Abstract
Tumours are complex entities, wherein cancer cells interact with myriad soluble, insoluble and cell associated factors. These microenvironmental mediators regulate tumour growth, progression and metastasis, and are produced by cancer cells and by stromal components such as fibroblast, adipocytes and immune cells. Through their ability to bind to extracellular matrix proteins, cell surface receptors and growth factors, matricellular proteins enable a dynamic reciprocity between cancer cells and their microenvironment. Hence, matricellular proteins play a critical role in tumour progression by regulating where and when cancer cells are exposed to key growth factors and regulatory proteins. Recent studies suggest that, in addition to altering Wingless (Wnt) signalling, certain members of the Secreted Frizzled Related Protein (sFRP) family are matricellular in nature. In this review, we outline the importance of matricellular proteins in cancer, and discuss how sFRPs may function to both inhibit and promote cancer progression in a context-dependent manner. By considering the matricellular functionality of sFRPs, we may better understand their apparently paradoxical roles in cancers.
Keywords: secreted Frizzled-Related Proteins, matricellular, cancer, microenvironment, stroma
The tumour microenvironment
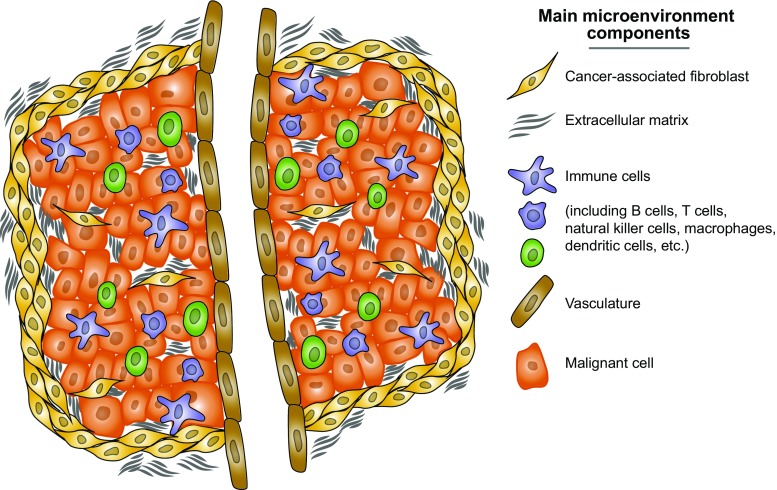
While tumours were historically thought of as insular masses of abnormally proliferative cells, it is becoming increasingly appreciated that this view is overly simplistic (Egeblad et al. 2010; Hanahan and Weinberg 2011). Instead, tumours are complex tissues that are composed of multiple distinct cell types, of which tumour cells are only one (Fig. 1). Within this complex ecosystem, these multiple cell types influence one another on a variety of levels. The tumour microenvironment is the entire cellular and acellular milieu in which the tumour exists. It consists of tumour vasculature, immune cells, fibroblasts, adipocytes, the resultant extracellular matrix and other biochemical macromolecules, as well as the physical properties of the environment itself (eg. pH and oxygen levels) (Polyak et al. 2009; Pietras and Östman 2010; Hanahan and Weinberg 2011; Quail and Joyce 2013). A complete understanding of tumourigenesis and successful therapeutic targeting will only be achieved when the entire microenvironment is taken into account.
Fig. 1.
Cellular components of tumour heterogeneity. Tumours involve the co-evolution of tumour cells with an extracellular matrix, and stromal, immune and endothelial cells. Taken together this forms a specialized tumour niche that supports neoplastic progression
Tumour stroma
During tumourigenesis, tumour cells recruit numerous normal cells, which together form tumour-associated stroma. This consists of various cell types including fibroblasts, endothelial cells, adipocytes and immune cells. These cell types have been shown to contribute to events critical in cancer progression, such as proliferation, loss of polarity, invasion, and angiogenesis (Olumi et al. 1999; Mueller and Fusenig 2004; Campbell et al. 2010; Mao et al. 2013; Quail and Joyce 2013; Taubenberger et al. 2016). In addition, given that the cellular components of the tumour stroma are genetically stable they provide appealing candidates for cancer therapies, with a decreased risk of acquired resistance. However, the tumour stroma has the capacity to both promote and obstruct tumourigenesis and specifically targeting the pro-tumourigenic ability is challenging. Understanding the interactions between tumour cells and stromal cells will be paramount to determining optimal therapeutic targets.
Cancer cells themselves can alter their adjacent stroma to produce a particular permissive environment for tumour progression, termed the “reactive” tumour stroma (Kalluri and Zeisberg 2006). Fibroblasts, the dominant cell type in connective tissue, fulfill a multi-functional role in normal tissue. Among other functions, they synthesize extracellular matrix and basement membrane components; play an important role in wound healing; and help mediate tissue homeostasis (Kalluri and Zeisberg 2006; Quail and Joyce 2013). However, when fibroblasts encounter a tumour microenvironment — complete with a range of tumour-derived growth factors and proteases (e.g. bFGF and PDGF) — they acquire an “activated phenotype” and are termed cancer-associated fibroblasts (CAFs) (Kalluri and Zeisberg 2006; Quail and Joyce 2013). In the tumour microenvironment, CAFs are present in abnormally high numbers and perform a pro-tumourigenic role distinct from their normal counterparts (Olumi et al. 1999; Quail and Joyce 2013). For example, in breast cancers, CAFs enhance the metastasis of pre-malignant and malignant mammary epithelial cells, whereas normal fibroblasts suppress this process (Dumont et al. 2013), highlighting the importance of tumour-stromal interactions in mediating tumour progression.
