Abstract
The direct neurotoxicity of HIV and neurotoxicity of combination antiretroviral therapy medications both contribute to the development of neuropathic pain. Activation of satellite glial cells (SGCs) in the dorsal root ganglia (DRG) plays a crucial role in mechanical and thermal hyperalgesia. The P2Y12 receptor expressed in SGCs of the DRG is involved in pain transmission. In this study, we explored the role of the P2Y12 receptor in neuropathic pain induced by HIV envelope glycoprotein 120 (gp120) combined with ddC (2′,3′-dideoxycytidine). A rat model of gp120+ddC-induced neuropathic pain was used. Peripheral nerve exposure to HIV-gp120+ddC increased mechanical and thermal hyperalgesia in gp120+ddC-treated model rats. The gp120+ddC treatment increased expression of P2Y12 receptor mRNA and protein in DRG SGCs. In primary cultured DRG SGCs treated with gp120+ddC, the levels of [Ca2+]i activated by the P2Y12 receptor agonist 2-(Methylthio) adenosine 5′-diphosphate trisodium salt (2-MeSADP) were significantly increased. P2Y12 receptor shRNA treatment inhibited 2-MeSADP-induced [Ca2+]i in primary cultured DRG SGCs treated with gp120+ddC. Intrathecal treatment with a shRNA against P2Y12 receptor in DRG SGCs reduced the release of pro-inflammatory cytokines, decreased phosphorylation of p38 MAPK in the DRG of gp120+ddC-treated rats. Thus, downregulating the P2Y12 receptor relieved mechanical and thermal hyperalgesia in gp120+ddC-treated rats.
Keywords: HIV-gp120-associated neuropathic pain, Antiretroviral therapy-associated neuropathic pain, P2Y12 receptor, Dorsal root ganglia, Satellite glial cells
Introduction
Infection by the human immunodeficiency virus (HIV) results in progressive failure of the immune system in humans. Distal symmetric polyneuropathy has been recognized as one of the most common neurologic complications of HIV [1–3]. The direct neurotoxicity of HIV and the neurotoxic effects of combination antiretroviral therapy medications are both thought to contribute to the development of HIV distal symmetric polyneuropathy [1–3]. Glycoprotein 120 (gp120) is involved in the indirect neurotoxic effects of HIV on the peripheral nervous system [2, 4–6]. As one type of neurotoxic combination antiretroviral therapy drugs, nucleoside analog reverse-transcriptase inhibitors have been shown to be associated with distal symmetric polyneuropathy [1, 3, 7]. HIV and nucleoside analog reverse-transcriptase inhibitors have synergistic neurotoxic effects. Indeed, distal symmetric polyneuropathy originating from HIV or use of nucleoside analog reverse-transcriptase inhibitors are clinically indistinguishable from one another [4, 7]. Animal studies have also demonstrated that the combination of HIV and nucleoside analog reverse-transcriptase inhibitors may lead to an increased risk of pathological findings [8–10]. However, the pathogenesis of HIV distal symmetric polyneuropathy in humans remains poorly understood.
The dorsal root ganglia (DRG) play a crucial role in pain pathways, as they are the first locus where pain sensation is generated [11]. SGCs enwrap the DRG neuronal soma; the neurons and their surrounding SGCs form a morphologically distinct functional unit [11–13]. When peripheral nerve injury occurs, SGCs are activated, and the expression of inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) is upregulated. These effects enhance the excitability of sensory neurons [11, 13–15]. SGCs have multiple receptors for neurotransmitters and other bioactive molecules [11, 13]. ATP is the major transmitter mediating neuronal-SGC communication [16–18]. ATP signals of sensory inputs can activate ionotropic P2X receptors and metabotropic P2Y receptors in the DRG. The P2Y12 receptor is expressed in DRG SGCs [19, 20]. However, the effects of the P2Y12 receptor on HIV added with combination antiretroviral therapy-associated neuropathic pain in rats are unclear. In this study, we investigated the role of the P2Y12 receptor in HIV-gp120 added with combination antiretroviral therapy-induced neuropathology in rat DRG afferent fibers.
Materials and methods
Animals and surgical methods
Healthy male Sprague-Dawley (SD) rats with a body mass of 180–250 g were provided by the Center of Laboratory Animal Science of Nanchang University. All procedures were approved by the Animal Care and Use Committee of Nanchang University Medical School. Experiments were performed according to the guidelines of the US NIH regarding the care and use of animals for experimental procedures. Animals were maintained in a quiet environment with adequate indoor ventilation and an air filtration system. The room was maintained at 22 °C and 50% humidity with a 12:12-h light/dark cycle and free feeding; cages and bedding were changed frequently. The rats were randomly divided into five groups (8–9 rats per group): the control group; the HIV-gp120 plus 2′,3′-dideoxycytidine (ddC) (intraperitoneal injection, i.p.) group (gp120+ddC group); the group of HIV-gp120+ddC rats treated with the P2Y12 receptor shRNA plasmid (Invitrogen) interference (gp120+ddC+P2Y12 shRNA group); the HIV-gp120+ddC rats treated with scrambled shRNA as a negative control (gp120+ddC+NC group); and the rats treated with a sham operation (sham group).
A previously described technique was used for perineural HIV-gp120 administration [21]. Briefly, the left sciatic nerve of the SD rats was exposed at the popliteal fossa under 10% chloral hydrate anesthesia (3 mL/kg, i.p., supplemented as necessary) without damaging the nerve structure. A 2 × 6 mm strip of oxidized regenerated cellulose had previously been soaked in 250 μl of a 0.1% rat serum albumin (RSA) saline solution containing 200 ng of gp120 (Sigma). For the sham surgery, the 0.1% RSA contained no gp120. A 3 to 4-mm length of the sciatic nerve proximal to the trifurcation was wrapped loosely with the soaked cellulose to avoid nerve constriction and left in situ. The incision was closed with 4–0 sutures [6, 10, 21].
Then, animals received intraperitoneal injection of the NRTI ddC (20 mg/kg, Sigma, St. Louis, MO). The ddC was prepared in saline on the day of the experiment. After surgery, the ddC group was given an i.p. injection of ddC (0.5 mL, 20 mg/kg) followed by injections three times per week at the same dose. Animals in the sham group received the same volume of saline injection immediately post-surgery. Animals showing motor deficits after surgery were excluded. On the 7th day after surgery, a single intrathecal injection of P2Y12 receptor shRNA plasmid (5 μg dissolved in Entrater™ in vivo, Engreen Biosystem Co., Ltd., Beijing) was performed. Meanwhile, scrambled shRNA was intrathecally injected into rats in the gp120+ddC+NC group.
Mechanical withdrawal threshold
The measurements of the mechanical withdrawal threshold were implemented on day 0 (before operation), as well as days 1, 3, 5, 7, 9, 11, and 14 after the operation. Determination of the mechanical withdrawal threshold was performed from 8:00 to 12:00 o’clock by using a BME-404 electronic mechanical stimulator (Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Tianjin, China). This device had a test needle with a 0.6-mm end face diameter, a pressure measurement range of 0.1–50 g, and a pressure measurement resolution of 0.05 g. An organic glass box (22 × 22 × 12 cm) was placed on the sieve of the metal frame. The rat was placed in the box for 30 min of adaptation. The left hind paws were touched with the test needle until escape behavior was observed. An effective escape behavior response was defined as a rapid withdrawal and/or licking of the paw immediately upon application of the stimulus. Whenever there was an effective response, the force of the test needle was adjusted to the next lowest setting. Whenever an invalid response occurred, the next highest force was applied. The pressure value was automatically recorded. Measurements were performed 2–3 times for each rat (interval ≥ 5 min), and the mechanical withdrawal threshold was calculated as the mean of these measurements [21–24].
