Abstract
Adenosine represents a powerful modulating factor, which has been shown to orchestrate the scope, duration, and remission of the inflammatory response through the activation of four specific receptors, classified as A1, A2A, A2B, and A3, all being widely expressed in a variety of immune cells. Several selective A2A receptor agonists have displayed anti-inflammatory effects, through the suppression of IL-12, TNF, and IFN-γ production by monocytes and lymphocytes, in the setting of chronic intestinal inflammation. However, the therapeutic application of A2A receptor agonists remains hindered by the risk of serious cardiovascular adverse effects arising from the wide systemic distribution of A2A receptors. The present study focused on evaluating the anti-inflammatory effects of the novel poorly absorbed A2A receptor agonist PSB-0777 in a rat model of oxazolone-induced colitis as well as to evaluate its cardiovascular adverse effects, paying particular attention to the onset of hypotension, one of the main adverse effects associated with the systemic pharmacological activation of A2A receptors. Colitis was associated with decreased body weight, an enhanced microscopic damage score and increased levels of colonic myeloperoxidase (MPO). PSB-0777, but not dexamethasone, improved body weight. PSB-0777 and dexamethasone ameliorated microscopic indexes of inflammation and reduced MPO levels. The beneficial effects of PSB-0777 on inflammatory parameters were prevented by the pharmacological blockade of A2A receptors. No adverse cardiovascular events were observed upon PSB-0777 administration. The novel A2A receptor agonist PSB-0777 could represent the base for the development of innovative pharmacological entities able to act in an event-specific and site-specific manner.
Keywords: A2A adenosine receptor, Colon, Colitis, Inflammation, Locally acting agent
Introduction
Inflammatory bowel diseases (IBDs) are chronic, idiopathic inflammatory disorders characterized by alternating periods of active disease or flare-ups and remission, with highly negative impact on well-being and patient’s quality of life [1]. Although the precise etiological factors leading to the onset of these chronic inflammatory conditions remain unclear, increasing evidence supports the concept that IBDs arise from altered and complex interactions of environmental, genetic, and immunological factors [2].
At present, an increasing interest is being focused on the pharmacological modulation of endogenous mediators, actively involved in the suppression and/or resolution of inflammation, as viable and innovative anti-inflammatory strategies [3]. On these bases, a number of novel anti-inflammatory and healing mediators have been identified. Among them, adenosine represents a powerful modulating factor, which has been shown to orchestrate the goal, duration, and remission of the inflammatory response through the activation of four specific receptors, classified as A1, A2A, A2B, and A3, all being widely expressed in a variety of immune cells (lymphocytes, neutrophils, monocytes, macrophages, and dendritic cells) [4–6]. On this ground, a number of preclinical and clinical investigations have been implemented to evaluate the potential beneficial effects of adenosine receptor ligands in the management of several inflammatory conditions (i.e., asthma, chronic obstructive pulmonary disorder, rheumatoid arthritis, psoriasis, sepsis, IBD), with fairly encouraging results [7–10].
Several selective A2A receptor agonists have displayed anti-inflammatory effects, through the suppression of IL-12, TNF, and IFN-γ production by monocytes and lymphocytes [11–13], in the setting of chronic intestinal inflammation [14, 15], thus supporting a possible employ of these drugs as novel tools for the management of IBDs. However, the therapeutic application of A2A receptor agonists remains hindered by the risk of serious cardiovascular adverse effects arising from the wide systemic distribution of A2A receptors [10]. For these reasons, intensive efforts are being focused on the design of novel agonists aimed at channeling the A2A-mediated anti-inflammatory/immunomodulatory effects specifically towards the inflammatory environment. Along this line, interesting results have been recently pursued by Müller and co-workers [16] through the development of PSB-0777, a novel highly polar A2A-selective agonist, which cannot be absorbed systemically by the digestive mucosa once administered by the oral route.
Based on the above background, the present study has been conceived to investigate the anti-inflammatory effects of PSB-0777 in a rat model of oxazolone-induced colitis as well as to evaluate its cardiovascular effects, paying particular attention to the onset of hypotension, one of the main adverse events associated with the systemic pharmacological activation of A2A receptors.
Materials and methods
Stability of PSB-0777 in simulated gastric acid
Preparation of simulated gastric acid
Pepsin 3.2 g (pig, 35,000, i.e., activity), 2.0 g NaCl, and 80 ml HCl (1 M) were mixed together, and pure water was added to a final volume of 1000 ml (pH = 1.2). Freshly prepared solutions of the test drugs were used for the measurements.
Liquid chromatography/mass spectrometry (LC/MS) measurements
Mass spectra were recorded on an API 2000 (Applied Biosystems, Darmstadt, Germany) mass spectrometer (turbo ion spray ion source) coupled with an Agilent 1100 HPLC system (Agilent, Böblingen, Germany) using a C18 column from Macherey & Nagel. Solution of PSB-0777 (1 mM) was added to the simulated gastric acid solution and incubated at 37 °C in a water bath. At specific time intervals of 10, 30, 60, 120, and 180 min, 100 μl of the solution were transferred to an autosampler vial and LC/MS analysis was immediately started. After 10 min of column equilibration with 90% H2O/10% MeOH (containing 2 mM ammonium acetate), 8 μl of the samples was injected to the LCMS instrument and chromatographed at a flow rate of 250 μl/min. A solvent gradient from 90% H2O/10% MeOH (containing 2 mM ammonium acetate) to 100% MeOH (containing 2 mM ammonium acetate) was applied within 10 min. The column was subsequently rinsed for 10 min with 100% methanol. Full scan mass spectra from 150 to 600 μ were recorded in both positive and negative modes.
Determination of solubility and Caco-2 cell membrane permeability
These studies were performed by Pharmacelsus, Saarbrücken, Germany.