The origin of CAFs in human tumours remains unclear. Studies suggest that they are derived from normal fibroblasts in surrounding stroma, from pericytes, mesenchymal stem cells, or via transition from alternative cell types like endothelial cells (via endothelial-to-mesenchymal transition [EndMT]), or tumour cells themselves (via epithelial-to-mesenchymal transition [EMT]) (Petersen et al. 2003; Zeisberg et al. 2007; Marsh et al. 2013; Quail and Joyce 2013). Regardless of their source, once CAFs encounter the tumour microenvironment, they become activated by the various factors present. Many groups have noted the similarity of these activated CAFs with myofibroblasts in cancer with respect to their morphology and the expression of marker proteins (including α-SMA and FAP) (Dvorak 1986; Sugimoto et al. 2006; Orimo and Weinberg 2007). In turn, these activated CAFs provide an important source of secreted growth factors that support tumour progression (Marsh et al. 2013). These factors include vascular endothelial growth factor (VEGF), a growth factor critical in mediating angiogenesis and the molecular target of select cancer anti-angiogenesis therapies such as bevacizumab. In addition, CAFs play a critical role in depositing and remodeling the tumour-associated extracellular matrix through deposition of select proteins and production of proteases (Campbell et al. 2010). For example, stromal-derived matrix metalloproteinases (MMPs) activate cell-surface and ECM-bound growth factors, contributing to the pro-tumourigenic effects of stromal-tumour crosstalk (McCawley and Matrisian 2001; Egeblad and Werb 2002). Additionally, CAFs have been shown to mediate tumour-enhancing inflammation by producing pro-inflammatory factors that activate NF-κB signalling, demonstrating a further level of crosstalk between fibroblasts and immune cells (Erez et al. 2010).
Extracellular matrix
All cell types in a tumour contribute to the formation of the extracellular matrix and associated proteins in the extracellular space. This protein scaffold provides architectural support for cells, which adhere to the ECM via a variety of different receptors including integrins (Hynes and Naba 2012). However, in addition to acting as a scaffold, the ECM provides important biochemical cues that regulate critical cellular processes including survival, proliferation, migration and invasion (Hynes 2009). During tumour initiation, the resident ECM appears to initially obstruct tumourigenesis, but in later stages of tumourigenesis, the remodelled matrix has been shown to drive tumour progression. For example, long before the molecular details of the ECM were starting to be appreciated, excessive ECM deposition (termed “desmoplasia”) was recognized and used as a marker of poor prognosis (Anastassiades and Pryce 1974). More recently, a proteomics approach has demonstrated that primary tumours with different metastatic potential also differ in terms of molecular ECM composition, and that this difference is a result of proteins differentially produced by both tumour stromal cells and cancer cells (Naba et al. 2014). Despite its critical role in regulating tumourigenesis, the extracellular matrix remains poorly understood and provides an additional level for therapeutic intervention.
Matricellular proteins
While the majority of the protein component of the ECM fulfills a structural role, there exists a second group of non-structural ECM proteins, or “matricellular” proteins. The term was first introduced by the Bornstein group in 1995 to define a group of dynamically expressed proteins that have the ability to bind to both matrix proteins and cell surface receptors or secreted growth factors (Bornstein 1995). Thus providing a dynamic and bidirectional way for cells to communicate with their microenvironment. Founding members of this group include SPARC (secreted protein, acidic and rich in cysteine), thrombospondin (TSP1) and tenascin-C (TN-C) (Sage and Bornstein 1991; Bornstein 1995). Given their predominantly regulatory role, matricellular proteins tend to be especially prevalent in areas of tissue remodelling and during embryogenesis (Bornstein and Sage 2002; Sangaletti and Colombo 2008).
Unsurprisingly, many of these proteins have also been implicated in tumour development and progression, albeit with complex and often controversial roles (Sangaletti and Colombo 2008). An example of a matricelllular protein with a multifaceted role in tumour progression is thrombospondin-1 (TSP1). It is clear from the literature that the function of TSP1 in cancer depends on the presence of other factors, since both overexpression and knockdown of TSP1 in tumours has been shown to inhibit tumour growth (Sargiannidou et al. 2001; Sangaletti and Colombo 2008). The effects of TSP1 on angiogenesis are dependent on the proteolytic form and localization of TSP1, as well as the presence of other angiogenic factors such as bFGF (Sargiannidou et al. 2001; Sangaletti and Colombo 2008). Taraboletti et al. have shown that in the rabbit cornea model of angiogenesis, both the amino terminus and full-length molecule of TSP1 had a stimulatory effect, while the 140 kDa carboxy terminus had no effect on angiogenesis (Taraboletti et al. 2000). However, both the full-length molecule and the carboxy fragment inhibited the effects of bFGF-induced angiogenesis. This indicates that the potential angiogenic effects of this protein are dictated by the specifics of the local microenvironment – an environment that is being constantly remodelled and changed during tumour progression. Thus, the potential effects of this, and other matricellular proteins, can be hijacked during tumour progression to their more pro-tumourigenic states.
Indeed, this phenomenon has been observed for TSP1. During tumour formation, TSP1 is generally downregulated compared to associated normal tissues, likely due to its observable initial anti-angiogenic and anti-proliferative activity (Sargiannidou et al. 2001). However, it appears that many tumours adapt to this negative control, with high levels and sustained expression of TSP1 in the tumour stroma (Fontana et al. 2005). Furthermore, increased stromal expression of TSP1 has been correlated with reduced relapse-free survival in breast cancer and tumour progression in esophogeal squamous cell carcinomas (Oshiba et al. 1999; Fontana et al. 2005). A further example is SPARC during breast cancer development: adenoviral mediated overexpression of SPARC in MDA-MB-231 breast cancer cells decreases in vivo metastatic ability, whereas selection of MDA-MB-231 lung-specific metastatic subpopulations results in higher expression of SPARC, an upregulation which is required for lung-tropic metastasis (Minn et al. 2005; Koblinski et al. 2005). Taken together, this suggests that tumours downregulate the expression of certain matricellular proteins during initial transformation, but later during tumour progression they can become resistant to that same protein, and may even use it to progress towards metastasis.