Measurement of the thermal withdrawal latency
The measurements of the thermal withdrawal latency were also implemented on day 0 (before the operation), as well as days 1, 3, 5, 7, 9, 11, and 14 after the operation. The latency to hind paw withdrawal from a thermal stimulus was determined by exposing the plantar surface of the hind paw to radiant heat using a Thermal Paw Stimulation System (BME-410C, Tianjin) [21–24]. Rats were placed in a transparent, square, bottomless acrylic box (22 × 12 × 22 cm) on a glass plate with a light located underneath. After a 30-min habituation period, the plantar surface of the paw was exposed to a beam of radiant heat applied through the glass floor. Activation of the bulb simultaneously activated a timer, and both were immediately turned off by paw withdrawal or at the 30-s cut-off time. The hind paws were tested by a blinded observer 2–3 times at 5-min intervals.
RNA extraction and real-time reverse transcription (RT) quantitative polymerase chain reaction (PCR)
On the 14th day after the operation, rats in the five groups were anesthetized by 10% chloral hydrate (3 mL/kg, i.p.). The L4–6 DRG were isolated immediately and flushed with ice-cold PBS. Total RNA samples were prepared from the L4–6 DRG of each group using the TRIzol Total RNA Reagent (Beijing Tiangen Biotech Co.). cDNA synthesis was performed with 2 μg of total RNA using a RevertAid First Strand cDNA SynthesisKit (Thermo Fisher Scientific, USA). Primers were designed with Primer Express 3.0 software (Applied Biosystems) using the following sequences: P2Y12 receptor forward 5′-CTTCGTTCCCTTCCACTTTG-3′; reverse 5′-AGGGTGCTCTCCTTCACGTA-3′; β-actin, forward 5′-TAAAGACCTCTATGCCAACACAGT-3′, reverse 5′-CACGATGGAGGGGCCGGACTCATC-3′. Quantitative PCR was performed using the SYBR® Green MasterMix in an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems, Inc., Foster City, CA). Quantification of gene expression was performed using the ΔΔCT calculation with CT as the threshold cycle. The relative levels of target genes, which are normalized to the sample with the lowest CT, are given as 2−ΔΔ CT [21, 25]. Relative expression levels of mRNA in the five groups were normalized to β-actin levels.
Western blotting
On the 14th day after the operation, the animals were anesthetized, and tissue collection was performed as described above, except that tissue was snap-frozen in tubes on dry ice during collection [21, 23]. Briefly, on the 14th day after the operation, the animals were anesthetized with chloral hydrate and the L4–6 DRG were dissected. The DRG were isolated immediately and rinsed in ice-cold phosphate-buffered saline (PBS). The ganglia were homogenized by mechanical disruption in lysis buffer containing the following: 50 mM Tris-Cl (pH = 8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, 0.02% sodium deoxycholate, 100 μg/mL phenylmethylsulfonyl fluoride, and 1 μg/mL aprotinin. The cells were incubated on ice for 50 min. The homogenates were then centrifuged at 12,000 rpm for 10 min, and the supernatants were collected. The quantity of total protein in the supernatants was determined using the Lowry method. After dilution with loading buffer (250 mM Tris-Cl, 200 mM dithiothreitol, 10% sodium dodecyl sulfate (SDS), 0.5% bromophenol blue, and 50% glycerol) and heating the samples up to 95 °C for 5 min, samples containing equal amounts of protein (20 μg) were separated by 10% SDS–polyacrylamide gel electrophoresis using a Bio-Rad system. The proteins were then transferred onto PVDF membranes by electrophoretic transfer using the same system. The membrane was blocked with 5% non-fat dry milk in 25 mM Tris-buffered saline (pH = 7.2) and 0.05% Tween 20 (TBST) for 2 h at room temperature; it was subsequently incubated with rabbit monoclonal anti-P2Y12 receptor (diluted to a 1:1000 ratio, Abcam, USA), rabbit polyclonal anti-tumor necrosis factor-α (anti-TNF-α), rabbit polyclonal anti-interleukin-1 beta (IL-1β) (1:1000 concentration, Abcam, USA), rabbit anti-phospho-p38 (p-p38) MAPK and rabbit anti-p38 MAPK (1:1000 dilutions, Abcam, USA), and mouse monoclonal anti-β-actin antibody (1:800 concentration, Beijing Zhongshan Biotech Co., China) at 4 °C overnight. The membranes were washed three times with TBST and incubated (1 h, room temperature) with a horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG or goat anti-rabbit IgG, 1:2000, Beijing Zhongshan Biotech Co.) in blocking buffer. After another wash cycle, the labeled proteins were visualized by enhanced chemiluminescence (ECL, Thermo Fisher Scientific, USA) on high-performance film (Shanghai Pufei Biotech Co.). Chemiluminescent signals were collected on autoradiography film, and the band intensity was quantified using Image-Pro Plus software. The relative band intensity of the target proteins was normalized against the intensity of the respective β-actin internal control.
Double-labeling immunofluorescence
On the 14th day after the operation, the animals were anesthetized with chloral hydrate, the L4–6 DRG were dissected and fixed in 4% paraformaldehyde (PFA) diluted in phosphate-buffered saline [PBS: 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 (pH = 7.2)] for 24 h. The ganglia were dehydrated in 20% sucrose overnight at 4 °C and then cut into 8-μm-thick sections. The sections were rinsed three times for 5 min in PBS and subsequently rinsed with 0.1% Triton X-100 in PBS for 30 min at room temperature. Nonspecific staining was blocked by incubation with 10% normal goat serum (Jackson ImmunoResearch Inc., West Grove PA, USA). The sections were then incubated with rabbit anti-P2Y12 receptor (1:50, Alomone Lab, Israel) and mouse anti-glial fibrillary acidic protein (GFAP) (1:150, Millipore, USA) overnight at 4 °C. Subsequently, sections were incubated for 1 h at 37 °C with the secondary antibodies, goat anti-rabbit IgG conjugated to tetraethyl rhodamine isothiocyanate (1:200, TRITC, EarthOx, USA) and goat anti-mouse IgG conjugated to fluorescein isothiocyanate (1:200, FITC, EarthOx, USA). The fluorescently stained sections were cover-slipped and examined under a fluorescence microscope (Olympus, Tokyo, Japan). The mean optical density value was calculated with the ImageJ software package and used to describe the staining intensity.
Isolation and primary culture of DRG satellite glial cells (SGCs)
DRG SGCs from neonatal Sprague-Dawley rats were prepared with a modification of previously described methods [26–28]. Briefly, 1 to 3-day-old neonatal rats were anesthetized, and the DRG were harvested in ice-cold Hank’s balanced salt solution (HBSS). The DRG were removed with fine dissecting forceps from the inner side of each half of the dissected vertebrae together with the dorsal and ventral roots and attached spinal nerves. After removing the attached nerves and the surrounding connective tissue, the DRG were incubated with trypsin (2.5 mg/mL; type III, Sigma), collagenase (1.0 mg/mL; type IA, Sigma), and DNase (0.1 mg/mL; type IV, sigma) at 37 °C in a shaking bath for 15 min. Subsequently, 10% fetal bovine serum (FBS) was added to stop enzymatic digestion. After centrifuging (5 min, 1000 rpm), the remaining ganglia were dissociated into single cells by trituration with heat-polished Pasteur pipettes and passed through nylon mesh with a pore diameter size of 100 μm. Isolated cells were suspended in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco by Life Technologies) supplemented with 10% FBS and 1% penicillin/streptomycin. These cells were seeded on uncoated 35 mm dishes at 37 °C with 5% CO2 for up to 3 days. The media were completely changed every day. Before electrophysiological recording, DRG SGCs were cultured with or without HIV-gp120 (500 pM) combined with ddC (50 μM) for 48 h. The experiments were performed at room temperature (20–30 °C) [26–28].
