Abstract
Breast cancer (BC) has emerged as a deadly disease that affects the lives of millions of women worldwide. It is the second leading cause of cancer-related deaths in the United States. Advancements in BC screening, preventive measures and treatment have resulted in significant decline in BC related deaths. However, unacceptable levels of racial disparity have been consistently reported, especially in African-American (AA) women compared to European American (EA). AA women go through worse prognosis, shorter survival time and higher mortality rates, despite higher cancer incidence reported in EA. These disparities are independent of socioeconomic status, access to healthcare or age, or even the stage of BC. Recent race-specific genetic and epigenetic studies have reported biological causes, which form the crux of this review. However, the developments are just the tip of the iceberg. Prioritizing primary research towards studying race-specific tumor microenvironment and biological composition of the host system in delineating the cause of these disparities is utmost necessary to ameliorate the disparity and design appropriate diagnosis/treatment regimen for AA women suffering from BC. In this review article, we discuss emerging trends and exciting discoveries that reveal how genetic/epigenetic circuitry contributed to racial disparity and discussed the strategies that may help in future therapeutic development.
Keywords: Breast cancer, Racial disparity, Tumor microenvironment, African-American, European American, microRNAs
Introduction
BC is the leading cause of cancer-related deaths worldwide in women aged 29–59 years (Siegel et al. 2016). In the United States, 255,180 new cases of invasive BC and 63,410 new cases of non-invasive (in situ) BC will be diagnosed in women in 2017. About 40,610 women will lose their lives to BC (ACS. 2017). The lifetime risk of developing BC is the highest in North America (Forouzanfar et al. 2011). BC is classified based on intrinsic subtyping as luminal A, luminal B, Her2 overexpressed, basal and normal-like (Cejalvo et al. 2017). Further based on expressions of hormone receptors (HR) namely estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor (Her2), BC is classified as ER+/PR+/Her2+, ER+/PR+/Her2-, ER−/PR−/Her2+, and ER−/PR−/Her2- (Onitilo et al. 2009).
Luminal subtypes of breast cancer are positive for ER and PR receptors. Luminal can be further classified into luminal A, and luminal B. Luminal A is the most common type of breast cancer with positive ER and PR, but the negative Her2 expression and a low expression of Ki67. Hence, luminal A has the best prognosis overall. But, AA gets this type of cancer less frequently than EA. In the Carolina Breast Cancer Cohort, luminal breast cancer accounted for 67% of breast cancer in postmenopausal EA, and 55% in postmenopausal AA (Dai et al. 2015). Luminal B, on the other hand, is ER+, PR+ and Her2+ and occasionally ER+ PR+ and Her2-. It also has a higher expression of Ki67 which leads to a worse prognosis (Thompson et al. 2016). Luminal A responds better to only hormone therapy, whereas luminal B needs a combination of hormone therapy and chemotherapy.
Her2 overexpressed BC is ER- and PR- and Her2+. This subtype has a worse prognosis than luminal types. However, significant progress has been made in the treatment of this type of BC. Her2 inhibitors combined with chemotherapy have resulted in the improved prognosis of Her2+ BC (Dawood et al. 2010; Swain et al. 2015). Despite improved prognosis, a majority of patients with Her2+ metastatic BC treated with the current standard of care has shown a high risk of relapse in 12–18 months of treatment (Baselga et al. 2012; Nahta et al. 2006; Swain et al. 2015). AA patients treated with trastuzumab showed significantly lower overall median survival and progression-free survival compared to EA patients receiving the same therapy (Rugo et al. 2013).
Basal type lacks ER, PR, and Her2 and therefore is also called as triple negative breast cancer (TNBC). This kind of cancer is the most fatal and has the worst prognosis overall. It is very aggressive and prone to metastasis with a lower survival rate. The size of the tumor in basal type BC is larger, and the tumor grows rapidly compared to HR positive BC. It is also known to be the most heterogeneous subtype of BC. The basal subtype BC is more common in AA than EA, and there are not many effective treatments available. Besides these, other two sub-types are also found, although less frequently. These are claudin-low (Dias et al. 2017; Katayama et al. 2017), and molecular apocrine types (Vranic et al. 2015).
