Abstract
Connective tissue growth factor (CTGF/CCN2) has strong inflammatory and profibrotic activities. Its expression is enhanced in skeletal muscular dystrophies such as Duchenne muscular dystrophy (DMD), a myopathy characterized by exacerbated inflammation and fibrosis. In dystrophic tissue, necrotic-regenerative foci, myofibroblasts, newly-regenerated muscle fibers and necrosis all occur simultaneously. To determine if CCN2 is involved in the appearance of the foci, we studied their presence and characteristics in mdx mice (DMD mouse model) compared to mdx mice hemizygous for CCN2 (mdx-Ccn2+/−). We used laser capture microdissection followed by gene expression and immunofluorescence analyses to investigate fibrotic, inflammation and regeneration markers in damaged and non-damaged areas in mdx and mdx-Ccn2+/− skeletal muscle. Mdx mice foci express elevated mRNAs levels of transforming growth factor type beta, collagen, fibronectin, the myofribroblast marker α-SMA, and the myogenic transcription factor myogenin. Mdx foci also show elevated levels of MCP-1 and CD-68 positive cells, indicating that CCN2 could be inducing an inflammatory response. We found a significant reduction in the number of foci in mdx-Ccn2+/− mice muscle. Fibrotic and inflammatory markers were also decreased in these foci. We did not observe any difference in Pax7 mRNA levels, a marker for satellite cells, in mdx mice compared to mdx-Ccn2+/− mice. Thus, CCN2 appears to be involved in the fibrotic response as well as in the inflammatory response in the dystrophic skeletal muscle.
Keywords: CTGF/CCN2, Muscular dystrophy, Fibrosis, Inflammation, Necrotic-regenerative focus
Introduction
Fibrotic disorders are characterized by excessive connective tissue and extracellular matrix (ECM) deposition that precludes normal healing and regeneration. Over-expression of pro-fibrotic factors, such as transforming growth factor type-β (TGF-β) (Pohlers et al. 2009) and connective tissue growth factor (CTGF/CCN-2) (Kubota and Takigawa 2015), are strongly linked to the pathogenesis of these diseases. For example, congestive heart failure, end-stage kidney disease, liver cirrhosis, pulmonary fibrosis and muscular dystrophies (Kubota and Takigawa 2015) all involve the abnormal formation of scar tissue. Scar formation includes the replacement of healthy tissue with α-smooth muscle actin (α-SMA)-positive myofibroblasts and the excessive accumulation of ECM proteins, such as collagen and fibronectin, which ultimately leads to organ failure and death (Contreras et al. 2016; Wynn 2008; Wynn and Ramalingam 2012).
Duchenne muscular dystrophy (DMD) is caused by the absence of the protein dystrophin, which causes defective connections between the cytoskeleton of muscle fibers and the surrounding extracellular matrix. In DMD patients and mdx mice (a murine model of DMD) this defect leads to increased vulnerability of muscle fibers and, through cycles of degeneration and imperfect regeneration, a progressive decrease in muscle mass, diminished muscle force, and fibrosis (Fadic et al. 2006; Porter et al. 2004). The accumulation of extracellular matrix that replaces normal tissue leads to severe impairment of the regenerative capacity of the skeletal muscle. Both TGF-β and CCN2 are known to be over-expressed in this disease (Bernasconi et al. 1999; Morales et al. 2013b; Sun et al. 2008). CCN2 expression is induced by TGF-β in skeletal muscle cells and increases the synthesis of ECM molecules in myoblasts, exerting an inhibitory effect on skeletal muscle differentiation and causing myoblast dedifferentiation (Vial et al. 2008).
We have shown that over-expression of CCN2 is directly involved in the induction of fibrosis in normal muscle, concomitant with inflammation (Morales et al. 2011). Additionally, the systemic administration of CCN2 has been reported to induce a marked increase of inflammatory cells in the renal interstitium, which leads to elevated renal NF-κB activity (Sanchez-Lopez et al. 2009). Moreover, the dystrophic phenotype of the mdx mice is significantly ameliorated when CCN2 is inhibited by injection of monoclonal antibodies against this factor (Morales et al. 2013b). Similar improvement was also observed in mdx-Ccn2+/− mice. In both cases, the animals showed improvement in exercise resistance tests, increased strength in isolated muscles and decreased fibrosis (Morales et al. 2013b).
The absence of dystrophin in mdx mice, as in DMD patients, produces muscle contraction-induced damage in the sarcolemma, which causes necrosis of the muscle fiber (Fargas et al. 2002). Between the third week and third month of life, the mdx skeletal muscle shows high numbers of different types of degenerative-regenerative fiber groups that can be categorized by the sequential expression of known key genes involved in the muscle regeneration phases (Roig-Quilis et al. 2004; Roig et al. 2004). This is an indication of the asynchronous process of skeletal muscle-degeneration-regeneration, characteristic of this animal model (Marotta et al. 2007).
Since CCN2 has important pro-inflammatory and pro-fibrotic properties, we decided to evaluate how CCN2 levels affect the necrotic-regenerative processes. Using Laser capture microdissection (LCM) we found elevated levels of myogenic, fibrotic and inflammatory mRNAs in the necrotic-regenerative foci compared to non-damaged areas. We also observed that diminished CCN2 activity in the mdx-Ccn2 +/− model leads to significant decrease in the number of these foci and reduced mRNA levels of fibrotic markers. Regeneration was analyzed by quantifying the expression of embryonic myosin, and we found that this marker is also lower in damaged areas of mdx-Ccn2+/− skeletal muscle compared to mdx- Ccn2+/+. These results indicate that diminished CCN2 activity does not only reduce the number of necrotic-regenerative foci, but it also decreases the severity of damage within these foci. In addition, mRNA levels of MCP1, the chemokine monocyte chemotactic protein-1, which plays a role in the recruitment of monocytes to sites of injury and infection, were elevated in damaged areas of mdx-Ccn2+/+ compared to mdx-Ccn2+/−, suggesting a role for CCN2 in the inflammatory response of the dystrophic muscle.