Intracellular Ca2+ imaging
The primary cultured SGCs were divided into four groups: control, gp120+ddC, gp120+ddC+P2Y12 receptor shRNA, and gp120+ddC+NC groups. All groups were treated with their corresponding reagent for 48 h. The dissociated DRG SGCs were then loaded with fluo-3AM (5 μM, Molecular Probes/Invitrogen Corporation, Carlsbad, CA) for 40 min at 37 °C temperature in a balanced salt solution (HBSS) (NaCl (140 mM), HEPES (10 mM), CaCl2 (2 mM), MgCl2 (1 mM), glucose (10 mM), and KCl (50 mM)). Cells were rinsed with HBSS and mounted in a chamber that was placed onto the inverted microscope; HBSS was perfused continuously at a rate of 1 mL/min. Intracellular calcium was measured by a xenon arc lamp, interference filters, an electronic shutter (MT 20, Germany), a × 20 objective (Olympus, Japan), and digital video microfluorometry with an intensified CCD camera coupled to a microscope (Olympus, Japan). The excitation wavelengths for the fluo-3AM (485 nm) were selected by a filter changer. The P2Y12 receptor-specific agonist 2-(Methylthio) adenosine 5′-diphosphate trisodium salt (2-MeSADP, Sigma) was applied directly to the cover slip bathing solution after perfusion was stopped. In all of the experiments, a concentration of 100 μM was used to ensure maximal activation. Cells were reconstituted in 0.1% BSA/PB. If no response was seen within 1 min, the 2-MeSADP was washed out. A minimum of 50 SGCs were analyzed for each group [26, 27]. The fluorescence ratio F/F0, where F0 is the baseline, was used to describe relative changes in [Ca2+]i with CellR Sens software (Olympus Soft Imaging Solutions (OSIS), Germany) [26, 27].
Statistical analysis
The data were analyzed using SPSS 20 software. The numerical values are reported as the mean ± SE. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by the Fisher’s post hoc test for multiple comparisons. A value of p < 0.05 was considered to be statistically significant.
Results
P2Y12 receptor expression in the DRG is upregulated in gp120+ddC-treated rats
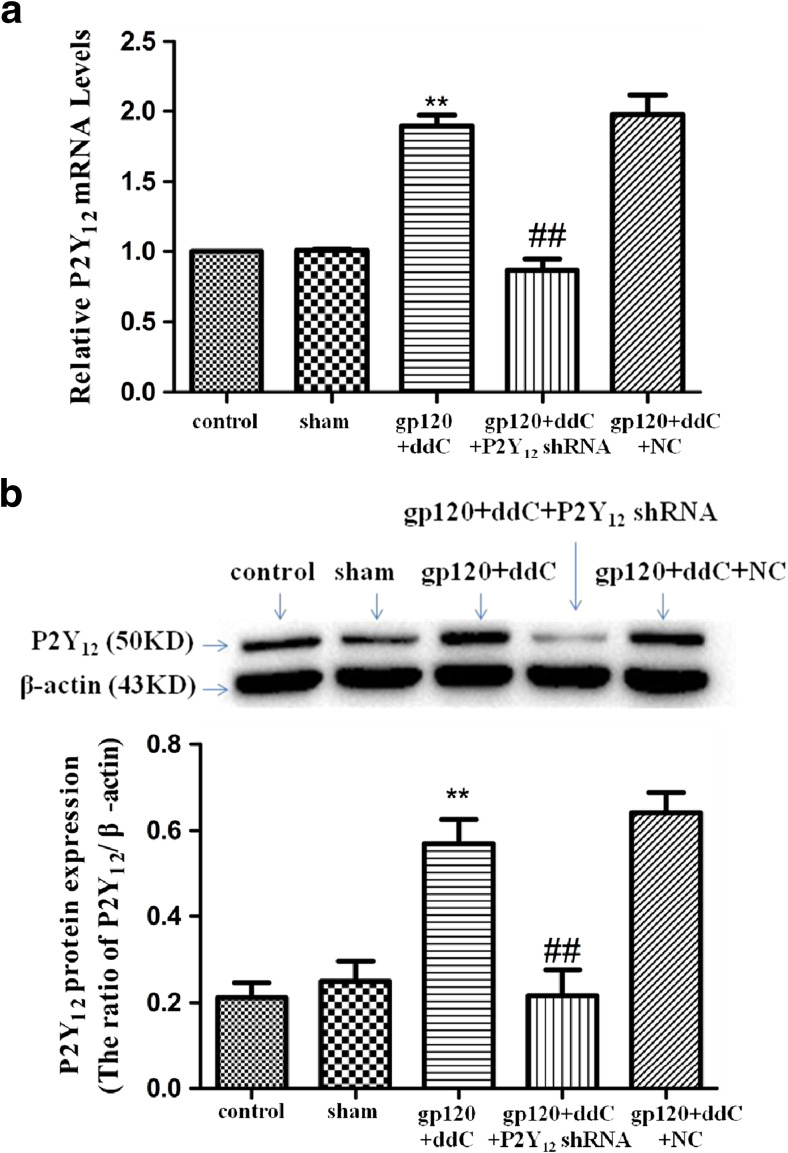
There was no difference in the relative levels of P2Y12 receptor mRNA between the sham group and the control group (p > 0.05). Expression levels of the P2Y12 receptor mRNA in the gp120+ddC group were significantly higher compared to the sham group (p < 0.01, n = 8 for each group). In gp120+ddC rats treated with P2Y12 receptor shRNA, expression levels of P2Y12 receptor mRNA were significantly lower than in the gp120+ddC rats (p < 0.01) (Fig. 1a, n = 8 for each group). There was no difference in expression levels of P2Y12 receptor mRNA between the gp120+ddC group and the gp120+ddC+NC group (p > 0.05).
Fig. 1.
P2Y12 receptor in the DRG is upregulated in gp120+ddC-treated rats. a Expression of P2Y12 receptor mRNA in the DRG was measured by RT-qPCR. Expression in the gp120+ddC group was higher than in the sham group (p < 0.01). In gp120+ddC rats treated with P2Y12 receptor shRNA, expression was significantly lower than in the gp120+ddC rats (p < 0.01). The experiment was performed three times (n = 8 per group). Data are presented as the means ± SE. **p < 0.01 compared to the sham group; ##p < 0.01 compared to the gp120+ddC-treated group. b Expression of P2Y12 receptor protein in the DRG was assessed by Western blotting. Protein expression in the gp120+ddC-treated group was increased compared to the sham group (p < 0.01). In gp120+ddC rats treated with P2Y12 receptor shRNA, expression was significantly lower than in the gp120+ddC rats (p < 0.01). Bar graphs show the ratio of the P2Y12 receptor protein level to β-actin level in each group. Data are displayed as the means ± SE. **p < 0.01 compared to the sham group; ##p < 0.01 compared to the gp120+ddC group
There was no difference in the relative levels of the P2Y12 receptor protein between the sham group and the control group (p > 0.05). Image analysis indicated that P2Y12 receptor protein expression (normalized to each β-actin internal control) in the gp120+ddC group was significantly enhanced compared to the sham group (p < 0.01, n = 9 for each group). The relative levels of P2Y12 receptor protein expression in the gp120+ddC+P2Y12 receptor shRNA group were lower than that in the gp120+ddC group (p < 0.01) (Fig. 1b). There was no difference in the expression levels of the P2Y12 receptor protein between the gp120+ddC group and the gp120+ddC+NC group (p > 0.05).
The results tested by RT-qPCR and Western blotting indicated that gp120 combined with ddC treatment upregulated the expression of P2Y12 receptor mRNA and protein, intrathecal treatment with a shRNA against P2Y12 receptor decreased the upregulated expression of P2Y12 receptor mRNA and protein in the gp120+ddC treatment group.
