Abstract
Vaccination against Ebola virus disease is a tool that may limit disease transmission and deaths in future outbreaks, integrated within traditional Ebola outbreak prevention and control measures. Although a licensed Ebolavirus vaccine (EV) is not yet available, the 2014–2016 West African Ebola outbreak has accelerated EV clinical trials and given public health authorities in Guinea, Liberia, and Sierra Leone experience with implementation of emergency ring vaccination. As evidence supporting the use of EV during an outbreak response has become available, public health authorities in at-risk countries are considering how to integrate EV into future emergency Ebola responses and for prevention in high-risk groups, such as healthcare workers and frontline workers (HCW/FLWs), even before an EV is licensed. This review provides an overview of Ebola epidemiology, immunology, and evidence to inform regional and country-level decisions regarding EV delivery during an emergency response and to at-risk populations before a licensed vaccine is available and beyond. Countries or regions planning to use EV will need to assess factors such as the likelihood of a future Ebolavirus outbreak, the most likely species to cause an out-break, the availability of a safe and effective EV (unlicensed or licensed) for the affected population, capacity to implement Ebola vaccination in conjunction with standard Ebola outbreak control measures, and availability of minimum essential resources and regulatory requirements to implement emergency Ebola vaccination. Potential emergency vaccination strategies for consideration include ring or geographically targeted community vaccination, HCW/FLW vaccination, and mass vaccination. The development of guidelines and protocols for Ebola vaccination will help ensure that activities are standardized, evidence-based, and well-coordinated with overall Ebola outbreak response efforts in the future.
Keywords: Ebola infection, Ebola virus vaccines, Emergencies, Outbreaks, Strategic planning, Vaccination
1. Introduction
The 2014–2016 West African Ebola virus outbreak was the largest ever filovirus outbreak. It lasted 24 months and resulted in more than 28,000 confirmed, probable and suspected cases, and more than 11,000 deaths in Guinea, Liberia, and Sierra Leone [1]. The outbreak was widespread and difficult to control due to multiple systemic factors including healthcare system capacity [2–5], lack of resources [2–4,6,7], challenges related to international coordination and communication [7], and community resistance to prevention measures [2,6]. To avoid another widespread outbreak, resources have been directed at improving health systems, surveillance for disease, and emergency response capacity. Vaccination against Ebola virus disease (EVD) could be a valuable adjunct to traditional measures to limit Ebola transmission and deaths.
Ebola vaccine (EV) delivery to at-risk populations under experimental protocols during the final stages of the outbreak was possible because of expedited vaccine development driven by the magnitude of the public health emergency. As evidence supporting the use of EV during an outbreak response is now available, public health authorities and partners are considering strategies to deliver EV during future emergency responses and for prevention in known high-risk groups such as healthcare and frontline workers (HCW/FLWs). In April 2017, the World Health Organization (WHO) Strategic Advisory Group of Experts (SAGE) recommended, “Should an Ebola disease outbreak occur before the candidate vaccine is licensed, . . . that the rVSVΔG-ZEBOV-GP vaccine be promptly deployed under the Expanded Access framework, with informed consent and in compliance with Good Clinical Practice” [8], as in clinical trial protocols. Practical guidance for EV implementation post-licensure has been drafted by the Global Ebola Vaccine Implementation Team (GEVIT) led by the WHO [9,10]. However licensure will not be completed until 2019 (Merck, personal communication). This review provides an overview of Ebola epidemiology and immunology as well as evidence and experience to inform decisions regarding EV delivery to at-risk populations both before and after a licensed vaccine is available. As we will illustrate, pre-licensure vaccination under the strict requirements of an investigational protocol is achievable but will require technical assistance and financial resource commitment from WHO and partners for preparedness planning and implementation during an emergency.
2. Decision framework for Ebola vaccination planning
The first step in planning is for regions and countries to determine if they will use EV in future emergency responses. The urgency to develop vaccination plans and protocols is driven by the likelihood of a future outbreak. Countries that have been affected by recent or past Ebola outbreaks should consider immediate vaccination planning. Countries that are geographically close to a country with a history of Ebola outbreaks may also consider vaccination planning.
Key factors that regions and countries should consider include:
-
-
The likelihood of an Ebolavirus outbreak occurring in the future (based on prior outbreaks).
-
-
The most likely Ebolavirus species to occur.
-
-
Availability of an EV (unlicensed or licensed) likely to prevent transmission of the species identified and safe for the population at-risk.
-
-
Country capacity to implement Ebola vaccination in addition to standard Ebola outbreak control measures (active case finding, isolation, contact tracing and monitoring, laboratory testing, social mobilization).
-
-
Available resources to implement Ebola vaccination (see Box 1).
Box 1 Emergency Ebola vaccination resource needs checklist.
-
✔
Team of experts prepared to coordinate response to a potential outbreak
-
✔
Regulatory (manufacturer and national regulatory authority) and ethical approvals for local use of the vaccine under an approved protocol
-
✔
During pre-licensure, country capacity to implement Ebola vaccination under an approved expanded access protocol, which would include provision of training according to Good Clinical Practice (GCP), informed consent, and regulatory monitoring of vaccine storage.
-
✔
Protocol and standard operating procedures outlined in emergency response plans
-
✔
Vaccine stock/supply
-
✔
Procurement mechanisms to ensure future vaccine stock
-
✔
Cold chain equipment and transport capacity
-
✔
Funding sources
Trained staff for planning, supervision, vaccination, monitoring and evaluation.
Subsequent sections of this paper will provide background and discuss the considerations for emergency vaccination planning during pre- and post-licensure phases as well as strategies for pre-emptive vaccination of high-risk individuals.
3. Ebola epidemiology
Ebola vaccination planning requires an understanding of Ebola-virus epidemiology, including Ebolavirus transmission patterns and populations at risk. Immunologic and clinical disease characteristics inform how quickly to initiate vaccination and how to differentiate EVD symptoms from symptoms of vaccine reactogenicity.