Materials and methods
Mice and tissue harvest
C57BL/10ScSn-Dmdmdx (mdx mice) and C57BL/10 (wild type mice) were purchased from the Jackson Laboratory (Bar Harbor, ME), mdx-Ccn2+/− mice were obtained as described previously (Morales et al. 2013b). Male mice were used in all studies. All mouse protocols were conducted in strict accordance and with formal approval of the Animal Ethics Committee of the Pontificia Universidad Católica de Chile. For tissue harvesting, animals were anesthetized and sacrificed by cervical dislocation. Muscles were quickly dissected for cryosectioning, flash-frozen in isopentane cooled in liquid nitrogen and stored at −80 °C until processing (Cabello-Verrugio et al. 2012; Morales et al. 2011).
Laser dissection microscopy and gene expression analysis
Seven-micrometer thick sections of frozen muscle sections were put in PALM MembraneSlide (Carl Zeiss MicroImaging, Munich, Germany), fixed with ethanol and stained with hematoxylin in RNAase-free media. The tissue was then cut and catapulted with a PALM MicroBeam into the cap of a 0.5-ml tube. About 20 cryosections (7 μm) of normal and fibrotic tissue were collected from each sample. Total RNA was extracted immediately using the PicoPure RNA isolation kit (Arcuturus Bioscience). mRNA concentrations were measured using a spectrophotometer. We used 3 μg of undiluted RNA for cDNA synthesis with a Superscript II reverse transcriptase system (Invitrogen, USA). Taqman quantitative real-time PCR reactions were performed in triplicate on an Eco Real-Time PCR System (Illumina, USA), using pre-designed primer sets for mouse myogenin, CCN2, α-SMA, fibronectin, Pax-7, MCP-1 and the housekeeping gene GAPDH (Taqman Assays-on-Demand, Applied Biosystems, USA). mRNA expression was quantified using the comparative DCt method (2-ΔΔCT), using GAPDH as the reference gene. mRNA levels were expressed relative to the mean expression in wild type mice.
Immunohistochemical staining
For immunohistochemical analysis, cryosections (7 μm) were fixed in acetone, incubated for 1 h with anti-CCN2 (Santa Cruz Biotechnology) or anti-CD68 (Serotec, USA) in 5% goat serum in PBS, and blocked for 15 min in methanol-H2O2 (3%). After 1 h of incubation with Poly-HRP anti-rabbit-IgG (Immunologic, the Netherlands), enzyme activity was measured using a 3′,3′-diaminobenzidine tetrahydrochloride liquid system (Dako, USA). Nuclei were stained with haematoxylin.
Immunofluorescence microscopy
For muscle immunofluorescence, cryosections (7 μm) were fixed in 4% paraformaldehyde, blocked for 1 h in 4% BSA in PBS, and incubated overnight at 4 °C with anti-Fibronectin (Sigma, USA), anti-collagen III (Rockland, USA), and anti-laminin (Sigma, USA) (Acuña et al. 2014; Contreras et al. 2016; Morales et al. 2013a). For embryonic myosin staining, monoclonal anti-embryonic myosin (Hibridoma Bank, USA) was used with the M.O.M. reagent kit (Vector laboratories, USA). The corresponding Alexa Fluor 568- and 488-conjugated anti IgGs were used as secondary antibodies. For nuclear staining, sections were incubated with 1 μg/mL Hoechst 33,258 in PBS for 10 min.
Statistical analysis
