Summary
Background
Chromatin organisation affects gene expression and regional mutation frequencies and contributes to carcinogenesis. Aberrant organisation of DNA has been correlated with cancer prognosis in analyses of the chromatin component of tumour cell nuclei using image texture analysis. As yet, the methodology has not been sufficiently validated to permit its clinical application. We aimed to define and validate a novel prognostic biomarker for the automatic detection of heterogeneous chromatin organisation.
Methods
Machine learning algorithms analysed the chromatin organisation in 461 000 images of tumour cell nuclei stained for DNA from 390 patients (discovery cohort) treated for stage I or II colorectal cancer at the Aker University Hospital (Oslo, Norway). The resulting marker of chromatin heterogeneity, termed Nucleotyping, was subsequently independently validated in six patient cohorts: 442 patients with stage I or II colorectal cancer in the Gloucester Colorectal Cancer Study (UK); 391 patients with stage II colorectal cancer in the QUASAR 2 trial; 246 patients with stage I ovarian carcinoma; 354 patients with uterine sarcoma; 307 patients with prostate carcinoma; and 791 patients with endometrial carcinoma. The primary outcome was cancer-specific survival.
Findings
In all patient cohorts, patients with chromatin heterogeneous tumours had worse cancer-specific survival than patients with chromatin homogeneous tumours (univariable analysis hazard ratio [HR] 1·7, 95% CI 1·2–2·5, in the discovery cohort; 1·8, 1·0–3·0, in the Gloucester validation cohort; 2·2, 1·1–4·5, in the QUASAR 2 validation cohort; 3·1, 1·9–5·0, in the ovarian carcinoma cohort; 2·5, 1·8–3·4, in the uterine sarcoma cohort; 2·3, 1·2–4·6, in the prostate carcinoma cohort; and 4·3, 2·8–6·8, in the endometrial carcinoma cohort). After adjusting for established prognostic patient characteristics in multivariable analyses, Nucleotyping was prognostic in all cohorts except for the prostate carcinoma cohort (HR 1·7, 95% CI 1·1–2·5, in the discovery cohort; 1·9, 1·1–3·2, in the Gloucester validation cohort; 2·6, 1·2–5·6, in the QUASAR 2 cohort; 1·8, 1·1–3·0, for ovarian carcinoma; 1·6, 1·0–2·4, for uterine sarcoma; 1·43, 0·68–2·99, for prostate carcinoma; and 1·9, 1·1–3·1, for endometrial carcinoma). Chromatin heterogeneity was a significant predictor of cancer-specific survival in microsatellite unstable (HR 2·9, 95% CI 1·0–8·4) and microsatellite stable (1·8, 1·2–2·7) stage II colorectal cancer, but microsatellite instability was not a significant predictor of outcome in chromatin homogeneous (1·3, 0·7–2·4) or chromatin heterogeneous (0·8, 0·3–2·0) stage II colorectal cancer.
Interpretation
The consistent prognostic prediction of Nucleotyping in different biological and technical circumstances suggests that the marker of chromatin heterogeneity can be reliably assessed in routine clinical practice and could be used to objectively assist decision making in a range of clinical settings. An immediate application would be to identify high-risk patients with stage II colorectal cancer who might have greater absolute benefit from adjuvant chemotherapy. Clinical trials are warranted to evaluate the survival benefit and cost-effectiveness of using Nucleotyping to guide treatment decisions in multiple clinical settings.
Funding
The Research Council of Norway, the South-Eastern Norway Regional Health Authority, the National Institute for Health Research, and the Wellcome Trust.
Introduction
Genetic alterations in tumours occur on many different levels, ranging from single nucleotide changes and gene amplifications, to chromosome translocations and loss or gain of whole chromosomes.1 Abnormal chromosome number is associated with poor prognosis in many common cancer types.2 Higher-order chromatin structure regulates gene expression and changes during cell differentiation,3 suggesting that chromatin reorganisation might contribute to disease pathogenesis. Chromatin organisation is also the main determinant of variation in regional (ie, on a megabase scale) mutation frequency in cancer cells.4, 5 Integrating chromatin analysis and DNA density measurements could provide an objective assessment of genetic instability and epigenetic aberrations.
Research in context.
Evidence before this study
In addition to sporadic and systematic review of relevant scientific literature over the past decades, we searched PubMed without language or time restrictions for articles published until Nov 18, 2017, using the terms “nuclear”, “chromatin”, “texture”, and “cancer” (full specification of the search criteria is provided in the appendix p 2), and systematically reviewed the titles and abstracts of the 701 search results. We also searched the digital publications collection at The University of Oslo (Oslo, Norway) using the terms “texture analysis” and “microscopy images” to locate relevant academic theses submitted to the University of Oslo in which different methods for detecting chromatin aberrations had been evaluated and compared.
Attempts to correlate changes in chromatin organisation with cancer diagnosis and prognosis have been made for many decades. Early findings suggested that accurate identification of disease and patient outcome could be obtained by applying complex image analysis methods on images that depict the chromatin organisation in cell nuclei, but there is an absence of independent validation in external cohorts.
We have previously shown that image patterns associated with aberrant chromatin organisation is associated with poor prognosis in many cancers, but none of the developed markers are suitable for validation on external cohorts of different cancer types.
Added value of this study
We have shown that heterogeneous chromatin organisation can be reliably assessed by machine learning algorithms without being adapted for various cancer types or distinct methods and equipment used to prepare the samples and image the nuclei. Validation of the chromatin heterogeneity marker, termed Nucleotyping, in external cohorts shows that chromatin heterogeneity predicts cancer-specific survival of patients with colorectal cancer, ovarian carcinoma, uterine sarcoma, and endometrial cancer independently of established prognostic markers. In stage II colorectal carcinoma, cancer-specific survival was stratified more precisely by chromatin heterogeneity than by microsatellite instability.
Implications of all the available evidence
The generic utility of Nucleotyping warrants study of its molecular basis and suggests that it could enhance selection of patients for adjuvant treatment.
DNA organisation can be described by the texture of cell nuclei stained specifically for DNA, and abnormal structure has been linked to poor prognosis in cancer.6 In previous assessments of chromatin aberrations, machine learning algorithms were trained on part of a patient cohort with one specific cancer type and validated on the complementary part of the same cohort. The analysed tissue samples were usually prepared and imaged using the same methods and equipment, and attempts to make the assessments of chromatin aberrations robust to moderate technical deviations have rarely been made, which severely limits their applicability, not only in the clinic, but also in research facilities. Despite obvious limitations, findings from previous studies of this type (eg, our own studies on ovarian carcinoma,7 uterine sarcoma,8 prostate carcinoma,9 and endometrial carcinoma10) suggest that such alterations in chromatin organisation could be a consistent finding in carcinogenesis. We hypothesised that generic texture patterns of aberrant chromatin could be detectable in most cancer types and wanted to reliably detect these patterns independently of moderate deviations in preparation methods and imaging equipment. To this end, we developed an automated method to robustly identify such patterns and independently validated this marker, termed Nucleotyping, in a range of cancer types.
Methods
Patient cohorts
Between 1993 and 2003, 494 consecutive patients with primary colorectal cancer at Aker University Hospital (Oslo, Norway) had resection of non-synchronous stage I and II tumours.11, 12 390 of these patients were included in our discovery cohort (figure 1A; table 1). Two (<1%) patients received adjuvant chemotherapy, and 324 (83%) patients did not; the information was missing for the remaining 64 (16%) patients. Total mesorectal excision was done in all patients with rectal cancer (n=118), and ten (8%) rectal cancer patients received neoadjuvant treatment. None of the colon cancer patients (n=272) received neoadjuvant treatment. The study was approved by the Regional Committees for Medical and Health Research Ethics (REK) in Norway (number 1.2005.1629). An experienced pathologist (AS) did the pathology analyses.