Normalization of cancer stroma
Given this association of an abnormal stroma with tumour progression, returning or “normalizing” the stroma to its non-malignant derivatives has been suggested as a way to slow or even reverse cancer progression (Mueller and Fusenig 2004; Joyce 2005; Jain 2013). The converse has been shown to be possible: normal epidermal melanocytes exposed to an ECM conditioned by metastatic melanoma cells transiently acquire gene expression changes that mimic melanoma cells, as well as the ability to form vasculogenic networks (Seftor et al. 2005). However, the attempt to produce a reciprocal effect of reprogramming melanoma cells to a more “normal” melanocytic phenotype upon exposure to an ECM conditioned by normal melanocytes has been unsuccessful (Seftor et al. 2005; Postovit et al. 2006).
Cancer has been described as a problem of developmental biology, or “development undone”. As such, an embryonic microenvironment may have a greater capacity to normalize or differentiate cancer cells (Gerschenson et al. 1986; Postovit 2006). This potential was first demonstrated by Illmensee et al. in 1975 when they showed that malignant mouse teratocarcinoma cells could develop into normal tissues and generate normal mice when injected into developing blastocysts (Mintz and Illmensee 1975). In 1986, implantation of B16 melanoma cells into the embryonic skin of developing mice was shown to inhibit melanoma tumour formation (Gerschenson et al. 1986). More recently, an embryonic chick model was used to revert melanoma cells towards their cell-of-origin neural crest-derived phenotype (Kulesa et al. 2006). These transplanted melanoma cells were able to respond to their embryonic microenvironment and follow neural crest migration pathways. Ultimately they lost their tumourigenicity and were able to migrate to and populate structures such as the brachial arches and dorsal root. Taken together, these results confirm that cancer cells can be reprogrammed or “normalized” by exposure to an embryonic microenvironment, suggesting that factors unique to this environment can be used therapeutically.
Postovit et al. determined that human embryonic stem cell (hESC)-conditioned matrices, but not hESC-conditioned media, had the ability to reprogram melanoma cells (Postovit et al. 2006). This suggests that the hESC reprogramming factor(s) are preferentially deposited or activated by the extracellular matrix. In an attempt to elucidate the matrix factors involved in this, Hughes et al. used a mass spectrometry-based proteomics approach to categorize the components of a pluripotent stem cell matrix and identified sFRP1 and sFRP2 to be previously unknown but major constituents of this matrix (Hughes et al. 2012).
Secreted Frizzled-related proteins (sFRPs)
sFRPs form an evolutionary conserved family of secreted proteins that have been shown to modulate Wnt signalling in the extracellular space (Bovolenta et al. 2008). sFRPs are important in development, and have been shown to play integral roles in dorsoventral patterning, brain and retinal development, gastrulation and formation of epithelial structures (Piccolo et al. 1997; Houart et al. 2002; Lee et al. 2006a; Satoh 2006; Muraoka et al. 2006; Satoh et al. 2008; Matsuyama et al. 2009; Ruiz et al. 2009; Ploper et al. 2011; Kong et al. 2012). In vertebrates, sFRPs form a family of five proteins (sFRP1–5), which, based on sequence homology, are split into two subfamilies: sFRP1, 2, and 5; and sFRP3 and 4 (Yan et al. 2014). Structurally, sFRP proteins fold into two independent domains: (1) an N-terminal cysteine-rich domain (CRD), and (2) a C-terminal netrin-like domain (NTR) (Chong et al. 2002). Due to the sequence similarity of the sFRP CRD domain to the Frizzled receptors, sFRPs were immediately recognized for their potential to modulate Wnt signalling.
Wnt ligands compose a large family of secreted cysteine-rich, hydrophobic glycoproteins. Currently, there are 19 known mammalian Wnt ligands. Wnts activate signalling pathways by binding to one of ten known Frizzled receptors and, in some cases, other co-receptors (e.g. LRP-5, LRP-6, Ryk, ROR). Broadly speaking, Wnt signalling can be divided into β-catenin dependent (canonical) and β-catenin independent (non-canonical) pathways. However, there is extensive evidence suggesting considerable crosstalk among the canonical and non-canonical branches (Weidinger and Moon 2003; De 2011). Intricacies of these signalling mechanisms have been extensively reviewed in (Miller et al. 2000; Gordon and Nusse 2006; Angers and Moon 2009; Komiya and Habas 2014). Altogether, these signalling pathways have been shown to play central roles in cell survival, cell proliferation, cell fate determination, cell polarity and tissue patterning and unsurprisingly, dysregulation of these pathways has been determined to be a key event in the development of many types of cancer.