Water solubility
Semi-thermodynamic solubility was determined by the shaking flask method. In brief, PSB-0777 was dissolved in DMSO at a concentration of 20 mM. This stock solution was used to spike three tubes containing PBS (cutoff concentration 200 μM) with a final solvent content of 1% DMSO. Tubes were shaken for 24 h, undissolved particles were removed by centrifugation, and the supernatant was used for quantification by LC/MS using a 5–8-point calibration curve.
Caco-2 permeability
In order to predict peroral absorption, bidirectional permeation studies using Caco-2 cells were performed. Caco-2 cells were differentiated for 21 days in Transwell® plates, and PSB-0777 was tested at a concentration of 10 μM.
Animals
Albino male Sprague-Dawley rats, 200 g body weight, were used throughout the study. The animals were fed with standard laboratory chow and tap water ad libitum and were not employed for at least 1 week after their delivery to the laboratory. They were housed, three in a cage, in a temperature-controlled rooms on a 12-h light cycle at 22–24 °C and 50–60% humidity. Their care and handling were in accordance with the provisions of the European Community Council Directive 210/63/UE, recognized and adopted by the Italian Government. The experiments were approved by the Ethical Committee for Animal Experimentation of the University of Pisa and by the Italian Ministry of Health.
Induction of colitis, drug treatments, and experimental design
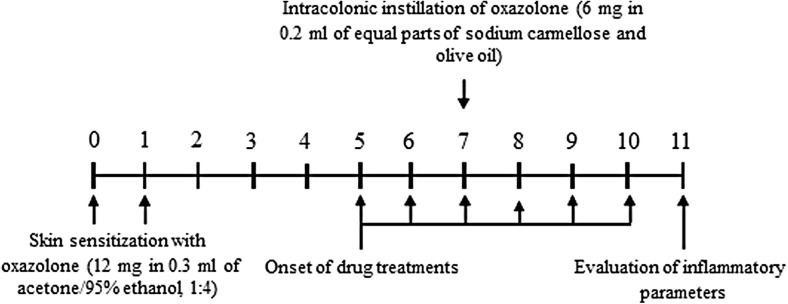
Colitis was induced in accordance with Ekstrom [17]. Briefly, during slight inhalation anesthesia with isoflurane, the animals underwent immune sensitization by painting 0.3 ml acetone/95% ethanol (1:4), containing 12 mg of oxazolone, on an 8-cm2 area of shaved abdomen on days 6 and 7 before challenge. Some animals, to be employed as controls for the sensitization procedure, were painted with the vehicle (acetone/ethanol) only. Six days after the second sensitization, the animals were challenged with oxazolone injected directly into the colon. For this purpose, under isoflurane anesthesia, a rubber catheter was inserted 8 cm into the colon via rectal route, and 200 ml of the challenge solution was injected. The challenge solution consisted of 6 mg oxazolone in a mixture consisting of equal parts of carmellose sodium and olive oil. Carmellose sodium was mixed with olive oil to emulsify oxazolone. Some animals were treated with carmellose sodium/olive oil, only as controls, to determine the putative proinflammatory properties of the vehicle. Test drugs were administered orally for 6 days starting 2 days before intracolonic injection of oxazolone (Fig. 1).
Fig. 1.
Diagram showing the design and time course of experiments on rats with oxazolone-induced colitis
A series of preliminary experiments were performed to select appropriate doses of PSB-0777 (perorally non-absorbable A2A-selective agonist) (Fig. 2). The dose of dexamethasone, employed as a standard anti-inflammatory comparator, was selected on the basis of a previous study performed on a rat model of colitis [18].
Fig. 2.
Chemical structure of PSB-0777
The first part of the study was aimed at testing the efficacy of the oral administration of agonist PSB-0777 (0.4 mg/kg/day) and dexamethasone (1 mg/kg/day) in ameliorating oxazolone-induced colitis. For this purpose, the effects of test drugs were evaluated on systemic (body weight) and tissue [microscopic score, myeloperoxidase (MPO) levels] inflammatory parameters. At the time of sacrifice, specimens of colonic tissues were fixed in cold 4% paraformaldehyde for the assessment of microscopic damage score or immediately snap-frozen in liquid nitrogen and stored at − 80 °C for subsequent MPO assays. In addition, in order to verify the selectivity of action on A2A receptors, the effects of PSB-0777 on the inflammatory parameters were evaluated either in the absence or in the presence of CSC, a selective A2A adenosine receptor antagonist (1 mg/kg/day). An effective and selective dose of the receptor antagonist was chosen on the basis of previous reports [19, 20].
Assessment of colitis
At the end of treatments, colonic tissues were excised, rinsed with saline, and scored for histological damage, in accordance with the criteria reported previously by Zhang et al. [21]. Histological evaluations were carried out by light microscopy on hematoxylin- and eosin-stained sections obtained from whole-gut specimens fixed in cold 4% neutral formalin, diluted in phosphate-buffered saline (PBS). Histological score included the following criteria: mucosal architecture loss (0–3), cellular infiltrate (0–3), muscle thickening (0–3), crypt abscess (0, absent; 1, present), and goblet cell depletion (0, absent; 1, present). All parameters of histological changes were recorded and scored for each rat by two observers blinded to treatments.
Assay of tissue myeloperoxidase
Myeloperoxidase (MPO) levels in colonic tissues were determined as previously reported by Antonioli et al. [22] and assumed as a quantitative index to estimate the degree of mucosal infiltration by polymorphonuclear cells. Briefly, colonic tissue samples (300 mg) were homogenized three times (30 s each) at 4 °C with a polytron homogenizer (Cole Parmer Homogenizer, Vernon Hills, IL, USA) in 1 ml of ice-cold 50 mmol/L phosphate buffer (pH 6.0), containing 0.5% of hexadecyltrimethylammonium bromide to prevent the pseudoperoxidase activity of hemoglobin as well as to solubilize membrane-bound MPO. The homogenate was sonicated for 10 s, frozen-thawed three times and spun by centrifugation for 20 min at 18,000×g. The supernatant was then recovered and used for determination of MPO by means of a kit for enzyme-linked immunosorbent assay (Bioxytech, Oxis International Inc., Portland, OR, USA). All samples were assayed within 2 days from collection. The results were expressed as nanograms of MPO per 100 mg of tissue.