3.1. At-risk countries
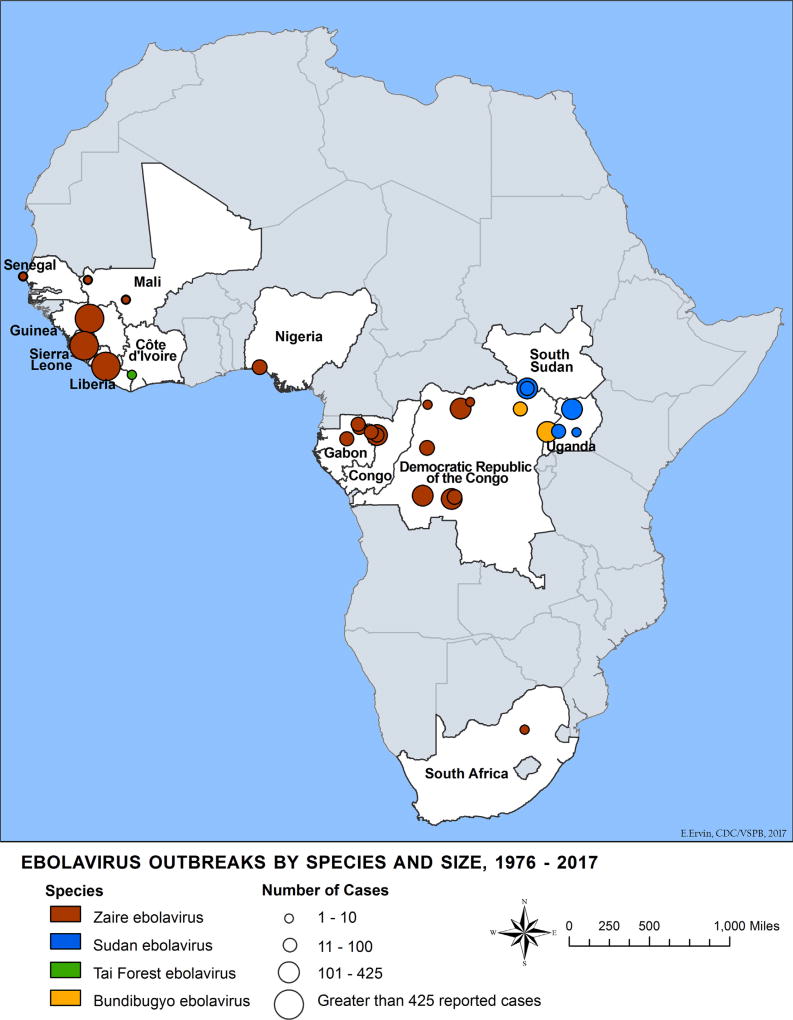
Thirteen African countries have documented at least one human case of one or more Ebolavirus species. Four species within the Ebola-virus genus are known to cause human disease: Zaire ebolavirus (EBOV), Sudan ebolavirus (SUDV), Tai Forest ebolavirus (TAFV), and Bundibugyo ebolavirus (BDBV). Outbreaks of more than 300 cases have occurred in the Democratic Republic of Congo (DRC), Guinea, Liberia, Sierra Leone, and Uganda (Fig. 1). EBOV, SUDV, and BDBV have caused multiple outbreaks in Africa with substantial case fatality [11] while TAFV has been documented in only one non-lethal human case related to a chimpanzee outbreak in Ivory Coast [12].
Fig. 1.
Ebola virus disease distribution map, 1976–2017.
3.2. Human transmission
Human-to-human Ebolavirus transmission occurs through percutaneous or mucous membrane contact with blood or other infected body fluids [13,14]. Skin swabs have yielded virus during periods of high viremia [15] and contaminated surfaces and aerosolized droplets may be infectious [16], necessitating strict infection prevention and control procedures. Household caregivers and HCWs face elevated risk of infection, especially when the infected individual is near death [3–5,7,14,17–26]. Corpses remain infectious after death, and participating in funeral rituals that include washing and handling corpses has been implicated in out-break transmission [13,22,24,27,28]. Additionally, EVD survivors have the potential to transmit virus due to viral persistence in select body fluids during convalescence [29] (see Contacts of survivors section below).
Ebolavirus transmission dynamics are important when considering control strategies and potential impact of vaccination. Although the basic reproductive number (Ro, the average number of cases generated by an index case), is relatively low for Ebolavirus transmission, significant variation in individual transmission exists. Ro estimates for the 2014 outbreak were 1.2–2.5, similar to prior EVD outbreaks [30]. Transmission chain analyses from Guinea showed that the majority of infected individuals transmitted to 0–1 secondary cases, while few transmitted to multiple secondary cases [31,32]. However, the phenomenon of superspreading, where a single case leads to many secondary cases, has been documented [7,27,33] and may be a key factor for sustained transmission. Analyses of community-level safe burial data in Sierra Leone and of surveillance data from all three affected West African countries (Epi Info Viral Hemorrhagic Fever application) suggest that a small number of superspreaders were responsible for 61% [34] and 73% [35] of all infections, respectively. Early vaccination of contacts and contacts of contacts may have the potential to suppress superspreading events and prevent epidemic spread.
3.3. Clinical manifestations & immune response
The incubation period for Ebola infection is 2–21 days. Infection results in a generalized viral illness with early signs and symptoms difficult to distinguish from other endemic febrile illnesses including malaria [7,11,36]; patients may develop “wet” symptoms including nausea/vomiting, diarrhea, and hemorragic signs [2,3,6,7,24, 25, 37]. The incubation and early infection periods are important in relation to vaccination, since Ebola-exposed vaccinees are monitored for EVD symptoms, but may experience similar symptoms (e.g., fever) related to vaccine reactogenicity, particularly for replicating vaccines. Participants in ring vaccination protocols were provided with paracetamol to prevent or treat fever related to reactogenicity. In addition, Ebolavirus PCR testing for the glycoprotein (GP) gene may be positive in vaccinees due to the replication of the vaccine itself.
Humoral and cellular immune correlates of disease and protection are essential to interpret vaccine immunogenicity. The evolution of EVD involves increasing viremia at the most severe and infectious stages of disease, when high titers of virus can be detected in blood and numerous body fluids. Ebolavirus-specific immunoglobulin G (IgG) antibody response has been associated with EVD survival both in primates receiving post-exposure anti-body prophylaxis [38] and in humans in outbreak settings [39]. High viral load is associated with poor outcomes [40]. While evidence shows protection to be primarily antibody based, the role of cellular immune protection is less well understood. CD4+ and CD8+ T-cell function may be important among EVD survivors [41,42], and vaccines that promote CD4+ and CD8+ T-cell responses may improve efficacy.
4. Candidate Ebola vaccines
No licensed vaccines are available to prevent Ebola; however, a number of candidate vaccines are being evaluated. Several recent publications have reviewed vaccine candidates in both preclinical development and human clinical trials [41,43–48]. Ebolavirus vaccine platforms being evaluated include nucleic acids, inactivated/subunit vaccines, viral replicons, replication-competent or -defective viral vectors, and virus-like particles (VLPs). Adenovirus, vesicular stomatitis virus (VSV), vaccinia virus, cytomegalovirus, paramyxovirus, rabies, and influenza virus vectors have been studied. Recombinant vaccines using either replication-competent or -defective viral vectors containing the EBOV GP have accelerated to human phase 2 and 3 trials. Only VSV and adenovirus vector candidates have made significant progress towards potential licensure.