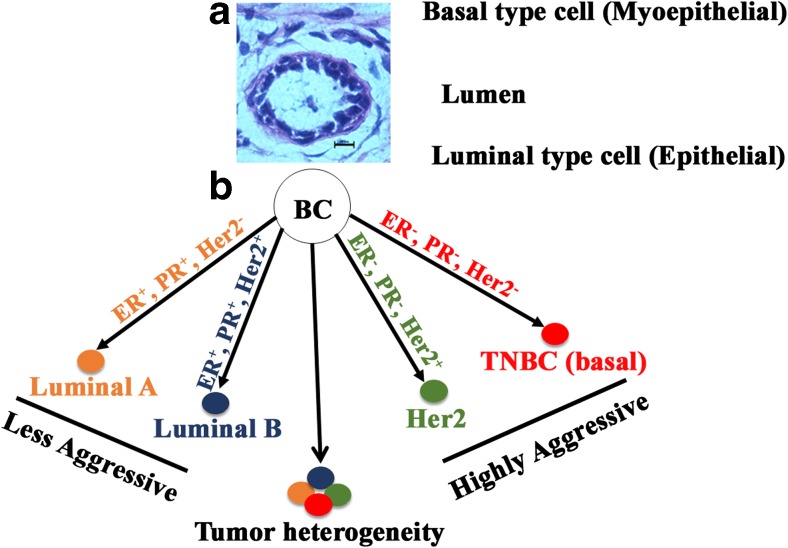
Hormone receptor (HR) positive BC has a good prognosis, as they can be treated using available hormone-based therapies. TNBC, on the other hand, has the worst prognosis as it does not succumb well to current treatments. Histology of normal duct and classification of BC subtypes have been depicted in Fig. 1. As illustrated in figure A, a normal mammary duct consists of a lumen, surrounded by an epithelial layer called as luminal epithelium which in turn rests on myoepithelial cells. The outermost layer is the basement membrane. Myoepithelial layer along with the basement membrane plays a critical role in separating the lumen and stromal compartments. Aberrations in the numbers or functions or both of myoepithelial cells cause the luminal epithelial cells to escape outside of the duct and turn invasive (Polyak and Kalluri 2010). The characteristics of different breast tumor subtypes, their origin, morphology and clinical significance are shown in Table 1. BC, in general, is a complex, heterogeneous disease that makes it challenging to unravel. In the past few years, several reports have highlighted an alarming rate of racial disparity in BC. The disparity in occurrence and severity of BC has been reported across several ethnic groups. Hispanic women have been found to be diagnosed with BC at a younger age (~11 years) compared to EA women, and they also had higher TNBC and poor cancer-specific survival as well as lower disease-free survival (Lara-Medina et al. 2011). On the other hand, the rate of development of BC and related death is reported to be lower in Hispanics, Asian and Native American women (ACS. 2017). AA form the third largest ethnic group in the United States and several reports consistently showed that BC is crueler to AA as compared to EA (Dietze et al. 2015). In addition, AA women show very poor BC associated survival rate and response to therapy. AA women suffering from BC show a higher percentage of recurrence, death rate, BMI and lower quality of life compared to EA patients with BC (Wu et al. 2017). Earlier, socioeconomic condition and poor access to health care were blamed for this disparity, but advanced research tools used to analyze population study showed that racial disparity exists between AA and EA at biological levels, which is independent of economic factors and lifestyle differences.
Fig. 1.
a. Histology of a normal human mammary duct and cell types. b. Subtypes of breast cancer and the heterogeneity within these types
Table 1.
Breast Cancer subtypes and their characteristics (modified from Dai et al. 2015
| Subtypes | ER | PR | HER2 | Origin | Morphology | Prognosis |
|---|---|---|---|---|---|---|
| Luminal A | + | + | – | Luminal | Epithelial | good |
| Luminal B | + | + | −/+ | Baso-luminal | Baso-luminal | average |
| Her2-overexpressed | – | – | + + | Baso-luminal | Baso-luminal | poor |
| Basal | – | – | – | Myo-epithelial | Myo-epithelial | poor |
| Claudin-low | – | – | – | Myo-epithelial | Myo-epithelial | poor |
| Apocrine | – | – | – | Myo-epithelial | Myo-epithelial | poor |
BC occurrence has been more or less stable in EA women during 2008–2012, whereas there was 0.4% per year increase in AA women in the same time period (DeSantis et al. 2016). Localized breast cancer incidence increased in EA women by 0.9% per year during 2004–2012, while the increase was more than double in AA patients. Early screening and improved treatment modalities have led to significant decline in BC related death rate, but this drop-in mortality is seen more in EA women compared to AA. Data from the Surveillance Epidemiology and End Results (SEER) program suggested that the five-year survival rate was 92% in EA women, but 82% in AA women (Howlader et al. 2015). Considering these facts, the seemingly marginal (0.4%) increase in breast cancer occurrence in AA women snow-balls into a scenario that is detrimental to their health and well-being.