While sFRPs have the Frizzled CRD domain implicated in Wnt binding, they lack the Frizzled (Fz) transmembrane domain and associated apparatus to transduce downstream signalling events. Thus, through molecular mimicry of the Fz CRD, sFRPs were recognized for their potential to sequester Wnt ligands away from receptor complexes and ultimately antagonize Wnt signalling. Some of the first studies conducted on sFRPs investigated this likelihood: in Xenopus embryos, ectopic expression of sFRP3/Frzb, normally localized to Spemann’s organizer, was able to prevent axis duplication of co-injected Wnt1, Wg and XWnt-8 (though not XWnt-3A) (Finch et al. 1997). Furthermore, biochemical analyses determined that sFRPs were able to bind Wnts when either protein was artificially bound to the plasma membrane (Leyns et al. 1997; Wang et al. 1997; Rattner et al. 1997; Finch et al. 1997; Lin et al. 1997). Since then, similar Wnt antagonistic effects have been observed for sFRPs in a plethora of contexts, suggesting that sFRPs can act as competitive inhibitors of Wnt-Fz binding.
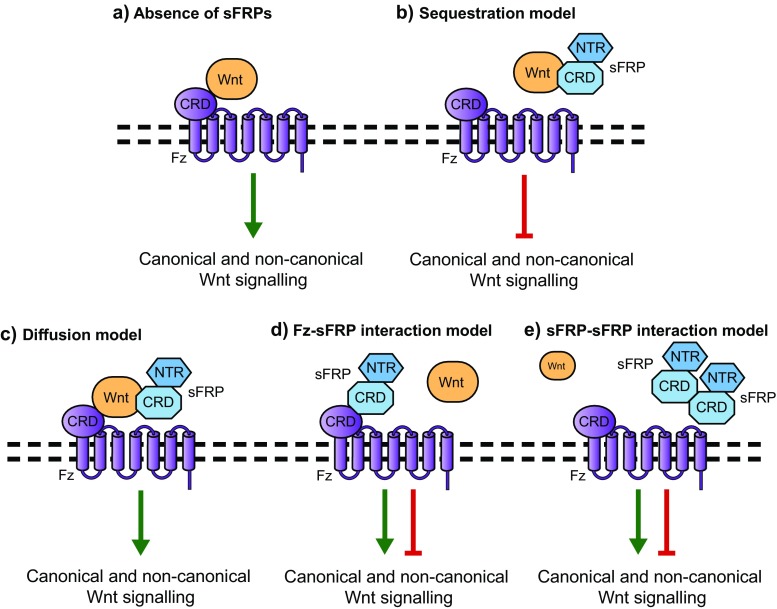
While the most popular model of sFRP-Wnt interaction remains inhibition through Wnt sequestration, other types of Wnt interactions have been proposed (Fig. 2). In Xenopus, sFRP3/Frzb and Crescent can activate canonical Wnt signalling by promoting the diffusion of Wnt8 and Wnt11 (Mii and Taira 2009). Thus, sFRPs may act as carriers for Wnt proteins in the extracellular space, expanding the range of Wnt signalling. Further models were proposed when crystal structures of the CRDs from mouse Fz8 and Sfrp3 revealed the potential for their CRDs to dimerize (Dann et al. 2001). This was supported by multiple groups who determined that sFRPs and Fz proteins have the ability to form homo- and hetero-meric complexes (Bafico 1999; Rodriguez et al. 2005). Given evidence for the direct interaction with Fzs, sFRPs may augment Wnt signalling by mimicking the Wnt ligands themselves. This model has been substantiated by various studies that have shown sFRPs can enhance Wnt signalling (Rodriguez et al. 2005; Yokota et al. 2008; Kress et al. 2009; Marschall von and Fisher 2010). In HEK293 and HSG cells, sFRP2 was shown to augment Wnt3a signalling, likely at the level of ligand/receptor interaction (Marschall von and Fisher 2010). In mouse intestine, sFRP2 was shown to activate β-catenin signalling and mechanistic studies implicated the direct interaction between sFRP2-Fz4/7 in this effect (Kress et al. 2009). Studies have also shown that sFRPs can have a biphasic effect on Wnt signalling: increasing β-catenin protein levels at low sFRP concentrations, but inhibiting it at high concentrations (Bhat et al. 2007). Another possible mechanism leading to enhanced Wnt signalling involves the interaction and antagonization of sFRPs by each other. Evidence for this model occurs in rat renal organogenesis, where sFRP1 and sFRP2 appear to compete locally to regulate Wnt signalling; with sFRP2 having an inhibitory effect on sFRP1-induced events but no independent effects (Yoshino et al. 2001). Deciphering the effects of sFRPs on Wnt signalling is further complicated by potential crosstalk between the different branches of the Wnt signalling pathway and the local complement of the Wnt pathway components themselves. Taken together, this suggests the effects of sFRP family members on Wnt signalling to be highly dynamic and dependent on the local molecular context.
Fig. 2.
Possible mechanisms by which sFRPs could modulate Wnt signalling. (a) In the absence of sFRPs, Wnt ligands are free to interact with Fz receptors and activate downstream Wnt signaling. (b) sFRPs can sequester Wnt ligands through the CRD or NTR domain, thereby acting as a classical Wnt antagonist. (c) sFRPs may bind Wnt ligands, and aid in their stability and diffusion in the extracellular space. (d) sFRPs may bind Fz receptors, and form signalling active or inactive complexes. (e) sFRPs interact with each other in the extracellular space and modulate one another’s activity
It is also becoming increasingly appreciated that the effects of sFRPs are not restricted to their impact on Wnt signalling; in multiple systems, sFRPs have been shown to modulate BMP, ERK, Hedgehog and TGFβ signalling (Yabe 2003; Lee et al. 2006a; Kobayashi et al. 2008; Scardigli et al. 2008; Nathan and Tzahor 2009; Esteve et al. 2011; Stuckenholz et al. 2013; Qu et al. 2013; Ma et al. 2015). Moreover, sFRPs can interact with a variety of non-Wnt ligands and receptors, as well as other matrix molecules. For example they can bind to other growth factors (e.g. EGF), and can act as proteinase inhibitors (Scardigli et al. 2008; Esteve et al. 2011; Mastri et al. 2014). Finally, sFRPs have been shown to play critical roles in regulating matrix remodelling and fibrosis – to the extent where sFRP2 inhibition is being investigated as a potential therapy to mitigate pathological fibrosis (Mastri et al. 2014).