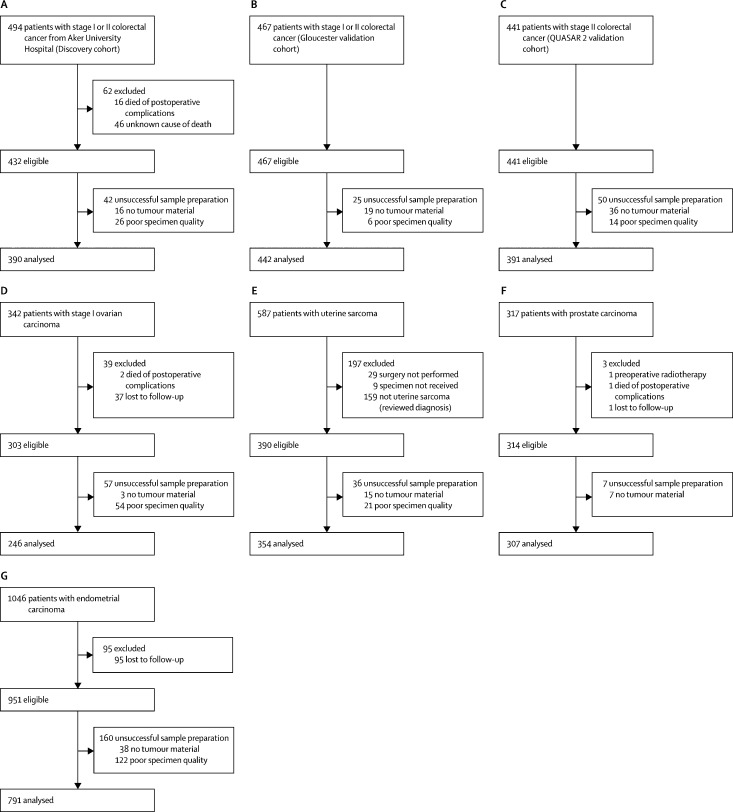
Figure 1.
CONSORT diagrams showing the origin of each patient cohort
(A) Colorectal cancer discovery cohort. (B) Gloucester colorectal cancer validation cohort. (C) QUASAR 2 colorectal cancer validation cohort. (D) Ovarian carcinoma cohort. (E) Uterine sarcoma cohort. (F) Prostate cancer cohort. (G) Endometrial carcinoma cohort.
Table 1.
Baseline characteristics of patients with colorectal carcinoma in the discovery and validation cohorts
| Discovery (n=390) | Gloucester validation (n=442) | QUASAR 2 validation (n=391) | ||
|---|---|---|---|---|
| Follow-up time, years | 6·9 (3·6–10·0) | 3·5 (1·7–5·3) | 4·8 (4·0–5·1) | |
| Age at surgery, years | 73 (64–79) | 72 (65–79) | 63 (57–70) | |
| ≤72 | 190 (49%) | 230 (52%) | 333 (85%) | |
| >72 | 200 (51%) | 212 (48%) | 58 (15%) | |
| Sex | ||||
| Female | 192 (49%) | 204 (46%) | 161 (41%) | |
| Male | 198 (51%) | 238 (54%) | 230 (59%) | |
| Stage | ||||
| I | 112 (29%) | 83 (19%) | 0 | |
| II | 278 (71%) | 359 (81%) | 391 (100%) | |
| Histological grade | ||||
| 1 | 37 (10%) | 120 (27%) | 15 (4%) | |
| 2 | 315 (82%) | 257 (58%) | 282 (77%) | |
| 3 | 34 (9%) | 65 (15%) | 68 (19%) | |
| Pathologic tumour (T) stage | ||||
| T1 | 23 (6%) | 14 (3%) | 0 | |
| T2 | 89 (23%) | 68 (15%) | 0 | |
| T3 | 261 (67%) | 236 (54%) | 190 (51%) | |
| T4 | 17 (4%) | 123 (28%) | 185 (49%) | |
| Microsatellite stability | ||||
| Unstable | 63 (17%) | NA | 62 (17%) | |
| Stable | 300 (83%) | NA | 306 (83%) | |
| Location | ||||
| Rectum | 118 (30%) | 131 (30%) | 44 (12%) | |
| Distal colon | 116 (30%) | 162 (37%) | 142 (38%) | |
| Proximal colon | 156 (40%) | 149 (34%) | 188 (50%) | |
| Surgery type | ||||
| Elective | 354 (91%) | 366 (85%) | NA | |
| Acute* | 36 (9%) | 65 (15%) | NA | |
| Chromatin heterogeneity | ||||
| Homogeneous | 235 (60%) | 308 (70%) | 244 (62%) | |
| Heterogeneous | 155 (40%) | 134 (30%) | 147 (38%) | |
Data are median (IQR) or number (%). NA=data not available.
Acute surgery was done because of obstruction or perforation of the bowel at presentation in the discovery cohort, and defined as either urgent or emergency surgery in the Gloucester validation cohort.
The Gloucester colorectal cancer validation cohort included 442 of the 467 patients with non-synchronous stage I or II colorectal cancer from the Gloucester Colorectal Cancer Study (UK), who were recruited between 1988 and 1996 (figure 1B, table 1).13, 14 Data on adjuvant radiotherapy and chemotherapy were available for 310 (70%) patients, of whom 23 (7%) received adjuvant treatment and 287 (93%) did not. Total mesorectal excision was done in most patients with rectal cancer (n=131), and six (5%) patients with rectal cancer received neoadjuvant treatment. None of the patients with colon cancer (n=311) received neoadjuvant treatment. This study was approved by the Gloucestershire Local Research Ethics Committee (number 01/21G) and REK in Norway (number 2015/1606), and the pathology analyses were done by an expert pathologist (NAS).
The QUASAR 2 trial (ISRCTN registry number ISRCTN45133151) established a biobank that included formalin-fixed, paraffin-embedded tumour tissue blocks from 441 patients with stage II colorectal cancer.15 Data from 391 patients were available for the second colorectal cancer validation cohort (figure 1C; table 1). Patients were recruited between 2005 and 2010, and all patients received adjuvant chemotherapy (capecitabine with or without bevacizumab), but none received neoadjuvant treatment. Approval for the study was obtained from the West Midlands Research Ethics Committee (number 04/MRE/11/18) and REK in Norway (number 2015/1607). The pathology assessments were done by pathologists at the participating hospitals in the trial.
246 patients treated for International Federation of Gynecology and Obstetrics (FIGO) stage I ovarian carcinoma between 1982 and 1989 (surgery at county hospitals and evaluation of further treatment at the Norwegian Radium Hospital, a tertiary referral comprehensive cancer centre in Oslo, Norway) were analysed as an ovarian cancer validation cohort (figure 1D; appendix p 6).7 All patients provided verbal informed consent, and the study was in accordance with Norwegian law. The surgical procedure consisted of peritoneal washing, hysterectomy, bilateral salpingo-oophorectomy, and omentectomy. The number of patients who received adjuvant treatments and the types of adjuvant treatments are described in the appendix (p 24). The FIGO stage was reviewed according to 1988 criteria, although para-aortic and pelvic lymphadenectomy was not routinely performed. A single pathologist (VMA) masked to patient outcome reviewed the histological sections using WHO criteria.
We confirmed the diagnosis and obtained adequate tumour material from 354 patients with uterine sarcoma reported to the Norwegian Cancer Registry between 1970 and 2000 to make up the uterine sarcoma validation cohort (figure 1E; appendix p 7).8, 16 All patients in this cohort had a hysterectomy, but records on the precise surgical procedure and additional treatment are not available. An experienced gynaecological pathologist (VMA) reviewed the tumours according to the WHO recommendations without knowledge of the clinical outcome. The study of the total population of uterine sarcoma patients in Norway was approved by REK (number S-04298).
From 1987 to 2005, 317 consecutive patients underwent open retropubic prostatectomy at the Norwegian Radium Hospital.9, 17 One investigator (HW) was responsible for treatment and follow-up. 307 patients were included in the prostate cancer validation cohort (figure 1F; appendix p 8), and the study was approved by REK (number S-07443a). Only one patient received adjuvant treatment. An experienced uropathologist (LV) reviewed all specimens to obtain a complete set of consistent pathological assessments using established recommendations.18, 19
791 tumour samples from consenting patients with endometrial carcinoma who were treated between 2001 and 2011 in the Molecular Markers in Treatment of Endometrial Cancer (MoMaTEC) trial (NCT00598845) were included in the endometrial carcinoma validation cohort (figure 1G; appendix p 9).10 767 patients had hysterectomy, three patients had tumour reduction, and 21 patients had curettage. The number of patients who received adjuvant treatment and the types of adjuvant treatments are described in the appendix (p 27). Curettage specimens were preoperatively assessed to obtain a histological risk classification. REK approved the study (number 052.01), and the 2009 FIGO staging criteria were applied to determine FIGO stage. The pathology analyses were done by pathologists at the participating centres in the trial.