While it is known that AA women suffer more from TNBC which is more lethal than ER/PR (+) cancer, recently it was reported that death hazard due to ER/PR (+) tumor was 4 times higher in AA women compared to EA, irrespective of their tumor stage, grade or therapy timeline (Rauscher et al. 2017). Thus, the lack of information about the mechanistic details that orchestrates the entire racial disparity is becoming evident in high magnitude. In addition, reports describing these disparities have been even contradicting each other as well as established theories. The above statement has led researchers to speculate that genetic/epigenetic factors strongly contribute to racial disparity and several reports have indicated that this indeed is the case. In the interest of providing appropriate breast cancer screening and treatment modalities to different ethnic groups, especially when personalized therapy is close to becoming a reality, it is crucial to unveil the biological causes of this racial disparity and design regimen to eliminate the same. This review will focus on summarizing reports specifically describing disparities seen in breast cancer initiation, advancement, tumor environment and treatment response between AA and EA women.
Racial disparity allied with breast cancer incidence and diversity
As mentioned earlier, AA women have been reported to have a higher rate of TNBC than EA, however other groups have reported that TNBC is not the only disparity causing factor. Interestingly, AA women suffering from ER/PR (+) breast cancer show higher mortality, indicating that AA women react differently to BC sub-types than EA. Between AA and EA women, EA women have a higher incidence of BC, but in the younger population (<45 years of age), it is seen that more AA women suffer from BC compared to EA. Several reports have adjusted socio-economic factors and access to healthcare and still found significant age and racial disparity about breast cancer initiation and progression between the two populations (Newman et al. 2006; Sweeney et al. 2014). An investigative study of women who were diagnosed with invasive BC as reported in SEER 18 registries database showed that AA women were less likely to be diagnosed with stage 1 BC compared to EA (37% vs. 50.8%) (Iqbal et al. 2015). However, a risk of death with stage 1 BC was higher among AA women compared to EA (6.2% vs. 3.0%). AA women were twice as likely to die due to small sized tumors as EA (9.0% vs 4.6%). We have summarized below, key observations based on HR expression:
-
I.
TNBC: A study was carried out in 91,908 women in California, who were diagnosed with invasive BC between 2006 and 2009, and categorized based on the tumor expression of HR and Her-2 (Clarke et al. 2012). It was found that there was no significant difference in the age of initiation of BC between AA and EA women when any of these sub-types were considered individually, but they did see a pattern when all these cancer types were analyzed collectively. Mainly, AA women over the age of 35 years had a higher incidence of TNBC and lower occurrence of HR+/Her2- a type of breast cancer compared to EA. An earlier report by the same group observed higher lifetime risk of TNBC among AA women compared to EA (Kurian et al. 2010). An extensive national level data on population-based BC categorized on molecular sub-types have also been reported (Clarke et al. 2012; Kohler et al. 2015). They showed that even at national level, in the United States, AA women had a higher incidence of TNBC compared to EA, who on the other hand had a higher occurrence of HR+/Her2- type cancer, accompanied with better prognosis.