The only candidate vaccine for which human efficacy data has been generated is rVSVΔG-ZEBOV-GP (rVSV-ZEBOV), a recombinant replicating VSV vector vaccine encoding the Kikwit 1995 strain EBOV GP. This single-dose vaccine was given to at-risk HCW/FLWs and at-risk adults and children during the 2014–2016 West African outbreak during clinical trials using investigational expanded access protocols. The Guinea Ebola Ça Suffit! ring vaccination trial used a cluster-randomized design and showed a primary outcome of 100% efficacy (95% confidence interval [CI] 68.9–100.0) in preventing EVD 10 or more days after vaccination. Clusters were composed of at-risk adults including contacts and contacts of contacts of an infected individual [49]. Following review of interim efficacy and safety results, randomization was halted and all subsequent clusters vaccinated [50]. Most adverse events were mild and self-limited. Two serious adverse events (fever and anaphylaxis) were judged vaccine-related; both participants recovered [49]. Data from the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE), which vaccinated nearly 8000 HCW/FLWs, showed a safety profile consistent with other studies of this vaccine with no serious adverse events related to vaccination [51]; the Partnership for Research on Ebola Virus in Liberia (PREVAIL) trial in Liberia showed a robust initial antibody response that was maintained over twelve months following vaccination and adverse events consistent with previous trials [52].
Single-dose and two-dose prime-boost regimens for other EVs are in development. In the prime-boost strategy, the first dose is the priming dose followed by a booster dose to generate a robust and long-term immune response. Heterologous prime-boost regimens use different vectors or antigens for the priming and the booster doses and aim to provide broad immunity across antigens. A prime-boost regimen being studied in phase 2 trials is a human adenovirus serotype 26 (Ad26) vaccine expressing ZEBOV GP (Ad26-ZEBOV) followed by a multivalent filovirus modified replication-incompetent vaccinia Ankara (MVA) boost dose [53].
5. Considerations for outbreak response vaccination
5.1. Ring vaccination implementation
Ring vaccination is the most likely vaccination strategy (Table 1) to be used in future community Ebolavirus outbreak responses given the experience in the 2014–2016 West African outbreak; this strategy, particularly in the pre-approval period, will require substantial effort and mobilization of resources. In October 2015, the WHO SAGE on Immunization provisionally recommended that EV continue to be used in outbreak response settings for those at high risk of infection [54]. Following publication of the interim Guinea ring vaccination trial results, ring vaccination was used in all three countries under amended clinical trial protocols for “compassionate use” or “expanded access” to give high-risk individuals access to vaccination, including HCW/FLWs. In April 2017, SAGE recommended pre-licensure use of rVSV-ZEBOV using the ring vaccination delivery strategy to include contacts, contacts of contacts, and local and international HCW/ FLWs in outbreak-affected areas and in areas at risk of spread [8]. SAGE also recommended that in case of an outbreak caused by a species other than EBOV, the use of a candidate vaccine designed to target the viral species identified should be considered. However, candidate vaccines for other Ebolavirus species are not yet available.
Table 1.
Ebola vaccination strategies.
| Ebola vaccination strategies | |
|---|---|
| Reactive: | Ring vaccination: |
| Emergency response vaccination following identification of an Ebola case or cases | Includes case contacts and contacts of contacts (identified through contact tracing). Community HCW/FLWs at-risk may also be included |
| Ring vaccination modified with a geographic boundary: | |
| Includes case contacts and contacts of contacts (identified through contact tracing), and individuals residing within designated natural or man-made geographic boundaries where contact with the case(s) is judged likely. Community HCW/FLWs at-risk are may also be included | |
| Geographically targeted vaccination: | |
| Includes a population within a designated geographic boundary determined to be at risk of outbreak spread. Geographic boundary is determined based on outbreak characteristics and existing natural or man-made barriers that are likely to curtail population movement | |
| Mass vaccination: | |
| Includes a large population (district, subnational division, country, region) determined to be at risk of outbreak spread. Requires large vaccine supply and is unlikely to be feasible using an unlicensed vaccine under an investigational protocol | |
| HCW/FLW vaccination (reactive): | |
| Targeted reactive vaccination of HCW/FLWs in an outbreak limited to a health facility or set of health facilities | |
| Preemptive: | HCW/FLW vaccination (preemptive): |
| Planned vaccination of a group of high-risk individuals before an outbreak occurs. | May include HCW/FLWs at a community, district, or national level in affected countries, international responders, and laboratory personnel |
| Contacts of EVD survivors: | |
| Contacts of male survivors post-outbreak have the greatest risk of infection based on current evidence. Both female and male sexual and household contacts of survivors may be included pending additional evidence of viral persistence and transmission |
Abbreviations: HCW/FLWs, healthcare and frontline workers; EVD, Ebolavirus disease.
5.2. Regulatory requirements
Licensure for rVSV-ZEBOV is not expected until 2019 at the earliest. Before licensure, outbreak response ring vaccination could take place within the expanded access framework in the context of appropriate regulatory approvals and a protocol approved by the manufacturer, the country’s national regulatory authority, and appropriate ethical review board(s) (Table 2). As with any clinical trial protocol, an expanded access protocol must include procedures for vaccine handling (including compliance with GCP, individual informed consent, safety monitoring, and adverse events management (follow-up, reporting, and provision of medical care). Duration of safety follow-up may be shorter than required for clinical trials if supported by available safety data for the populations included. Building on experience from prior ring vaccination, a standard protocol for all at-risk countries will facilitate planning across countries. After licensure, vaccine safety will need to be monitored through an adverse events following immunization surveillance system [55].
Table 2.
Ebola vaccine pre- and post-licensure delivery pathways.
| Regulatory mechanisms | International expert advisory | Vaccine supply & procurement | Delivery strategies | |
|---|---|---|---|---|
| Pre-licensure |
|
|
|
|
| Neither agency has issued these approvals for an Ebola vaccine. | ||||
| Licensed vaccine |
|
|
|
|
Abbreviations: NRA, national regulatory authorities; EUA, Emergency Use Authorization; SAGE, Strategic Advisory Group of Experts; EUAL, WHO Emergency Use Assessment and Listing; WHO, World Health Organization; ICG EBOV, International Coordinating Group for the provision of Ebola vaccine; HCW/FLWs, healthcare and frontline workers; EVD, Ebolavirus disease.