Sample preparation and imaging
Images of cell nuclei were acquired from curettage specimens for patients with endometrial carcinoma and from the surgically resected tumours for all other patients. One of several pathologists selected a representative tumour region for each patient from haematoxylin and eosin-stained sections of the formalin-fixed, paraffin-embedded tumour tissue blocks. To account for heterogeneity,20 three regions (IQR three to four) from different tumour blocks were included for prostate carcinoma patients. One or more 50 μm sections of each selected tumour region was used to obtain isolated nuclei using a modification of Hedley's method.21 After rehydration, the sections were enzymatically digested at room temperature at 200 rotations per min (rpm) for 70 min (for colorectal and prostate specimens) or 60 min (for other specimens) with 0·5 mg/mL protease (Sigma protease type XXIV [P5380] or type VIII [P8038]; Sigma Chemical, St Louis, MO, USA) to disaggregate the cells. The cell suspension was filtered through a 60 μm mesh nylon filter, washed, and cytospun (600 rpm for 5 min) onto a poly-l-lysine-coated slide.22 The nuclei were stained using Feulgen's method, and slides were incubated in 5 M HCl for 60 min at room temperature for hydrolysis, stained with Schiff's solution for 2 h in the dark, rinsed in a fresh solution of 0·5% sodium metabisulfite in 0·05 M hydrochloric acid (three times 10 min), dehydrated, and coverslipped.21, 22 Feulgen-stained nuclei were imaged by a Zeiss Axioplan microscope equipped with a 546 nm green filter and a monochrome high-resolution digital camera (AxioCam MrM, Zeiss, Jena, Germany, or C4742-95, Hamamatsu Photonics, Hamamatsu, Japan) with a depth of field of about 1·5 μm. In the resulting images, the value of each pixel reflects the DNA density at that location and is referred to as the pixel grey level. Although the sample preparation method and imaging equipment were similar within each cohort except for the prostate carcinoma cohort, methodological and equipment updates were implemented between work on different sample series, and images from the entire set of cohorts thus have notably different technical features (eg, in image contrast and number of nuclear pixels). Additionally, a single pathologist selected the tumour regions for all patients in the three colorectal carcinoma cohorts, the ovarian carcinoma cohort, and the uterine sarcoma cohort (although the pathologist was not the same across these cohorts), whereas the selections were done by multiple pathologists in each of two other cohorts.
The imaged nuclei were assessed to exclude non-representative cells (eg, cut or connected nuclei and non-tumour cells). Trained personnel identified the nuclei of interest in the ovarian carcinoma cohort as those that appeared to be whole, isolated, and epithelial.7 In all other cohorts, the Ploidy Work Station (PWS, Room4, Sussex, UK) was applied to automatically discard non-intact nuclei (eg, cut, folded, and connected) and to detect cell types. The initial method23 was used in the uterine sarcoma cohort, and only non-necrotic, intact nuclei were kept for further analysis.8 The analysis was further restricted to epithelial nuclei in the colorectal, prostate, and endometrial carcinoma cohorts. Trained personnel verified the automatic nucleus classifications in all cohorts, except in the two colorectal cancer validation cohorts because by then we considered the method in PWS to be both robust and accurate enough to allow completely automated identification of non-necrotic, intact epithelial nuclei. If less than 200 nuclei were classified as applicable for further analysis then the specimen was considered of insufficient quality for analysis and excluded. 4·3 million images of cell nuclei from the 2921 analysed patients were included in further analysis, giving an average of about 1200 images per tumour region and about 1500 images per patient. Each image comprised an average of about 3700 nuclear pixels. Because images in different cohorts deviated in contrast and size due to differences in sample preparation methods and imaging equipment, the set of images from each single tumour region was independently normalised using a previously described algorithm that automatically standardises the optical and spatial scales of the images.9 This normalisation method does not depend on external controls and automatically finds internal controls by estimating which nuclei are diploid. The resulting images had a physical resolution of about 160 nm/pixel and a pixel depth of ten bits.
Nuclear texture analysis
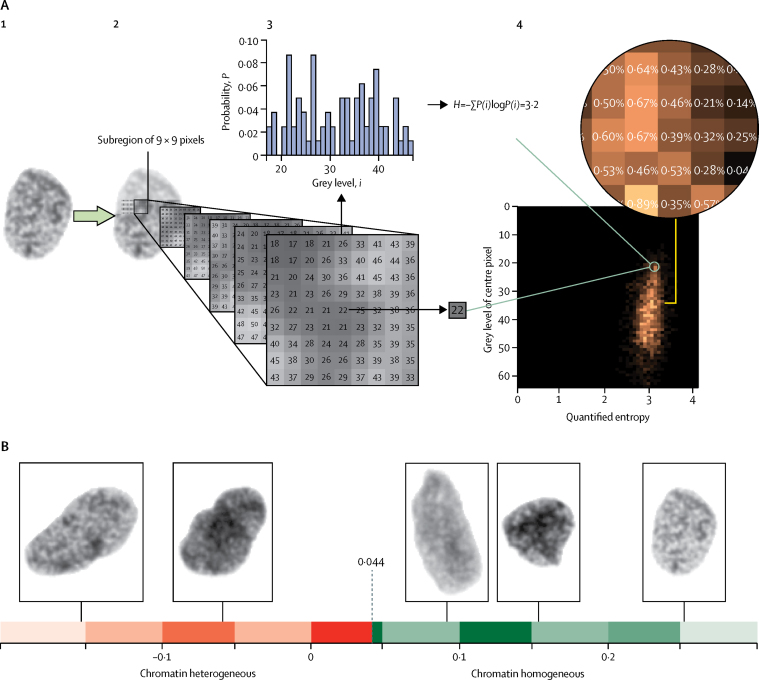
Chromatin organisation was quantified by computing the entropy of pixel grey levels in a subregion of a nucleus (figure 2). Entropy is a measure of disorder commonly used in thermodynamics but applied here to assess whether the chromatin organisation is disordered in the sense of more interleaved chromatin compartments with different condensations. The subregion was taken to be a square region, and the entropy of the region was coupled with the grey level value of the region's centre pixel to integrate measurements of disordered chromatin organisation and DNA content. The frequency in which each pair of entropy and centre grey level occur throughout a nucleus was stored in a two-way table, known as the grey level entropy matrix (GLEM; figure 2A).24 Each chromatin pattern (ie, a subregion with concrete values and arrangement of pixel grey levels) corresponded to a specific element in the GLEM.
Figure 2.
Computation of the grey level entropy matrix (GLEM) and visualisation of nuclear images
(A) Illustration of GLEM computation. (1) A nuclear image. (2) Each nuclear pixel is taken to be the centre of a square subregion, here with a side length of nine pixels. (3) For each subregion, two quantities are extracted (the grey level of the centre pixel [here 22] and the entropy of the grey levels in the subregion [here 3·2]); the entropy H is a variability characteristic of the probability mass function P(i) (ie, the histogram that gives the probability P that grey level i occurs in the subregion). (4) The two quantities extracted from the subregion will together identify a position in a two-way table. The table cell position corresponding to the subregion in figure part 3 of panel A is marked by a green circle in part 4 of panel A. The occurrence is counted by incrementing the value at the table cell position (initially, all table cell values are 0), and the computation of the two quantities and incrementation of the corresponding table cell value is performed for every subregion of the nuclear image. The resulting table describes the frequency of each pair of centre grey level and surrounding entropy and is normalised by its total count to provide the bivariate probability mass function called the GLEM. The two-way table visualised in part A4 is the GLEM of the nuclear image in part A1. (B) Depiction of five nuclear images and their chromatin value. The threshold applied to dichotomise the chromatin value was 0·044.