A recent report showed that there was a significant increase in the number of AA women who were diagnosed with BC at a younger age (<40 years) (Komenaka et al. 2010). They also found that even when the age at diagnosis was similar in EA and AA women, AA women presented advanced clinical stage when compared to EA. This indicates that BC progression is more rapid in AA women than in EA. Furthermore, in their study cohort, significantly higher number of AA women had HR-negative tumors and suffered higher death rate in comparison to their European counterparts. AA women were thrice as likely to have TNBC as EA, irrespective of their age and body mass index (Stead et al. 2009). The Higher predisposition of AA women, to TNBC, advanced stage tumor, and poor prognosis have also been reported by others (Amirikia et al. 2011). The 2017 ACS Cancer facts and figures, supports the occurrence of this trend (ACS. 2017). Previous studies have also described that AA breast cancer patients show a higher grade tumor with negative ER, PR expression (Porter et al. 2004). Her2, however, was not significantly different between AA and EA. Cell cycle components such as cyclin D1, cyclin E, p53 were overexpressed in AA breast cancer patients, and they also had a higher mitotic index, and their tumors were more necrotic. Interestingly, race/ethnicity appears to play a more important role in determining breast cancer-specific survival than Her2 status in ER (−) and PR (−) patients (Brown et al. 2008). AA breast cancer patients were majorly shown to have a basal-like phenotype which is more aggressive and resistant to therapy. Thus, BC incidence exhibits a striking level of racial disparity, with the scale tipping against AA women.
-
II.
ER/PR positive: A histological analysis found that the risk of luminal A, luminal B, basal-like and Her2+/ER- cancer subtypes varied considerably between EA and AA, as shown in Table 2 (O'Brien et al. 2010). However, the racial effect was seen only in luminal A. Luminal A are ER/PR+ breast cancer types, and thus considered to have a good prognosis. However, in their cohort, they saw that AA women carrying this type of cancer faced higher mortality rates compared to EA, even though the percentage risk of this type of BC was less in AA. On the other hand, a higher mortality was seen in EA women with basal-like BC. This suggested that though basal-like subtype is associated with poor prognosis, it did not play a role in increasing the aggressiveness in AA women.
Table 2.
Percentage risk of various BC sub-types in EA and AA women (modified from O’Brien et al. (2010)
| Race | Luminal A | Luminal B | Basal-like | Her2+/ER- |
|---|---|---|---|---|
| EA | 64% | 11% | 11% | 5% |
| AA | 48% | 8% | 22% | 7% |
Interestingly, PAM50 gene expression assay showed that AA women were more likely to harbor basal-like sub-type of BC (Sweeney et al. 2014). On the other hand, there was a recent report that death rate in AA women with ER/PR (+) breast cancer is four times more than in EA. This alarming disparity confirmed that HR+ breast cancer does not confer same “desirable” effect in AA, as it does in EA women, in terms of treatment response and survival (Rauscher et al. 2017).
This further suggests that higher occurrence of TNBC in AA is not the only cause of the racial disparity, but HR+ breast cancer too demonstrates significant disparate behavior in AA women, leading to overall reduced quality of life and prognosis. It is discouraging to see that the seemingly “good prognosis” hormone receptor positive BC is more lethal in AA. Overall, it was seen that though cancer occurrence is higher in EA women compared to AA, cancer-related death rate is higher in AA (Adams et al. 2012; Cunningham and Butler 2004).
Racial disparity influences on prognosis and treatment
While variation in access to healthcare has been reported as one of the factors that cause the disparity between AA and EA, a study on women receiving health care in Department of Defense (DOD) carries great strength as it negates that variable. They observed that there was no significant difference in AA and EA BC patients receiving surgery (mastectomy, breast-conserving surgery plus radiation) or chemotherapy and hormone therapy in case of a local tumor (Enewold et al. 2012). However, among patients who had regional tumors, significantly less AA opted for chemotherapy and hormone therapy when compared to EA. This is definitive evidence that stage related racial disparity increases with advancement of BC and the disparity persists even when there is equal