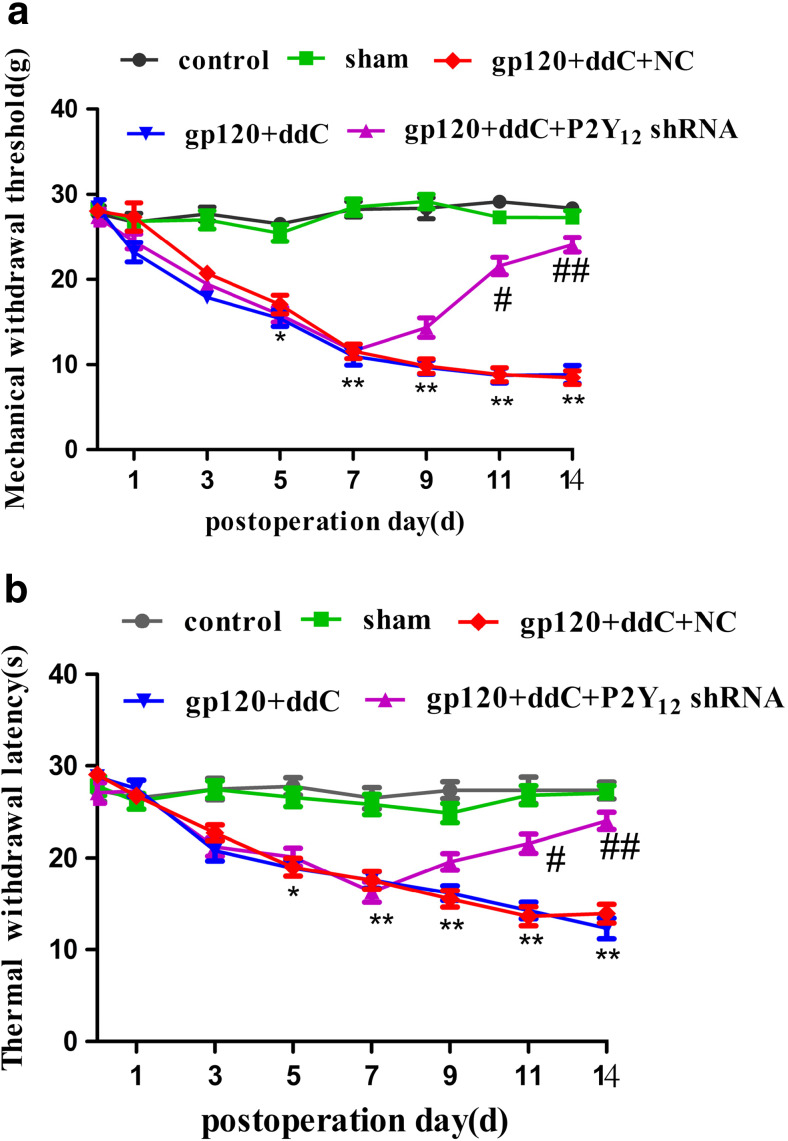
P2Y12 receptor upregulation in the DRG is involved in mechanical and thermal hyperalgesia in gp120 and ddC-treated rats
There was no difference in the mechanical withdrawal threshold between the sham group and control group (p > 0.05). At 5 to 14 days post-surgery, the mechanical withdrawal threshold in the gp120+ddC group was gradually lower than that in the sham group (p < 0.01). The mechanical withdrawal threshold in the gp120+ddC+P2Y12 receptor shRNA group was gradually higher compared to the gp120+ddC group after day 7 (p < 0.01) (Fig. 2a, n = 8 for each group). There was no difference in the mechanical withdrawal threshold between the gp120+ddC group and the gp120+ddC+NC group (p > 0.05).
Fig. 2.
Intrathecal treatment with a shRNA against P2Y12 receptor relieves mechanical and thermal hyperalgesia in gp120+ddC-treated rats. a The mechanical withdrawal threshold in the gp120+ddC group was lower than in the control group (p < 0.01). No difference was found between the sham group and the control group (p > 0.05). In gp120+ddC rats treated with P2Y12 receptor shRNA, the mechanical withdrawal threshold was higher than in the gp120+ddC group that did not receive P2Y12 receptor shRNA (p < 0.01). No difference was found between the gp120+ddC+NC group and the gp120+ddC group (p > 0.05). Each group consisted of eight rats. Data are displayed as the means ± SE. *p < 0.05 compared to the control group; **p < 0.01 compared to the control group; #p < 0.05 compared to the gp120+ddC-treated group; ##p < 0.01 compared to the gp120+ddC-treated group. b The thermal withdrawal latency in the gp120+ddC group was gradually shorter than in the sham group (p < 0.05 or p < 0.01). No difference was found between the sham group and the control group (p > 0.05). In gp120+ddC rats treated with P2Y12 receptor shRNA, the thermal withdrawal latency was higher than in the gp120+ddC group (p < 0.01). No difference was found between the gp120+ddC+NC group and the gp120+ddC group (p > 0.05). Each group consisted of eight rats. The data are displayed as the means ± SE. *p < 0.05 compared to the sham group; **p < 0.01 compared to the sham group; #p < 0.05 compared to the gp120+ddC-treated group; and ##p < 0.01 compared to the gp120+ddC-treated group
There was no difference in the thermal withdrawal latency between the sham group and the control group (p > 0.05). At 5 to 14 days after the operation, the thermal withdrawal latency in the gp120+ddC group was gradually shorter than thermal withdrawal latency in the sham group (p < 0.01). The thermal withdrawal latency in the gp120+ddC+P2Y12 receptor shRNA group was gradually longer compared to the gp120+ddC group after day 7 (p < 0.01) (Fig. 2b, n = 8 for each group). There was no difference in the thermal withdrawal latency between the gp120+ddC group and the gp120+ddC+NC group (p > 0.05).
The results suggested that gp120 combined with ddC treatment induced mechanical hyperalgesia and thermal hyperalgesia. After intrathecal treatment with a shRNA against P2Y12 receptor, the hyperalgesia was diminished. This indicated that P2Y12 receptor was involved in the gp120 combined with ddC treatment induced hyperalgesia.
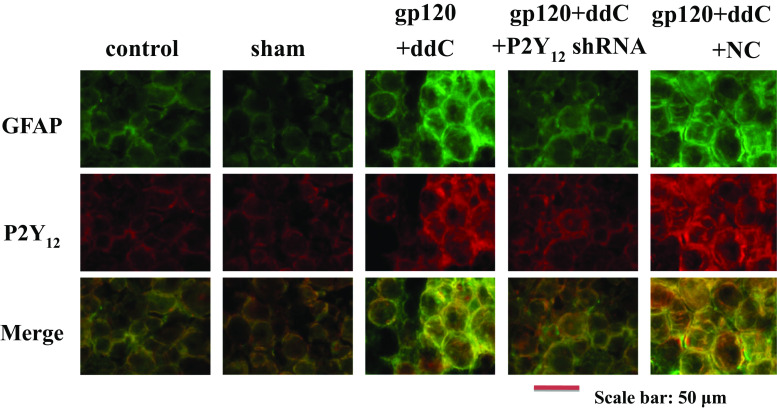
Intrathecal treatment with a shRNA against P2Y12 receptor reduced the upregulated co-localization values of P2Y12 receptor and GFAP in the DRG of gp120+ddC-treated rats.
GFAP is a marker of satellite glial cells (SGCs) [13]. The upregulation of GFAP in SGCs suggests the activation of SGCs after gp120+ddC treatment. Co-localization values of P2Y12 receptor and GFAP in the gp120+ddC group were higher than those in the sham group. No difference was found between the control rats and the sham rats. Intrathecal treatment with a shRNA against P2Y12 receptor was associated with lower co-localization values for P2Y12 receptor and GFAP in gp120+ddC+P2Y12 receptor shRNA-treated rats compared to gp120+ddC-treated rats. There was no significant difference between the gp120+ddC group and the gp120+ddC+NC group (Fig. 3, n = 8 for each group). The results indicated that the P2Y12 receptor in SGCs of gp120+ddC rats was involved in the activation of SGCs and related to pathological injury.
Fig. 3.