The statistical significance of the differences between the means of the experimental groups was evaluated using one-way analysis of variance (ANOVA) with a post-hoc Bonferroni multiple-comparison test (Prism 3.0, GraphPad). A difference was considered statistically significant at p value <0.05.
Results
Current evidence suggests that CCN2 is a matricellular protein with strong relevance in the pathophysiology of muscular dystrophies. Loss- and gain-of-function experiments have demonstrated its critical role suggesting that targeting CCN2 has significant potential for the development of novel therapies for DMD and related diseases (Morales et al. 2011, 2013b).
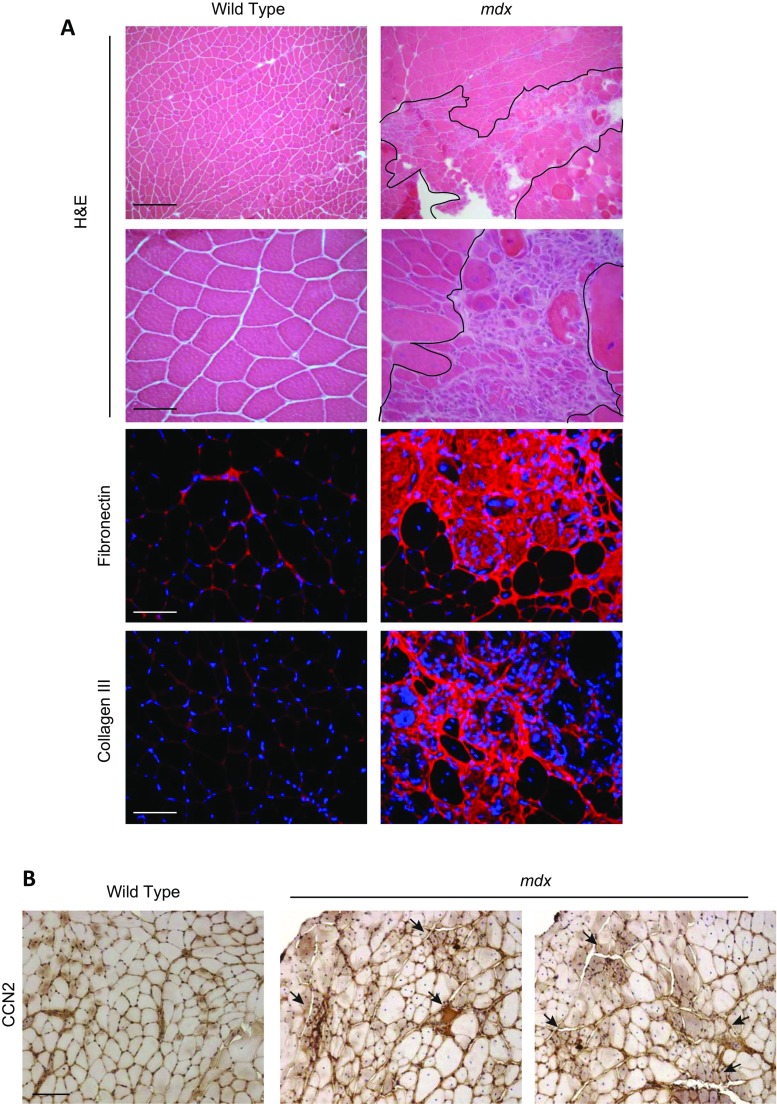
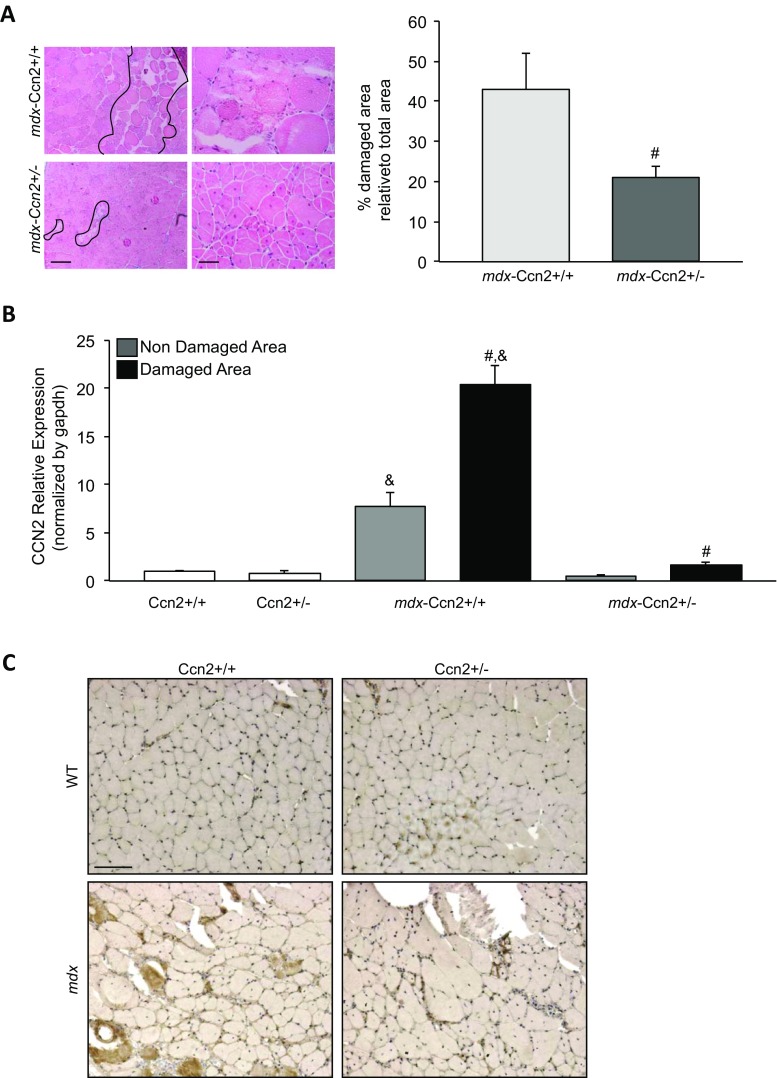
The presence of necrotic-regenerative foci in mdx skeletal muscle has been identified in previous LCM-based studies (Marotta et al. 2007). Figure 1a shows the presence of many of these foci in mdx muscle sections. These necrotic-regenerative foci are characterized by a significant accumulation of fibronectin, collagen type III and interstitial cells, compared to wild type muscle sections (Fig. 1a). mdx muscle specific immunostaining shows elevated levels of CCN2 compared to wild type muscle. Besides, the staining is more intense in necrotic-regenerative foci, suggesting that CCN2 is increased in these damaged areas (Fig. 1b). We have already shown that mdx mice with reduced CCN2 levels develop less severe muscular dystrophy (Morales et al. 2013b). To evaluate if the presence of the damaged areas is connected to the enhanced presence of CCN2 in mdx muscles, we used the mdx mice with a hemizygous CCN2 deletion (mdx-Ccn2+/−) in which the expression of CCN2 is reduced by approximately 50% (Morales et al. 2013b). H&E staining in Fig. 2a (left) shows that mdx-Ccn2+/− muscle has fewer necrotic-regenerative foci compared to mdx- Ccn2+/+ muscle. The right-hand side of Fig. 2 shows a significant 50% decrease of damaged areas of mdx-Ccn2+/− skeletal muscle compared to mdx- Ccn2+/+. Fig. 2b shows that mdx skeletal muscle express higher level of CCN2 mRNA in damaged areas compared to non-damaged areas. The same figure also shows that there are decreased levels of CCN2 mRNAs in the damaged areas of mdx-Ccn2+/− skeletal muscle compared to mdx muscle. We also observed less intense immunostaining for CCN2 in these areas (Fig. 2c). These results show the number of damaged areas present in skeletal muscle is decreased in dystrophic mice with diminished levels of CCN2 (mdx-Ccn2+/−) compared to mdx- Ccn2+/+ mice. Results also show that the expression levels of CCN2 within the damaged areas in mdx-Ccn2+/− muscle are lower than in mdx-Ccn2+/+.
Fig. 1.
Features of necrotic-regenerative foci in mdx mice. a Upper panels: H&E staining of gastrocnemius crossections from wild type and mdx mice. Magnifications: upper panel bar: 200 μm; lower panel bar: 50 μm. The necrotic-regenerative foci are shown delimeted by black lines. Lower panels: Immunostaining for the fibrotic markers fibronectin and collagen III. Bar 50 μm. b CCN2 immunostaining of gastrocnemius crossections from wild type and mdx mice. Arrows indicate the necrotic-regenerative foci of mdx muscle where CCN2 staining is more intense. Bar 100 μm
Fig. 2.