GLEMs stratified on nuclear area (grouped at 1–999 pixels, 1000–1999 pixels, …, 9000–9999 pixels, and 10 000 pixels or more) and computed on different scales (subregions of 3 × 3 pixels, 5 × 5 pixels, …, 31 × 31 pixels) were concatenated to form a four-dimensional expansion of the GLEM called GLEM4D.7 Each pixel in a nucleus is thus the centre of 15 subregions representing the chromatin organisation near the pixel on different magnifications, and the frequency of these chromatin patterns for all pixels in the nucleus is stored in the GLEM4D. The GLEM4D was calculated for each of the 461 000 nuclear images in the discovery cohort, and each patient was represented by the average GLEM4D of the patient's nuclei. Aberrant chromatin patterns were discovered as patterns corresponding to elements in the GLEM4D that were associated with poor prognosis in the discovery cohort. This association was computed for each GLEM4D element as a constant scaling factor multiplied by the statistic of a two-sample t test that tested for the difference between good and poor prognosis in the specific GLEM4D element in the discovery cohort. The applied adaptive machine learning algorithm25 could then compute the compliance between the GLEM4D representation of a new patient and the discovered patterns of chromatin aberrations by multiplying each GLEM4D element with the corresponding scaled t statistic and summing the products. The result is a continuous value termed the chromatin value, which describes the overall amount of chromatin disorder in a given patient. Finally, the robust minimum Euclidean distance classification method26, 27 was applied to calculate a fixed threshold with which to dichotomise the chromatin value into a classification of the tumour as either chromatin homogeneous or chromatin heterogeneous. The threshold was computed using the discovery cohort (0·044), but other thresholds provided markers with similar accuracy in the discovery cohort when measured by hazard ratio, although with different abilities to correctly identify patients as good and poor prognosis (appendix p 23). Complete specification of the method is provided in the appendix (pp 3–4). Details of Nucleotyping and its testing and validation in clinical cohorts can be found in a webvideo. Example nuclear images and their chromatin values are shown in figure 2B. Nucleotyping could subsequently be applied blindly to label new, individual patients as chromatin homogeneous or chromatin heterogeneous on the basis of the GLEM4Ds computed from its nuclear images.
In an average chromatin heterogeneous tumour, 63% (IQR 52–73) of the nuclei expressed aberrant chromatin patterns. The proportion of nuclei required for a tumour to be labelled as chromatin heterogeneous was not fixed because the classification of a tumour sample was based on the average estimated severity of its nuclei (the scaled t statistic of the chromatin patterns in the nucleus), which in turn was calculated as the average severity of all observed chromatin patterns in the nuclear image. Thus, a relatively small proportion of nuclei (minimum in the analysed cohorts was 36%) expressing highly severe chromatin patterns could define the tumour as chromatin heterogeneous, whereas a chromatin homogeneous tumour could have a relatively large proportion of nuclei (maximum in the analysed cohorts was 57%) with chromatin patterns associated with mild disorganisation.
Statistical analysis
We measured cancer-specific survival because it was considered the most clinically relevant endpoint that was common to all patient cohorts. Events are defined, as proposed by Punt and colleagues,28 exclusively as death from the same cancer. All recurrences are ignored, and patients are censored at all other deaths or loss to follow-up. The follow-up time is computed from the date of entry to date of death or loss to follow-up. Mantel-Cox log-rank test was used in univariable survival analysis, and Wald χ2 test with Cox proportional hazards model in multivariable analysis. Each analysis included only patients with complete data for the variables in question, but imputation for missing data was subsequently done using multiple imputation by chained equations to assess all patients. The clinical and pathological markers included in multivariable analyses were the same as those that had been used in the previous studies of the individual patient cohorts7, 8, 9, 10, 12 or, in case of the colorectal cancer validation cohorts, were the same prognostic markers as those applied to the discovery cohort. Subsequently, number of investigated lymph nodes (<12 vs ≥12) and tumour sidedness (left vs right) were separately added to the multivariable model.
We did sensitivity analyses by repeating analyses without patients who had received neoadjuvant or adjuvant treatment, or both. Associations were evaluated with Spearman's correlation coefficients.
A likelihood ratio test was used to assess whether inclusion of chromatin heterogeneity improved the prediction of cancer-specific survival compared with a multivariable model without chromatin heterogeneity. The proportion of patients with chromatin heterogeneous tumours who died of their cancer (positive predictive value), the proportion of patients with chromatin homogeneous tumours who did not die of their cancer (negative predictive value), and the proportion of patients who were either both chromatin heterogeneous and died of their cancer or chromatin homogeneous and did not die of their cancer (correct classification rate) were computed to quantify how well chromatin heterogeneity corresponded with the final patient outcome.
Category-free net reclassification improvement can be defined as the sum of the event-net reclassification improvement and non-event-net reclassification improvement.29 Event-net reclassification improvement is the probability of the new model to estimate worse cancer-specific survival than the old model in the case of an event, minus the probability of the new model to estimate better cancer-specific survival than the old model in the case of an event. Non-event-net reclassification improvement is the probability of the new model to estimate better cancer-specific survival than the old model in the case of no event, minus the probability of the new model to estimate worse cancer-specific survival than the old model in the case of no event. We used category-free net reclassification improvement to evaluate the reclassification from using only microsatellite stability status to using both Nucleotyping and microsatellite stability status to predict the outcome for patients with stage II colorectal cancer.
A two-sided p value of less than 0·05 was considered statistically significant. We used MATLAB R2012b for the texture analysis and Stata/SE 14.2 for the survival analyses. Category-free net reclassification improvement was computed using the survIDINRI package (version 1.1-1) in R (version 3.4.2).
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or in the writing of the report. AN had access to the raw data for the discovery cohort, NAS for the Gloucester validation cohort, DJK for the QUASAR 2 validation cohort, GBK for the ovarian carcinoma and uterine sarcoma cohorts, HW for the prostate carcinoma cohort, and JT for the endometrial carcinoma cohort. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
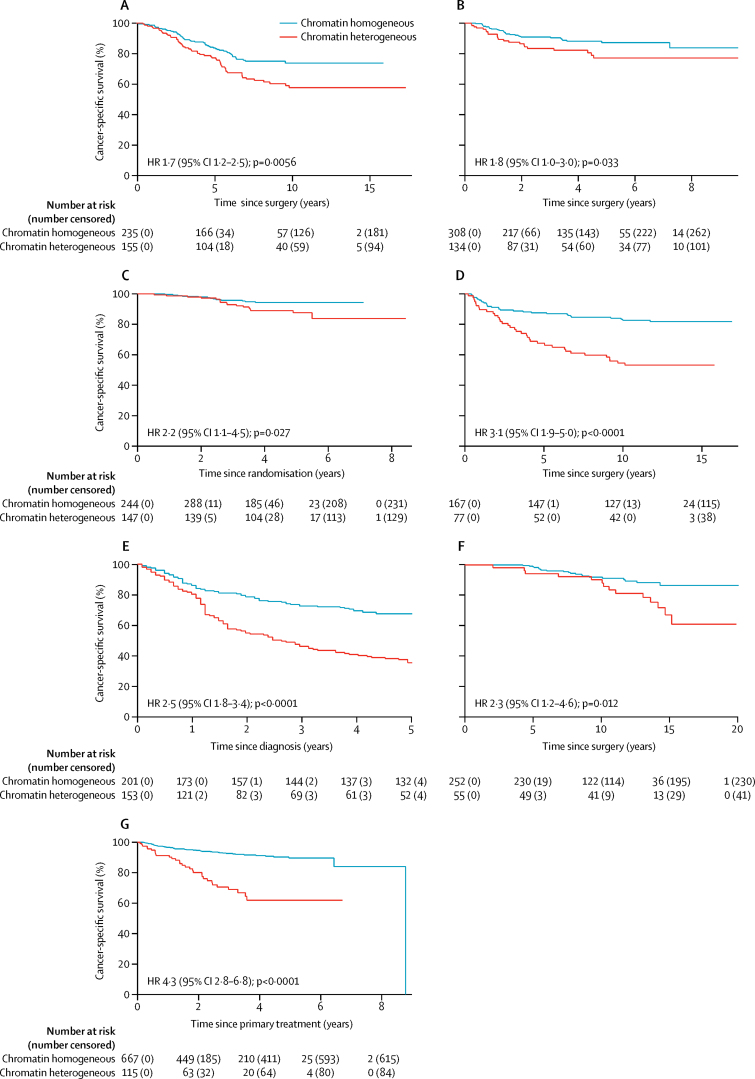
In the colorectal cancer discovery cohort (n=390), 155 (40%) patients had chromatin heterogeneous tumours and 235 (60%) had chromatin homogeneous tumours. Patients with chromatin heterogeneous tumours had shorter cancer-specific survival than patients with chromatin homogeneous tumours, both in univariable analysis (hazard ratio [HR] 1·7, 95% CI 1·2–2·5) and multivariable analysis (1·7, 1·1–2·5; figure 3A; table 2; appendix p 10), and when imputing missing data for variables in the multivariable model (1·7, 1·2–2·5). Adding tumour sidedness (left vs right) to the multivariable model did not substantially alter the results (HR 1·6, 95% CI 1·1–2·4), nor did removing the 11 patients (all with chromatin homogeneous tumours) who received neoadjuvant or adjuvant treatment, or both (1·7, 1·2–2·6). In patients with stage II colorectal cancer, 5-year cancer-specific survival was 83% (95% CI 76–88) for patients with chromatin homogeneous tumours (26 [16%] cancer-specific deaths out of 164 patients) and 72% (62–79) for patients with chromatin heterogeneous tumours (30 [26%] cancer-specific deaths out of 114 patients).