Intrathecal treatment with a shRNA against P2Y12 receptor reduces the upregulation of P2Y12 receptor and GFAP co-localization values in the DRG of gp120+ddC-treated rats. Co-localization of P2Y12 receptor and GFAP in the gp120+ddC group was higher than in the sham group. No difference was found between control and sham rats. Silencing of P2Y12 receptor reduced the co-localization values of P2Y12 receptor and GFAP in gp120+ddC+P2Y12 receptor shRNA-treated rats compared with gp120+ddC-treated rats that did not receive P2Y12 receptor shRNA. There was no significant difference between the gp120+ddC group and the gp120+ddC+NC group
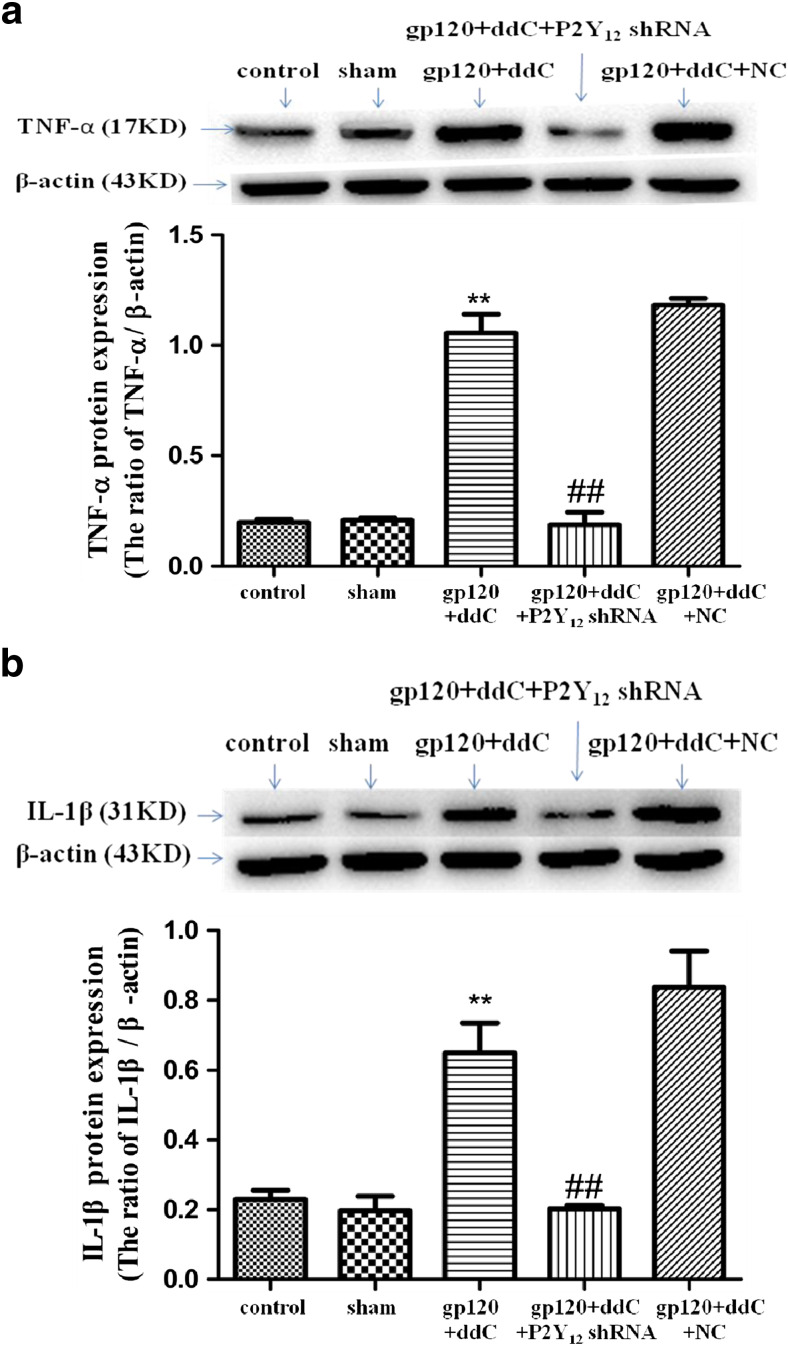
Intrathecal treatment with a shRNA against P2Y12 receptor reduced expression of pro-inflammatory cytokines in gp120+ddC-treated rats
Image analysis indicated that TNF-α and IL-1β protein expression values (normalized to each β-actin internal control) in the gp120+ddC group were significantly higher than those in the sham group (p < 0.01, n = 9 for each group). There was no difference in TNF-α and IL-1β protein expression between the sham group and the control group (p > 0.05). The relative levels of TNF-α and 1L-1β protein expression in the gp120+ddC+P2Y12 receptor shRNA group were lower than those in the gp120+ddC group (p < 0.01) (Fig. 4). There was no difference in TNF-α and IL-1β protein expression between the gp120+ddC group and the gp120+ddC+NC group (p > 0.05). The results showed that gp120 combined with ddC treatment increased the release of pro-inflammatory cytokines, intrathecal treatment with a shRNA against P2Y12 receptor decreased the release of pro-inflammatory cytokines in the gp120+ddC group.
Fig. 4.
Intrathecal treatment with a shRNA against P2Y12 receptor decreases the expression of TNF-α and IL-1β protein in the DRG of gp120+ddC-treated rats. a Expression of the TNF-α protein (normalized to each β-actin internal control) in the gp120+ddC group was higher compared to sham group (p < 0.01). No difference was found between the sham group and the control group (p > 0.05). In gp120+ddC rats treated with P2Y12 receptor shRNA, TNF-α protein expression was lower compared to gp120+ddC group (p < 0.01). No difference was found between the gp120+ddC+NC group and the gp120+ddC group (p > 0.05). Bar graphs show the ratio of TNF-α protein level to β-actin level in each group. Data are displayed as the means ± SE (n = 9 per group). **p < 0.01 compared to the control group; ##p < 0.01 compared to the gp120+ddC-treated group. b Expression of the IL-1β protein (normalized to each β-actin internal control) in the gp120+ddC group was higher than in the sham group (p < 0.01). No difference was found between the sham group and the control group (p > 0.05). In gp120+ddC rats treated with P2Y12 receptor shRNA, expression levels of the IL-1β protein were lower than in the gp120+ddC group that did not receive P2Y12 receptor shRNA (p < 0.01). No difference was found between the gp120+ddC+NC group and the gp120+ddC group (p > 0.05). Bar graphs show the ratio of IL-1β protein to β-actin in each group. The data are displayed as the means ± SE (n = 8 per group). **p < 0.01 compared to the control group; ##p < 0.01 compared to the gp120+ddC-treated group
Intrathecal treatment with a shRNA against P2Y12 receptor inhibits activation of the p38 MAPK pathway in the DRG of gp120+ddC-treated rats
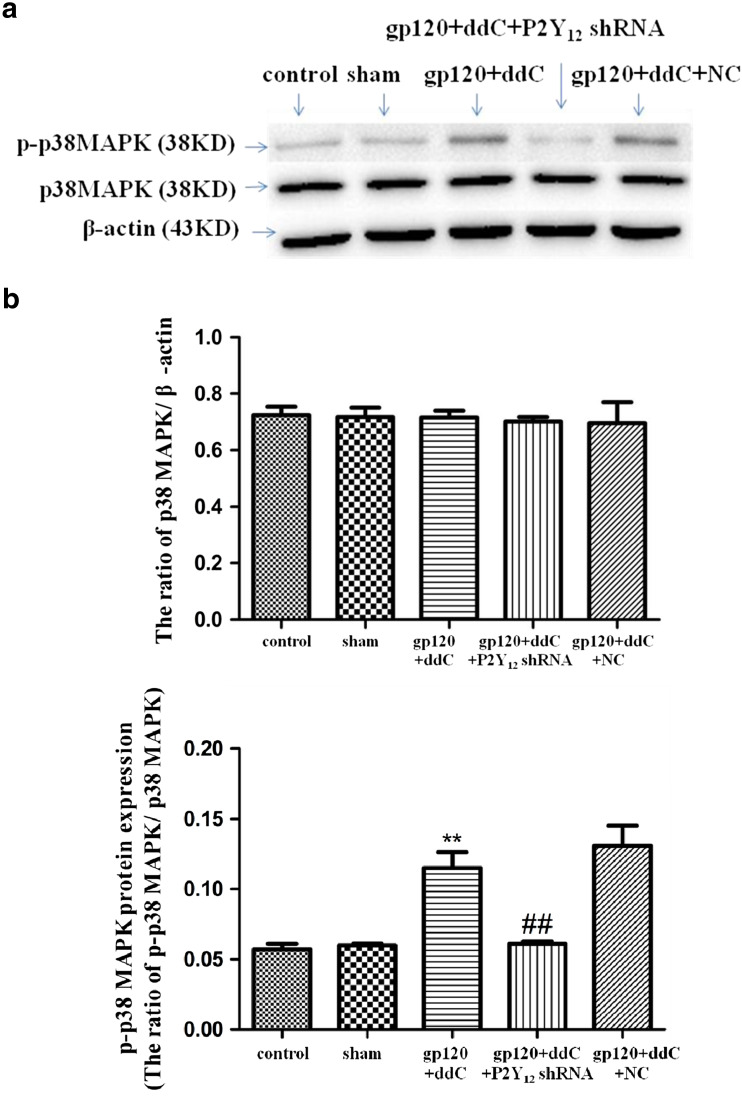
Phosphorylation and activation of p38 MAPK are involved in inflammatory pain. The integrated optical density (IOD) ratio of p38 MAPK to β-actin was not significantly different between the gp120+ddC and sham groups (p > 0.05). There was likewise no difference in the p38 MAPK/β-actin IOD ratio between the sham and control groups (p > 0.05). However, the p-p38 MAPK/p38 MAPK IOD ratio was higher in the gp120+ddC group than the sham group (p < 0.01, n = 8 for each group) (Fig. 5). There was no difference in the p-p38 MAPK/p38 MAPK IOD ratio between the sham group and control group (p > 0.05). These results indicated that p38 MAPK phosphorylation in the DRG is related to P2Y12 receptor-mediated hyperalgesia in the gp120+ddC-treated rats.