5.3. Vaccine stock and procurement
For prelicensure supply needs for expanded access or emergency use, Gavi, the Vaccine Alliance, recently entered into an advance purchase agreement with Merck®, committing $5 million USD towards a stockpile of 300,000 rVSV-ZEBOV doses [56]. After licensure, countries will make requests to the International Coordinating Group (ICG) for the provision of Ebola Vaccine, an international partnership to manage, deploy, finance, and monitor emergency vaccine stockpiles [57]. If, in the future, vaccine is available at the country-level, doses of vaccine should be monitored for quantity and expiration.
5.4. Cold chain capacity
The rVSV-ZEBOV vaccine must be stored at −60 °C, requiring specialized cold chain equipment not used for routine immunization programs in Africa. Specialized equipment for storage and transport of vaccine, including −80 °C freezers, modified Arktek passive storage devices and Creedo cold store boxes were used to support ring vaccination activities in Guinea, Liberia and Sierra Leone; these countries are transitioning these resources to government management for emergency planning. Maintenance of the −60 °C cold chain requires careful coordination to allow effective vaccination in remote areas. Resource needs include equipment (−80 °C freezers and passive storage devices for transport and maintenance of thermostability over several days to weeks) with adequate space, power, backup supply, and trained personnel for monitoring the EV. Vaccine manufacturers aim to provide data to support stability at higher temperatures. Until such data become available EV delivery will be challenging, and in the meantime countries should ensure the availability of ultra-cold freezer capacity either. This could be centrally within the country or at an established regional hub shared by multiple countries with shipping, import/export agreements, and plans in place to rapidly transport vaccine to remote locations.
5.5. Social mobilization
Experience with EV has shown that successful vaccination depends on community vaccine knowledge, acceptance, and cooperation. Just after publication of the interim ring trial results, about 86% of community members in Guinea responded positively about the need for an EV and 84% reported that their family would accept a safe and effective vaccine if offered. Acceptability and interest were highest among those who were more educated, had children who had received immunizations in the routine childhood immunization schedule, understood Ebola virus transmission modes, had witnessed response teams, or knew Ebola-affected persons [58]. Studies in Sierra Leone [59] and Nigeria [60] during the epidemic period also showed good community acceptance of a hypothetical EV. Qualitative research among HCW/FLWs in two regions of Nigeria showed that willingness to receive an EV was significantly higher among individuals who had interacted with EVD cases; fear and hesitancy related to misconceptions about EV safety were mitigated through an education program [61]. In Liberia, ring vaccination activities during an Ebola virus cluster response were difficult to implement due to mistrust and misconceptions about EVD and EV. In one vaccination effort, none of the community contacts, half of HCW contacts, and only a third of the targeted population accepted vaccination, despite community engagement activities (J. Mann, personal communication). Because no community contacts accepted vaccination, the ring vaccination strategy was modified to include a geographically bounded ring to define contacts of contacts.
Broadly targeted education and advocacy may help to avoid vaccine hesitancy in both the pre- and post-licensure phases. Social mobilization activities for vaccines require collaboration among the national ministry of health, the United Nations Children’s Fund and other non-governmental organizations and partners. Coordination with the national immunization program is recommended to avoid confusion between sensitization messages for EV and other childhood immunizations [16]. Targeting at risk populations is particularly important before EV is licensed, since reactive ring vaccination, not mass vaccination, has been recommended by SAGE [8].
5.6. Alternate emergency vaccination strategies
While most EV experience is in the context of ring vaccination, certain scenarios may warrant alternate emergency vaccination strategies including geographic or mass vaccination and reactive HCW/FLW vaccination (Table 1). Both geographically-targeted and mass vaccination strategies might miss individuals who are not present at the time of vaccination, whereas careful listing of contacts as part of ring vaccination might locate those individuals. In instances of widespread transmission, limited vaccine and supplies may be a barrier to mass vaccination. Geographically-targeted vaccination may be most feasible if areas of transmission are well-defined, but high-quality contact tracing is not possible. In a geographically-targeted strategy, a post-vaccination household coverage survey is recommended. In some situations, a combination of strategies may be justified.
5.7. Implementation research
To inform policy decisions, vaccine implementation research is needed to determine the feasibility of introduction in various target populations and settings and sources of vaccine acceptance or hesitancy. Implementation research has been valuable for the introduction for other new vaccines [62].
6. Preemptive vaccination in special populations
6.1. Healthcare and frontline workers
Risk of EVD transmission to HCW/FLWs is known to be greater than risk to the general population [23], especially early in an outbreak if infection prevention and control practices are not strong. Preemptive vaccination for HCWs in high-risk countries may avert another large-scale outbreak. In the 13 countries in Africa with history of Ebola outbreaks, there are more than 236,000 HCWs [63], and HCW vaccination coverage against other endemic diseases is limited. Considering resource limitations in these countries, HCW/FLW EV implementation would require external funding sources. A strategy for phased vaccination in HCW/FLW subgroups or regions may be needed. Skrip and Galvani suggest prioritizing subgroups using indicators such as recency of transmission, country capacity for vaccination and monitoring of safety and effectiveness in the population, followed by phased vaccination [63]. International responders, medevac teams, and personnel from high containment laboratories could also benefit from preemptive vaccination.
Data to support duration of immunogenicity of single dose vaccines have not yet been published, but results are expected from clinical trials in Guinea, Liberia, and Sierra Leone up to 12 months post-vaccination, and in the PREVAIL study up to 5 years post-vaccination. Multi-dose boosted regimens may also be promising for long-term protection (see Continued vaccine development: duration of effectiveness). Results will inform whether prophylactic strategies for HCWs will be useful in preventing future outbreaks and the needed frequency of revaccination.
6.2. Contacts of survivors
Prophylactic vaccination of contacts of EVD survivors may prevent new cases. Some EVD survivors may continue to transmit virus in the convalescent phase due to viral persistence over long periods in areas of the body considered “immune privileged”, where the virus is sequestered by a barrier that allows it to evade removal by the immune system [64]. Most evidence on viral persistence comes from semen testing in survivors following recovery after acute infection [36,65–70]. Epidemiologic and molecular investigations suggest rare but important sexual transmission from male survivors during the 2014–2016 epidemic. The virus was documented to persist more than 500 days in the semen of a survivor in Guinea, resulting in sexual transmission and resurgence of disease [29]. Viral persistence and risk of transmission from the eye [71], CNS [72], placenta/amniotic fluid [73], and breast milk [15,74,75] continue to be studied but data are currently limited [76].