mdx-Ccn2+/− muscle shows reduced necrotic-regenerative foci and is correlated with reduced CCN2 levels. a Left panel: H&E staining of gastrocnemius crossections from mdx-Ccn2+/+ and mdx-Ccn2+/− mice at different magnifications. Left bar: 100 μm, right bar: 50 μm. The necrotic-regenerative foci are shown delimited by black lines. Right panel: Quantitation of the degree of damage: % damaged area relative to the total area of mdx-Ccn2+/+ and mdx-Ccn2+/− crossections. # T-test p < 0.05 mdx-Ccn2+/+ vs mdx-Ccn2+/−. b Quantitative qPCR of CCN2 from LMD samples of damaged (black bars) and non-damaged (gray bars) areas of gastrocnemius from mdx-Ccn2+/+ and mdx-Ccn2+/− mice. White bars correspond to wild type (Ccn2+/+) or wild type hemizygous for CCN2 (Ccn2+/−). # One way ANOVA p < 0.05 vs non-damaged areas. & p < 0.05 vs Ccn2+/+. c CCN2 immunostaining of gastrocnemius crossections from Ccn2+/+, Ccn2+/−, mdx-Ccn2+/+ and mdx-Ccn2+/− mice. Bar 100 μm
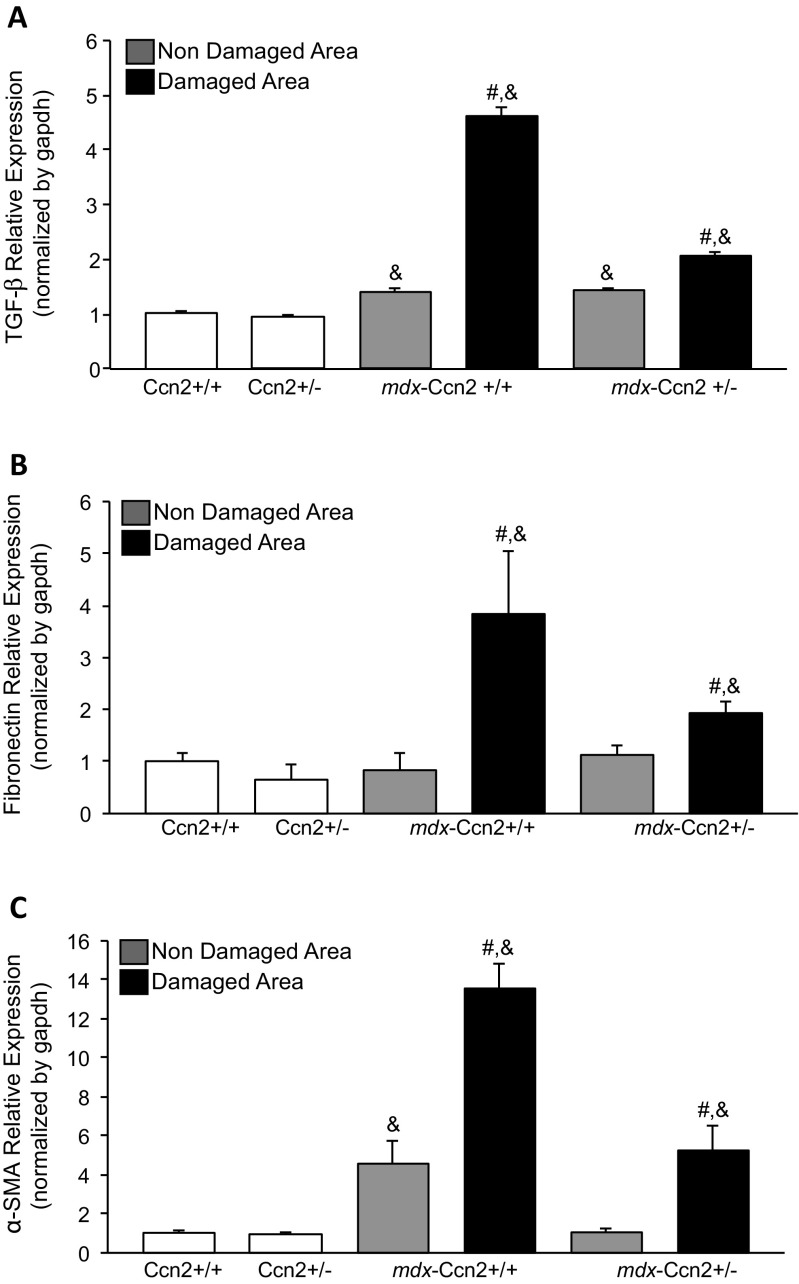
Next, we evaluated mRNAs levels from three fibrotic components. Figure 3 shows that expression of TGF-β (Fig. 3a), fibronectin (Fig. 3b) and α-SMA (Fig. 3c) are significantly elevated in damaged areas compared to non-damaged areas in dystrophic muscle obtained from mdx and mdx-Ccn2 +/−. Furthermore, as expected all three markers are increased in dystrophic muscle compared to wild type muscle. Figure 3 also shows that the levels of expression of these three fibrotic components are significantly reduced in damaged areas of mdx-Ccn2+/− compared to mdx-Ccn2+/+ (Fig. 3). These observations suggest an important increase in pro-fibrotic and fibrotic components in damaged areas of dystrophic muscle compared to similar areas of dystrophic muscle hemizygous for Ccn2.
Fig. 3.