Fig. 5.
a, b Intrathecal treatment with a shRNA against P2Y12 receptor inhibits activation of the p38 MAPK pathway in the DRG of gp120+ddC-treated rats. The integrated optical density (IOD) ratio of p38 MAPK to β-actin was not significantly different between the gp120+ddC group and the sham group (p > 0.05). There was no difference in the IOD ratio of p38 MAPK to β-actin between the sham group and the control group (p > 0.05). The IOD ratio of p-p38 MAPK to p38 MAPK was higher in the gp120+ddC group than the sham group (p < 0.01, n = 8 for each group). There was no difference in the IOD ratio of p-p38 MAPK to p38 MAPK between the sham group and control group (p > 0.05). The IOD ratio of p-p38 MAPK to p38 MAPK in the gp120+ddC+P2Y12 receptor shRNA-treated rats was significantly lower than in the gp120+ddC group (p < 0.01, n = 8 for each group). The data are displayed as the means ± SE, n = 8. **p < 0.01 compared to the control group; ##p < 0.01 compared to the gp120+ddC group
In addition, we tested whether the administration of P2Y12 receptor shRNA affected the phosphorylation of p38 MAPK in the DRG of the gp120+ddC group. The p-p38 MAPK/p38 MAPK IOD ratio in the gp120+ddC+P2Y12 receptor shRNA group was significantly lower than that of the gp120+ddC group (p < 0.01, n = 8 for each group) (Fig. 5). There was no difference in the p-p38 MAPK/p38 MAPK IOD ratio between the gp120+ddC and gp120+ddC+NC groups (p > 0.05). These results suggest that intrathecal treatment with a shRNA against P2Y12 receptor decreased the phosphorylation and activation of p38 MAPK in the DRG of the gp120+ddC-treated rats and then decreased the hyperalgesia in the gp120+ddC-treated rats.
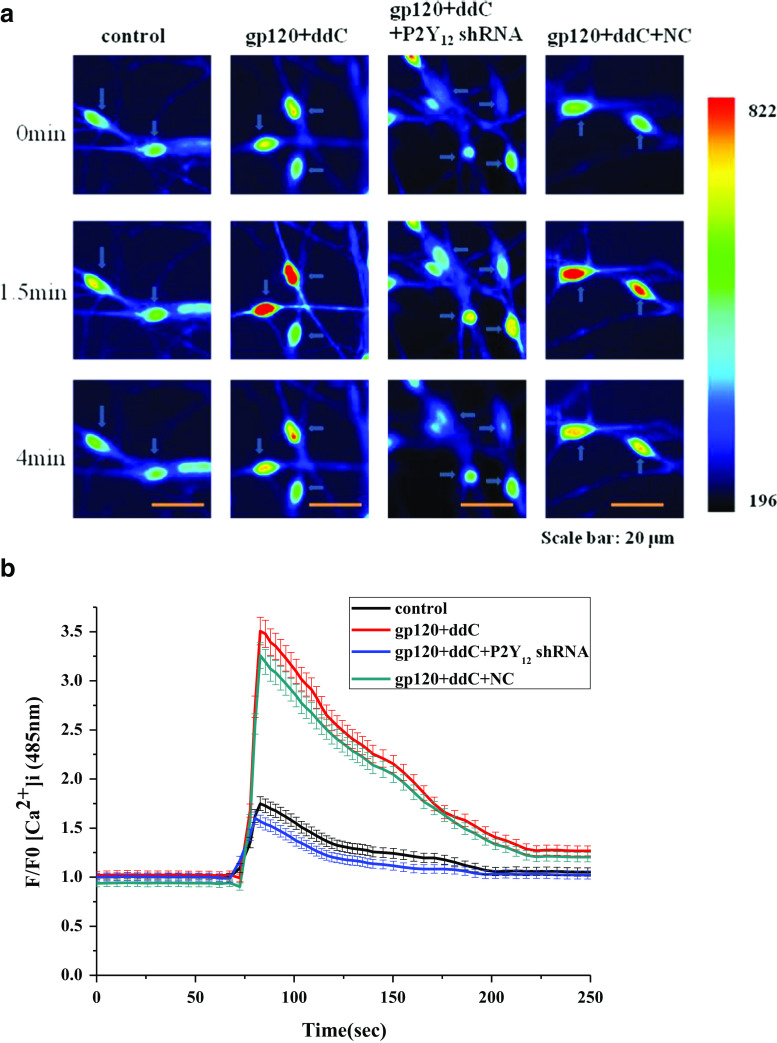
Treatment with a shRNA against P2Y12 receptor inhibits P2Y12-agonist-activated [Ca2+]i in primary cultured DRG SGCs treated with gp120 and ddC
The dynamic change of fluorescence of [Ca2+]i activated by the P2Y12 receptor agonist 2-MeSADP (100 μM) in primary cultured DRG SGCs were shown by Fig. 6a, b. The change of [Ca2+]i in gp120+ddC treatment SGCs was larger than that in the control SGCs (n = 50). P2Y12 receptor shRNA treatment inhibited 2-MeSADP-induced [Ca2+]i in primary cultured DRG SGCs treated with gp120+ddC (n = 50). These results indicated that gp120 combined with ddC treatment caused activation of the P2Y12 receptor in primary cultured SGCs, then followed by larger [Ca2+]i. P2Y12 receptor shRNA treatment inhibited [Ca2+]i by downregulating of P2Y12 receptor.
Fig. 6.
Treatment with a shRNA against P2Y12 receptor inhibits P2Y12 receptor agonist-activated [Ca2+]i in DRG SGCs cultured with gp120+ddC. P2Y12 receptor agonist-activated [Ca2+]i in DRG SGCs was recorded by calcium fluorescence imaging. The ratio of F/F0 fluorescence for [Ca2+]i activated by the P2Y12 receptor agonist 2-MeSADP (100 μM) in DRG SGCs cultured with gp120+ddC was higher than in controls (a, b). P2Y12 receptor shRNA treatment inhibited 2-MeSADP-induced [Ca2+]i in DRG SGCs cultured with gp120+ddC treatment (p < 0.01). The dynamic change of fluorescence of [Ca2+]i activated by the P2Y12 receptor agonist 2-MeSADP (100 μM) in primary cultured DRG SGCs were shown in b. Data are displayed as the means ± SE, n = 50 cells for each group
Discussion
The present study demonstrated that the levels of P2Y12 receptor mRNA and protein in the DRG were upregulated in gp120+ddC-treated rats. The P2Y12 receptor is involved in inflammatory and neuropathic pain [19, 29]. Meanwhile, mechanical and thermal hyperalgesia in gp120+ddC-treated rats was increased when the upregulation of P2Y12 receptor in the DRG. Our findings indicated that the treatment of HIV-gp120+ddC on the rats can induce neuropathic pain, this may attribute to the upregulation of the P2Y12 receptor in the DRG. After the administration of P2Y12 receptor shRNA decreased expression levels of the P2Y12 receptor and rescued the mechanical withdrawal threshold and thermal withdrawal latency in gp120+ddC-treated rats. Our findings suggested that the upregulation of the P2Y12 receptor in the DRG was related to the mechanical and thermal hyperalgesia induced by HIV-gp120+ddC treatment.