In the 2014–2016 epidemic resources to support survivors were directed towards programs for semen testing, sexual transmission prevention education (abstinence, condom use), and clinical care. In early 2016, WHO initiated vaccination of contacts of male survivors in Guinea as part of an investigational protocol. Implementation challenges for vaccinating this population include: (1) risk of transmission may be greater with informal sexual partners (e.g., short-term partners, male partners of men, sex workers) who are not aware of the survivor’s past EVD history; (2) identification and sensitization of sexual partners without causing stigmatization may be difficult; and, (3) the at-risk population likely includes minors who require parental consent for receipt of counseling and vaccine administration. Despite these challenges, this population may be considered for vaccination in a future outbreak if a licensed EV becomes available.
7. Impact of Ebola vaccination
Case identification and isolation, contact tracing and monitoring, safe burials, and social mobilization are considered essential control measures in Ebola outbreak response. Vaccination is expected to provide an additional marginal benefit. Early introduction of EV is predicted to have the greatest impact on epidemic control [77–79]. In a study modeling data from Guinea, assuming large quantities of available vaccine, pre-emptive mass vaccination was predicted to control both early and late phases of a large outbreak, while the relative impact of ring vaccination depends upon effective contact tracing to identify contacts and contacts of contacts [78]. Merler and colleagues [79] modeled Sierra Leonean data to compare effectiveness of ring versus mass vaccination (25–50% of the population) versus a reactive geographically-targeted vaccination within 20 km around an index case. They found that geographically-targeted reactive vaccination may be more effective than mass vaccination and both strategies more effective than ring vaccination, especially when Ro is high.
A modified ring vaccination strategy with a tight geographic community boundary, as tried in Liberia, would be expected to effectively capture more contacts of contacts without requiring strong contact tracing. Another model based on data from Liberia and Sierra Leone suggested that ring vaccination would be most beneficial in situations where cases have many contacts in areas with crowded homes [80]. The model showed that a vaccine conferring post-exposure prophylaxis provides added benefit compared to a prophylactic agent. Assuming a vaccine conferred post-exposure prophylaxis, ring vaccination would decrease the number of cases only 8% beyond other control strategies.
8. Continued vaccine development
In the absence of another large-scale epidemic, human vaccine efficacy data beyond the rVSV-ZEBOV data published from the Guinea ring trial are not expected. Ideally, licensure of a single candidate would not halt progress towards an EV with an improved safety and effectiveness profile. An ideal EV would be thermostable, easy to administer, safe in multiple populations (including pregnant women and children), provide multi-species coverage, and provide fast-acting and long-term protection (Table 3).
Table 3.
Key Ebola vaccine characteristics and considerations for use.
| Vaccine characteristics | Considerations for use | |
|---|---|---|
| Vaccine type | Replication-competent viral vectors | Appropriate for healthy adults and children, non-pregnant women |
| Non-replicating vectors and protein subunit vaccines | Appropriate for the general population including immunocompromised and pregnant women | |
| Species coverage | Monovalent protection against Zaire ebolavirus | Useful only for outbreak response to a known Zaire ebolavirus outbreak |
| Broad protection against Zaire ebolavirus, Sudan ebolavirus, and Bundibugyo ebolavirus | Useful in multiple outbreak settings and for preemptive vaccination of international aid workers and healthcare/frontline workers in regions with multiple endemic species | |
| Dosing regimen | Single dose | Ideal regimen for outbreak response |
| Primary dose plus booster dose(s) |
Difficult to implement as part of outbreak response where vaccinees may not be accessible or compliant for follow-up dose(s) Feasible for preemptive vaccination of
|
|
| Durability of protection | At least 3 months | Useful for outbreak response |
| At least 1 year (ideally 3–5 years) |
Ideal for preemptive vaccination of
|
|
| Temperature stability | Current experimental candidates:
|
Feasible for
|
Ideal candidates:
|
Best scenario for efficient vaccine delivery in all settings, especially endemic regions | |
8.1. Multi-species coverage
The ability for a vaccine to provide protective immunity against multiple Ebolavirus species is particularly important, given that three Ebola species have caused human outbreaks (EBOV, SUDV, and BDBV) and are likely to cause future outbreaks. A broadly protective EV would allow at-risk countries to plan around the characteristics of a single product for rapid distribution from global or regional stockpiles. This would be desirable in regions where outbreaks of more than one type of filovirus or Ebolavirus outbreak has occurred, for instance in the DRC and Uganda, which have documented previous outbreaks from multiple filovirus species.
While some evidence of Ebolavirus species serologic cross-reactivity in humans [81] and cross-protection in animal models [82–85] suggests potential for a cross-protective monovalent vaccine, more promising for broad protective immunity are vaccine candidates that are multivalent or contain blended antigens from multiple Ebolavirus species [86]. The MVA booster dose in the Ad26-ZEBOV and MVA-BN-Filo vaccine regimen in clinical trials includes pooled proteins from EBOV, SUDV, TAFV, and Marburg virus. This approach has the potential to provide broad protection, but additional evidence of safety and effectiveness is needed. Additional blended and multivalent vaccines are in early clinical trials.
8.2. Duration of protection
Rapid and durable protection is highly desirable for vaccination during an outbreak, especially to provide prophylactic protection for HCW/FLWs. Two-dose boosted regimens are promising for prolonged protection. Prime boost clinical trials of Ad26-EBOV and MVA-BN-Filo have found European participants to have sustained immune responses 12 months after vaccination at varied prime-boost intervals (15, 29, or 57 days) [87]. Additional immunogenicity results are expected from African trials of these regimens. Longer intervals between the first and second dose are expected to elicit a more robust and prolonged response but possibly at the cost of early protective immunity and the chance of loss to follow-up among vaccinees.
8.3. Safety in special populations
With a replication-competent vaccine such as rVSV-ZEBOV, vaccinees have a period of transient viremia that could be more profound and prolonged in immunocompromised individuals. HIV-infected individuals are being evaluated in rVSV-ZEBOV and Ad26-EBOV/MVA-BN-Filo prime-boost trials. VSV infection in a pregnant vaccinee is also a theoretical risk. Platforms such as non-replicating recombinant vectors, nucleic acid, or VLP vaccines, would be preferable for use in populations with a high prevalence of immunodeficiency (e.g., HIV infection), or where ruling out pregnancy is not feasible before vaccination. HIV-infected individuals are being evaluated in rVSV-ZEBOV and Ad26-EBOV/MVA-BN-Filo prime-boost trials.
