Abstract
Background
Desmoplastic small round cell tumor (DSRCT) is a rare sarcoma that primarily affects adolescents and young adults. Patients can present with many peritoneal implants. We conducted a Phase II clinical trial utilizing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) with Cisplatin for DSRCT and pediatric type abdominal sarcomas.
Methods
A prospective cohort study was performed on 20 patients, who underwent CRS-HIPEC procedures, with Cisplatin from 2012–2013. All patients were enrolled in the phase 2 clinical trial. Patients with extra-abdominal disease and in whom a complete cytoreduction (CCR0-1) could not be achieved were excluded. All outcomes were recorded.
Results
Fourteen patients had DSRCT while 5 patients had other sarcomas. One patient had repeat HIPEC. Patients with DSRCT had a significantly longer median overall survival after surgery than patients with other tumors (44.3 months vs. 12.5 months, p = 0.0013). The 3-year overall survival from time of diagnosis for DSRCT patients was 79%. The estimated median recurrence-free survival (RFS) was 14.0 months. However, RFS for patients with DSRCT was significantly longer than for non-DSRCT patients (14.9 months vs. 4.5 months, p = 0.0012). Among DSRCT patients, those without hepatic or portal metastases had a longer median RFS than those with tumors at these sites (37.9 months vs. 14.3 months, p = 0.02). In 100% of patients without hepatic or portal metastasis, there was no peritoneal disease recurrence after CRS-HIPEC.
Conclusions
Complete CRS-HIPEC with Cisplatin is effective in select DSRCT patients. DSRCT patients with hepatic or portal metastasis have poorer outcomes.
INTRODUCTION
Desmoplastic small round cell tumor (DSRCT) is an aggressive, rare form of sarcoma that primarily affects children, adolescents and young adults. DSRCT is a malignancy for which the organ or tissue of origin is unknown. When diagnosed, dozens to hundreds of intra-abdominal implants are identified throughout the peritoneal cavity. Five-year survival remains 15–25% despite multimodal treatment including chemotherapy, radiation therapy and surgical resection.1–3 DSRCT has a characteristic translocation (11;22p12;13q), resulting in a fusion protein joining EWS with WT1. It is this unique genetic fusion protein that distinguishes it from other sarcomas.
Previous reports have shown improved overall survival with aggressive surgical resection of all of the intra-abdominal implants when combined with neoadjuvant and adjuvant chemotherapy and postoperative radiation.4 Because DSRCT patients almost always present with sarcomatosis, we hypothesized, hyperthermic intraperitoneal chemotherapy (HIPEC) following cytoreductive surgery (CRS) would be an effective local control tool to improve survival, as in adult carcinomatosis.3,5–9
Here, we describe our results of a phase two study of CRS and HIPEC in pediatric, adolescent and young adult aged sarcoma patients, most of whom had DSRCT. This research expands on phase one results demonstrating the safety of the CRS and HIPEC technique in the pediatric population.10
MATERIAL AND METHODS
From January 2012 through December 2013, accrual was met and 20 patients were enrolled. (None of these patients have been published elsewhere, except for one patient with granulosa cell tumor.) This was an investigator-initiated, single-institution, IRB-approved protocol (IRB #2009-0528) at the University of Texas MD Anderson Cancer Center only. All operations were performed by two surgeons who specialize in HIPEC; one surgeon for those patients under 21 years of age and another surgeon for those over age 21. Patients were included who were at least one year of age, had excellent performance status, had no liver or renal dysfunction, no cardiovascular contraindications to general anesthetic and no detectable FDG-avid disease by PET (Positron Emission Tomography) imaging outside the abdominal cavity at the time of CRS and HIPEC. Liver metastasis, if present, needed to be resectable at the time of CRS and HIPEC, or without metabolic activity on PET at the time of enrollment. All patients under age 21 years had DSRCT or other histologies of sarcomatosis. DSRCT was the only diagnosis for which adult patients were enrolled. All patients demonstrated a response to neoadjuvant chemotherapy with at least four months of prior chemotherapy. Chemotherapy regiments varied, but all chemotherapies were those commonly used to treat Ewing’s sarcoma. Patients were not eligible unless at least a partial response (PR) to chemotherapy was identified by cross sectional imaging. All DSRCT patients underwent post-operative whole abdominal radiation therapy (WART), while RMS patients, who had previous abdominal or pelvic radiation therapy, did not.11
Among the 20 patients enrolled, one patient enrolled twice. This patient enrolled in the study again after recurrence following the 1st CRS-HIPEC procedure. The patient had a recurrence after the 2nd procedure and succumbed. In our analysis, for the 1st procedure, the overall survival was calculated from the date of diagnosis to one day before the date of 2nd surgery, the patient was censored, and RFS time was calculated as the time from date of the 1st surgery to date of 1st recurrence. For the 2nd procedure, the overall survival was calculated from the date of the 2nd surgery to date of death, and RFS time was calculated as the time from date of the 2nd surgery to date of 2nd recurrence. Clinical factors and age at the time of each surgery were included in the analysis. We treated the two enrollments as two independent patients in our analysis, no within patient association was adjusted. However we performed a sensitivity analysis by excluding this patient’s second enrollment. The sensitivity analysis revealed that exclusion of the second enrollment had no significant effect on comparisons of overall survival or recurrent-free survival between patients with DSRCT and those with other histologies.