Evaluation of tissue TNF levels
TNF levels in colonic tissues were measured by an ELISA kit (Abcam), as previously described [1]. For this purpose, colonic tissue samples, stored previously at − 80 °C, were weighed, thawed, and homogenized in 0.4 ml of pH 7.2 PBS/20 mg of tissue at 4 °C and centrifuged at 10,000×g for 5 min. Aliquots (100 μl) of the supernatants were then used for assay. Tissue TNF levels were expressed as picograms per milligram of tissue.
Evaluation of the systemic effects and plasma concentrations of test drugs
A second set of experiments was aimed at evaluating putative correlations between the effects of PSB-0777 on blood pressure and its plasma concentration, after its administration at doses displaying beneficial effects on oxazolone-induced colitis. For this purpose, a subgroup of animals was treated with test drugs by oral gavage or intraperitoneal route. Systolic blood pressure was measured before euthanization (30, 60, 120, and 240 min after test drug administration) by the tail-cuff method at baseline and after injection of experimental drugs or vehicle (BP recorder 58600; Ugo Basile, Comerio, Italy). An average of three pressure readings was obtained. At the time of sacrifice, blood samples were collected in order to evaluate plasma concentrations of test drugs as described below.
Assay of test drugs in plasma samples by LC-MS
Pre-treatment of plasma samples
Acetonitrile (LCMS quality) was added to each plasma sample (1:1). Samples were mixed with a vortexer for 30 s and subsequently sonicated in an ultrasonic bath for 5 min followed by centrifugation (Savant SpeedVac, SPD111V) for 10 min. In order to obtain more concentrated samples, 700 μl from the above samples was vacuum-dried (40 °C, 3 h, 200 mbar; Savant SpeedVac, SPD111V). The resulting residues were dissolved in 70 μl acetonitrile/water (1:1) and centrifuged. The viscous supernatants were subsequently diluted with acetonitrile (1:1), homogenized, and transferred to an LC/MS auto sampler vials and subjected to quantitative analysis.
Quantitative analyses
Mass spectra were recorded on a micrOTOF-Q mass spectrometer (Bruker) with an electrospray ionization source coupled with an HPLC Dionex Ultimate 3000 instrument (Thermo Scientific) using an EC50/2 Nucleodur C18 Gravity 3-μm column (Macherey-Nagel). The column temperature was 25 °C. Samples (5 μl) were injected and a flow rate of 0.3 ml/min was applied. HPLC analysis started with 90% water containing 0.1% acetic acid for 1 min, and a gradient was applied after 1 min reaching 100% acetonitrile (containing 0.1% acetic acid) after 10 min. The column was further flushed with 100% acetonitrile containing 0.1% acetic acid for 5 min. Positive full scan MS was set and recorded from 50 to 1000 m/z (optimized for the target mass 261.181). For identification and quantification, the extract ion chromatogram (EIC) of 261.181 ± 0.005% m/z was used. The employed acetonitrile was of LCMS quality from Riedel de Haen (Seelze, Germany). The retention time (RT) was 3.8 min. The limit of detection for PSB-0777 was 1 nM.
Drugs and reagents
Acetonitrile, of LCMS quality, was purchased from Riedel de Haen (Seelze, Germany). Water was purified by a PURELAB flex 2 from Veolia water, Celle, Germany. Ammonium acetate, acetic acid, acetone, carmellose sodium, dexamethasone, ethanol, formalin, hexadecyltrimethylammonium bromide, and 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone) pepsin were purchased from Sigma Chemicals (Saint Louis, MO, USA). CSC ((E)-8-[2-(3-Chlorophenyl)ethenyl]-3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione) was purchased from Tocris (Bristol, UK).
The synthesis of PSB-0777 was performed as previously reported by El Tayeb et al. [16]. For oral gavage administration, PSB-0777 and dexamethasone were suspended in 1% methocel (Sigma Chemicals, Saint Louis, MO, USA) and administered in a volume of 0.5 ml per rat. For i.p. administration, test drugs were dissolved in sterile dimethyl sulfoxide (Sigma Chemicals, Saint Louis, MO, USA).
Statistical analysis
The results are given as mean ± S.E.M. The statistical significance of differences was assessed by one-way analysis of variance followed by post hoc analysis by Student-Newman-Keuls test, and p values lower than 0.05 were considered significant. All statistical procedures were performed using Prism 3.0 software (GraphPad, San Diego, CA).
Results
Solubility, stability, and Caco-2 permeability of PSB-0777
Semi-thermodynamic solubility measurements showed that PSB-0777 is well soluble in phosphate-buffered saline containing 1% of DMSO, displaying a solubility of 95.3 ± 5.5 μM. Moreover, PSB-0777 displayed high stability in simulated gastric acid at 37 °C for at least 3 h. No degradation was observed (data not shown). Caco-2 cell transport assays indicated no penetration (data not shown).