9. Conclusions
The 2014–2016 West African Ebola outbreak accelerated vaccine testing which demonstrated efficacy and safety of the rVSV-ZEBOV vaccine. Guinea, Liberia and Sierra Leone gained valuable experience with implementation of vaccine clinical trials, and ring vaccination as part of emergency response. Countries at risk for future Ebola outbreaks should consider implementation of Ebola vaccine as an effective strategy for disease prevention and control alongside traditional outbreak control strategies. Prior to licensure of the vaccine, countries will need to engage in considerable planning and preparedness activities including regulatory review of investigational protocols and evaluation of cold chain capacity. Technical and financial support will be needed from global partners. The development of guidelines and protocols for Ebola vaccination will ensure that activities are standardized, evidence-based, and well-coordinated with overall Ebola outbreak response efforts. Advance social mobilization and education at multiple levels are essential to ensure community engagement and cooperation during future outbreaks. Although licensure of the rVSV-ZEBOV vaccine will greatly facilitate emergency vaccination, licensure of a single candidate vaccine should not halt progress towards an ideal candidate for all at-risk areas and populations. Continued planning, engagement and financing from international stakeholders are essential to support further Ebola vaccine development, delivery strategies, and evaluation activities.
Acknowledgments
We would like to acknowledge the CDC Guinea, Liberia, and Sierra Leone country offices as well as the Ebola vaccine clinical trial teams and local partners in each country who have worked to provide access to Ebola vaccination for the populations at risk during the 2014–2016 outbreak. We would also like to acknowledge Dr. Pierre Rollin, CDC subject matter expert in the Division of High-Consequence Pathogens and Pathology and Dr. Barbara Mahon, STRIVE trial lead, National Center for Immunizations and Respiratory Diseases, CDC, for their helpful input on this manuscript.
Footnotes
Conflict of interest
All authors report no conflicts of interest. No financial support has been provided for this work.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Contributor Information
Jenny A. Walldorf, Email: jwalldorf@cdc.gov.
Emily A. Cloessner, Email: ecloessner@cdc.gov.
Terri B. Hyde, Email: thyde@cdc.gov.
Adam MacNeil, Email: amacneil1@cdc.gov.
References
- 1.Team WER. After Ebola in West Africa — unpredictable risks, preventable epidemics. N Engl J Med. 2016;375:587–96. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- 2.Borchert M, Mutyaba I, Van Kerkhove MD, Lutwama J, Luwaga H, Bisoborwa G, et al. Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned. BMC Infect Dis. 2011;11:357. doi: 10.1186/1471-2334-11-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Matanock A, Arwady MA, Ayscue P, Forrester JD, Gaddis B, Hunter JC, et al. Ebola virus disease cases among health care workers not working in Ebola treatment units-Liberia, June–August, 2014. MMWR Morbidity Mortality Weekly Rep. 2014;63:1077–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Kerstiens B, Matthys F. Interventions to control virus transmission during an outbreak of Ebola hemorrhagic fever: experience from Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S263–7. doi: 10.1086/514320. [DOI] [PubMed] [Google Scholar]
- 6.Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 7.Khan AS, Tshioko FK, Heymann DL, Le Guenno B, Nabeth P, Kerstiens B, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl 1):S76–86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 8.Summary of the April 2017 meeting of the Strategic Advisory Group of Experts on Immunization. Releve epidemiologique hebdomadaire/Weekly epidemiological record. 2017;92:241. [Google Scholar]
- 9.Henao-Restrepo AM, Preziosi MP, Wood D, Moorthy V, Kieny MP. On a path to accelerate access to Ebola vaccines: the WHO’s research and development efforts during the 2014–2016 Ebola epidemic in West Africa. Curr Opin Virol. 2016;17:138–44. doi: 10.1016/j.coviro.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Global Ebola Vaccine Implementation Team (GEVIT) Practical Guidance on the Use of Ebola Vaccine in an outbreak response. 2016 May; Draft Guidance: < http://www.who.int/csr/resources/publications/ebola/gevit-guide/en/>.
- 11.Centers for Disease Control and Prevention. Outbreaks Chronology: Ebola Virus Disease. < http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html>.
- 12.Le Guenno B, Formenty P, Wyers M, Gounon P, Walker F, Boesch C. Isolation and partial characterisation of a new strain of Ebola virus. Lancet. 1995;345:1271–4. doi: 10.1016/s0140-6736(95)90925-7. [DOI] [PubMed] [Google Scholar]
- 13.Velasquez GE, Aibana O, Ling EJ, Diakite I, Mooring EQ, Murray MB. Time from infection to disease and infectiousness for Ebola virus disease, a systematic review. Clin Infect Dis. 2015;61:1135–40. doi: 10.1093/cid/civ531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl 1):S87–91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 15.Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–7. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. World Health Organization ARO. Informational Note. 2015. Guidance for Immunization Programmes in the Africa Region in the context of Ebola, Informational Note, 30 March 2015. [Google Scholar]
- 17.Glynn JR. Age-specific incidence of Ebola virus disease. Lancet. 2015;386:432. doi: 10.1016/S0140-6736(15)61446-5. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Outbreak of Ebola hemorrhagic fever Uganda, August 2000-January 2001. MMWR Morbidity Mortality Weekly Rep. 2001;50:73–7. [PubMed] [Google Scholar]
- 19.World Health Organization. Ebola haemorrhagic fever in Sudan, 1976. Bull World Health Organ. 1978;56:247–70. [PMC free article] [PubMed] [Google Scholar]
- 20.Tomori O, Bertolli J, Rollin PE, Fleerackers Y, Guimard Y, De Roo A, et al. Serologic survey among hospital and health center workers during the Ebola hemorrhagic fever outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S98–S101. doi: 10.1086/514307. [DOI] [PubMed] [Google Scholar]
- 21.Sadek RF, Khan AS, Stevens G, Peters CJ, Ksiazek TG. Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995: determinants of survival. J Infect Dis. 1999;179(Suppl 1):S24–7. doi: 10.1086/514311. [DOI] [PubMed] [Google Scholar]
- 22.Roels TH, Bloom AS, Buffington J, Muhungu GL, Mac Kenzie WR, Khan AS, et al. Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis. 1999;179(Suppl 1):S92–7. doi: 10.1086/514286. [DOI] [PubMed] [Google Scholar]