Figure 3.
Kaplan-Meier analysis of cancer-specific survival in patients with chromatin homogeneous and chromatin heterogeneous tumours
(A) Discovery cohort for colorectal cancer. (B) Gloucester validation cohort for colorectal cancer. (C) QUASAR 2 validation cohort for colorectal cancer. (D) Ovarian carcinoma cohort. (E) Uterine sarcoma cohort. (F) Prostate carcinoma cohort. (G) Endometrial carcinoma cohort. HR=hazard ratio.
Table 2.
Chromatin heterogeneity in analysis of cancer-specific survival
|
Univariable analysis |
Multivariable analysis* |
Likelihood ratio test* |
|||||
|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | p value | n | HR (95% CI) | p value | p value | |
| Colorectal cancer, discovery | 390 | 1·7 (1·2–2·5) | 0·0056 | 386 | 1·7 (1·1–2·5) | 0·0096 | 0·0096 |
| Colorectal cancer, Gloucester validation | 442 | 1·8 (1·0–3·0) | 0·033 | 431 | 1·9 (1·1–3·2) | 0·026 | 0·030 |
| Colorectal cancer, QUASAR 2 validation | 391 | 2·2 (1·1–4·5) | 0·027 | 365 | 2·6 (1·2–5·6) | 0·016 | 0·015 |
| Ovarian carcinoma | 246 | 3·1 (1·9–5·0) | <0·0001 | 246 | 1·8 (1·1–3·0) | 0·022 | 0·021 |
| Uterine sarcoma | 354 | 2·5 (1·8–3·4) | <0·0001 | 301 | 1·6 (1·0–2·4) | 0·038 | 0·035 |
| Prostate carcinoma | 307 | 2·3 (1·2–4·6) | 0·012 | 301 | 1·4 (0·7–3·0) | 0·34 | 0·35 |
| Endometrial carcinoma | 791 | 4·3 (2·8–6·8) | <0·0001 | 776 | 1·9 (1·1–3·1) | 0·013 | 0·014 |
HR=hazard ratio.
In each colorectal cancer cohort, chromatin heterogeneity was added to the multivariable model consisting of age, stage, histological grade, and surgery type, although stage was not relevant and surgery type data were not available for the QUASAR 2 validation cohort. For the ovarian carcinoma cohort, the model consisted of stage and histological grade. For the uterine sarcoma cohort, the model consisted of histological subtype, mitotic index, tumour extent, tumour size, tumour margins, cellular atypia, tumour necrosis, hyaline necrosis, and vascular invasion. For the prostate cancer cohort, the model consisted of age, preoperative prostate-specific antigen, Gleason grade, surgical margins, extracapsular extension, seminal vesicle invasion, and pathological node stage. For the endometrial carcinoma cohort, the model consisted of age and curettage histology classification. Patients without complete data for model variables were omitted from the multivariable analyses.
Of 442 patients in the Gloucester colorectal cancer validation cohort, 308 (70%) had chromatin homogeneous tumours and 134 (30%) had chromatin heterogeneous tumours. Analysis of the Gloucester cohort replicated the results from the discovery cohort (figure 3B; table 2; appendix p 11), and chromatin heterogeneity was also prognostic when imputing missing data (HR 1·9, 95% CI 1·1–3·3) or including number of investigated lymph nodes (<12 vs ≥12; 1·8, 1·0–3·2) or tumour sidedness (1·9, 1·1–3·2) in the multivariable model. Excluding the 29 patients who received neoadjuvant or adjuvant treatment, or both (23 patients with chromatin homogeneous tumours and six with chromatin heterogeneous tumours), gave similar estimates in univariable analysis (HR 1·9, 95% CI 1·1–3·5) and multivariable analysis (2·0, 1·1–3·6). In patients with stage II colorectal cancer, 5-year cancer-specific survival was 85% (95% CI 78–89) for patients with chromatin homogeneous tumours (31 [12%] deaths out of 254 patients) and 72% (59–81) for patients with chromatin heterogeneous tumours (22 [21%] deaths out of 105 patients).
In the QUASAR 2 colorectal cancer validation cohort, 147 (38%) of 391 patients had chromatin heterogeneous tumours and 244 (62%) had chromatin homogeneous tumours. Cancer-specific survival was shorter in patients with chromatin heterogeneous tumours than in those with chromatin homogeneous tumour (HR 2·2, 95% CI 1·1-4·5 in univariable analysis; 2·6, 1·2-5·6 in multivariable analysis; figure 3C; table 2; appendix p 12). Chromatin heterogeneity remained prognostic when missing data in the multivariable model were imputed (HR 2·3, 95% CI 1·1–4·7). The multivariable result was not substantially altered by additionally adjusting for the number of investigated lymph nodes (HR 2·7, 95% CI 1·2–5·9) or tumour sidedness (2·8, 1·3–6·3). 5-year cancer-specific survival was 94% (95% CI 90–97) for patients with chromatin homogeneous tumours (13 [5%] deaths out of 244 patients) and 88% (80–92) for patients with chromatin heterogeneous tumours (16 [11%] deaths out of 147 patients).
