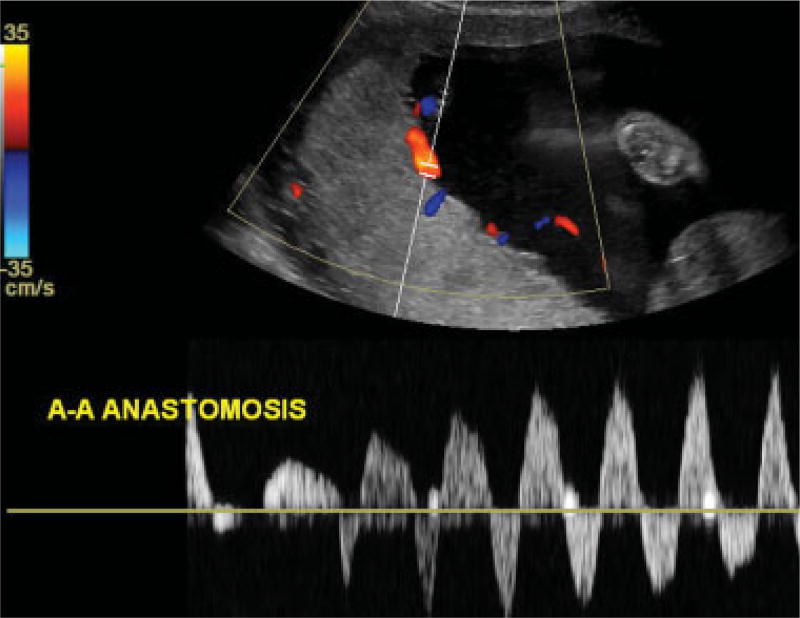
Sonographic placental mapping was undertaken for all patients in our cohort using targeted color and spectral duplex Doppler interrogation to evaluate for the presence of an A-A anastomosis along the fetal surface of the monochorionic placenta.10,14,15 An A-A anastomosis was recognized as a vessel coursing between the cord insertion sites along the fetal surface of the shared placenta with pulsatile bidirectional arterial flow (Fig. 3). This evaluation is routinely undertaken at our institution for all MCDA twins referred for possible TTTS.
Fig. 3.
Color and spectral duplex Doppler ultrasound image showing an arterioarterial (A-A) anastomosis. Coursing between the cord insertion sites along the fetal surface of the shared posterior placenta, a vessel is shown with pulsatile bidirectional flow.
Patients were followed up with serial ultrasounds at our center. In general, MCDA twin pregnancies with PART were followed up with ultrasounds every 1 to 2 weeks from 16 to 26 weeks’ gestation and every 2 to 4 weeks thereafter if stable. If there was any concern for developing TTTS or other fetal pathology, more frequent ultrasounds were performed. If a patient remained stable or was deemed low risk by their provider, they were subsequently followed up by their referring physician. However, if there was later concern for evolving TTTS or other complicating conditions, these patients were re-referred to the UCSF FTC.
Additional sonographic information including percentage of discordance in estimated fetal weight (EFW) [(EFWlarger twin − EFWsmaller twin)/EFWlarger twin × 100] at the time of diagnosis of PART, Doppler findings, placental location, and placental cord insertion sites were also recorded. Percentage of birth weight discordance was calculated in the same manner and recorded. FGR was defined as EFW <10%ile for one or both of the twins at any point in the pregnancy. We additionally considered the percentage of EFW discordance independently from FGR: twins were considered discordant if they had an EFW discordance >20% but were only diagnosed with FGR if at least one twin’s EFW was <10%ile for GA. The difference in DVPs between twins at the time of diagnosis was calculated and recorded for each twin pair (ΔDVP = DVPpoly − DVPnonpoly).
The FTC database and UCSF electronic medical record were used to collect additional information regarding demographic characteristics, treatment interventions, and delivery and neonatal outcomes for those pregnancies delivered at our institution. For patients who ultimately delivered at an outside institution, the FTC staff contacted the patients and outside hospital providers to collect information regarding clinical course, delivery, and neonatal outcomes.