- 23.Kilmarx PH, Clarke KR, Dietz PM, Hamel MJ, Husain F, McFadden JD, et al. Ebola virus disease in health care workers-Sierra Leone, 2014. MMWR Morbidity Mortality Weekly Rep. 2014;63:1168–71. [PMC free article] [PubMed] [Google Scholar]
- 24.Wamala JF, Lukwago L, Malimbo M, Nguku P, Yoti Z, Musenero M, et al. Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007–2008. Emerg Infect Dis. 2010;16:1087–92. doi: 10.3201/eid1607.091525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maganga GD, Kapetshi J, Berthet N, Kebela Ilunga B, Kabange F, Mbala Kingebeni P, et al. Ebola virus disease in the Democratic Republic of Congo. New England J Med. 2014;371:2083–91. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- 26.Forrester JD, Hunter JC, Pillai SK, Arwady MA, Ayscue P, Matanock A, et al. Cluster of Ebola cases among Liberian and U.S. health care workers in an Ebola treatment unit and adjacent hospital - Liberia, 2014. MMWR Morbidity Mortality Weekly Rep. 2014;63:925–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Victory KR, Coronado F, Ifono SO, Soropogui T, Dahl BA. Ebola transmission linked to a single traditional funeral ceremony - Kissidougou, Guinea, December 2014-January 2015. MMWR Morbidity Mortality Weekly Rep. 2015;64:386–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Yamin D, Gertler S, Ndeffo-Mbah ML, Skrip LA, Fallah M, Nyenswah TG, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Internal Med. 2015;162:11–7. doi: 10.7326/M14-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diallo B, Sissoko D, Loman NJ, Bah HA, Bah H, Worrell MC, et al. Resurgence of Ebola virus disease in guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis. 2016;63:1353–6. doi: 10.1093/cid/ciw601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Kerkhove MD, Bento AI, Mills HL, Ferguson NM, Donnelly CA. A review of epidemiological parameters from Ebola outbreaks to inform early public health decision-making. Sci Data. 2015;2:150019. doi: 10.1038/sdata.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faye O, Boelle PY, Heleze E, Faye O, Loucoubar C, Magassouba N, et al. Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet Infect Dis. 2015;15:320–6. doi: 10.1016/S1473-3099(14)71075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Althaus CL. Ebola superspreading. Lancet Infect Dis. 15:507–8. doi: 10.1016/S1473-3099(15)70135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacNeil A, Farnon EC, Morgan OW, Gould P, Boehmer TK, Blaney DD, et al. Filovirus outbreak detection and surveillance: lessons from Bundibugyo. J Infect Dis. 2011;204(Suppl 3):S761–7. doi: 10.1093/infdis/jir294. [DOI] [PubMed] [Google Scholar]
- 34.Lau MS, Dalziel BD, Funk S, McClelland A, Tiffany A, Riley S, et al. Spatial and temporal dynamics of superspreading events in the 2014–2015 West Africa Ebola epidemic. Proc Natl Acad Sci United States of America. 2017;114:2337–42. doi: 10.1073/pnas.1614595114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Ebola Response T. Agua-Agum J, Ariyarajah A, Aylward B, Bawo L, Bilivogui P, et al. Exposure patterns driving ebola transmission in West Africa: A retrospective observational study. PLoS Med. 2016;13:e1002170. doi: 10.1371/journal.pmed.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe AK, Bertolli J, Khan AS, Mukunu R, Muyembe-Tamfum JJ, Bressler D, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl 1):S28–35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 37.MacNeil A, Farnon EC, Wamala J, Okware S, Cannon DL, Reed Z, et al. Proportion of deaths and clinical features in Bundibugyo Ebola virus infection, Uganda. Emerg Infect Dis. 2010;16:1969–72. doi: 10.3201/eid1612.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci United States of America. 2012;109:5034–9. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–6. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 40.Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–41. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmunity. 2014;55:1–9. doi: 10.1016/j.jaut.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Ruibal P, Oestereich L, Ludtke A, Becker-Ziaja B, Wozniak DM, Kerber R, et al. Unique human immune signature of Ebola virus disease in Guinea. Nature. 2016;533:100–4. doi: 10.1038/nature17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Exp Rev Vaccines. 2014;13:521–31. doi: 10.1586/14760584.2014.885841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mire CE, Geisbert TW, Feldmann H, Marzi A. Ebola Virus Vaccines - reality or fiction? Exp Rev Vaccines. 2016 doi: 10.1080/14760584.2016.1178068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozak RA, Kobinger GP. Vaccines against ’the other’ Ebolavirus species. Exp Rev Vaccines. 2016:1–8. doi: 10.1586/14760584.2016.1170597. [DOI] [PubMed] [Google Scholar]
- 46.Sridhar S. Clinical development of Ebola vaccines. Therap Adv Vaccines. 2015;3:125–38. doi: 10.1177/2051013615611017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mennechet FJ, Tran TT, Eichholz K, van de Perre P, Kremer EJ. Ebola virus vaccine: benefit and risks of adenovirus-based vectors. Exp Rev Vaccines. 2015;14:1471–8. doi: 10.1586/14760584.2015.1083429. [DOI] [PubMed] [Google Scholar]
- 48.Martins KA, Jahrling PB, Bavari S, Kuhn JH. Ebola virus disease candidate vaccines under evaluation in clinical trials. Exp Rev Vaccines. 2016;15:1101–12. doi: 10.1080/14760584.2016.1187566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!) Lancet. 2016 doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. The Lancet. 2015;386:857–66. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 51.Samai M, Schuchat A, Fofanah AB. Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE). American Society of Tropical Medicine and Hygiene Annual Meeting; Atlanta, Georgia. November 17, 2016. [Google Scholar]
- 52.Davey RT. PREVAIL I: A randomized controlled safety and immunogenicity trial of two different vaccines against Ebola virus. Presented at 8th International Symposium on Filoviruses; September 12–15, 2016; Antwerp, Belgium. 2016. [Google Scholar]
- 53.Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia ankara-vectored ebola vaccines: a randomized clinical trial. Jama. 2016;315:1610–23. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 54.Meeting of the Strategic Advisory Group of Experts on immunization, October 2015 - conclusions and recommendations. Releve epidemiologique hebdomadaire / Weekly epidemiological record. 2015;90:681–99. [PubMed] [Google Scholar]
- 55.WHO. Global manual on surveillance of adverse events following immunization. Geneva, Switzerland: Mar, 2016. [Google Scholar]
- 56.Gavi Ebola vaccine purchasing commitment from Gavi to prepare for future outbreaks. 2016 Jan 20; < http://www.gavi.org/library/news/press-releases/2016/ebola-vaccine-purchasing-commitment-from-gavi-to-prepare-for-future-outbreaks/>.