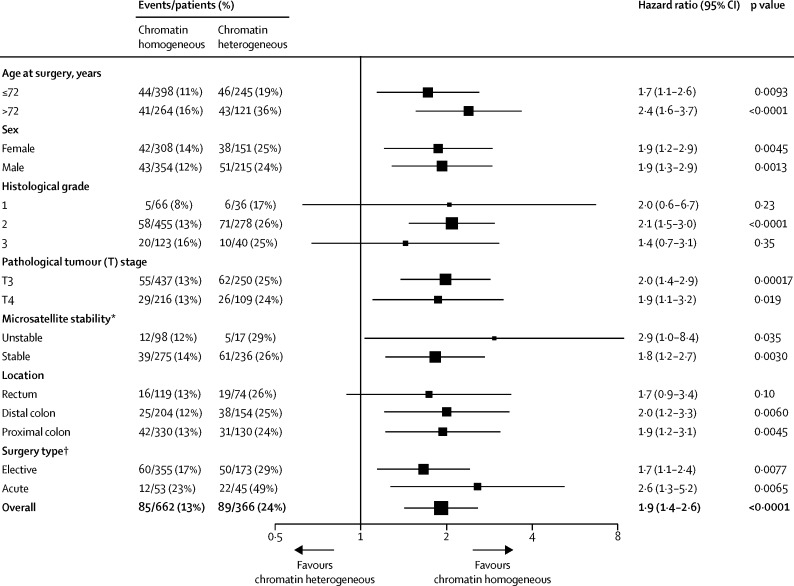
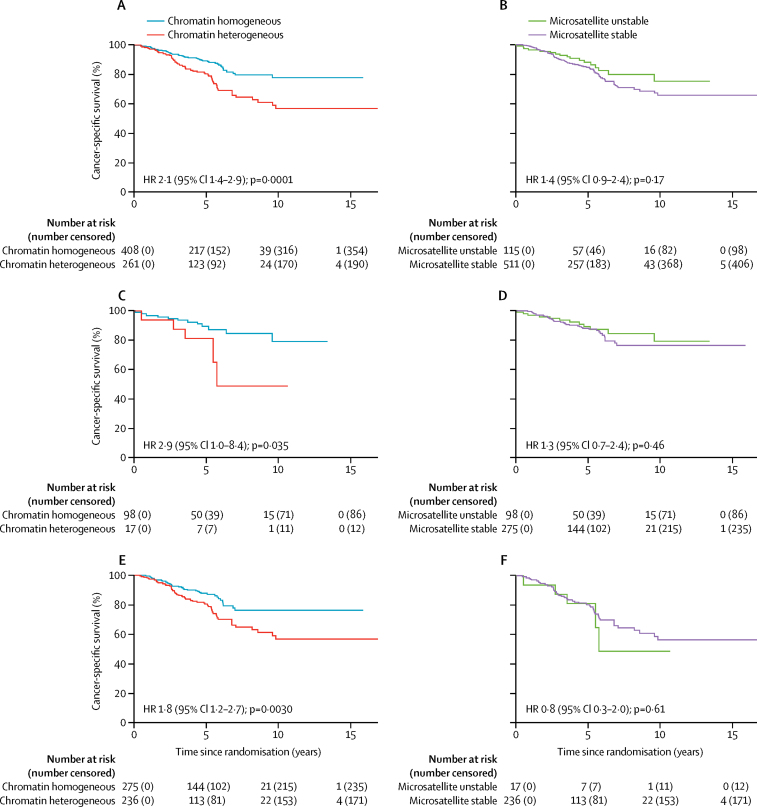
In a pooled analysis of all three colorectal cancer cohorts, chromatin heterogeneity was independent of sex, stage (II vs I), and pathological tumour stage (T4 vs T3 vs T2 vs T1; appendix p 13). No association was found for colon versus rectum (p=0·48), but chromatin heterogeneity correlated weakly with tumour sidedness (p=0·12, 95% CI 0·07–0·18; p<0·0001) and therefore tumour location (p=0·0010; appendix p 13). Similar weak correlations were found for age, histological grade, microsatellite stability, and surgery type (appendix p 13). In a pooled analysis of stage II colorectal cancer, the HR between patients with chromatin heterogeneous and chromatin homogeneous tumours was similar across subgroups of each patient characteristic (figure 4). Chromatin heterogeneity predicted cancer-specific survival more accurately than microsatellite stability status (stable vs unstable) and provided prognostic information for patients with microsatellite unstable and microsatellite stable stage II colorectal cancer (figure 5; appendix p 14). In a multivariable model with microsatellite stability status, cancer-specific survival was shorter in patients with stage II colorectal cancer with chromatin heterogeneous tumours compared with patients with chromatin homogeneous tumours (HR 1·9, 95% CI 1·3–2·8). The category-free net reclassification improvement of supplementing microsatellite stability status with Nucleotyping for prediction of 5-year cancer-specific survival in stage II colorectal cancer patients was 31·1% (95% CI 2·7–54·5); the event-net reclassification improvement was 7·5%, and the non-event-net reclassification improvement was 23·6%.
Figure 4.
Forest plot of chromatin heterogeneity for all stage II colorectal cancer patients in analysis of cancer-specific survival
*Microsatellite stability data were not available for the Gloucester validation cohort. †Surgery type data were not available for the QUASAR 2 validation cohort.
Figure 5.
Cancer-specific survival of stage II colorectal cancer patients according to Nucleotyping and microsatellite stability
Kaplan-Meier curves according to (A) Nucleotyping, (B) microsatellite stability, (C) Nucleotyping in microsatellite unstable tumours, (D) microsatellite stability in chromatin homogeneous tumours, (E) Nucleotyping in microsatellite stable tumours, and (F) microsatellite stability in chromatin heterogeneous tumours. HR=hazard ratio.
Of 246 patients in the ovarian carcinoma cohort, 77 (31%) had chromatin heterogeneous tumours and 169 (71%) had chromatin homogenous tumours. Patients with chromatin heterogeneous tumours had shorter cancer-specific survival than those with chromatin homogeneous tumours (figure 3D). Chromatin heterogeneity was consistently prognostic across a range of clinicopathological subgroups and was significant in multivariable analysis (table 2; appendix pp 15, 24). 5-year cancer-specific survival was 88% (95% CI 82–92) for patients with chromatin homogeneous tumours (21 [12%] deaths out of 169 patients) and 68% (56–77) for patients with chromatin heterogeneous tumours (25 [32%] deaths out of 77 patients).
Of the 354 patients in the uterine sarcoma cohort, 201 (57%) had chromatin homogeneous tumours and 153 (43%) had chromatin heterogeneous tumours. Chromatin heterogeneity predicted 5-year cancer-specific survival in univariable analysis (figure 3E), performed consistently across a range of clinicopathological subgroups, and was significant in multivariable analyses (table 2; appendix pp 16, 25). Chromatin heterogeneity remained prognostic in both univariable (HR 2·4, 95% CI 1·8–3·1) and multivariable (1·4, 1·0–2·0) analysis of cancer-specific survival, also when missing data were imputed. 5-year cancer-specific survival was 67% (95% CI 61–74) for patients with chromatin homogeneous tumours (65 [32%] deaths out of 201 patients) and 35% (28–43) for patients with chromatin heterogeneous tumours (97 [63%] deaths out of 153 patients).
55 (18%) of the 307 patients in the prostate carcinoma cohort had chromatin heterogeneous tumours; the other 252 (82%) patients had chromatin homogeneous tumours. In univariable analysis, cancer-specific survival was shorter in patients with chromatin heterogeneous tumours than in patients with chromatin homogeneous tumours, but there was no significant difference in multivariable analysis (figure 3F; table 2; appendix pp 17, 26) or when performing imputation for missing data (HR 1·43, 95% CI 0·71–2·90; p=0·32). 5-year cancer-specific survival was 99% (95% CI 96–100) for patients with chromatin homogeneous tumours (three [1%] deaths out of 252 patients) versus 94% (83–98) for patients with chromatin heterogeneous tumours (three [5%] deaths out of 55 patients).
In the endometrial carcinoma cohort, 118 (15%) of 791 patients had chromatin heterogeneous tumours and 673 (85%) had chromatin homogeneous tumours. Patients with chromatin heterogeneous tumours had shorter cancer-specific survival than those with chromatin homogeneous tumours (figure 3G). Chromatin heterogeneity was prognostic independent of age at surgery and curettage histology classification (table 2; appendix pp 18, 27), also when imputing missing data (HR 1·9, 95% CI 1·2–3·2). 5-year cancer-specific survival was 90% (95% CI 86–92) for patients with chromatin homogeneous tumours (50 [7%] deaths out of 673 patients) and 62% (49–72) for patients with chromatin heterogeneous tumours (32 [27%] deaths out of 118 patients). Exclusion of the 260 patients who received adjuvant treatment (192 with chromatin homogeneous tumours and 68 with chromatin heterogeneous tumours) increased the HR in the univariable analysis (HR 10·9, 95% CI 4·8–24·8) and multivariable analysis (4·6, 1·8–11·3; appendix pp 19, 28).
The negative predictive value exceeded 90% in the two colorectal cancer validation cohorts, the prostate cancer cohort, and the endometrial carcinoma cohort, but the positive predictive value in these cohorts was not higher than 27% (appendix p 20). The positive predictive value was higher in the two remaining validation cohorts (47% in the ovarian carcinoma cohort and 63% in the uterine sarcoma cohort), but the negative predictive value was 82% and 68%, respectively (appendix p 20). Sensitivity, specificity and correct classification rate for all cohorts are shown in the appendix (p 20).