The peritoneal carcinomatosis index as described by Sugarbaker was calculated at the time of abdominal exploration.12 Complete (CR0) or near complete (CR1 =< 2.5 cm of tumor remaining) cytoreduction was achieved before HIPEC. Standard peritonectomy technique was used. All patients underwent closed technique HIPEC using 100 mg/M2 of cisplatin for 90 minutes at 41 degrees Celsius. Nephroprotection was employed in all patients. Specifically, preoperatively patients were admitted the day before for continuous intravenous hydration. In the operating room a bolus of intravenous sodium thiosulfate was delivered beginning 30 minutes into the 90 minute perfusion, followed by a continuous infusion for 12 hours. This was followed by postoperative delivery of 1.5 times maintenance intravenous fluids and replacement of all fluid losses. This regimen, and the advantages of vigorous pre-, intra-, and postoperative hydration in this setting, was previously published by our group.10 To verify intraperitoneal temperatures, 4 fiberoptic temperature probes were sutured to the peritoneum in the following positions: right abdominal wall, left abdominal wall, sigmoid colon mesentery in the pelvis and ligament of Treitz. As a control for core body temperature, a needle temperature probe was placed in the right lobe of the liver to verify the core temperature. These probes were connected to a standard computer, giving a constant reading of the temperature in each area for the duration of the 90 minute HIPEC.
Types of operations performed during CRS included peritonectomy, small and large bowel resections with primary anastomosis, pelvic tumor and rectal resection without colostomy, partial diaphragm resection and primary closure, resection of the pelvic tumor and pouch-of-Douglas, distal pancreatectomy and splenectomy.
Portal disease was defined as nodal, and/or a plaque-like 1–2mm thick sheet of tumor at the porta hepatis. Portal node dissection was completed when nodal enlargement was encountered. However, plaque-like disease was dissected distally up to the confluence of the liver edge.
Descriptive statistics and tabulation were used to summarize the data. The overall survival time was calculated from the date of surgery to the date of death or the date of last follow-up for patients alive. The recurrence-free survival were calculated as the time from the date of surgery to the date of death or date of recurrence whichever occurred first for patients who experienced an event, and to the date of last follow-up for patients alive without recurrence. The Kaplan-Meier method was used to estimate the probability of recurrence-free survival and overall survival.13 The log rank test and Cox model were applied to determine the association between relapse-free survival (RFS) or overall survival (OS) and patient characteristics. Variables with a p value < 0.05 were considered statistically significant. S-Plus software v8.2 (TIBCO Software Inc. Palo Alto, CA) was used for data analysis.
RESULTS
Patients aged 23 months to 50 years. Data was evaluated for 36 months after the last enrollee. The median follow-up time for all enrolled patients was 44.6 months. Twenty-two patients were consented on the protocol. Two patients could not be completely cytoreduced and therefore were not enrolled. There were no protocol violations. All 20 enrolled patients completed the protocol. Among these 20 patients, 14 had DSRCT, 2 RMS, 2 undifferentiated sarcomas, 1 juvenile granulosa cell tumor with sarcomatous elements and 1 Ewing’s sarcoma. There were 14 males and 6 females. All but one of the DSRCT patients were male. The median patient age was 20.8 years. The median PCI was 15 (highest 37). Three patients had liver metastasis, which were resected at the time of HIPEC. All patients had a complete cytoreduction (CCR0-1).