It is the practice at our center to offer fetoscopic placental laser ablation of intertwin vascular connections to patients who develop stage II, III, or IV TTTS prior to 26 weeks GA. Amnioreduction for the twin with polyhydramnios is performed routinely at the conclusion of a laser ablation procedure. Selective reduction of one twin with percutaneous ultrasound-guided radiofrequency ablation (RFA) is also offered as a treatment option to patients who develop severe growth discordance with selective FGR or nonreassuring status of one twin prior to 24 weeks GA.
Wilcoxon’s rank-sum and Fisher’s exact tests were used to compare nonparametric continuous variables and proportions, respectively. Multivariate logistic regression was then performed to adjust for possible confounding variables, including the presence of A-A anastomosis, GA at the time of diagnosis of PART, DVP of each twin at diagnosis, and percentage of EFW discordance at diagnosis. These variables were chosen as clinically relevant characteristics that could impact the development of PART and the primary outcomes of interest: TTTS and neonatal survival. Results were considered statistically significant if the p-value was <0.05. All statistics were performed using Stata (version 14.0; StataCorp, College Station, TX). This study was approved by the Committee on Human Research at UCSF (Institutional Review Board Study Number 10-04093). Based on the retrospective and deidentified nature of this study, a waiver of patient consent was granted.
Results
A total of 241 MCDA twin pairs were identified during the study time period, 72 of which met criteria for PART. Eight of these patients lacked sufficient follow-up to determine the outcomes of interest and were thus excluded. This led to a final cohort of 64 patients with PART that were included in analyses.
Demographic characteristics of our cohort are displayed in Table 1. Forty-nine patients (76.6%) remained stable or had resolution of the polyhydramnios and 15 (23.4%) progressed to TTTS including 10 (15.6%) that progressed to stage II TTTS or above. The median time to progression to TTTS was 9 days (interquartile range of 13 days).
Table 1.
Cohort characteristics
| Variable | All PART pregnancies (n = 64) |
By progression to TTTS | ||
|---|---|---|---|---|
| PART without progression to TTTS (n = 49) |
PART with progression to TTTS (n = 15) |
p-Value | ||
| Maternal age, ya | 32.0 (7.0) | 32.0 (7.0) | 34.0 (7.0) | 0.18 |
| Nulliparity, n (%)b | 21 (32.8) | 15 (30.6) | 6 (40.0) | 0.54 |
| Spontaneous, n (%)b,c | 58 (90.6) | 44 (89.8) | 14 (93.3) | >0.99 |
| GA at the time of diagnosis of PART, wka | 21.2 (2.8) | 21.3 (3.3) | 21.0 (1.9) | 0.68 |
| GA at delivery, wka,d | 33.8 (5.4) | 34.3 (3.7) | 31.3 (6.1) | 0.16 |
Abbreviations: GA, gestational age; PART, polyhydramnios affecting a recipient-like twin; TTTS, twin–twin transfusion syndrome.
Note: Wilcoxon’s rank-sum and Fisher’s exact tests were used to compare nonparametric continuous variables and proportions, respectively.
p-Values correspond to comparisons between the group progressing to TTTS and the group that did not progress.
Results for continuous variables are displayed as median (interquartile range).
Results for proportions are displayed as n (percentage).
Spontaneous refers to spontaneous conception without the help of assisted reproductive technology.
Excluding patients who underwent elective termination or dilation and curettage.
Of the 10 patients who progressed to ≥ stage II TTTS, three elected termination of the entire pregnancy and one chose RFA of the cotwin after developing stage III TTTS at 21.9 weeks GA. Four additional patients underwent fetoscopic laser ablation. The remaining two patients developed ≥ stage II TTTS after 26 weeks GA and were ineligible for placental laser ablation or RFA at our institution.
Sonographic characteristics of the PART patients are shown in Table 2. In comparison to those patients who remained stable with PART, the patients who progressed to TTTS had a higher DVP of the polytwin and lower DVP of the cotwin at the time of PART diagnosis. Those who progressed also demonstrated a larger ΔDVP at PART diagnosis with a median ΔDVP of 7.5 cm (± interquartile range of 2.1 cm) for the progression group versus 5.4 cm (±2.7 cm) for the group that did not progress (p = 0.01). Those who progressed to TTTS were also less likely to have an identifiable A-A anastomosis (20.0 vs. 67.4%, p = < 0.01). The observation of a marginal or velamentous placental cord insertion for either twin was not associated with risk of progression. However, the cotwin with normal DVP was significantly more likely than the polytwin to have a marginal or velamentous cord insertion (62.5 vs. 26.6%, p = < 0.01).