sFRPs in cancer
SFRP1 was initially implicated in tumourigenesis when expression was found to be downregulated by loss of heterozygosity or promoter methylation in breast and colorectal cancer cell lines (Ugolini et al. 1999; Suzuki et al. 2002). Given their recognized ability to antagonize Wnt signalling — a pathway whose overactivity is generally thought to contribute to tumourigenesis — and their observed downregulation, SFRPs were initially described as tumour suppressor genes. Many studies in a variety of cancers have supported this proposed functional role (reviewed in Surana et al. 2014). Moreover, methylation of SFRP promoter regions is currently being investigated for clinical utility as a cancer biomarker (Harada et al. 2014; Liu et al. 2015; Andresen et al. 2015; Amornpisutt et al. 2015).
However, evidence is accumulating that sFRPs can contribute to tumour progression in certain contexts. sFRP1 is highly expressed in basal-like breast cancers and is associated with brain-specific metastases (Smid et al. 2008). In canine mammary gland tumours, sFRP2 was found to be overexpressed and induced cancerous transformation in normal mammary epithelial cells. In this case, sFRP2 interacted with a fibronectin-integrin extracellular matrix protein complex, and this association was shown to block apoptosis (Lee et al. 2004a; Lee et al. 2004b; Lee et al. 2006b). In renal cancer, both sFRP1 and sFRP2 have shown oncogenic potential, by increasing cellular invasion or proliferation and in vivo tumour growth (Saini et al. 2009; Yamamura et al. 2010). In gastric cancer, high sFRP1 expression correlates with poor patient prognosis and induces proliferative and invasive phenotypes. Deciphering the pro- and anti-tumourigenic abilities of these complex matricellular proteins is likely confounded by the local context, the particular sFRP of interest and the unknown impact of the NTR domain.
Additionally, unraveling the context-specific effects of sFRPs is likely dependent on the entire tumour microenvironment. As a component of the extracellular matrix, sFRPs have the potential to be deposited by any number of cell types present, and in addition, their effects are likely mediated by this entire environmental molecular context. Thus, completely modelling this environment is a particularly important, but difficult task.
sFRPs as matricellular proteins
Stromal-derived and matrix proteins have been shown to play critical roles in cancer progression (reviewed in (Quail and Joyce 2013)). Matricellular proteins are dynamic non-structural proteins in the extracellular matrix that have demonstrated importance in cancer progression, albeit in a complex and content-specific manner (Sage and Bornstein 1991; Bornstein 1995; Bornstein and Sage 2002; Sangaletti and Colombo 2008; Campbell et al. 2010). Founding members of this group of proteins include SPARC and thrombospondin-1 (TSP1). Though secreted Frizzled-related proteins have classically been recognized as secreted or soluble Wnt antagonists (Kawano and Kypta 2003; Cruciat and Niehrs 2013), we posit that they fulfill a more complex role as matricellular proteins. Initial work by Hughes et al. using a mass spectrometry-based approach to characterize the human embryonic stem cell conditioned matrix led to the discovery of abundant sFRPs in this depositome (Hughes et al. 2012). As well, sFRPs have many of the defining characteristics of matricellular proteins, including: (1) secretion by diverse types of cells (Leimeister et al. 1998; Heller et al. 2002; Yam et al. 2005; Satoh 2006; Ehrlund et al. 2013); (2) association with the extracellular matrix (Lee et al. 2006b; Martin-Manso et al. 2011; Hughes et al. 2012); (3) high prevalence in areas of tissue remodeling (Alfaro et al. 2008; Zhang et al. 2009; Alfaro et al. 2010; Mastri et al. 2014); and (4) importance in development (Leimeister et al. 1998; Yoshino et al. 2001; Satoh 2006; Satoh et al. 2008; Warr et al. 2009; Misra and Matise 2010). We anticipate that future studies will continue to reveal the matricellular nature of the sFRP family of proteins.
As such, it is highly likely that sFRPs fulfill complex roles in tumour development and progression, and that accurately modelling them in vitro will be challenging. Two separate recent studies on sFRP2 in melanoma depict a clear example of the importance of accurately considering and accounting for the microenvironment in cancer progression. The first study by Luo et al. looked at sFRP2 expression in tumour samples compared to normal skin, and melanoma cell lines compared to a non-transformed melanocyte cell line (Luo et al. 2016). They determined that sFRP2 expression was lower in cancer samples than associated normal samples, and that these expression differences were dictated by promoter methylation. Furthermore, they found that treating cells with 5-Azacytidine induced sFRP2 expression and suppressed cellular invasion, and concluded that sFRP2 inhibits melanoma pathogenesis. By contrast, Kaur et al. determined that sFRP2 was expressed by aged fibroblasts in a melanoma microenvironment, and that this expression helped drive melanoma metastasis, therapy resistance and poor outcomes in elderly patients (Kaur et al. 2016). This study incorporated models that accounted for matrix-sFRP2 interactions, such as reconstructed skin models. When they included these complex models, they implicated SFRP2 as factor contributing to poor patient outcomes, the exact opposite conclusion from the Luo et al. study.