Evaluation of anti-inflammatory effects
Body weight
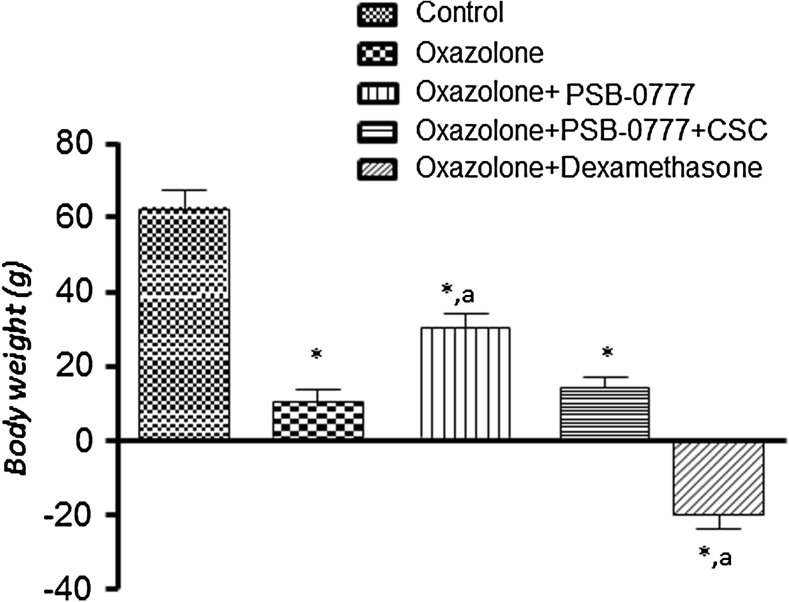
Rats with oxazolone-induced colitis displayed a lower body weight gain as compared with control animals (Fig. 3). Significant increments of body weight were observed in rats with colitis under treatment with PSB-0777 (Fig. 3): These effects were significantly counteracted by CSC (Fig. 3). By contrast, a significant body weight loss was recorded after administration of dexamethasone (Fig. 3). This effect was not altered by co-administration of dexamethasone with CSC (not shown).
Fig. 3.
Effects of PSB-0777 (0.4 mg/kg/day) alone or in combination with CSC (1 mg/kg/day) or dexamethasone (1 mg/kg/day) on body weight in rats with oxazolone-induced colitis. Each column represents the mean ± S.E.M (n = 6). *p < 0.05, significant difference versus control group; a p < 0.05, significant difference versus oxazolone group
Colonic microscopic damage score
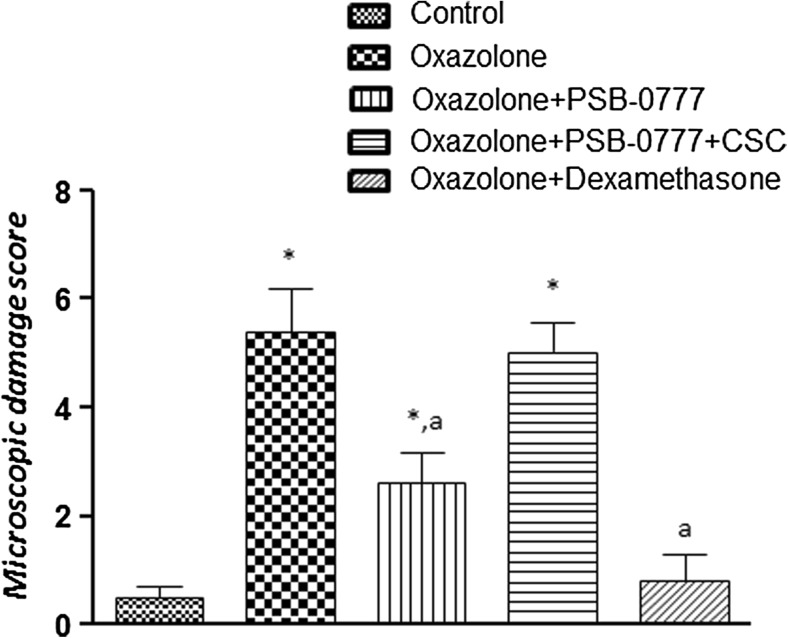
Histological examination of colonic specimens from oxazolone-treated rats showed the presence of an inflammatory reaction at the colonic level, characterized by disruption of the epithelial layer and lamina propria, accompanied by mucosal edema, infiltration by inflammatory cells into the mucosa, along with goblet cell disruption and disappearance (Fig. 4b), as compared with colonic specimens from control rats (Fig. 4a). Colonic sections from rats with colitis displayed a marked increase in microscopic damage score (Fig. 5). In colonic tissues from inflamed animals treated with PSB-0777 or dexamethasone, a marked reduction of inflammatory cell infiltration and an amelioration of colonic mucosal architecture were found (Fig. 4c, d), with a consequent improvement of the microscopic damage score (Fig. 5). The effect of PSB-0777 was significantly counteracted the administration of the A2A antagonist CSC (Fig. 5). No significant alteration was observed in the beneficial effects of dexamethasone upon co-administration with CSC (not shown).
Fig. 4.
Hematoxylin and eosin-stained sections of rat colon. Microscopic images refer to control rats (a) or oxazolone-treated animals, either alone (b) or in the presence of PSB-0777 (0.4 mg/kg/day) (c) or dexamethasone (1 mg/kg/day) (d)
Fig. 5.