Table 2.
Sonographic characteristics by progression to TTTS
| Variable | All PART pregnancies (n = 64) |
By progression to TTTS | ||
|---|---|---|---|---|
| PART without progression to TTTS (n = 49) |
PART with progression to TTTS (n = 15) |
p-Value | ||
| DVP of polytwin, cma,b | 9.5 (2.4) | 9.2 (1.9) | 10.5 (3.3) | 0.03 |
| DVP of cotwin, cma,b | 3.5 (1.5) | 3.7 (1.3) | 3.0 (1.3) | 0.02 |
| ΔDVP, cma,b | 6.2 (2.9) | 5.4 (2.7) | 7.5 (2.1) | 0.01 |
| Anterior placenta, n (%)c | 25 (39.1) | 19 (38.8) | 6 (40.0) | >0.99 |
| Posterior placenta, n (%)c | 39 (60.9) | 30 (61.2) | 9 (60.0) | |
| EFW discordance, %a,b | 16.5 (18.0) | 20.0 (17.0) | 11.0 (8.0) | 0.05 |
| EFW discordance >20%, n (%)c | 29 (45.3) | 26 (53.1) | 3 (20.0) | 0.04 |
| FGRe of cotwin, n (%)b,c | 6 (10.5) | 6 (12.2) | 0 (0.0) | 0.32 |
| Birth weight discordance, %a,d | 19.4 (12.2) | 21.8 (12.0) | 17.1 (5.5) | 0.40 |
| AREDF of cotwin, n (%)b,c | 5 (7.8) | 4 (8.2) | 1 (6.7) | >0.99 |
| A-A anastomosis present, n (%)c | 36 (56.3) | 33 (67.4) | 3 (20.0) | <0.01 |
| Marginal or velamentous cord insertion of poly twin, n (%)c | 17 (26.6) | 13 (26.5) | 4 (26.7) | >0.99 |
| Marginal or velamentous cord insertion of cotwin, n (%)c | 39 (60.9) | 30 (61.2) | 9 (60.0) | >0.99 |
Abbreviations: A-A, arterioarterial; AREDF, absent or reversed end-diastolic flow in the umbilical artery on in utero Doppler; DVP, deepest vertical pocket; EFW, estimated fetal weight; FGR, fetal growth restriction; GA, gestational age; PART, polyhydramnios affecting a recipient-like twin; TTTS, twin–twin transfusion syndrome; ΔDVP, difference in deepest vertical pocket at diagnosis.
Note: Wilcoxon’s rank-sum and Fisher’s exact tests were used to compare nonparametric continuous variables and proportions, respectively.
p-Values correspond to comparisons between the group progressing to TTTS and the group that did not progress.
Results for continuous variables are displayed as median (interquartile range).
Results for proportions are displayed as n (percentage).
At the time of diagnosis of PART.
Excluding patients who underwent elective termination and/or dilation and curettage.
Defined as cotwin EFW ≤ 10%ile for GA at any time from diagnosis of PART to delivery.
Interestingly, the pregnancies that remained stable with PART demonstrated a greater EFW discordance at the time of diagnosis compared with those that progressed (20.0 ± 17.0 vs. 11.0% ± 8.0, p = 0.05) as well as a greater proportion with EFW discordance >20% (53.1 vs. 20.0%, p = 0.04). In all cases, the twin with polyhydramnios was the larger twin. These differences in EFW, however, did not translate to significant differences in birth weights. The presence of an A-A anastomosis also was related to EFW discordance as 86.7% of patients with an A-A anastomosis had >20% EFW discordance at diagnosis versus 13.3% without an A-A anastomosis (p = < 0.01).
Of the 29 pregnancies with EFW discordance >20% at the time of diagnosis of PART, 6 had FGR of the cotwin (Table 2). In this subset of patients, none progressed to TTTS and neonatal survival was 11 out of 12 fetuses (91.7%). In addition, there were four patients who had EFW discordance >20% and Doppler findings of absent UA end-diastolic flow in the cotwin at the time of diagnosis of PART. In contrast to stage III TTTS, these cotwins did not have oligohydramnios. Of these patients with abnormal UA Doppler studies at the time of diagnosis of PART, one developed FGR and only one other progressed to TTTS (Table 2). All four of these patients had survival of both twins.