We recently characterized the context-specific associations of SFRPs in over 8000 tumour samples from 29 different cancers (Vincent and Postovit 2017) and found that SFRP2 and 4 strongly associate with stromal, EMT and angiogenic signatures. Furthermore, their expression often associated with poor patient outcomes, distinct from their proposed tumour suppressive roles. Recent mechanistic and functional studies also support the stromal production of sFRP2 and 4. For example, in January of 2016, Sun et al. found that sFRP2 was one of the top genes that was induced in tumour stroma after genotoxic treatments (Sun et al. 2016). Instead of antagonizing the Wnt signalling pathway, they showed that in this capacity, sFRP2 augmented β-catenin signalling initiated by Wnt16B. In a separate study in ovarian cancer, high expression of sFRP2 in the stromal compartment, independent of malignant cell production, was associated with poor patient survival (Yeung et al. 2014). Clearly, these stromal-derived proteins are important in tumour progression, and future studies modelling them in relevant extracellular matrix systems will help elucidate their functional role.
Conclusion
Tumours are not insular masses of proliferating cells; rather tumour cells evolve within a complex local environment complete with matrix, stromal, and immune components. Therefore, successful tumour growth and eventual metastasis is not determined solely by tumour cells themselves, but also by the fitness advantage or disadvantage conferred by their microenvironment. Matricellular proteins are non-structural components of the extracellular matrix that are integral to cancer development and progression. We propose that secreted Frizzled-related proteins display key characteristics of matricellular proteins and should be considered as such. In cancer, sFRPs are often supplied by tumour stroma and are associated with pro- or anti-tumourigenic phenotypes in a context- and cancer-specific manner. Given that the cellular components of the tumour stroma are genetically stable, they provide appealing candidates for cancer therapies.
Acknowledgements
This work was supported by an Alberta Innovates Health Solutions Translational Health Chair in cancer and a Canadian Breast Cancer Foundation operating grant awarded to LMP. LMP was the recipient of the Peter-Lougheed Premier New Investigator Award from the Canadian Institutes of Health Research. KMV is a Vanier Scholar.
Contributor Information
Krista Marie Vincent, Email: kvincent@ualberta.ca.
Lynne-Marie Postovit, Email: postovit@ualberta.ca.
References
- Alfaro MP, Pagni M, Vincent A, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro MP, Vincent A, Saraswati S, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem. 2010;285:35645–35653. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amornpisutt R, Proungvitaya S, Jearanaikoon P, Limpaiboon T. DNA methylation level of OPCML and SFRP1: a potential diagnostic biomarker of cholangiocarcinoma. Tumor Biol. 2015;36:4973–4978. doi: 10.1007/s13277-015-3147-2. [DOI] [PubMed] [Google Scholar]
- Anastassiades OT, Pryce DM. Fibrosis as in indication of time in infiltrating breast cancer and its importance in prognosis. Br J Cancer. 1974;29:232–239. doi: 10.1038/bjc.1974.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen K, Boberg KM, Vedeld HM, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61:1651–1659. doi: 10.1002/hep.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Bafico A. Interaction of Frizzled Related Protein (FRP) with Wnt Ligands and the Frizzled Receptor Suggests Alternative Mechanisms for FRP Inhibition of Wnt Signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- Bhat RA, Stauffer B, Komm BS, Bodine PVN. Structure-function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J Cell Biochem. 2007;102:1519–1528. doi: 10.1002/jcb.21372. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, et al. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Campbell NE, Kellenberger L, Greenaway J, et al. Extracellular Matrix Proteins and Tumor Angiogenesis. J Oncol. 2010;2010:1–13. doi: 10.1155/2010/586905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JM, Uren A, Rubin JS, Speicher DW. Disulfide bond assignments of secreted Frizzled-related protein-1 provide insights about Frizzled homology and netrin modules. J Biol Chem. 2002;277:5134–5144. doi: 10.1074/jbc.M108533200. [DOI] [PubMed] [Google Scholar]
- Cruciat C-M, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, Hsieh J-C, Rattner A, et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sinica. 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- Dumont N, Liu B, Defilippis RA, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013;15:249–262. doi: 10.1593/neo.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: Wounds That Do Not Heal. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z. Tumors as Organs: Complex Tissues that Interface with the Entire Organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlund A, Mejhert N, Lorente-Cebrián S, et al. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab. 2013;98:E503–E508. doi: 10.1210/jc.2012-3416. [DOI] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-κB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Esteve P, Sandonìs A, Cardozo M, et al. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci. 2011;14:562–569. doi: 10.1038/nn.2794. [DOI] [PubMed] [Google Scholar]
- Finch PW, He X, Kelley MJ, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci U S A. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Filleur S, Guglielmi J, et al. Human breast tumors override the antiangiogenic effect of stromal thrombospondin-1 in vivo. Int J Cancer. 2005;116:686–691. doi: 10.1002/ijc.20584. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Graves K, Carson SD, et al. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci U S A. 1986;83:7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harada T, Yamamoto E, Yamano H-O, et al. Analysis of DNA methylation in bowel lavage fluid for detection of colorectal cancer. Cancer Prev Res (Phila) 2014;7:1002–1010. doi: 10.1158/1940-6207.CAPR-14-0162. [DOI] [PubMed] [Google Scholar]