Fibrotic markers expression levels are reduced in the damaged areas of mdx-Ccn2+/− skeletal muscle. Quantitative qPCR of a TGF-β, b Fibronectin and c α-SMA, from LMD samples of damaged (black bars) and non-damaged (gray bars) areas in gastrocnemius muscle from mdx-Ccn2+/+ and mdx-Ccn2+/− mice. White bars correspond to wild type (Ccn2+/+) or wild type hemizygous for CCN2 (Ccn2+/−). # One way ANOVA p < 0.05 vs non-damaged areas, & p < 0.05 vs Ccn2+/+
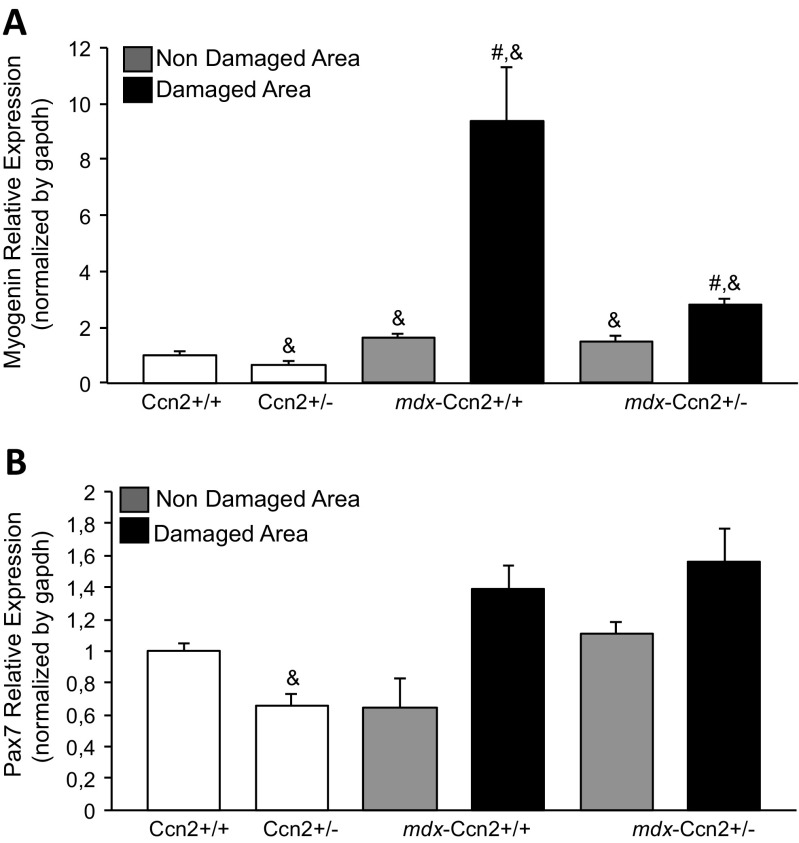
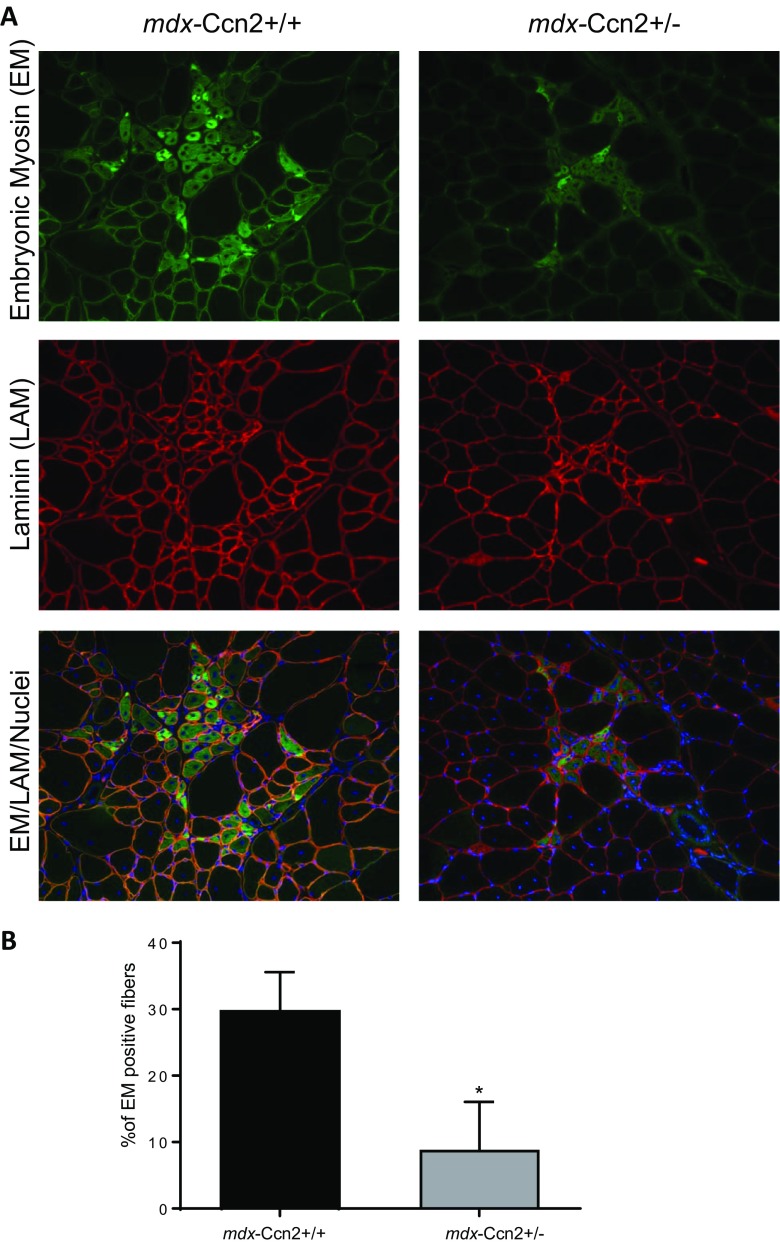
Next, we evaluated mRNAs levels of myogenin and Pax7, markers of muscle differentiation and satellite cells respectively, in damaged and non-damaged muscle areas obtained from wild type, mdx and mdx-Ccn2+/− mice. Figure 4a shows that mRNA expression levels for myogenin are significantly elevated in damaged areas of mdx muscle compared to mdx-Ccn2+/−, suggesting increased skeletal muscle regeneration in those areas. By contrast, no changes in Pax7 transcript levels were observed (Fig. 4b). Figure 5a shows immunostaining for embryonic myosin, a marker of muscle regeneration. We found that the percentage of fibers expressing embryonic myosin is significantly higher in mdx compared to mdx-Ccn2+/− muscle, indicating that under conditions of low CCN2 expression there is less skeletal muscle damage and consequently less skeletal muscle regeneration. Figure 5a (middle panels) also shows that laminin staining, a basal lamina constituent, is similar in mdx and mdx-Ccn2+/− skeletal muscle sections. However, the size of the fibers in the mdx-Ccn2+/− mice is less heterogeneous than in mdx, as we have shown previously (Morales et al. 2013b). The bottom panels of Fig. 5 show the merge of embryonic myosin, laminin and total nuclei staining, also consistent with reduced muscle regenerative activity in mdx-Ccn2+/− compared to mdx skeletal muscle. Figure 5b shows the quantitative analysis of embryonic myosin immunostaining in mdx-Ccn2+/− compared to mdx-Ccn2+/+.These results suggest that elevated levels of CCN2, characteristic of dystrophic muscle, are specifically related to the number and activity of necrotic-regenerative foci.
Fig. 4.
Myogenic markers expression levels are reduced in the damaged areas of mdx-Ccn2+/− skeletal muscle. Quantitative qPCR of a Pax7 and b Myogenin, from LMD samples of damaged (black bars) and non-damaged (gray bars) areas in gastrocnemius muscle from mdx-Ccn2+/+ and mdx-Ccn2+/− mice. White bars correspond to wild type (Ccn2+/+) or wild type hemizygous for CCN2 (Ccn2+/−). # One way ANOVA p < 0.05 vs non-damaged areas, & p < 0.05 vs Ccn2+/+
Fig. 5.