In the complete cohort, three pregnancies ended in termination and one was treated with RFA. Overall fetal survival for the PART cohort was 88.3% (113 out of 128 fetuses). Survival for the fetuses of pregnancies that remained stable with PART was 93.9% (92 out of 98). Neonatal survival by pregnancy is displayed in Table 3. Of the 64 PART pregnancies, 85.9% (55 out of 64) resulted in survival of both neonates, 4.7% (3 out of 64) had survival of one neonate, and 9.4% (6 out of 64) had no surviving neonates. This corresponded to a 90.6% overall survival of at least one twin. Of the six patients with no surviving neonates, three had elective terminations for ≥ stage II TTTS, two delivered at a previable GA (<23 weeks GA), and one had dual IUFD after progression to ≥ stage II TTTS. Of the pregnancies that remained stable with PART, 95.9% (47 out of 49) had survival of at least one twin. In the subset of PART patients with an A-A anastomosis—regardless of progression status—88.9% (32 out of 36 twin pairs) had survival of both neonates and 94.4% (34 out of 36) had survival of at least one twin.
Table 3.
Neonatal survival based on progression to TTTS
| Surviving neonates per pregnancy |
All PART pregnancies (n = 64) |
PART without progression to TTTS (n = 49) |
PART with progression to TTTS (n = 15) |
|---|---|---|---|
| 2 | 55 (85.9) | 45 (91.8) | 10 (66.7) |
| 1 | 3 (4.7) | 2 (4.1) | 1 (6.7) |
| 0 | 6 (9.4) | 2 (4.1)a | 4 (26.7)b |
| ≥1 | 58 (90.6) | 47 (95.9) | 11 (73.3) |
Abbreviations: PART, polyhydramnios affecting a recipient-like twin; TTTS, twin–twin transfusion syndrome.
Note: Results are displayed as n (percentage).
Both had spontaneous preterm labor and delivered at <23 weeks GA.
Including three elective terminations.
Finally, in multivariate analyses adjusting for the presence of an A-A anastomosis, GA at the time of diagnosis of PART, DVP of each twin at diagnosis, and percentage of EFW discordance at diagnosis, the only variable that remained significantly associated with progression to TTTS was the presence of an identifiable A-A anastomosis which conferred a significantly decreased risk of progression to TTTS (odds ratio: 0.12, p = 0.03, 95% confidence interval: 0.02–0.78). These findings do not significantly change if DVP and/or EFW discordance >20% at diagnosis were considered in the model in place of DVP of each twin and/or percentage of EFW discordance at diagnosis, respectively.
Discussion
In this cohort of MCDA twins with PART, less than 25% progressed to TTTS and more than 90% had survival of at least one twin. The presence of an identifiable A-A anastomosis was protective and found to be an independent predictor of lack of progression to TTTS. In the subset of PART patients with an A-A anastomosis, more than 90% remained stable.
The overall 23.4% rate of progression from PART to TTTS— and 15.6% to ≥ stage II TTTS—observed in this cohort are consistent with rates seen in prior studies,5,6 strengthening the understanding of the natural history of PART. Although this rate is notably higher than the 8 to 10% baseline prevalence of TTTS in MCDA twins,1,2 76.6% of patients with PART remained stable or improved, 85.9% had survival of both twins, and 90.6% had survival of at least one twin. These findings are important as many patients with PART are counseled that the observed amniotic fluid discrepancy is a precursor to TTTS and confers a high risk of progression. This may lead to unnecessary fetal intervention with associated risks of iatrogenic complications.
The severity of amniotic fluid discrepancy may correlate with the risk of progression to TTTS as ΔDVP at diagnosis was higher for those patients who progressed versus those who remained stable. However, this did not remain significant in the multivariate analysis. Although early diagnosis (<20 weeks GA) and FGR have previously been suggested as risk factors for progression to TTTS,6 this was not found in our study.