DRG neurons are surrounded by an envelope of SGCs [12, 13, 15]. Damage causes SGCs to contribute to neuropathic pain [12, 13, 15, 30]. GFAP was elevated in DRG SGCs after gp120+ddC treatment since GFAP is considered a marker of SGCs activation, the finding suggested the activation of SGCs after nerve injury. The P2Y12 receptor is expressed in the DRG SGCs [19, 20]. Co-localization values of P2Y12 receptor and GFAP in the gp120+ddC group were higher than those in the sham group. Thus, P2Y12 receptor in DRG SGCs of the gp120+ddC rats is likely to participate in signal transmission of nerve injury. Intrathecal treatment with a shRNA against P2Y12 receptor decreased co-localization values of P2Y12 receptor and GFAP in gp120+ddC-treated rats. These data indicated that the treatment of HIV-gp120+ddC on the rats can activate SGCs in the DRG, through enhancing the expression of GFAP and P2Y12 receptor in the SGCs and increasing the transmission of nociception, which resulted in neuropathic pain. Therefore, when the rats were treated with P2Y12 receptor shRNA, the co-localization of GFAP and P2Y12 receptor were decreased, and the pain behaviors in the rats were relieved.
HIV-gp120 also has direct and indirect effects on nerves by stimulating the nervous system to release pro-inflammatory cytokines [1, 22, 31, 32]. Pro-inflammatory cytokines can activate SGCs, as a feedback, SGC activation may cause more release of pro-inflammatory cytokines, that will increase abnormal neuronal excitability and contribute to neuropathic pain. TNF-α and IL-1β play important roles in neuropathic pain [33, 34]. Our results showed that expression levels of P2Y12 receptor mRNA and protein were enhanced in gp120+ddC-treated rats and were accompanied by upregulated IL-1β protein. TNF-α in DRG neurons enhances neuronal excitability [35] and IL-1β release during neuropathic pain requires P2Y12 receptor activation [36]. Consistent with these reports, expression levels of TNF-α and IL-1β protein were increased in the DRG of the gp120+ddC-treated rats. Therefore, the upregulation of IL-1β protein and TNF-α in the DRG may induce abnormal neuronal excitability in the DRG, and this excitability may have enhanced hyperalgesia in gp120+ddC-treated rats. Our results also showed that hyperalgesia in gp120+ddC rats treated with P2Y12 receptor shRNA was decreased by downregulation of IL-1β protein and TNF-α in the DRG. The data further indicated that the upregulation of P2Y12 receptor induced the release of IL-1β and TNF-α, which promoted the neuropathic pain in gp120+ddC-treated rats.
The p38 MAPK signaling is critical in neuropathic pain [37]. Thus, blockade of p38 MAPK activation in the DRG can decrease mechanical and thermal hypersensitivity. Prior research suggests that the P2Y12 receptor may activate p38 MAPK after peripheral nerve injury [38, 39]. In the present study, the integrated optical density (IOD) ratio of p-p38 MAPK to p38 MAPK in the gp120+ddC group was higher than in the sham group. Thus, we conclude that p38 MAPK phosphorylation in the DRG of gp120+ddC-treated rats is involved in hyperalgesia that induced by gp120+ddC treatment. After treatment with P2Y12 receptor shRNA, the IOD ratios of p-p38 MAPK to p38 MAPK in the gp120+ddC+P2Y12 receptor shRNA-treated rats were significantly lower than that in the gp120+ddC group. Simultaneously, P2Y12 receptor shRNA treatment decreased the expression of P2Y12 receptor as well as phosphorylation and activation of p38 MAPK in the DRG of gp120+ddC-treated rats. The subsequent reduction of p-p38 MAPK in gp120+ddC-treated rats correlated well with changes in mechanical and thermal hyperalgesia after P2Y12 receptor shRNA treatment. Therefore, the increasing phosphorylation and activation of p38 MAPK in the DRG of gp120+ddC-treated rats aggravated mechanical and thermal hyperalgesia, and the P2Y12 receptor was involved in hyperalgesia through the p38 MAPK signaling pathway.
Activation of the P2Y12 receptor contributes to Ca2+ elevation [40]. The endogenous agonists of P2Y12 receptor are ADP and ATP. Because 2-MeSADP is a P2Y12 receptor-specific agonist, we used it to induce [Ca2+]i in primary cultured DRG SGCs. In this study, the fluorescence resulting from [Ca2+]i activated by the P2Y12 receptor agonist 2-MeSADP was significantly increased in primary cultured DRG SGCs treated with gp120 and ddC. On the contrary, P2Y12 receptor shRNA treatment inhibited 2-MeSADP-induced [Ca2+]i in these cells. Treatment of the rat paw with TNF-α and IL-1β decreases nociceptive thresholds and produces nociceptive hypersensitivity [11, 41]. Treatment with TNF-α and IL-1β can increase intracellular Ca2+ transients, depolarize membrane potentials, reduce threshold currents for action potentials, and elicit spontaneous firing in DRG neurons [15, 42, 43]. The administration of P2Y12 receptor shRNA in the SGCs may reduce the release of pro-inflammatory cytokines. SGCs and neurons can communicate through transmission of Ca2+ signals in the peripheral ganglia [44]. ATP and its metabolite ADP are used for communicating between glial cells and neurons [45, 46]. Either ATP or its metabolite ADP activates the P2Y12 receptor in DRG SGCs [45, 46]. Our results suggested that the inhibition of the P2Y12 receptor in the gp120+ddC-treated rats was accompanied by reduced communication between SGCs and neurons.
In conclusion, the current study demonstrated that peripheral nerve exposure to HIV-gp120+ddC increased mechanical and thermal hyperalgesia in rats. The gp120+ddC treatment increased expression of the P2Y12 receptor in DRG SGCs. This upregulation promoted transmission of [Ca2+]i between the SGCs and the neurons, as well as the release of pro-inflammatory cytokines (IL-1β and TNF-α). IL-1β and TNF-α increased the sensitivity of neurons in the DRG, which resulted in neuropathic pain behaviors induced by gp120+ddC treatment. The administration of P2Y12 receptor shRNA in DRG SGCs reduced the release of pro-inflammatory cytokines, and decreased the phosphorylation of p38 MAPK in the DRG of gp120+ddC-treated rats. Therefore, inhibition of the P2Y12 receptor relieved mechanical and thermal hyperalgesia in gp120+ddC-induced neuropathic pain.
Acknowledgements
These studies were supported by grants (nos. 31560276, 81570735, 81560219, 81701114, 81760152, 81760152, 81360140, and 81560529) from the National Natural Science Foundation of China, a grant (nos. 20151BBG70250 and 20151BBG70253) from the Technology Pedestal and Society Development Project of Jiangxi Province, a grant (no. 20142BAB205028, 20142BAB215027, and 20121512040234) from the Natural Science Foundation of Jiangxi Province, and grants (nos. GJJ13155 and GJJ14319) from the Educational Department of Jiangxi Province.
Compliance with ethical standards
This article does not contain any studies with human participants. All animal experiments were approved by the approved by the Animal Care and Use Committee of Nanchang University Medical School. The IASP’s ethical guidelines for pain research in animals were followed.
Conflict of interest
Matteo Fornai declares that he has no conflict of interest.
Zhihua Yi declares that she has no conflict of interest.
Lihui Xie declares that he has no conflict of interest.
Congfa Zhou declares that he has no conflict of interest.
Huilong Yuan declares that he has no conflict of interest.
Shuai Ouyang declares that he has no conflict of interest.
Zhi Fang declares that he has no conflict of interest.
Shanhong Zhao declares that she has no conflict of interest.
Tianyu Jia declares that she has no conflict of interest.
Lifang Zou declares that she has no conflict of interest.
Shouyu Wang declares that she has no conflict of interest.
Yun Xue declares that she has no conflict of interest.