There was no association of age or PCI to overall or relapsed-free survival on multivariate analysis. The estimated median RFS duration for the 20 patients was 13.99 months (95% CI: 7.26 m – NA). The estimated 3-year overall survival rate from diagnosis was 83% (95% CI: 67% – 100%) (Figure 1a and b). When divided by histology, DSRCT patients had a longer RFS time (median = 14.87 months with a 95%CI of 13.99 months to NA) than patients with other histologies (median = 4.5 months with a 95%CI of 2.96 months to NA) (p = 0.0012, Figure 2a). Similarly, DSRCT patients had a longer OS following surgery (median = 44.32 months with a 95%CI of 34.53 m to NA) than patients with other histologies (median = 12.52 months with a 95%CI of 11.47 m to NA) (p = 0.0013, Figure 2B). From the time of diagnosis for DSRCT patients, the estimated median OS was 58.44 months (95%CI: 40.14 months to NA), the estimated 3-year OS was 79% (95%CI: 0.6–1).
Figure 1.
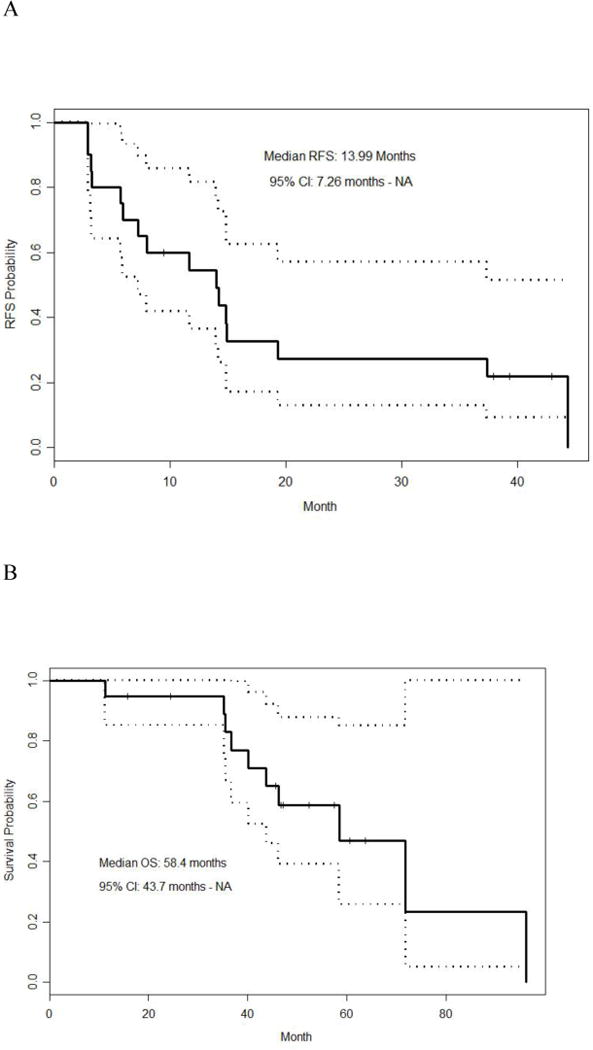
A = Recurrence-free survival in all patients
B = Overall survival in all patients
Figure 2.