The PART patients who did not progress to TTTS had a greater proportion of intertwin EFW discordance >20% at diagnosis but no observed difference in birth weight discordance. This finding was also associated with the presence of an A-A anastomosis, with a significantly higher percentage of patients with A-A anastomosis having EFW discordance >20% at diagnosis. Although interpretation of these findings is limited without postnatal placental pathologic evaluation, these observations are consistent with prior placental dye injection studies showing that early-onset growth discordance in MCDA twins without TTTS is associated with an increased degree of unequal placental sharing16 and that A-A anastomoses both reduce the risk of TTTS10,11,13 and help normalize growth resulting in decreased birth weight discordance for a given placental territory discordance.17 These observations suggest that unequal placental sharing may contribute to amniotic fluid discordance in MCDA twin pregnancies.
Strengths of this study include the relatively large cohort of PART pregnancies and inclusion of novel placental mapping data that may inform patient counseling regarding the prognosis of PART.
However, there are several limitations to acknowledge. As a tertiary referral center, there is the possibility of referral bias as all included patients were sent for concern for TTTS and not all delivered at this institution. Although information was collected regarding delivery and neonatal outcomes for patients who delivered at outside institutions, this information was limited and long-term infant and childhood outcomes are not known. In addition, while prenatal ultrasound has been shown to detect A-A anastomoses with high specificity (97–100%),18,19 the sensitivity of this technique is lower (75–85%)18,19 and postnatal confirmation was not available. Therefore, while an identifiable A-A anastomosis was associated with a decreased risk of progression to TTTS, the absence of an A-A anastomosis is less informative. In addition, the lack of placental angioarchitecture studies limits our ability to understand, in more detail, the underlying etiology of PART and its relationship to unequal placental sharing and TTTS.
Finally, while the primary outcome for this study was progression toTTTS, this is only one type of possible complication in MCDA gestations. Monochorionic pregnancies are very complex and dynamic, and there is increasing recognition of the spectrum of complications that can occur—including varying combinations of amniotic fluid discrepancy, growth discordance, twin anemia polycythemia sequence, and neonatal demise—many of which are not captured in the current Quintero et al’s staging system. Similarly, while it was necessary to define clear cutoffs for amniotic fluid levels in this study, these are artificial thresholds and may not adequately reflect the complex three-dimensional anatomy, varied configuration of intrauterine MCDA twin sacs, and more nuanced clinical picture. Therefore, while the majority of patients with PART do not progress to TTTS, our ability to predict development of TTTS and other complications of monochorionicity remains limited, and these patients warrant ongoing close clinical and sonographic surveillance.
In conclusion, most MCDA twin pregnancies with PART do not progress to TTTS and have a favorable prognosis. Therefore, PART is not equivalent to “pre” or “early” TTTS and this distinction should be reflected in the counseling and management of patients. Progression rates are higher than observed in uncomplicated MCDA twins; however, so close surveillance is warranted. The presence of an identifiable A-A anastomosis appears to be protective and confers decreased risk of progression to TTTS. Further studies are needed to elucidate the underlying pathophysiology of PART, risk factors for TTTS and other poor obstetric outcomes in MCDA twins, and the role of A-A anastomoses in TTTS and intertwin fluid and growth discordance.
Acknowledgments
Funding
None.
Footnotes
This study was presented at the 36th Annual Pregnancy Meeting of the Society for Maternal-Fetal Medicine in Atlanta, GA, February 1–6, 2016.
Conflict of Interest
None.