Microscopic damage scores estimated for colon in rats under normal conditions or after oxazolone treatment, either alone or in the presence of PSB-0777 (0.4 mg/kg/day) alone or in combination with CSC (1 mg/kg/day), or dexamethasone (1 mg/kg/day) administration. Each column represents the mean S.E.M. (n = 6). *p < 0.05, significant difference versus control group; a p < 0.05, significant difference versus oxazolone group
Colonic tissue myeloperoxidase levels
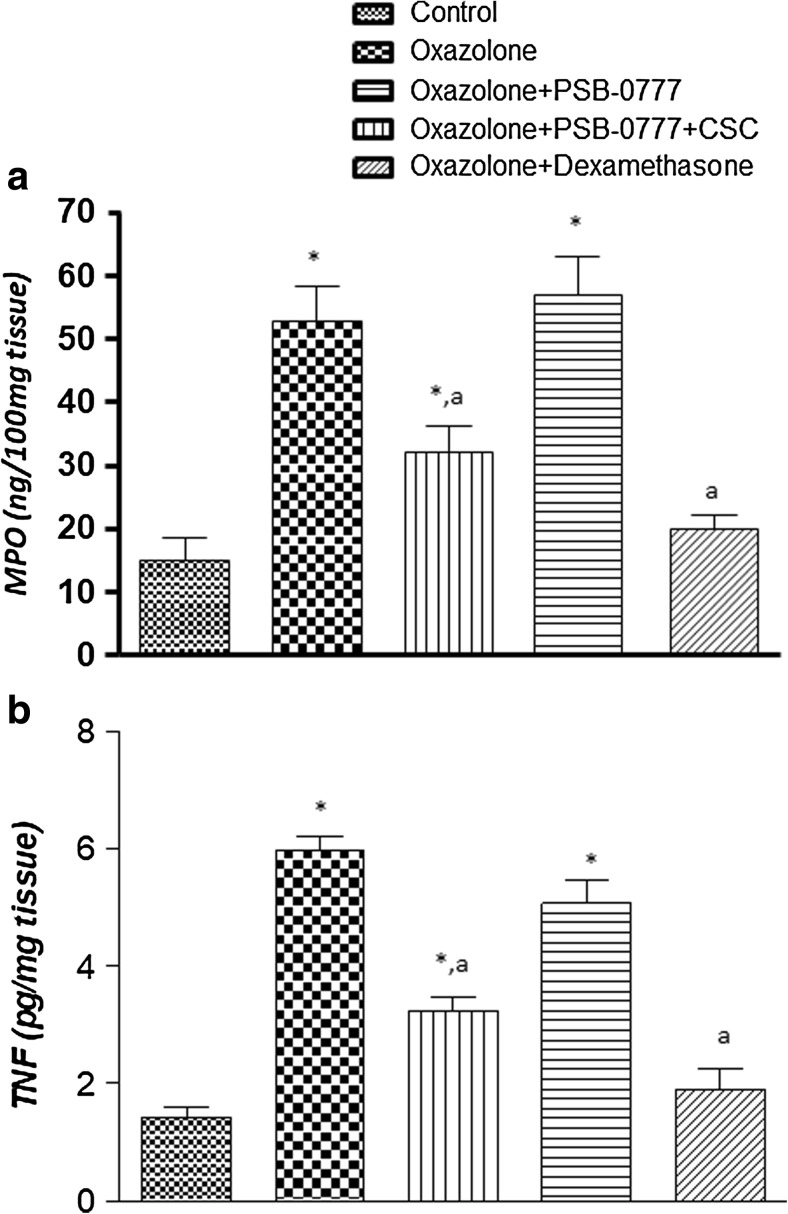
Oxazolone-treated rats displayed a marked increase in colonic MPO levels, as compared with control animals (Fig. 6). Treatment with PSB-0777 counteracted significantly the increment of colonic MPO levels associated with colitis (Fig. 6). A significant decrease in MPO levels was also obtained following treatment with dexamethasone (Fig. 6). The effects of PSB-0777 on colonic MPO levels were blunted by the A2A receptor antagonist CSC (Fig. 6), whereas no significant effects were observed upon co-treatment with dexamethasone (not shown).
Fig. 6.
a MPO and b TNF levels in colonic tissues from control rats or in animals treated with oxazolone, either alone or in combination with PSB-0777 (0.4 mg/kg/day) alone or in combination with CSC (1 mg/kg/day), or dexamethasone (1 mg/kg/day). Each column represents the mean S.E.M. (n = 6). *p < 0.05, significant difference versus control group; a p < 0.05, significant difference versus oxazolone group
Colonic tissue TNF levels
Colonic inflammation induced by oxazolone was associated with a significant increase in tissue TNF levels (Fig. 6b). Treatments with PSB-0777 significantly decreased the concentration of this cytokine in colonic tissues (Fig. 6b). Dexamethasone-treated animals showed a significant decrease in TNF colonic tissue levels (Fig. 6b). The A2A receptor antagonist CSC prevented the effects of PSB-0777 (Fig. 6b) but was ineffective on the effect of dexamethasone (not shown).
Plasma concentrations
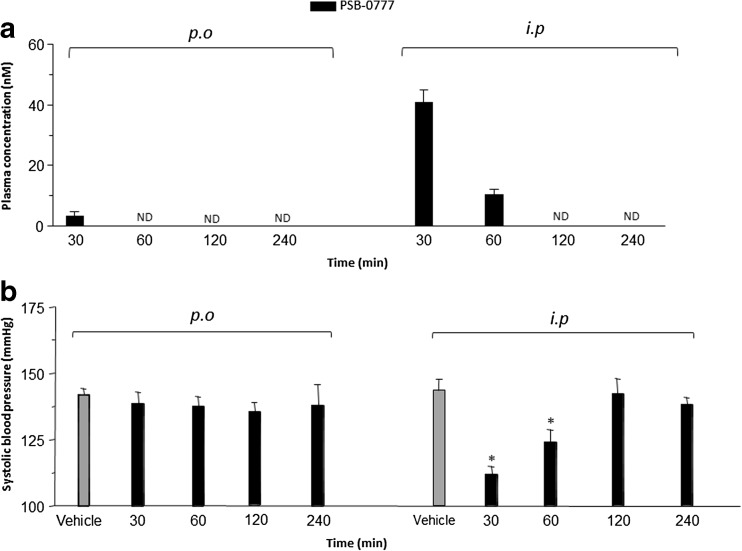
Only very low plasma concentrations of PSB-0777 in perorally treated rats were detectable at 30 min (below 5 nM), but not 60 min after administration, indicating a very low systemic absorption by the oral route (limit of detection 1 nM) (Fig. 7a). This compound was not detectable in plasma samples 60 min after its administration (Fig. 7a). After i.p administration, PSB-0777 plasma concentrations were well evident at 30 min, decreased after 60 min, and were not detectable at 120 and 240 min (Fig. 7a).