There are fewer regenerating fibers in the muscle of mdx-Ccn2+/− mice. a Embryonic myosin (EM), and Laminin (LAM) immunostaining to evaluate regenerating fibers and basement membrane respectively, in gastrocnemius crossections from mdx-Ccn2+/+ and mdx-Ccn2+/− mice. Nuclei were visualized with Hoechst. Bar: 100 μm. b The percentage of EM positive fibers was quantified by counting 5 different fields for three mdx-Ccn2+/− and mdx-Ccn2+/+ mice (a mean of 600 fibers per animal were counted). Data corresponds to mean ± standard deviation. *p < 0.05 T-test mdx-Ccn2+/− vs mdx-Ccn2+/+
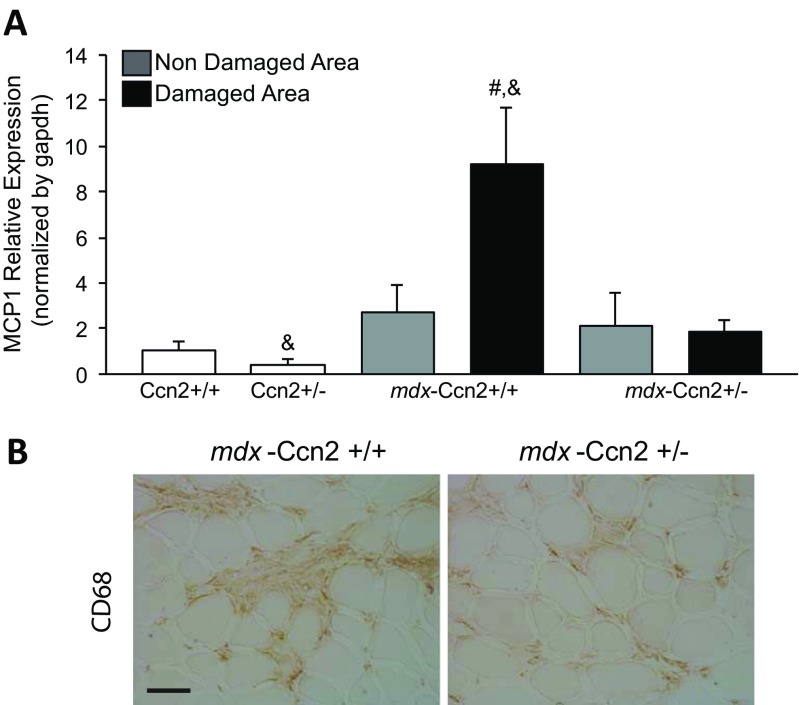
One of the features of DMD is the invasion of damaged muscle areas by inflammatory cells (Haslett et al. 2002). We found that the expression of monocyte chemoattractant protein-1 (MCP1), a marker of monocytes/macrophages, in damaged areas of mdx muscle is higher than in damaged areas of mdx-Ccn2+/− muscle (Fig. 6a). We also observed that the increased expression of MCP1 correlates with an elevated number of macrophages in the tissue, as evidenced by increased CD68 staining, which is reduced in damaged areas of mdx-Ccn2+/− muscle compared to mdx. Thus, increased levels of CCN2 correlate with increased necrotic regenerative and inflammatory response in mdx skeletal muscle.
Fig. 6.
Inflammation markers are reduced in the damaged areas of mdx-Ccn2+/− mice. a Quantitative qPCR of MCP1, from LMD samples of damaged (black bars) and non-damaged (gray bars) areas of gastrocnemius muscle from mdx-Ccn2+/+ and mdx-Ccn2+/− mice. White bars correspond to wild type (Ccn2+/+) or wild type hemizygous for CCN2 (Ccn2+/−). # One way ANOVA p < 0.05 vs non-damaged areas, & p < 0.05 vs Ccn2+/+. b CD68 immunostaining to asses monocytes/macrophages in gastrocnemius crossections from mdx-Ccn2+/+ and mdx-Ccn2+/− mice. Bar: 50 μm
Discussion
CCN2, a matricellular protein, is normally expressed during development in various tissues, including myocardium (Chuva de Sousa Lopes et al. 2004), pancreas (Charrier and Brigstock 2013), bone (Hall-Glenn et al. 2013; Takigawa 2013) and teeth (Pacheco et al. 2008). The participation of CCN2 in chronically damaged tissue is very well known and a strong correlation between the levels of CCN2 and the severity of damage has been documented. CCN2 has been associated with the induction of fibrosis in several chronic diseases, including multiple organs in systemic sclerosis (Leask et al. 2009), kidney fibrosis (Ito et al. 1998), lung fibrosis in response to bleomycin (Ponticos et al. 2009) and liver cirrhosis (Chen et al. 2011). We and other researchers have previously shown elevated levels of CCN2 in dystrophic skeletal muscle, both in human patients and in animal models (Morales et al. 2013a, b; Sun et al. 2008). CCN2’s role in skeletal muscle fibrosis was well established in gain- and loss-of-function experiments. When CCN2 is overexpressed in normal skeletal muscle, a transient fibrotic response is observed (Morales et al. 2011). On the other hand, there is a significant reduction in the amount of collagen and fibronectin when CCN2 is inhibited with blocking antibodies or in dystrophic mice hemizygous for CCN2 (Morales et al. 2013b).
CCN2 expression is also associated with an inflammatory response. CCN2 exposure via systemic administration induces an important infiltration of inflammatory cells in the renal interstitium and produces a prominent NF-kB response in mice (Sanchez-Lopez et al. 2009). Cardiomyocytes exposed to CCN2 increase the levels the pro-inflammatory cytokines TNF-alpha, IL-6, MCP-1 and IL-8 (Wang et al. 2010). Moreover, overexpression of CCN2 induces a strong inflammatory response in skeletal muscle (Morales et al. 2011).