Chromatin heterogeneity showed low positive correlation with histological grade in the three gynaecological cancer cohorts and in the prostate cancer cohort, low negative correlation with histological grade in the Gloucester validation cohort, and no correlation with histological grade in the discovery cohort or the QUASAR 2 validation cohort (appendix p 21). The HR of the chromatin heterogeneity marker from the multivariable analysis with grade (appendix p 21) was similar to the HR derived in the full multivariable analyses (table 2).
Of the 2858 patients with assessable DNA ploidy, 1354 (47%) had diploid tumours, of which eight (<1%) had chromatin heterogeneous tumours; the remaining 1346 (>99%) patients had chromatin homogeneous tumours. Of the 1504 non-diploid tumours, 683 (45%) were chromatin homogeneous, and 821 (55%) were chromatin heterogeneous. In the three colorectal cancer cohorts, chromatin heterogeneity divided the non-diploid tumours into two groups of similar size (354 [45%] of 785 patients with chromatin homogeneous tumours and 431 [55%] of 785 patients with chromatin heterogeneous tumours) and was not associated with tetraploidy versus aneuploidy (p=0·36). Correspondingly, chromatin homogeneity was detected in 68 (47%) of 144 non-diploid tumours in the ovarian carcinoma cohort, 93 (38%) of 244 non-diploid tumours in the uterine sarcoma cohort, 73 (57%) of 127 in the prostate carcinoma cohort, and 95 (47%) of 204 in the endometrial carcinoma cohort. Diploid chromatin homogeneous tumours were associated with longer cancer-specific survival than were non-diploid chromatin homogeneous tumours in all cohorts, whereas patients with chromatin heterogeneous tumours consistently had the worst survival (appendix p 22).
Discussion
In all six independent validation cohorts, across a range of tumour types, and in the discovery cohort, chromatin heterogeneity correlated with cancer-specific survival. This suggests that chromatin heterogeneity might be a novel pan-prognostic factor irrespective of tumour histogenesis, and could add value to the traditional TNM staging system. Nucleotyping provided independent prognostic information beyond most conventional markers in multivariable analyses. Our data permitted more in-depth analysis of stage II colorectal cancer, for which Nucleotyping was found to be weakly associated with other patient characteristics and predicted cancer-specific survival more accurately than microsatellite instability. However, much remains to be discovered of the biology underpinning chromatin heterogeneity and whether these descriptive changes are associated with specific drivers and carcinogenic pathways or are reflective of a relatively non-specific burden of accumulated DNA damage.
Beside the fundamental biological differences between the analysed cancer types, the images from the various cohorts were acquired using different sample preparation methods and imaging equipment, which cause notable dissimilarities between the images, particularly when using rigid analytical nomograms. It was thus imperative for the developed marker to be invariant to technical features of the images. The consistent prognostic ability of Nucleotyping across all cohorts empirically shows that the recently developed normalisation techniques successfully handled technical variations. Similarly, the pathologist who selected the tumour region and the bioengineers who prepared the samples differed between cohorts, thus the consistent validation results suggest that the marker is independent of individual human influence. This altogether indicates that the marker could easily be applied in other laboratories and still be expected to perform consistently, which is a necessary requirement for mainstream clinical application. We have begun the process of obtaining ISO certification for the Nucleotyping procedure.
Earlier methods for assessing aberrant chromatin organisation have been reported for the ovarian carcinoma,7 uterine sarcoma,8 prostate carcinoma,9 and endometrial carcinoma cohorts.10 The major difference from the present study is that in those studies, a different marker was developed in each cohort, which allowed adaption to the particular cancer type, sample preparation methods, and imaging equipment. None of the earlier developed markers are suitable for validation on external cohorts of different cancer types, and they were only validated internally in the same patient series used to develop the marker. We therefore find it remarkable that the generic marker developed in this study offered similar prognostic ability in analysis of ovarian and endometrial carcinoma and depicted the prognosis of uterine sarcoma and prostate carcinoma with only slightly less accuracy compared with the pooled analyses of the discovery and internal validation cohorts that were reported in those studies. It thus appears that the technical standardisation applied in the generic marker preserves prognostic information and that texture patterns identifying chromatin heterogeneity are essentially identical in most cancer types.
The proportion of patients with chromatin homogeneous and heterogeneous tumours who died of their cancer differed between cancer types, suggesting that appropriate use of the chromatin heterogeneity marker in the clinic might differ between clinical settings. The role of chromatin heterogeneity, as well as options for treatment and survival benefits, must therefore be investigated in conjunction with disease type and characteristics.
In stage II colorectal cancer, our finding of a somewhat improved survival in the QUASAR 2 cohort compared with the other two colorectal cancer cohorts perhaps reflects modern advances in chemotherapy and a more consistent application of adjuvant chemotherapy. The correlation between Nucleotyping and other patient characteristics were either weak or absent, and the similar prognostic ability in univariable and multivariable analysis of each colorectal cancer cohort further indicates that the correlations do not substantially affect Nucleotyping's ability to predict cancer-specific survival and might therefore be unimportant in practice. This could explain why the proportions of patients with chromatin homogeneous and heterogeneous tumours were similar in all three cohorts despite the fact that the QUASAR 2 series represents a different clinical setting than the discovery and Gloucester validation cohorts.
The investigators of original QUASAR trial found that adjuvant chemotherapy reduces the odds of tumour recurrence and death by about 20% in stage II colorectal cancer, leading to a 5-year absolute survival advantage of about 4% assuming that the 5-year mortality without chemotherapy is 20%.30 Microsatellite stability status is often used in clinical practice to identify a subgroup of patients with stage II colorectal cancer for whom adjuvant treatment can be omitted because these patients are at low risk of recurrence and would therefore have a small (around 2%) absolute benefit of adjuvant treatment.31, 32, 33 Our data suggest that patients with chromatin heterogeneous tumours have poor cancer-specific survival irrespective of microsatellite stability status, and the slight difference in mortality between microsatellite unstable and microsatellite stable tumours in patients with chromatin homogeneous tumours was not significant. Individually, the chromatin homogeneous subgroup and the microsatellite unstable subgroup were associated with equally good prognosis (5-year cancer-specific survival 89% for chromatin homogeneous tumours and 88% in microsatellite unstable tumours), but the chromatin homogeneous subgroup was several times larger (about 60% of patients with stage II colorectal cancer had chromatin homogeneous tumours vs 10–15% of patients who had microsatellite unstable tumours), thus allowing substantially more patients to be identified as low risk and with a small absolute benefit of adjuvant chemotherapy. Moreover, the patients with chromatin heterogeneous tumours have lower cancer-specific survival than patients with microsatellite stable tumours and could therefore be expected to have a greater absolute survival benefit from adjuvant treatment, assuming, reasonably, that the proportional benefits of chemotherapy remain constant over the different prognostic groups.
Our findings suggest that heterogeneous chromatin organisation is found in many cancer types and that its presence signifies increased risk of death due to cancer. Such generic applicability is a rare feature for a tumour biomarker that can be measured objectively using automated microscope systems. This assay might potentially be cost-effective as it could possibly reduce adjuvant overtreatment of patients who are at relatively low risk of recurrence and death, thus diminishing the burden of toxicity on patients treated unnecessarily and the direct costs of cancer therapy. Focusing or intensifying adjuvant treatment on those most at risk of death due to cancer is consistent with the wider precision medicine agenda in oncology.
The main limitation of this study is that it investigates the prognostic ability of chromatin heterogeneity but not the survival benefit and cost-effectiveness of utilising Nucleotyping to guide treatment decisions. However, we expect positive findings in this respect because of the preserved or enhanced accuracy of Nucleotyping in sensitivity analyses where patients who received adjuvant therapy were excluded, and our experience with the sample preparation method relative to the costs of assessing other markers. We also see a potential to improve the cost-benefit ratio by assessing chromatin heterogeneity using routine histological sections with a DNA-specific stain. Another limitation is that tumour heterogeneity was not considered except in prostate cancer where it was partly handled by analysing about three regions from each tumour; sampling the entire tumour should increase the prognostic accuracy of the marker.