access to healthcare options. Another group considered the fact that AA women discontinued or delayed their treatments more in comparison to EA, and found that even after negating this factor, AA still shows inferior disease-free survival. Interestingly, there have been reports that AA and EA show a variable response to the same type of treatment (Hershman et al. 2009). In case of stage 2–3 tumors in a cohort of AA and EA women receiving similar treatment it was seen that in long-term, AA women with HR+ tumors showed inferior outcome (Tichy et al. 2015). Another recent report showed poor survival in AA women after BC diagnosis and reported greater disparity in first two years post-diagnosis in ER+ cancer (Warner et al. 2015). This clearly points towards the need to dissect the molecular mechanism behind TNBC and ER/PR positive BC. We have shown that MCF-7 cells, which are ER/PR positive, show higher expression of WISP2/CCN5, whereas MDA-MB-231 cells which are TNBC, show no expression of WISP2/CCN5 (Banerjee and Banerjee 2012; Haque et al. 2015; Haque et al. 2011). Further, we reported that introduction of WISP2/CCN5 in TNBC cells caused slow tumor cell growth (Das et al. 2017; Haque et al. 2015; Sarkar et al. 2017), and also ameliorated invasiveness of breast cancer cells (Banerjee et al. 2008). It will be interesting to determine whether WISP2/CCN5 plays any role in BC racial disparity. Pathologic complete response (pCR) to chemotherapy and neo-adjuvant chemotherapy in BC patients from the national cancer database revealed that AA women showed lower pCR compared to EA, although they were given chemotherapy in larger numbers than EA. Both TNBC and ER/PR-, Her2+ AA women, showed same pattern (Killelea et al. 2015). This raises a possibility that AA women respond differently to chemotherapy than EA. Impressive supporting evidence to this came from an observation that TNBC cells from AA and EA respond to treatment in a different manner (Martinez et al. 2016). TNBC cells from AA women were more sensitive to nitrosative stress-induced apoptosis than EA TNBC cells. Recently, it has been shown that cardiac glucosides inhibit cell clonogenicity, migration, invasion and viability more selectively in cell lines derived from AA breast cancer tumor than EA breast cancer tumor (Kaushik et al. 2017). Collectively, these reports have focused on the importance of considering racial disparity when treating BC patients and placing a high priority on racial disparity-centric research.
Racial disparity in tumor microenvironment
Variable response to breast cancer treatment does not sound surprising when we consider the knowledge that the tumor microenvironment and the host’s biological composition vary immensely between AA and EA women suffering from BC (Martin et al. 2009). At the genetic level, it was found that tumors from AA women expressed significantly higher levels of several cell cycle regulating genes, i.e., CDKN2A, CCNA2, CCNB1, and CCNE2. Other important and differentially regulated genes were β- crystallin B2 (CRYBB2), TMPO, AMFR and putative phosphoserine phosphatase-like protein (PSPHL). Apart from these differences, AA tumors also carried a pronounced interferon signature. The tumor stroma as well contained differentially expressed genes, the three most important genes being: PSPHL, CXCL10, and CXCL11. Tumors from AA women also expressed higher levels of angiogenesis promoting genes (VEGF and syndecan-1). Further, it was successfully demonstrated that CRYBB2 and PSPHL could be used as a two-gene classifier of tumor tissues between AA and EA breast cancer patients. Thus, the stroma environment in AA breast cancer patients overall was more inflammatory and pro-angiogenetic than in EA women. CRYBB2 is a major structural protein in the eye lens and has recently been shown to be over-expressed in AA patients who have prostate cancer (Faruque et al. 2015). On the other hand, higher levels of Insulin-like growth factor 2 (IGF2) in AA cell lines, as well as breast tissues, was reported (Kalla Singh et al. 2010b). IGF2 upregulated anti-apoptotic proteins (Bcl-2, Bcl-xL and Survivin), which causes cell death inhibition, increased cell proliferation and metastasis. Further, insulin-like growth factor 1 receptor (IGF1R) was present in significantly higher levels in normal AA compared to normal EA women. IGF1R levels were comparable between normal AA and malignant AA breast cancer. While IGFR2 was upregulated in EA tumors, phosphorylation of IGF1R, IRS-1 and Shc was higher in AA breast cancer (Kalla Singh et al. 2010a).