References
- 1.Acosta-Rojas R, Becker J, Munoz-Abellana B, Ruiz C, Carreras E, Gratacos E Catalunya and Balears Monochorionic Network. Twin chorionicity and the risk of adverse perinatal outcome. Int J Gynaecol Obstet. 2007;96(02):98–102. doi: 10.1016/j.ijgo.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol. 2008;199(05):514.e1–514.e8. doi: 10.1016/j.ajog.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Gul A, Aslan H, Polat I, et al. Natural history of 11 cases of twin-twin transfusion syndrome without intervention. Twin Res. 2003;6(04):263–266. doi: 10.1375/136905203322296584. [DOI] [PubMed] [Google Scholar]
- 4.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. J Perinatol. 1999;19(8 Pt 1):550–555. doi: 10.1038/sj.jp.7200292. [DOI] [PubMed] [Google Scholar]
- 5.Swiatkowska-Freund M, Pankrac Z, Allen MH, Bornick PW, Chmait RH, Quintero RA. P06.06: Natural history of amniotic fluid volume discordance in monochorionic-diamniotic twins. Ultrasound Obstet Gynecol. 2005;26(04):418–419. [Google Scholar]
- 6.Chon AH, Korst LM, Llanes A, Miller DA, Ouzounian JG, Chmait RH. Midtrimester isolated polyhydramnios in monochorionic diamniotic multiple gestations. Am J Obstet Gynecol. 2014;211(03):303.e1–303.e5. doi: 10.1016/j.ajog.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Van Mieghem T, Eixarch E, Gucciardo L, et al. Outcome prediction in monochorionic diamniotic twin pregnancies with moderately discordant amniotic fluid. Ultrasound Obstet Gynecol. 2011;37(01):15–21. doi: 10.1002/uog.8802. [DOI] [PubMed] [Google Scholar]
- 8.Benoit RM, Baschat AA. Twin-to-twin transfusion syndrome: prenatal diagnosis and treatment. Am J Perinatol. 2014;31(07):583–594. doi: 10.1055/s-0034-1372428. [DOI] [PubMed] [Google Scholar]
- 9.Machin GA. Placental vascular anatomy and twin transfusion syndrome. Am J Obstet Gynecol. 1996;174(02):799–800. doi: 10.1016/s0002-9378(96)70476-3. [DOI] [PubMed] [Google Scholar]
- 10.Denbow ML, Cox P, Talbert D, Fisk NM. Colour Doppler energy insonation of placental vasculature in monochorionic twins: absent arterio-arterial anastomoses in association with twin-to-twin transfusion syndrome. Br J Obstet Gynaecol. 1998;105(07):760–765. doi: 10.1111/j.1471-0528.1998.tb10208.x. [DOI] [PubMed] [Google Scholar]
- 11.Denbow ML, Cox P, Taylor M, Hammal DM, Fisk NM. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol. 2000;182(02):417–426. doi: 10.1016/s0002-9378(00)70233-x. [DOI] [PubMed] [Google Scholar]
- 12.Simpson LL Society for Maternal-Fetal Medicine. Twin-twin transfusion syndrome. Am J Obstet Gynecol. 2013;208(01):3–18. doi: 10.1016/j.ajog.2012.10.880. [DOI] [PubMed] [Google Scholar]
- 13.de Villiers SF, Slaghekke F, Middeldorp JM, Walther FJ, Oepkes D, Lopriore E. Arterio-arterial vascular anastomoses in monochorionic placentas with and without twin-twin transfusion syndrome. Placenta. 2012;33(08):652–654. doi: 10.1016/j.placenta.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Erskine RL, Ritchie JW, Murnaghan GA. Antenatal diagnosis of placental anastomosis in a twin pregnancy using Doppler ultrasound. Br J Obstet Gynaecol. 1986;93(09):955–959. doi: 10.1111/j.1471-0528.1986.tb08015.x. [DOI] [PubMed] [Google Scholar]
- 15.Hecher K, Jauniaux E, Campbell S, Deane C, Nicolaides K. Artery-to-artery anastomosis in monochorionic twins. Am J Obstet Gynecol. 1994;171(02):570–572. doi: 10.1016/0002-9378(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 16.Lewi L, Gucciardo L, Huber A, et al. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early- and late-onset discordant growth. Am J Obstet Gynecol. 2008;199(05):511.e1–511.e7. doi: 10.1016/j.ajog.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Lewi L, Cannie M, Blickstein I, et al. Placental sharing, birth weight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am J Obstet Gynecol. 2007;197(06):587.e1–587.e8. doi: 10.1016/j.ajog.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Fichera A, Mor E, Soregaroli M, Frusca T. Antenatal detection of arterio-arterial anastomoses by Doppler placental assessment in monochorionic twin pregnancies. Fetal Diagn Ther. 2005;20(06):519–523. doi: 10.1159/000088043. [DOI] [PubMed] [Google Scholar]
- 19.Taylor MJ, Denbow ML, Tanawattanacharoen S, Gannon C, Cox PM, Fisk NM. Doppler detection of arterio-arterial anastomoses in monochorionic twins: feasibility and clinical application. Hum Reprod. 2000;15(07):1632–1636. doi: 10.1093/humrep/15.7.1632. [DOI] [PubMed] [Google Scholar]