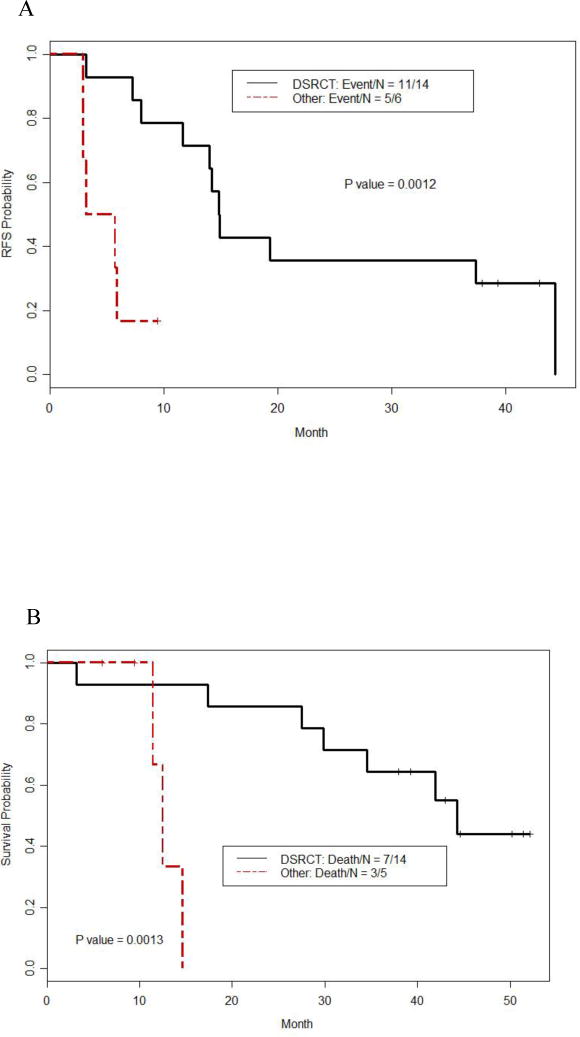
A = Recurrence-free survival in DSRCT vs. other tumors after cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy, CRS-HIPEC with Cisplatin
B = Overall survival in DSRCT vs. other tumors after cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy, CRS-HIPEC with Cisplatin
Among the 14 patients with DSRCT, eight patients had hepatic or portal disease, while six patients had neither hepatic nor portal disease. Of the patients without liver or portal disease, there were two recurrences, one patient developed intrahepatic and pulmonary metastases while the other patient recurred within his inguinal lymph nodes. We observed no peritoneal-based recurrences within among these six patients. In contrast, 87.5% (7/8) of patients with intrahepatic or portal disease eventually developed an intra-abdominal recurrence, with 4 being intrahepatic and 3 being peritoneal. However, there were no recurrences in patients with portal disease. All of the liver recurrences were parenchymal. In addition, DSRCT patients without intrahepatic, portal or distant disease had a significantly longer disease-free interval (37.9 months vs. 14.3 months, p = 0.02) than those who did have intrahepatic or portal disease at the time of CRS-HIPEC. Two of the six patients without intrahepatic or portal disease died, one from liver failure unrelated to his disease and one due to developing intrahepatic and pulmonary metastases. Five of the eight patients with hepatic or portal disease died, all of which were attributed to recurrent peritoneal or hepatic disease. There was no significant difference in OS between patients who did and did not have intrahepatic or portal disease at the time of CRS-HIPEC (p = 0.34).
There were no deaths or serious adverse events (SAE) related to cytoreductive surgery and HIPEC (perioperative). Two patients had a total of 2 grade 4 adverse events (AE). Both were elevation of creatinine of more than 1.5 times normal that spontaneously resolved within 2 weeks of HIPEC. Of 35 AEs possibly or probably related to the study drug delivery at HIPEC, 16 were spontaneously resolved grade 1–3 leukopenia or thrombocytopenia. At 30 days post-HIPEC, 7 patients had no complications. Major postoperative complications occurred in 8 patients (40%): three patients had temporary neurogenic bladder, 2 developed urinary tract infections and a wound infection, abscess and enterocutaneous fistula each occurred in one patient. The patient who developed an enterocutaneous fistula had previously been treated with high-dose abdominal radiation therapy.
DISCUSSION
DSRCT is a rare, highly aggressive sarcoma subtype that usually presents with massive tumor burden spread throughout the abdomen. Our phase two clinical trial results indicate that aggressive surgical therapy such as CRS and HIPEC with WART led to improved disease control and short-term survival in a highly selected subset of patients when combined with coordinated multidisciplinary care. These results reflect the ability of CRS, HIPEC and WART to provide effective local control of DSRCT. Of patients without liver disease, 100% had no peritoneal-disease recurrence after CRS, HIPEC and WART within the observed follow up period. All of these patients had a substantial reduction in tumor volume with neoadjuvant chemotherapy. All had postoperative radiation and adjuvant chemotherapy.
As demonstrated in this manuscript, at approximately 3 years post CRS, HIPEC and WART, extra-abdominal recurrences are seen as is recurrent liver disease, even in patients in whom liver disease was quiescent by PET or resected at the time of HIPEC. Extra-peritoneal recurrences persist despite long-term local control from CRS, HIPEC and WART. To that effect, 33% (2/6) DSRCT patients without liver or portal disease eventually developed disease within the inguinal lymph nodes, lung and liver parenchyma, despite the fact that they never developed a peritoneal recurrence. This highlights the need for improved systemic chemotherapies for DSRCT and that the local control from CRS, HIPEC and WART appears to be effective.