In this study, we found that in the mdx mouse there is a significant increase of CCN2 levels in the necrotic-regenerative foci, compared to non-damaged areas, correlating CCN2 levels with the degree of damage and fibrosis in skeletal muscle. The necrotic-regenerative foci also show increased levels of the fibrotic markers collagen I and fibronectin. In line with these results we found increased levels of α-SMA, which is produced by activated myofibroblasts, contributing to the generation and secretion of ECM components (Contreras et al. 2016). Additionally, we found incremented levels of MCP-1 in the necrotic-regenerative foci in both mouse models and elevated levels of CD-68 positive cells, indicating that CCN2 could also be inducing an inflammatory response. It would be interesting to also evaluate levels of inflammasome complex, IL1β and NLRP3 (Guo et al. 2015). Thus, high levels of CCN2 in both models seem to be accompanied by important inflammatory and fibrotic responses. The inflammatory and fibrotic responses observed in models of CCN2 loss of function presented in this work, are consistent with the properties already described for CCN2 (Kubota and Takigawa 2015).
The necrotic-regenerative areas in mdx mice were studied and characterized by Marotta et al., and found that markers of myogenesis such as MyoD, Myf-5 and myogenin are increased in these structures (Marotta et al. 2007). Here, we show that these foci have increased myogenin levels, corroborating the previous results; and we also found increased expression of embryonic myosin, a marker of newly regenerated fibers. Thus, a focus enriched in CCN2 is likely to induce an inflammatory reaction followed by necrosis, transforming it into an exquisite milieu for inducing fibrotic damage, which is in turn followed by activation of satellite cells and the formation of new fibers. Interestingly, the levels of Pax7, a marker of satellite cells, indicate no significant differences between damaged or non-damaged areas in the dystrophic mice, suggesting that local factors in the CCN2 expressing focus induce activation of satellite cells to form regenerating fibers.
Inhibition of CCN2 in deteriorated dystrophic muscle reduces the dystrophic phenotype as consequence of decreased inflammation and fibrosis (Morales et al. 2013a, b). The infusion of monoclonal antibodies against CCN2 (FG-3019), which block its activity, improves muscle architecture and function (Morales et al. 2013b). It is not known whether this improvement of the dystrophic muscle is triggered by a decrease in inflammation followed by a decrease in fibrosis. We previously showed that there is an important increase of fibrotic proteins levels in denervated skeletal muscle (Brandan et al. 1992; Fadic et al. 1990); however, we only observed a weak inflammatory reaction, suggesting that these processes might be independent. On the other hand, when dystrophic mice are treated with andrographolide, a potent and specific inhibitor of NF-kB signaling, we observed a reduction of fibrotic proteins in the deteriorated muscle (Cabrera et al. 2014). Are the same CCN2 receptors involved in the inflammatory and fibrotic response or, are there specific domains in the CCN2 molecule responsible for these differential responses? Certainly, future research is required to understand how CCN2 induces inflammatory and fibrotic responses.
Another important question is how CCN2 is induced in the skeletal muscle necrotic-regenerative foci. A potential inducer of CCN2 is NF-kB. Elevated levels of this transcription factor are observed in the skeletal muscle of dystrophic animal models as well as in DMD patients (Acharyya et al. 2007; Cabrera et al. 2014; Peterson et al. 2011). As mentioned before, an inhibitor of NF-kB activity reduces the amount of CCN2 in dystrophic muscle, resulting in decreased inflammation and fibrosis (Cabrera et al. 2014). CCN2 is also induced by TGF-β in several tissues including skeletal muscle cells (Vial et al. 2008). Smad response elements are present in the promoter region of CCN2 (Cordova et al. 2015; Chen et al. 2002; Leask et al. 2003), acting downstream of TGF-β signaling. TGF-β expression and signaling are augmented in denervated muscle and in dystrophic skeletal muscle (Ceco and McNally 2013; Cohn et al. 2007; Fanbin et al. 2011), therefore this pleiotropic factor can be a potential inducer of CCN2 in diseased skeletal muscle. Thus, there are strong inducers of CCN2 present in dystrophic or denervated skeletal muscle, and elevated TGF-β levels in the damaged zone correlate with increased CCN2 levels.
Summarizing, in this paper we have shown that the elevated CCN2 levels found in necrotic-regenerative foci in dystrophic muscle appear to induce both inflammatory and fibrotic responses. However, a direct effect of CCN2 in skeletal muscle dedifferentiation cannot be excluded as already suggested by us and other investigators (Nishida et al. 2015; Vial et al. 2008). Therefore, CCN2 is becoming an attractive therapeutical target for improving skeletal muscle physiology under pathological conditions.
Acknowledgements
The authors are grateful to Victor Troncoso, Darling Vera and Lina Correa for technical support. This study received financial support from Fondecyt grant 1150106, the CARE-PFB-12/2007 grant and Fondecyt grant 3140323.
Abbreviations
- α-SMA
α-smooth muscle actin
- CTGF/CCN-2
Connective Tissue Growth Factor
- DMD
Duchenne muscular dystrophy
- ECM
Extracellular matrix
- HIF-1α
Hypoxia inducible factor- 1α
- LCM
Laser capture microdissection
- MCP1
Monocyte chemoattractant protein-1
- TGF-β
Transforming growth factor type-β
Footnotes
María Gabriela Morales and María José Acuña contributed equally to this work.
Contributor Information
María Gabriela Morales, Email: maria.moralesfrance@utsouthwestern.edu.
María José Acuña, Email: mjacuna1@uc.cl.
Daniel Cabrera, Email: dacabrer@uc.cl.
Roel Goldschmeding, Email: R.Goldschmeding@umcutrecht.nl.
Enrique Brandan, Email: ebrandan@bio.puc.cl.