Bing Wu declares that he has no conflict of interest.
Yun Gao declares that she has no conflict of interest.
Guilin Li declares that she has no conflict of interest.
Shuangmei Liu declares that she has no conflict of interest.
Hong Xu declares that she has no conflict of interest.
Changshui Xu declares that he has no conflict of interest.
Chunping Zhang declares that he has no conflict of interest.
Shangdong Liang declares that he has no conflict of interest.
Footnotes
Zhihua Yi and Lihui Xie contributed equally to this work.
Change history
11/11/2020
A Correction to this paper has been published: 10.1007/s11302-020-09740-z
References
- 1.Hao S. The molecular and pharmacological mechanisms of HIV-related neuropathic pain. Curr Neuropharmacol. 2013;11(5):499–512. doi: 10.2174/1570159X11311050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. J Int AIDS Soc. 2014;17:18719. doi: 10.7448/IAS.17.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schutz SG, Robinson-Papp J. HIV-related neuropathy: current perspectives. HIV AIDS (Auckl) 2013;5:243–251. doi: 10.2147/HIV.S36674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB. Peripheral neuropathy in HIV: prevalence and risk factors. Aids. 2011;25(7):919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116(1):29–39. doi: 10.1016/S0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 6.Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133(1–3):47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshinaike O, Akinbami A, Ojo O, Ogbera A, Okubadejo N, Ojini F, Danesi M. Influence of age and neurotoxic HAART use on frequency of HIV sensory neuropathy. AIDS Res Treat. 2012;2012:961510. doi: 10.1155/2012/961510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107(1–2):147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26(40):10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130(Pt 10):2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33(6):784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Costa FA, Moreira Neto FL. Satellite glial cells in sensory ganglia: its role in pain. Rev Bras Anestesiol. 2015;65(1):73–81. doi: 10.1016/j.bjan.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48(3):457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Blum E, Procacci P, Conte V, Sartori P, Hanani M. Long term effects of lipopolysaccharide on satellite glial cells in mouse dorsal root ganglia. Exp Cell Res. 2017;350(1):236–241. doi: 10.1016/j.yexcr.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129(1–2):155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Fields R, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;276(6):423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verderio C, Matteoli M. ATP in neuron-glia bidirectional signalling. Brain Res Rev. 2011;66(1–2):106–114. doi: 10.1016/j.brainresrev.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104(23):9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ, Iwata K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain. 2012;8:23. doi: 10.1186/1744-8069-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi K, Yamanaka H, Noguchi K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int. 2013;88(1):10–16. doi: 10.1007/s12565-012-0163-9. [DOI] [PubMed] [Google Scholar]
- 21.Yi Z, Rao S, Ouyang S, Bai Y, Yang J, Ma Y, Han X, Wu B, Zou L, Jia T, Zhao S, Hu X, Lei Q, Gao Y, Liu S, Xu H, Zhang C, Liang S, Li G. A317491 relieved HIV gp120-associated neuropathic pain involved in P2X3 receptor in dorsal root ganglia. Brain Res Bull. 2017;130:81–89. doi: 10.1016/j.brainresbull.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Nasirinezhad F, Jergova S, Pearson JP, Sagen J. Attenuation of persistent pain-related behavior by fatty acid amide hydrolase (FAAH) inhibitors in a rat model of HIV sensory neuropathy. Neuropharmacology. 2015;95:100–109. doi: 10.1016/j.neuropharm.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Li G, Den X, Xu C, Liu S, Gao Y, Liu H, Zhang J, Li X, Liang S. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X(2)(/)(3) receptor of primary sensory neurons. Brain Res Bull. 2010;83(5):284–291. doi: 10.1016/j.brainresbull.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Liu X, Lu S, Yu A, Fu Z. Increased pain in response to mechanical or thermal stimulation in a rat model of incision-induced pain with nicotine dependence and withdrawal. Exp Ther Med. 2013;5(4):1063–1066. doi: 10.3892/etm.2013.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu G, Li G, Peng H, Hu J, Liu J, Kong F, Liu S, Gao Y, Xu C, Xu X, Qiu S, Fan B, Zhu Q, Yu S, Zheng C, Wu B, Peng L, Song M, Wu Q, Liang S. P2X(7) inhibition in stellate ganglia prevents the increased sympathoexcitatory reflex via sensory-sympathetic coupling induced by myocardial ischemic injury. Brain Res Bull. 2013;96:71–85. doi: 10.1016/j.brainresbull.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Feldman-Goriachnik R, Hanani M (2017) The effects of endothelin-1 on satellite glial cells in peripheral ganglia. Neuropeptides. 10.1016/j.npep.2017.03.002 [DOI] [PubMed]
- 27.Castillo C, Norcini M, Martin Hernandez LA, Correa G, Blanck TJ, Recio-Pinto E. Satellite glia cells in dorsal root ganglia express functional NMDA receptors. Neuroscience. 2013;240:135–146. doi: 10.1016/j.neuroscience.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Zhang C, Shi Q, Li G, Song M, Gao Y, Xu C, Xu H, Fan B, Yu S, Zheng C, Zhu Q, Wu B, Peng L, Xiong H, Wu Q, Liang S. Puerarin blocks the signaling transmission mediated by P2X3 in SG and DRG to relieve myocardial ischemic damage. Brain Res Bull. 2014;101:57–63. doi: 10.1016/j.brainresbull.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G. Purinergic mechanisms and pain––an update. Eur J Pharmacol. 2013;716(1–3):24–40. doi: 10.1016/j.ejphar.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 30.Ying M, Liu H, Zhang T, Jiang C, Gong Y, Wu B, Zou L, Yi Z, Rao S, Li G, Zhang C, Jia T, Zhao S, Yuan H, Shi L, Li L, Liang S, Liu S (2017) Effect of artemisinin on neuropathic pain mediated by P2X4 receptor in dorsal root ganglia. Neurochem Int. 10.1016/j.neuint.2017.02.004 [DOI] [PubMed]
- 31.Yuan S, Shi Y, Chen J, Zhou X, Li G, Gelman BB, Lisinicchia JG, Carlton SM, Ferguson MR, Tan A. Gp120 in the pathogenesis of human HIV-associated pain. Ann Neurol. 2014;75(6):837–850. doi: 10.1002/ana.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFα in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano R, Ma L, Nagai J, Ueda H. Interleukin-1β plays key roles in LPA-induced amplification of LPA production in neuropathic pain model. Cell Mol Neurobiol. 2013;33(8):1033–1041. doi: 10.1007/s10571-013-9970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer C, Lindenlaub T, Teuteberg P, Schafers M, Hartung T, Toyka KV. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913(1):86–89. doi: 10.1016/S0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- 35.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8(3):629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath G, Goloncser F, Csolle C, Kiraly K, Ando RD, Baranyi M, Kovanyi B, Mate Z, Hoffmann K, Algaier I, Baqi Y, Muller CE, Von Kugelgen I, Sperlagh B. Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol Dis. 2014;70:162–178. doi: 10.1016/j.nbd.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, Noguchi K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia. 2015;63(2):216–228. doi: 10.1002/glia.22745. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28(11):2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinheiro AR, Paramos-de-Carvalho D, Certal M, Costa C, Magalhaes-Cardoso MT, Ferreirinha F, Costa MA, Correia-de-Sa P. Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun Signal. 2013;11:70. doi: 10.1186/1478-811X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain. 2002;96(1–2):89–97. doi: 10.1016/S0304-3959(01)00433-X. [DOI] [PubMed] [Google Scholar]
- 42.Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-α receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42(1):93–106. doi: 10.1016/S0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- 43.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23(7):3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 2010;6(1):43–51. doi: 10.1017/S1740925X09990408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magni G, Ceruti S. P2Y purinergic receptors: new targets for analgesic and antimigraine drugs. Biochem Pharmacol. 2013;85(4):466–477. doi: 10.1016/j.bcp.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 46.Ceruti S, Fumagalli M, Villa G, Verderio C, Abbracchio MP. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell Calcium. 2008;43(6):576–590. doi: 10.1016/j.ceca.2007.10.003. [DOI] [PubMed] [Google Scholar]