- Heller RS, Dichmann DS, Jensen J, et al. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225:260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, et al. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/S0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Hughes C, Radan L, Chang WY, et al. Mass spectrometry-based proteomic analysis of the matrix microenvironment in pluripotent stem cell culture. Mol Cell Proteomics. 2012;11:1924–1936. doi: 10.1074/mcp.M112.020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Naba A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kaur A, Webster MR, Marchbank K, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532:250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, et al. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol. 2008;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinski JE, Kaplan-Singer BR, VanOsdol SJ, et al. Endogenous osteonectin/SPARC/BM-40 expression inhibits MDA-MB-231 breast cancer cell metastasis. Cancer Res. 2005;65:7370–7377. doi: 10.1158/0008-5472.CAN-05-0807. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organ. 2014;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Yang Y, Zhang T, et al. Characterization of sFRP2-like in amphioxus: insights into the evolutionary conservation of Wnt antagonizing function. Evol Dev. 2012;14:168–177. doi: 10.1111/j.1525-142X.2012.00533.x. [DOI] [PubMed] [Google Scholar]
- Kress E, Rezza A, Nadjar J, et al. The Frizzled-related sFRP2 Gene Is a Target of Thyroid Hormone Receptor 1 and Activates β-Catenin Signaling in Mouse Intestine. J Biol Chem. 2009;284:1234–1241. doi: 10.1074/jbc.M806548200. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Park S-J, Abraham SC, et al. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646–4654. doi: 10.1038/sj.onc.1207588. [DOI] [PubMed] [Google Scholar]
- Lee J-L, Lin C-T, Chueh L-L, Chang C-J. Autocrine/paracrine secreted Frizzled-related protein 2 induces cellular resistance to apoptosis: a possible mechanism of mammary tumorigenesis. J Biol Chem. 2004;279:14602–14609. doi: 10.1074/jbc.M309008200. [DOI] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic Dorsal-Ventral Signaling: Secreted Frizzled-Related Proteins as Inhibitors of Tolloid Proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-L, Chang C-J, Chueh L-L, Lin C-T. Secreted frizzled related protein 2 (sFRP2) decreases susceptibility to UV-induced apoptosis in primary culture of canine mammary gland tumors by NF-κB activation or JNK suppression. Breast Cancer Res Treat. 2006;100:49–58. doi: 10.1007/s10549-006-9233-9. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Bach A, Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech Dev. 1998;75:29–42. doi: 10.1016/S0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, et al. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/S0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Wang S, Julius MA, et al. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci U S A. 1997;94:11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li N, Lu H, et al. Circulating SFRP1 promoter methylation status in gastric adenocarcinoma and esophageal square cell carcinoma. Biomed Rep. 2015;3:123–127. doi: 10.3892/br.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Wei B, Chen A, et al. Methylation-mediated loss of SFRP2 enhances melanoma cell invasion via Wnt signaling. Am J Transl Res. 2016;8:1502–1509. [PMC free article] [PubMed] [Google Scholar]
- Ma J, Cheng J, Gong Y, et al. Downregulation of Wnt signaling by sonic hedgehog activation promotes repopulation of human tumor cell lines. Dis Model Mech. 2015;8:385–391. doi: 10.1242/dmm.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Keller ET, Garfield DH, et al. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall von Z, Fisher LW. Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem Biophys Res Commun. 2010;400:299–304. doi: 10.1016/j.bbrc.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832:1070–1078. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Manso G, Calzada MJ, Chuman Y, et al. sFRP-1 binds via its netrin-related motif to the N-module of thrombospondin-1 and blocks thrombospondin-1 stimulation of MDA-MB-231 breast carcinoma cell adhesion and migration. Arch Biochem Biophys. 2011;509:147–156. doi: 10.1016/j.abb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastri M, Shah Z, Hsieh K, et al. Secreted Frizzled-related protein 2 as a target in antifibrotic therapeutic intervention. Am J Physiol, Cell Physiol. 2014;306:C531–C539. doi: 10.1152/ajpcell.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama M, Aizawa S, Shimono A. Sfrp Controls Apicobasal Polarity and Oriented Cell Division in Developing Gut Epithelium. PLoS Genet. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/S0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene. 2000;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Matise MP. A critical role for sFRP proteins in maintaining caudal neural tube closure in mice via inhibition of BMP signaling. Dev Biol. 2010;337:74–83. doi: 10.1016/j.ydbio.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, et al. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Lamar JM, et al. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. elife. 2014;3:e01308. doi: 10.7554/eLife.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan E, Tzahor E. sFRPs: a declaration of (Wnt) independence. Nat Cell Biol. 2009;11:13–13. doi: 10.1038/ncb0109-13. [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Heterogeneity of Stromal Fibroblasts in Tumors. Cancer Biol Ther. 2007;6(4):6180619. doi: 10.4161/cbt.6.4.4255. [DOI] [PubMed] [Google Scholar]
- Oshiba G, Kijima H, Himeno S, et al. Stromal thrombospondin-1 expression is correlated with progression of esophageal squamous cell carcinomas. Anticancer Res. 1999;19:4375–4378. [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, et al. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/S0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K, Östman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Ploper D, Lee HX, De Robertis EM. Dorsal-ventral patterning: Crescent is a dorsally secreted Frizzled-related protein that competitively inhibits Tolloid proteases. Dev Biol. 2011;352:317–328. doi: 10.1016/j.ydbio.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Postovit LM. Influence of the Microenvironment on Melanoma Cell Fate Determination and Phenotype. Cancer Res. 2006;66:7833–7836. doi: 10.1158/0008-5472.CAN-06-0731. [DOI] [PubMed] [Google Scholar]