In this report, all patients had complete cytoreduction, likely contributing to the 79% three-year OS and estimated median OS of 58.4 months in DSRCT. In previous reports, of CRS and HIPEC in DSRCT, we noted a difference in median survival of 26.7 months compared to 63.4 months when incomplete or complete cytoreduction, respectively, was done with HIPEC.9 Other authors have shown in colon cancer and appendiceal carcinomatosis, complete cytoreduction improves survival.8,14,15 Complete cytoreduction is a critical component to the success of CRS and HIPEC in DSRCT.16,17 Complete cytoreduction impacts short term outcome, but long term survival remains a challenge due to lack of completely effective chemotherapy to address microscopic systemic disease.
In a report by Gil et al, median survival for DSRCT patients was 18 months.18 In this report, as well as the current study, complete or near cytoreduction was achieved. However, the main difference was lack of coordinated neoadjuvant and adjuvant chemotherapy and radiotherapy in the Gil report. In 2005, Lal et al. reported 55% 3-year survival for 29 DSRCT patients treated with P6 induction chemotherapy, surgical debulking and radiotherapy.17 Similarly, gross tumor resection was highly significant in prolonging overall survival (P <00001). HIPEC was not employed in this study. By comparison, we observed a 79% 3-year overall survival when adding HIPEC to the same multimodal treatment regimen employed by Lal and colleagues. Thus, HIPEC remains an intriguing local-control strategy that may help prolong survival in DSRCT patients.
Most recently, Honorè et al. reviewed 48 DSRCT patients who underwent CRS, including 11 patients who received intraperitoneal chemotherapy.19 Median overall survival was 42 months and was not significantly increased with intraperitoneal chemotherapy. However, there was non-uniform utilization of neoadjuvant chemotherapy (77%), adjuvant chemotherapy (79%), postoperative WART (48%) as well as intraperitoneal chemotherapy in this study. In addition, there was widespread heterogeneity amongst the chemotherapeutic agents employed for intraperitoneal treatment for these patients. Of the 11 patients receiving intraperitoneal chemotherapy, only 4 underwent HIPEC with cisplatin. Cisplatin is the preferred HIPEC drug in sarcoma.
It is unclear why patients with diagnoses other than DSRCT had poorer outcomes after surgery, even with complete cytoreduction. Perhaps a different drug other than Cisplatin is necessary. We hypothesize that these other sarcoma histologies are less responsive to the Cisplatin chemotherapy. In addition, these were, in general, more heavily pretreated patients who had sustained two or more relapses and recurrences. It is important to note, HIPEC has not been found to be effective in other sarcomas of adults.20–22 The Ewing’s type biology of DSRCT, may make it more sensitive to chemotherapy and radiation therapy, compared to other sarcomas, which perhaps is the explanation. Given these results, we have now initiated a phase I trial utilizing HIPEC with doxorubicin for pediatric patients with non-DSRCT sarcomas at our institution.
A limitation of this phase two trial is the fact it was non-randomized. Therefore, one cannot decipher the relative contribution to improved outcome from complete surgical excision of intra-abdominal implants, versus with the addition of hyperthermic intraperitoneal cisplatin. Similar outcomes may or may not be obtained with complete CRS, without HIPEC.
CONCLUSION
In conclusion, the results of this phase two trial show complete CRS, HIPEC and WART are an effective local control therapy in DSRCT patients. More effective chemotherapy is necessary to improve long-term outcomes.
SYNOPSIS.
This phase 2 trial of cytoreductive surgery and HIPEC in DSRCT and other pediatric-type sarcomas highlights the efficacy of CRS and HIPEC, using Cisplatin, in DSRCT. Worse outcomes are seen in patients with liver or peri-portal metastasis.
Acknowledgments
Supported in part by MD Anderson’s Cancer Center Support (Core) Grant, NIH/NCI P30 CA016672, and by Young Texans Against Cancer (YTAC).