Fig. 7.
a Plasma concentration of PSB-0777 (0.4 mg/kg/day) 30, 60, 120, and 240 min after oral or intraperitoneal administration in rats with oxazolone-induced colitis. b Effects of PSB-0777(0.4 mg/kg/day) 30, 60, 120, 240 min after oral or intraperitoneal administration on systolic blood pressure in rats with oxazolone-induced colitis. Each column represents the mean S.E.M. (n = 6). *p < 0.05, significant difference versus vehicle-treated animals
Systolic blood pressure evaluation
Rats treated perorally with PSB-0777 did not show any significant variation of systolic blood pressure as compared with control animals (Fig. 7b). By contrast, in rats treated intraperitoneally (i.p.) with PSB-0777, the systolic blood pressure significantly decreased after 30 and 60 min, but not after 120 and 240 min, of treatment, as compared to control (Fig. 7b). Of note, the hypotensive effect of PSB-0777 after i.p. administration was prevented by the administration of the A2A receptor antagonist (data not shown).
Discussion
It is being increasingly appreciated that adenosine holds a crucial role in the modulation of inflammatory and immune responses, thus spurring the progress of medicinal chemistry towards the development of novel compounds acting on this pathway, as promising pharmacological entities against intestinal inflammatory disorders [10]. In this context, compelling evidence supports A2A receptors as the major immunoregulatory arm of the adenosine system [10]. For this reason, consistent efforts are being dedicated to the development of A2A receptor agonists for the therapeutic management of several inflammatory disorders, such as asthma [23, 24], arthritis [25, 26], glomerulonephritis [27, 28], sepsis [29, 30], and intestinal inflammation [14, 31, 32]. However, the great expectation about the therapeutic use of A2A receptor agonists as a viable anti-inflammatory strategy is being challenged by several concerns, mainly depending on their severe hypotensive effects related to the wide distribution of A2A receptors in the cardiovascular system [33].
Based on the above background, the present study was performed as follows: (i) to investigate, by means of in vitro experiments, the stability of PSB-0777 (a novel A2A receptor agonist conceived to act as a poorly absorbed locally acting compound) in simulated gastric acid fluid as well as its cell membrane permeability; (ii) to evaluate the potential anti-inflammatory effects of PSB-0777 in a rat model of colitis; and (iii) to assess the impact of this ligand on blood pressure following its oral administration.
The first set of experiments allowed to demonstrate a significant stability of PSB-0777 in an in vitro gastric simulated environment, indicating the suitability of the oral administration of this compound. In parallel, experiments performed on CaCo-2 cell monolayers, aimed at investigating the level of PSB-0777 penetration, displayed a lack of absorption of this A2A agonist. This evidence is consistent with the chemical properties of PSB-0777 [16]. Indeed, as reported by El-Tayeb et al. [16], this compound, besides being endowed with high affinity for A2A receptors and high selectivity (> 225-fold) over the other adenosine receptors, is endowed with a high polar structure, which confers to the compound high solubility in water and a lack of permeability across cell membranes. These physico-chemical features of PSB-0777 account for its scarce systemic bioavailability upon oral administration, as documented by our in vivo experiments. On these bases, PSB-0777 may represent a useful candidate for exerting a local anti-inflammatory action on bowel mucosa, while sparing a concomitant decrease in the risk of hypotension and other systemic adverse effects.
Following the above positive findings, we decide on going with the second set of experiments to evaluate the putative anti-inflammatory effects of PSB-0777 in the experimental model of oxazolone-induced colitis. This model is regarded as remnant of ulcerative colitis, characterized by weight loss, diarrhea, loss of epithelial cells, erosion of the mucosal layer, ulcers, decrease in goblet cell number and gland density, and pronounced MPO tissue activity [17, 21]. In particular, the inflammatory process develops in or beneath the epithelial mucosa layer, although in some cases the muscle layer too appears to be infiltrated by immune/inflammatory cells [17, 21]. In our hands, the suitability of this model was demonstrated by the ability of dexamethasone to ameliorate the histological colonic damage as well as to reduce tissue TNF and MPO levels, in line with previous reports showing similar beneficial effects of this glucocorticoid drug [34]. When considering the effects of PSB-0777 on body weight, microscopic damage score, and tissue TNF and MPO levels, as an index of inflammatory cell infiltrate, we observed an amelioration of all these inflammatory parameters. In particular, animals treated with PSB-0777 displayed a significant increase in body weight gain as well as an improvement of the histological damage score and a decrease in TNF and MPO levels, thus providing additional evidence that the pharmacological stimulation of A2A receptors at inflammatory sites can result in a significant attenuation of the phlogistic processes [15, 35, 36]. The increase in body weight resulted very likely from the local action of PSB-0777 in the intestine, since the systemic administration of PSB-0777 was previously found to lead to a decrease in body weight in mice fed with high-fat diet due to the activation of A2A adenosine receptors in adipocytes [37].
A2A receptors, widely expressed on innate and adaptive immune cells [38], have been shown to curb their activity, thus reducing their cytotoxic functions, adhesion, production of oxygen radicals, degranulation, and release of proinflammatory cytokines (i.e., TNF, IL-1β, IL-6, IL-12, IL-17) as well as counteracting the activation of nuclear factor (NF)-κB [39]. Of note, neutrophils are a critical source of adenosine [40]. Indeed, once activated, these cells release massively ATP via connexin 43 hemichannels, which is quickly converted into AMP and adenosine by the ectoenzymes CD39 and CD73, respectively, expressed on the neutrophil surface [40]. The activation of A2A receptors expressed on neutrophils can influence the broader inflammatory response by modulating production and release of pro- and anti-inflammatory mediators, such as chemokines, leukotrienes, and prostaglandins [40], pointing out a critical role for this receptor subtype in shaping the immune response.