- Postovit L-M, Seftor EA, Seftor REB, Hendrix MJC. A Three-Dimensional Model to Study the Epigenetic Effects Induced by the Microenvironment of Human Embryonic Stem Cells. Stem Cells. 2006;24:501–505. doi: 10.1634/stemcells.2005-0459. [DOI] [PubMed] [Google Scholar]
- Qu Y, Ray PS, Li J, et al. High levels of secreted frizzled-related protein 1 correlate with poor prognosis and promote tumourigenesis in gastric cancer. Eur J Cancer. 2013;49:3718–3728. doi: 10.1016/j.ejca.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Esteve P, Weinl C, et al. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci. 2005;8:1301–1309. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- Ruiz JM, Rodriguez J, Bovolenta P. Growth and differentiation of the retina and the optic tectum in the medaka fish requires ol Sfrp5. Devel Neurobio. 2009;69:617–632. doi: 10.1002/dneu.20731. [DOI] [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Saini S, Liu J, Yamamura S, et al. Functional significance of secreted Frizzled-related protein 1 in metastatic renal cell carcinomas. Cancer Res. 2009;69:6815–6822. doi: 10.1158/0008-5472.CAN-09-1254. [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Colombo MP. Matricellular proteins at the crossroad of inflammation and cancer. Cancer Lett. 2008;267:245–253. doi: 10.1016/j.canlet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Sargiannidou I, Zhou J, Tuszynski GP. The role of thrombospondin-1 in tumor progression. Exp Biol Med (Maywood) 2001;226:726–733. doi: 10.1177/153537020222600803. [DOI] [PubMed] [Google Scholar]
- Satoh W. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133:989–999. doi: 10.1242/dev.02274. [DOI] [PubMed] [Google Scholar]
- Satoh W, Matsuyama M, Takemura H, et al. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46:92–103. doi: 10.1002/dvg.20369. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Gargioli C, Tosoni D, et al. Binding of sFRP-3 to EGF in the Extra-Cellular Space Affects Proliferation, Differentiation and Morphogenetic Events Regulated by the Two Molecules. PLoS One. 2008;3:e2471. doi: 10.1371/journal.pone.0002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor EA, Brown KM, Chin L. Epigenetic Transdifferentiation of Normal Melanocytes by a Metastatic Melanoma Microenvironment. Cancer Res. 2005;65:10164–10169. doi: 10.1158/0008-5472.CAN-05-2497. [DOI] [PubMed] [Google Scholar]
- Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- Stuckenholz C, Lu L, Thakur PC, et al. Sfrp5 Modulates Both Wnt and BMP Signaling and Regulates Gastrointestinal Organogensis in the Zebrafish, Danio rerio. PLoS One. 2013;8:e62470. doi: 10.1371/journal.pone.0062470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of Fibroblast Heterogeneity in the Tumor Microenvironment. Cancer Biol Ther. 2006;5(12):1620–1626. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhu D, Chen F et al (2016) SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene. doi:10.1038/onc.2015.494 [DOI] [PMC free article] [PubMed]
- Surana R, Sikka S, Cai W, et al. Secreted frizzled related proteins: Implications in cancers. Biochim Biophys Acta. 2014;1845:53–65. doi: 10.1016/j.bbcan.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Gabrielson E, Chen W, et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Morbidelli L, Donnini S, et al. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB J. 2000;14:1674–1676. doi: 10.1096/fj.99-0931fje. [DOI] [PubMed] [Google Scholar]
- Taubenberger AV, Bray LJ, Haller B, et al. 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments. Acta Biomater. 2016;36:73–85. doi: 10.1016/j.actbio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Ugolini F, Adélaïde J, Charafe-Jauffret E, et al. Differential expression assay of chromosome arm 8p genes identifies Frizzled-related (FRP1/FRZB) and Fibroblast Growth Factor Receptor 1 (FGFR1) as candidate breast cancer genes. Oncogene. 1999;18:1903–1910. doi: 10.1038/sj.onc.1202739. [DOI] [PubMed] [Google Scholar]
- Vincent KM, Postovit L-M. A pan-cancer analysis of secreted Frizzled-related proteins: re-examining their proposed tumour suppressive function. Sci Rep. 2017;7:42719. doi: 10.1038/srep42719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, et al. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/S0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Warr N, Siggers P, Bogani D, et al. Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol. 2009;326:273–284. doi: 10.1016/j.ydbio.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Moon RT. When Wnts antagonize Wnts. J Cell Biol. 2003;162:753–755. doi: 10.1083/jcb.200307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- Yam JWP, Chan KW, Ngan ESW, Hsiao WLW. Genomic structure, alternative splicing and tissue expression of rFrp/sFRP-4, the rat frizzled related protein gene. Gene. 2005;357:55–62. doi: 10.1016/j.gene.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Kawakami K, Hirata H, et al. Oncogenic functions of secreted Frizzled-related protein 2 in human renal cancer. Mol Cancer Ther. 2010;9:1680–1687. doi: 10.1158/1535-7163.MCT-10-0012. [DOI] [PubMed] [Google Scholar]
- Yan J, Jia H, Ma Z, et al. The evolutionary analysis reveals domain fusion of proteins with Frizzled-like CRD domain. Gene. 2014;533:229–239. doi: 10.1016/j.gene.2013.09.083. [DOI] [PubMed] [Google Scholar]
- Yeung T-L, Leung CS, Wong K-K, Mok SC. Identification and characterization of stromal factors with clinical significance in the ovarian tumor microenvironment. Cancer Res. 2014;74:4799–4799. doi: 10.1158/1538-7445.AM2014-4799. [DOI] [Google Scholar]
- Yokota T, Oritani K, Garrett KP, et al. Soluble frizzled-related protein 1 is estrogen inducible in bone marrow stromal cells and suppresses the earliest events in lymphopoiesis. J Immunol. 2008;181:6061–6072. doi: 10.4049/jimmunol.181.9.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino K, Rubin JS, Higinbotham KG, et al. Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev. 2001;102:45–55. doi: 10.1016/S0925-4773(01)00282-9. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta S, Xie L, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Deb A, Zhang Z, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370–377. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]