In conclusion, our results indicate that chromatin heterogeneity behaves similarly in several human cancers and identifies patients at increased risk of cancer-specific death, independently of established prognostic markers, and could possibly aid clinical decision making around use of adjuvant or neoadjuvant therapy.
Acknowledgments
Acknowledgments
This study was funded by The Research Council of Norway, through its IKTPLUSS Lighthouse program (259204) and through its Centres of Excellence funding scheme (179571), the South-Eastern Norway Regional Health Authority (2012027 and 2015070), the National Institute for Health Research (to the Oxford NIHR Comprehensive Biomedical Research Centre), and the Wellcome Trust (to the Wellcome Trust Centre for Human Genetics, 090532/Z/09/Z). We thank Ragnhild A Lothe for her contribution to the colorectal cancer dataset from Aker University Hospital, Vera M Abeler for pathological review of the uterine sarcoma and ovarian carcinoma cohorts, Aud Svindland for pathological assessment of the colorectal carcinoma specimens in the discovery cohort, Paul Callaghan for animating the appendix video, Marian Seiergren for creating figure 2 and editing Figure 3, Figure 4, Figure 5, Wanja Kildal, Marna Lill Kjæreng, Elin Ersvær, John Arne Nesheim, Ilyá Kostolomov, Rolf Anders Syvertsen, and the laboratory personnel at the Institute for Cancer Genetics and Informatics for assistance, and the reviewers for valuable suggestions. We also thank the participating centres in the QUASAR 2 trial and the MoMaTEC trial as well as the staff at Aker University Hospital, west Gloucestershire, and the Norwegian Radium Hospital. Finally, we thank all participating patients for making this study possible.
Contributors
AK, FA, DJK, and HED were involved in study design. HAA, GBK, AN, JT, HW, NAS, and DJK collected the data. LV, MP, and NAS did the pathological evaluations. AK, FA, BN, TSH, IT, MN, DJK, and HED analysed and interpreted the data. AK wrote the first draft, and all authors reviewed, contributed to, and approved the manuscript.
Declaration of interests
The University of Oxford (to DJK) received an educational grant from Roche to support the QUASAR 2 trial. All other authors declare no competing interests.
Supplementary Material
References
- 1.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 2.Danielsen HE, Pradhan M, Novelli M. Revisiting tumour aneuploidy—the place of ploidy assessment in the molecular era. Nat Rev Clin Oncol. 2016;13:291–304. doi: 10.1038/nrclinonc.2015.208. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JR, Jung I, Selvaraj S. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster-Böckler B, Lehner B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature. 2012;488:504–507. doi: 10.1038/nature11273. [DOI] [PubMed] [Google Scholar]
- 5.Polak P, Karlić R, Koren A. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature. 2015;518:360–364. doi: 10.1038/nature14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen B, Albregtsen F, Danielsen HE. Statistical nuclear texture analysis in cancer research: a review of methods and applications. Crit Rev Oncog. 2008;14:89–164. doi: 10.1615/critrevoncog.v14.i2-3.10. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen B, Albregtsen F, Kildal W, Abeler VM, Kristensen GB, Danielsen HE. The prognostic value of adaptive nuclear texture features from patient gray level entropy matrices in early stage ovarian cancer. Anal Cell Pathol (Amst) 2012;35:305–314. doi: 10.3233/ACP-2012-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen B, Hveem TS, Kildal W. Entropy-based adaptive nuclear texture features are independent prognostic markers in a total population of uterine sarcomas. Cytometry A. 2015;87:315–325. doi: 10.1002/cyto.a.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hveem TS, Kleppe A, Vlatkovic L. Chromatin changes predict recurrence after radical prostatectomy. Br J Cancer. 2016;114:1243–1250. doi: 10.1038/bjc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hveem TS, Njølstad TS, Nielsen B. Changes in chromatin structure in curettage specimens identifies high-risk patients in endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:61–67. doi: 10.1158/1055-9965.EPI-16-0215. [DOI] [PubMed] [Google Scholar]
- 11.Merok MA, Ahlquist T, Røyrvik EC. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274–1282. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hveem TS, Merok MA, Pretorius ME. Prognostic impact of genomic instability in colorectal cancer. Br J Cancer. 2014;110:2159–2164. doi: 10.1038/bjc.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes' B colon cancer. Gut. 2002;51:65–69. doi: 10.1136/gut.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchard JR, Love SB, Baxter KJ, Shepherd NA. How important is peritoneal involvement in rectal cancer? A prospective study of 331 cases. Histopathology. 2010;57:671–679. doi: 10.1111/j.1365-2559.2010.03687.x. [DOI] [PubMed] [Google Scholar]
- 15.Kerr RS, Love S, Segelov E. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): an open-label, randomised phase 3 trial. Lancet Oncol. 2016;17:1543–1557. doi: 10.1016/S1470-2045(16)30172-3. [DOI] [PubMed] [Google Scholar]
- 16.Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 17.Wæhre H, Vlatkovic L, Cvancarova M, Paus E, Fosså SD, Danielsen HE. Fifteen-year mortality after radical prostatectomy: Which factors are available for patient counselling? Scand J Urol. 2014;48:123–130. doi: 10.3109/21681805.2013.817483. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Allsbrook WCJ, Amin MB, Egevad LL, the ISUP Grading Committee The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Srigley J, Grignon D, Humphrey P, Association of Directors of Anatomic and Surgical Pathology Recommendations for the reporting of prostate carcinoma. Am J Clin Pathol. 2008;129:24–30. doi: 10.1309/59U8R6N5R7BKCWLV. [DOI] [PubMed] [Google Scholar]
- 20.Cyll K, Ersvær E, Vlatkovic L. Tumour heterogeneity poses a significant challenge to cancer biomarker research. Br J Cancer. 2017;117:367–375. doi: 10.1038/bjc.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradhan M, Abeler VM, Danielsen HE, Tropé CG, Risberg BÅ. Image cytometry DNA ploidy correlates with histological subtypes in endometrial carcinomas. Mod Pathol. 2006;19:1227–1235. doi: 10.1038/modpathol.3800641. [DOI] [PubMed] [Google Scholar]
- 22.Cyll K, Callaghan P, Kildal W, Danielsen HE. Preparing for image based DNA ploidy. 2015. http://bit.ly/1OOnPV2 (accessed Sept 10, 2017).
- 23.Maddison J. University of Huddersfield; 2005. Digital image processing for prognostic and diagnostic clinical pathology. PhD thesis. [Google Scholar]
- 24.Yogesan K, Jørgensen T, Albregtsen F, Tveter KJ, Danielsen HE. Entropy-based texture analysis of chromatin structure in advanced prostate cancer. Cytometry. 1996;24:268–276. doi: 10.1002/(SICI)1097-0320(19960701)24:3<268::AID-CYTO10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen B, Albregtsen F, Danielsen HE. Low dimensional adaptive texture feature vectors from class distance and class difference matrices. IEEE Trans Med Imaging. 2004;23:73–84. doi: 10.1109/TMI.2003.819923. [DOI] [PubMed] [Google Scholar]
- 26.Raudys SJ, Jain AK. Small sample size effects in statistical pattern recognition: recommendations for practitioners. IEEE Trans Pattern Anal Mach Intell. 1991;13:252–264. [Google Scholar]
- 27.Michiels S, Koscielny S, Hill C. Prediction of cancer outcome with microarrays: a multiple random validation strategy. Lancet. 2005;365:488–492. doi: 10.1016/S0140-6736(05)17866-0. [DOI] [PubMed] [Google Scholar]
- 28.Punt CJA, Buyse M, Köhne C-H. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99:998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, Steyerberg EW, D'Agostino RB. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray R, Barnwell J, McConkey C. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 31.Ribic CM, Sargent DJ, Moore MJ. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr DJ, Midgley R. Defective mismatch repair in colon cancer: a prognostic or predictive biomarker? J Clin Oncol. 2010;28:3210–3212. doi: 10.1200/JCO.2010.28.9322. [DOI] [PubMed] [Google Scholar]
- 33.Hutchins G, Southward K, Handley K. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.