The beneficial effects of A2A receptor agonists have been documented in several models of bowel inflammation, where A2A activation was found to prevent the trafficking of immune cells into the enteric mucosa [14]. In these models, consistently with our results, the decrease in leukocyte infiltration following A2A receptor stimulation was associated with a significant amelioration of the disease course, as indicated also by the preservation of mucosal architecture and villi [14]. In a recent paper, El Tayeb et al. [16], by means of in vitro studies, documented the efficacy of PSB-0777 in reverting the alterations of acetylcholine-induced contractions in the presence of intestinal inflammation induced via incubation of ileal tissues with 2,4,6-trinitrobenzenesulfonic acid colitis, thus strengthening the hypothesis about the potential usefulness of this non-absorbable A2A-selective agonist to counteract enteric motor dysfunctions associated with bowel inflammation. In the present study, we have shown, for the first time, that a highly polar A2A receptor agonist, which penetrates poorly cell membranes, exerted a significant effect in a model of colitis. In this regard, it is noteworthy that the murine model of oxazolone-induced colitis, as observed also in UC patients, is characterized by a laxity of epithelial tight junctions leading to an increase in mucosal permeability [41–43]. In particular, a pivotal role of NK cells in the alteration of epithelial layer, which may contribute significantly to the onset and development of oxazolone-induced colitis, has been reported [43]. Thus, based on this body of evidence, it is conceivable that the inflammatory impairments of the enteric mucosal barrier allow PSB-0777 to reach the lamina propria, where it can exert its immunomodulatory effects, without acting at a systemic level. In this context, the relevant role of A2A adenosine receptors in mediating the deep immunosuppressive actions of adenosine on several immune cell populations, including NK cells, is widely recognized [6, 39].
These observations might pave the way to the clinical development of non-absorbable A2A antagonists also for treatment of other intestinal disorders, such as the irritable bowel syndrome, characterized by alterations of the enteric epithelial barrier [44].
As anticipated above, systemically acting A2A receptor agonists can exert remarkable hemodynamic effects, which so far have severely limited their development for therapeutic employment, as anti-inflammatory drugs [26]. For this reason, the second part of the present study was aimed at evaluating plasma concentrations of PSB-0777, to verify its systemic bioavailability and to investigate its putative effects on blood pressure. As expected, our findings displayed a lack of significant systemic absorption of PSB-0777 after oral administration, thus indicating a negligible bioavailability by this route. By contrast, following its intraperitoneal administration, the compound could be detected in plasma samples at concentrations that are expected to activate A2A receptors. Of note, these pharmacokinetic features are in line with a lack of systemic effects by PSB-0777 following its oral administration. Indeed, animals treated perorally with PSB-0777 did not show any significant variation of systolic blood pressure, at variance with rats treated intraperitoneally with this compound, displaying a significant decrease in systolic blood pressure. In addition, the selective A2A antagonist CSC prevented the PSB-0777-induced hypotensive effect after intraperitoneal administration, thus corroborating further the evidence that this effect resulted from the activation of A2A receptors.
Our data about the efficacy of PSB-0777 in counteracting colonic inflammation, in parallel with a very low systemic bioavailability of PSB, provide sufficient, although indirect, demonstration that, upon oral administration, PSB achieves local concentrations in the intestinal lumen that were fairly sufficient to counteract mucosal inflammation. However, the lack of a direct evaluation about the concentration achieved by PSB in the luminal content represents a point of limitation of the present study that need to be resolved in future studies.
In conclusion, there is no question that the field of drugs acting on the adenosine pathway represents a huge opportunity for the development of novel anti-inflammatory/immunomodulatory agents. In particular, the passionate interest currently revolving around the adenosine system points out the discovery of several innovative molecules, for which the performance of preclinical and clinical studies is needed to define and understand their putative value in the management of immune/inflammatory disorders [10]. In this context, the novel locally acting A2A receptor agonist PSB-0777 could represent the base for the development of innovative pharmacological entities able to act both in an event-specific and site-specific manner, thus harnessing the beneficial effects arising from the stimulation of A2A receptors, without the limitation of adverse effects associated with the systemic stimulation of these receptors.
Acknowledgements
We thank Marion Schneider for the LCMS analyses.
Funding information
The present was supported by Nexus award “Marcello Tonini” (L.A.) and by the IBD Research Foundation (mini grant 2012) (L.A.).
Compliance with ethical standards
The care and handling of the animals were in accordance with the provisions of the European Community Council Directive 210/63/UE, recognized and adopted by the Italian Government. The experiments were approved by the Ethical Committee for Animal Experimentation of the University of Pisa and by the Italian Ministry of Health.
Conflict of interest
Matteo Fornai declares that he has no conflict of interest.
Luca Antonioli declares that he has no conflict of interest.
Alì El-Tayeb declares that he has no conflict of interest.
Carolina Pellegrini declares that she has no conflict of interest.
Oriana Awwad declares that she has no conflict of interest.
Giulio Giustarini declares that he has no conflict of interest.
Gianfranco Natale declares that he has no conflict of interest.
Larisa Ryskalin declares that she has no conflict of interest.
Zoltan H Németh declares that he has no conflict of interest.
Christa E Müller declares that she has no conflict of interest.
Corrado Blandizzi declares that he has no conflict of interest.
Rocchina Colucci declares that he has no conflict of interest.