Footnotes
Disclosures: None
References
- 1.Gerald WL, Ladanyi M, de Alava E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998;16(9):3028–3036. doi: 10.1200/JCO.1998.16.9.3028. [DOI] [PubMed] [Google Scholar]
- 2.Hayes-Jordan A, Anderson P. Desmoplastic small cell round tumor: review of therapy including surgery followed by continuous hyperthermic peritoneal perfusion of chemotherapy. Oncol Rev. 2009;3(3):195–200. [Google Scholar]
- 3.Hayes-Jordan A, Anderson PM. The diagnosis and management of desmoplastic small round cell tumor: a review. Curr Opin Oncol. 2011;23(4):385–389. doi: 10.1097/CCO.0b013e3283477aab. [DOI] [PubMed] [Google Scholar]
- 4.Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14(5):1526–1531. doi: 10.1200/JCO.1996.14.5.1526. [DOI] [PubMed] [Google Scholar]
- 5.Hayes-Jordan A, Anderson P, Curley S, et al. Continuous hyperthermic peritoneal perfusion for desmoplastic small round cell tumor. Journal of pediatric surgery. 2007;42(8):E29–32. doi: 10.1016/j.jpedsurg.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Hayes-Jordan A. Cytoreductive Surgery Followed by Hyperthermic Intraperitoneal Chemotherapy in DSRCT: Progress and Pitfalls. Current oncology reports. 2015;17(8):38. doi: 10.1007/s11912-015-0461-1. [DOI] [PubMed] [Google Scholar]
- 7.Hayes-Jordan A, Green H, Fitzgerald N, Xiao L, Anderson P. Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. Journal of pediatric surgery. 2010;45(5):1000–1006. doi: 10.1016/j.jpedsurg.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14(1):128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 9.Hayes-Jordan A, Green HL, Lin H, et al. Complete Cytoreduction and HIPEC Improves Survival in Desmoplastic Small Round Cell Tumor. Ann Surg Oncol. 2014;21(1):220–224. doi: 10.1245/s10434-013-3269-y. [DOI] [PubMed] [Google Scholar]
- 10.Hayes-Jordan A, Green H, Ludwig J, Anderson P. Toxicity of hyperthermic intraperitoneal chemotherapy (HIPEC) in pediatric patients with sarcomatosis/carcinomatosis: early experience and phase 1 results. Pediatr Blood Cancer. 2012;59(2):395–397. doi: 10.1002/pbc.24160. [DOI] [PubMed] [Google Scholar]
- 11.Osborne EM, Briere TM, Hayes-Jordan A, et al. Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol. 2016;119(1):40–44. doi: 10.1016/j.radonc.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker PH. Peritonectomy procedures. Annals of surgery. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 14.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker PH, Alderman R, Edwards G, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13(5):635–644. doi: 10.1245/ASO.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 16.Dufresne A, Cassier P, Couraud L, et al. Desmoplastic small round cell tumor: current management and recent findings. Sarcoma. 2012;2012:1–5. doi: 10.1155/2012/714986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of multimodal treatment for desmoplastic small round cell tumors. Journal of pediatric surgery. 2005;40(1):251–255. doi: 10.1016/j.jpedsurg.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Gil A, Gomez Portilla A, Brun EA, Sugarbaker PH. Clinical perspective on desmoplastic small round-cell tumor. Oncology. 2004;67(3–4):231–242. doi: 10.1159/000081323. [DOI] [PubMed] [Google Scholar]
- 19.Honore C, Amroun K, Vilcot L, et al. Abdominal Desmoplastic Small Round Cell Tumor: Multimodal Treatment Combining Chemotherapy, Surgery, and Radiotherapy is the Best Option. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4123-6. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner JM, Ahrendt SA, Pingpank JF, et al. Aggressive locoregional management of recurrent peritoneal sarcomatosis. Journal of surgical oncology. 2013;107(4):329–334. doi: 10.1002/jso.23232. [DOI] [PubMed] [Google Scholar]
- 21.Karakousis CP, Kontzoglou K, Driscoll DL. Intraperitoneal chemotherapy in disseminated abdominal sarcoma. Ann Surg Oncol. 1997;4(6):496–498. doi: 10.1007/BF02303674. [DOI] [PubMed] [Google Scholar]
- 22.Lim SJ, Cormier JN, Feig BW, et al. Toxicity and outcomes associated with surgical cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with sarcomatosis. Ann Surg Oncol. 2007;14(8):2309–2318. doi: 10.1245/s10434-007-9463-z. [DOI] [PubMed] [Google Scholar]