References
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-beta signalling. Hum Mol Genet. 2014;23:1237–1249. doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R. Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord. 1999;9:28–33. doi: 10.1016/S0960-8966(98)00093-5. [DOI] [PubMed] [Google Scholar]
- Brandan E, Fuentes ME, Andrade W. Decorin, a chondroitin/dermatan sulfate proteoglycan is under neural control in rat skeletal muscle. J Neurosci Res. 1992;32:51–59. doi: 10.1002/jnr.490320107. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E. Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med. 2012;16:752–764. doi: 10.1111/j.1582-4934.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera D, Gutierrez J, Cabello-Verrugio C, Morales MG, Mezzano S, Fadic R, Casar JC, Hancke JL, Brandan E. Andrographolide attenuates skeletal muscle dystrophy in mdx mice and increases efficiency of cell therapy by reducing fibrosis. Skelet Muscle. 2014;4:6. doi: 10.1186/2044-5040-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceco E, McNally EM. Modifying muscular dystrophy through transforming growth factor-beta. FEBS J. 2013;280:4198–4209. doi: 10.1111/febs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier A, Brigstock DR. Regulation of pancreatic function by connective tissue growth factor (CTGF, CCN2) Cytokine Growth Factor Rev. 2013;24:59–68. doi: 10.1016/j.cytogfr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62:1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Charrier AL, Leask A, French SW, Brigstock DR. Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor. J Hepatol. 2011;55:399–406. doi: 10.1016/j.jhep.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuva de Sousa Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, ten Dijke P, Lyons KM, Goldschmeding R, Doevendans P, Mummery CL. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras O, Rebolledo DL, Oyarzun JE, Olguin HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016;364:647–660. doi: 10.1007/s00441-015-2343-0. [DOI] [PubMed] [Google Scholar]
- Cordova G, Rochard A, Riquelme-Guzman C, Cofre C, Scherman D, Bigey P, Brandan E. SMAD3 and SP1/SP3 Transcription Factors Collaborate to Regulate Connective Tissue Growth Factor Gene Expression in Myoblasts in Response to Transforming Growth Factor beta. J Cell Biochem. 2015;116:1880–1887. doi: 10.1002/jcb.25143. [DOI] [PubMed] [Google Scholar]
- Fadic R, Brandan E, Inestrosa NC. Motor nerve regulates muscle extracellular matrix proteoglycan expression. J Neurosci. 1990;10:3516–3523. doi: 10.1523/JNEUROSCI.10-11-03516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E. Increase in decorin and biglycan in Duchenne Muscular Dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med. 2006;10:758–769. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanbin M, Jianghai C, Juan L, Yang W, Yuxiong W, Yanhua C, Tao L, Zhenbing C. Role of transforming growth factor-beta1 in the process of fibrosis of denervated skeletal muscle. J Huazhong Univ Sci Technolog Med Sci. 2011;31:77–82. doi: 10.1007/s11596-011-0154-4. [DOI] [PubMed] [Google Scholar]
- Fargas A, Roma J, Roig M. Muscle regeneration: the effect of the basal lamina, size of the lesion and inflammatory response in C57BL10/ScSn mice. Rev Neurol. 2002;34:328–338. [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Glenn F, Aivazi A, Akopyan L, Ong JR, Baxter RR, Benya PD, Goldschmeding R, van Nieuwenhoven FA, Hunziker EB, Lyons KM. CCN2/CTGF is required for matrix organization and to protect growth plate chondrocytes from cellular stress. J Cell Commun Signal. 2013;7:219–230. doi: 10.1007/s12079-013-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci U S A. 2002;99:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin Sci (Lond) 2015;128:181–196. doi: 10.1042/CS20140264. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta M, Sarria Y, Ruiz-Roig C, Munell F, Roig-Quilis M. Laser microdissection-based expression analysis of key genes involved in muscle regeneration in mdx mice. Neuromuscul Disord. 2007;17:707–718. doi: 10.1016/j.nmd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E. CTGF/CCN-2 overexpression can directly induce features of skeletal muscle dystrophy. J Pathol. 2011;225:490–501. doi: 10.1002/path.2952. [DOI] [PubMed] [Google Scholar]
- Morales MG, Cabrera D, Cespedes C, Vio CP, Vazquez Y, Brandan E, Cabello-Verrugio C. Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2) Cell Tissue Res. 2013;353:173–187. doi: 10.1007/s00441-013-1642-6. [DOI] [PubMed] [Google Scholar]
- Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22:4938–4951. doi: 10.1093/hmg/ddt352. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Aoyama E, Janune D, Lyons KM, Takigawa M. CCN family protein 2 (CCN2) promotes the early differentiation, but inhibits the terminal differentiation of skeletal myoblasts. J Biochem. 2015;157:91–100. doi: 10.1093/jb/mvu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MS, Reis AH, Aguiar DP, Lyons KM, Abreu JG. Dynamic analysis of the expression of the TGFbeta/SMAD2 pathway and CCN2/CTGF during early steps of tooth development. Cells Tissues Organs. 2008;187:199–210. doi: 10.1159/000112640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JM, Bakkar N, Guttridge DC. NF-kappaB signaling in skeletal muscle health and disease. Curr Top Dev Biol. 2011;96:85–119. doi: 10.1016/B978-0-12-385940-2.00004-8. [DOI] [PubMed] [Google Scholar]
- Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- Porter JD, Merriam AP, Leahy P, Gong B, Feuerman J, Cheng G, Khanna S. Temporal gene expression profiling of dystrophin-deficient (mdx) mouse diaphragm identifies conserved and muscle group-specific mechanisms in the pathogenesis of muscular dystrophy. Hum Mol Genet. 2004;13:257–269. doi: 10.1093/hmg/ddh033. [DOI] [PubMed] [Google Scholar]
- Roig M, Roma J, Fargas A, Munell F. Longitudinal pathologic study of the gastrocnemius muscle group in mdx mice. Acta Neuropathol. 2004;107:27–34. doi: 10.1007/s00401-003-0773-3. [DOI] [PubMed] [Google Scholar]
- Roig-Quilis M, Roma J, Marotta M, Sarria Y, Fargas A. Muscle regeneration following glycerol injection mimic that of mdx-mice degenerative-regenerative groups. Rev Neurol. 2004;38:1101–1108. [PubMed] [Google Scholar]
- Sanchez-Lopez E, Rayego S, Rodrigues-Diez R, Rodriguez JS, Rodrigues-Diez R, Rodriguez-Vita J, Carvajal G, Aroeira LS, Selgas R, Mezzano SA, Ortiz A, Egido J, Ruiz-Ortega M. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. J Am Soc Nephrol. 2009;20:1513–1526. doi: 10.1681/ASN.2008090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, Sato Y, Takigawa M, Iinuma K, Tsuchiya S. Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci. 2008;267:48–56. doi: 10.1016/j.jns.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Takigawa M. CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal. 2013;7:191–201. doi: 10.1007/s12079-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Zuniga LM, Cabello-Verrugio C, Canon P, Fadic R, Brandan E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol. 2008;215:410–421. doi: 10.1002/jcp.21324. [DOI] [PubMed] [Google Scholar]
- Wang X, McLennan SV, Allen TJ, Twigg SM. Regulation of pro-inflammatory and pro-fibrotic factors by CCN2/CTGF in H9c2 cardiomyocytes. J Cell Commun Signal. 2010;4:15–23. doi: 10.1007/s12079-009-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]