References
- 1.Antonioli L, et al. The blockade of adenosine deaminase ameliorates chronic experimental colitis through the recruitment of adenosine A2A and A3 receptors. J Pharmacol Exp Ther. 2010;335(2):434–442. doi: 10.1124/jpet.110.171223. [DOI] [PubMed] [Google Scholar]
- 2.Torres MI, Rios A. Current view of the immunopathogenesis in inflammatory bowel disease and its implications for therapy. World J Gastroenterol. 2008;14(13):1972–1980. doi: 10.3748/wjg.14.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward SG. New drug targets in inflammation: efforts to expand the anti-inflammatory armoury. Br J Pharmacol. 2008;153(Suppl 1):S5–S6. doi: 10.1038/sj.bjp.0707628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hasko G, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonioli L, et al. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120(3):233–253. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Polosa R. Finding better therapeutic targets for patients with asthma: adenosine receptors? Br J Pharmacol. 2008;155(4):441–443. doi: 10.1038/bjp.2008.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koscso B, et al. Investigational A(3) adenosine receptor targeting agents. Expert Opin Investig Drugs. 2011;20(6):757–768. doi: 10.1517/13543784.2011.573785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman P, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17(7–8):359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonioli L, et al. Adenosine and inflammation: what’s new on the horizon? Drug Discov Today. 2014;19(8):1051–1068. doi: 10.1016/j.drudis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Hasko G, et al. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157(10):4634–4640. [PubMed] [Google Scholar]
- 12.Hasko G, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14(13):2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 13.Csoka B, et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110(7):2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odashima M, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129(1):26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Naganuma M, et al. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177(5):2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 16.El-Tayeb A, et al. Development of polar adenosine A2A receptor agonists for inflammatory bowel disease: synergism with A2B antagonists. ACS Med Chem Lett. 2011;2(12):890–895. doi: 10.1021/ml200189u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekstrom GM. Oxazolone-induced colitis in rats: effects of budesonide, cyclosporin A, and 5-aminosalicylic acid. Scand J Gastroenterol. 1998;33(2):174–179. doi: 10.1080/00365529850166914. [DOI] [PubMed] [Google Scholar]
- 18.Motavallian A, et al. Involvement of 5HT3 receptors in anti-inflammatory effects of tropisetron on experimental TNBS-induced colitis in rat. Bioimpacts. 2013;3(4):169–176. doi: 10.5681/bi.2013.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golembiowska K, Dziubina A. Effect of the adenosine A2A receptor antagonist 8-(3-chlorostyryl) caffeine on L-DOPA biotransformation in rat striatum. Brain Res. 2004;998(2):208–217. doi: 10.1016/j.brainres.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Golembiowska K, Dziubina A. Effect of adenosine A(2A) receptor antagonists and L-DOPA on hydroxyl radical, glutamate and dopamine in the striatum of 6-OHDA-treated rats. Neurotox Res. 2012;21(2):222–230. doi: 10.1007/s12640-011-9263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HQ, et al. Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol. 2009;15(15):1821–1828. doi: 10.3748/wjg.15.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonioli L, et al. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther. 2007;322(2):435–442. doi: 10.1124/jpet.107.122762. [DOI] [PubMed] [Google Scholar]
- 23.Fozard JR, et al. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur J Pharmacol. 2002;438(3):183–188. doi: 10.1016/S0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- 24.Bonneau O, et al. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- 25.Mazzon E, et al. CGS 21680, an agonist of the adenosine (A2A) receptor, reduces progression of murine type II collagen-induced arthritis. J Rheumatol. 2011;38(10):2119–2129. doi: 10.3899/jrheum.110111. [DOI] [PubMed] [Google Scholar]
- 26.Flogel U, et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci Transl Med. 2012;4(146):146ra108. doi: 10.1126/scitranslmed.3003717. [DOI] [PubMed] [Google Scholar]
- 27.Garcia GE, et al. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. FASEB J. 2008;22(2):445–454. doi: 10.1096/fj.07-8430com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia GE, et al. Adenosine A(2A) receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int. 2011;80(4):378–388. doi: 10.1038/ki.2011.101. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan GW, et al. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189(10):1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 30.Moore CC, et al. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalcante IC, et al. Effect of novel A2A adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect Immun. 2006;74(5):2606–2612. doi: 10.1128/IAI.74.5.2606-2612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren CA, et al. Effects of adenosine A2A receptor activation and alanyl-glutamine in Clostridium difficile toxin-induced ileitis in rabbits and cecitis in mice. BMC Infect Dis. 2012;12:13. doi: 10.1186/1471-2334-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasko G, et al. Adenosine A2A receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med. 2006;34(4):1119–1125. doi: 10.1097/01.CCM.0000206467.19509.C6. [DOI] [PubMed] [Google Scholar]
- 34.Kojima R, et al. Oxazolone-induced colitis in BALB/C mice: a new method to evaluate the efficacy of therapeutic agents for ulcerative colitis. J Pharmacol Sci. 2004;96(3):307–313. doi: 10.1254/jphs.FP0040214. [DOI] [PubMed] [Google Scholar]
- 35.Kuno M, et al. Anti-inflammatory activity of non-nucleoside adenosine deaminase inhibitor FR234938. Eur J Pharmacol. 2006;534(1–3):241–249. doi: 10.1016/j.ejphar.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Adanin S, et al. Inhibiting adenosine deaminase modulates the systemic inflammatory response syndrome in endotoxemia and sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1324–R1332. doi: 10.1152/ajpregu.00373.2001. [DOI] [PubMed] [Google Scholar]
- 37.Gnad T, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516(7531):395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 38.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008;83(3):447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonioli L, et al. Pharmacological modulation of adenosine system: novel options for treatment of inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14(4):566–574. doi: 10.1002/ibd.20316. [DOI] [PubMed] [Google Scholar]
- 40.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol. 2015;1(2):154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heller F, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Heller F, et al. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17(5):629–638. doi: 10.1016/S1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 44.Piche T. Tight junctions and IBS—the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil. 2014;26(3):296–302. doi: 10.1111/nmo.12315. [DOI] [PubMed] [Google Scholar]