- 57.WHO. International Coordinating Group (ICG) on Vaccine Provision, Online Q&A. Jun 17, 2016. [Google Scholar]
- 58.Irwin KL, Jalloh MF, Corker J, Alpha Mahmoud B, Robinson SJ, Li W, et al. Attitudes about vaccines to prevent Ebola virus disease in Guinea at the end of a large Ebola epidemic: results of a national household survey. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Huo X, Shi G, Li X, Lai X, Deng L, Xu F, et al. Knowledge and attitudes about Ebola vaccine among the general population in Sierra Leone. Vaccine. 2016;34:1767–72. doi: 10.1016/j.vaccine.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 60.Ughasoro MD, Esangbedo DO, Tagbo BN, Mejeha IC. Acceptability and willingness-to-pay for a hypothetical Ebola virus vaccine in Nigeria. PLoS Neglect Trop Dis. 2015;9:e0003838. doi: 10.1371/journal.pntd.0003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esangbedo DO, Ughasoro MD, Tagbo BN, Olowu A, Anikene C, Iwegbulam CC. Health-care workers’ perspectives on Ebola virus vaccine: a focus group and in-depth interview interventional study. Am J Trop Med Hyg. 2016;95:654–62. doi: 10.4269/ajtmh.16-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Brien KL, Binka F, Marsh K, Abramson JS. Mind the gap: jumping from vaccine licensure to routine use. The Lancet. 387:1887–9. doi: 10.1016/S0140-6736(16)30394-4. [DOI] [PubMed] [Google Scholar]
- 63.Skrip LA, Galvani AP. Next steps for Ebola vaccination: deployment in nonepidemic, high-risk settings. PLoS Neglect Trop Dis. 2016;10:e0004802. doi: 10.1371/journal.pntd.0004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372–81. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 65.Christie A, Davies-Wayne GJ, Cordier-Lasalle T, Blackley DJ, Laney AS, Williams DE, et al. Possible sexual transmission of Ebola virus - Liberia, 2015. MMWR Morbidity Mortality Weekly Rep. 2015;64:479–81. [PMC free article] [PubMed] [Google Scholar]
- 66.Deen GF, Knust B, Broutet N, Sesay FR, Formenty P, Ross C, et al. Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med. 2015 doi: 10.1056/NEJMoa1511410. 151014140118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, Cordier-Lassalle T, et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med. 2015 doi: 10.1056/NEJMoa1509773. 151014140151006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez LL, De Roo A, Guimard Y, Trappier SG, Sanchez A, Bressler D, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S170–6. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 69.Sonnenberg P, Field N. Sexual and mother-to-child transmission of Ebola virus in the postconvalescent period. Clin Infect Dis. 2015;60:974–5. doi: 10.1093/cid/ciu981. [DOI] [PubMed] [Google Scholar]
- 70.Sow MS, Etard JF, Baize S, Magassouba N, Faye O, Msellati P, et al. New evidence of long-lasting persistence of Ebola virus genetic material in semen of survivors. J Infect Dis. 2016 doi: 10.1093/infdis/jiw078. [DOI] [PubMed] [Google Scholar]
- 71.Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola virus in ocular fluid during convalescence. New Engl J Med. 2015;372:2423–7. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Billioux BJ, Nath A, Stavale EJ, Dorbor J, Fallah MP, Sneller MC, et al. Cerebrospinal fluid examination in survivors of Ebola virus disease. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2017.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamali A, Jamieson DJ, Kpaduwa J, Schrier S, Kim M, Green NM, et al. Pregnancy, Labor, and delivery after Ebola virus disease and implications for infection control in obstetric services, United States. Emerg Infect Dis. 2016;22 doi: 10.3201/eid2207.160269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nordenstedt H, Bah EI, de la Vega M-A, Barry M, N’Faly M, Barry M, et al. Ebola virus in breast milk in an Ebola virus-positive mother with twin babies, Guinea, 2015. Emerg Infect Dis. 2016;22:759–60. doi: 10.3201/eid2204.151880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreau M, Spencer C, Gozalbes JG, Colebunders R, Lefevre A, Gryseels S, et al. Lactating mothers infected with Ebola virus: EBOV RT-PCR of blood only may be insufficient. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.es2015.20.3.21017. [DOI] [PubMed] [Google Scholar]
- 76.Heeney JL. Ebola: Hidden reservoirs. Nature. 2015;527:453–5. doi: 10.1038/527453a. [DOI] [PubMed] [Google Scholar]
- 77.Shen M, Xiao Y, Rong L. Modeling the effect of comprehensive interventions on Ebola virus transmission. Sci Rep. 2015;5:15818. doi: 10.1038/srep15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kucharski AJ, Eggo RM, Watson CH, Camacho A, Funk S, Edmunds WJ. Effectiveness of ring vaccination as control strategy for Ebola virus disease. Emerg Infect Dis. 2016;22:105–8. doi: 10.3201/eid2201.151410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merler S, Ajelli M, Fumanelli L, Parlamento S, Pastore YPA, Dean NE, et al. Containing Ebola at the source with ring vaccination. PLoS Neglect Trop Dis. 2016;10:e0005093. doi: 10.1371/journal.pntd.0005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wells C, Yamin D, Ndeffo-Mbah ML, Wenzel N, Gaffney SG, Townsend JP, et al. Harnessing case isolation and ring vaccination to control Ebola. PLoS Neglect Trop Dis. 2015;9:e0003794. doi: 10.1371/journal.pntd.0003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Macneil A, Reed Z, Rollin PE. Serologic cross-reactivity of human IgM and IgG antibodies to five species of Ebola virus. PLoS Neglect Trop Dis. 2011;5:e1175. doi: 10.1371/journal.pntd.0001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warfield KL, Dye JM, Wells JB, Unfer RC, Holtsberg FW, Shulenin S, et al. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS One. 2015;10:e0118881. doi: 10.1371/journal.pone.0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flyak AI, Shen X, Murin CD, Turner HL, David JA, Fusco ML, et al. Cross-Reactive and potent neutralizing antibody responses in human survivors of natural ebolavirus infection. Cell. 2016;164:392–405. doi: 10.1016/j.cell.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol. 2009;83:7296–304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hensley LE, Mulangu S, Asiedu C, Johnson J, Honko AN, Stanley D, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus Species. PLoS Pathogens. 2010;6:e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol: CVI. 2010;17:572–81. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winslow RL, Milligan ID, Voysey M, Luhn K, Shukarev G, Douoguih M, et al. Immune responses to novel adenovirus type 26 and modified vaccinia virus ankara-vectored Ebola vaccines at 1 year. Jama. 2017;317:1075–7. doi: 10.1001/jama.2016.20644. [DOI] [PubMed] [Google Scholar]