Twenty differentially expressed genes in breast cancer tissue samples obtained from AA and EA women were observed in a recent study, where AA samples showed alterations in the G1/S cycle, cell cycle regulatory genes, reduced cell adhesion, negligible ESR1, PGR, ERBB2 and estrogen pathway targets (Grunda et al. 2012). These play a role in imparting aggressive phenotype to the tumor, drug resistance, increased metastasis and poor survival. Several other genes that were differentially expressed between AA and EA women, not only in BC tissues but also in normal breast tissues were involved in cancer toxin detoxification, cell growth, proliferation and metastasis (Field et al. 2012). Molecular differences between AA and EA TNBC tumors determined by gene expression profiling and immunohistochemistry showed that AA tumors had higher levels of genes involved in proliferation (AURKB, CDCA5, CENPM, DDX11, and MK767) (Lindner et al. 2013) . Over-expression of VEGF in AA tumors correlated with increased vascularization observed in immunohistochemistry. On the other hand, BRCA1 and GATA-3 were under-expressed in AA tumors. GATA-3 acts together with BRCA1 to suppress the basal subtype genes, thereby building grounds for good prognosis (Tkocz et al. 2012). Using next-generation sequencing data from the cancer genomic atlas (TCGA) to determine differential expression of certain genes in age and stage-matched AA and EA BC patients, it was found that the number of differentially expressed genes increased with advanced disease stage (Stewart et al. 2013). Out of 342 genes and other transcripts, 110 were upregulated, and 232 were down-regulated in AA. A high fold difference was seen in 37 genes, which were relevant to BC. Adenylyl Cyclase-Associated Protein 1 (Resistin 1) and some components of p53 and BRCA1 pathways were highly expressed in AA tumors in stage 1. Stage 2 had more genes that were differentially expressed. A tumor protein p73, Aurora kinase B, polo-like kinase, associated with cancer aggressiveness were also found to be highly expressed in AA. A transcript LOC90784, whose expression is inversely proportional to tumor aggressiveness, was expressed in low amounts in AA tumors of all stages. ADAM metalloprotease with thrombospondin type 1 motif (ADAMTS15) known to inhibit breast cancer cell migration was also reduced in AA tumors. The expression of CRYBB2 has increased in stage 2 AA tumors as compared to EA breast cancer patients. Interestingly, CRYBB2 was also reported earlier as a marker to differentiate between AA and EA breast tumor epithelium (Martin et al. 2009). Stage 3 cancer analyses showed a much higher number of genes that were differentially expressed, most important being ESR1, which was reduced in in AA tumors. This indicates that AA women may have less ER at the later stage of BC which has been correlated with an earlier report (Grunda et al. 2012). Overall, initial stage tumors are identical between AA and EA, but exhibit increased diversity at later stages (Table 3).
Table 3.
Increasing number of genes were differentially expressed in AA women compared to EA as cancer stage advanced (modified from Stewart et al. 2013
| Stage | No. of upregulated genes | No. of downregulated genes |
|---|---|---|
| 1 | 19 | 7 |
| 2 | 134 | 27 |
| 3 | 156 | 67 |
AA breast tumors were found to have more TP53 mutations and more intra-tumor heterogeneity compared to EA tumors in an exome sequencing and gene expression study (Keenan et al. 2015). PAM50 analysis indicated that AA had more basal tumors, both overall and when only TNBC were considered. Tumors in AA TNBC patients leaned more towards basal-like and mesenchymal stem cell-like, collectively contributing towards more aggressive characteristics.
Recently, it was shown that p53 mutation was associated with high centromere amplification, which in turn increased the aggressiveness of breast cancer. AA women were found to have higher centromere amplification and thus higher TNBC, as observed by several others as well (Ogden et al. 2017b). Table 4 summarizes the major genes that various investigators found to be differentially expressed in AA women with BC.
Table 4.
Differentially regulated genes in AA women with breast cancer
| Gene symbol | Gene name | Regulation | References |
|---|---|---|---|
| CDKN2A | Cyclin Dependent Kinase Inhibitor 2A | Up | (Martin et al. 2009) |
| CCNA2 | Cyclin-A2 | Up | (Martin et al. 2009) |
| CCNB1 | Cyclin-B1 | Up | (Martin et al. 2009) |
| CCNE2 | Cyclin-E2 | Up | (Martin et al. 2009) |
| CRYBB2 | β-crystallin B2 | UP | (Martin et al. 2009) |
| TMPO | Thymopoietin | Up | (Martin et al. 2009) |
| AMFR | Autocrine Motility Factor Receptor | Up | (Martin et al. 2009) |
| PSPHL | Putative phosphoserine phosphatase-like protein | Up | (Martin et al. 2009) |
| CXCL10 | C-X-C motif chemokine 10 | Up | (Martin et al. 2009) |
| CXCL11 | C-X-C motif chemokine 11 | Up | (Martin et al. 2009) |
| VEGF | Vascular endothelial growth factor | Up | (Lindner et al. 2013; Martin et al. 2009) |
| SDC1 | Syndecan-1 | Up | (Martin et al. 2009) |
| IGF2 | Insulin-like growth factor 2 | Up | (Kalla Singh et al. 2010b) |
| Bcl-2 | B-cell lymphoma 2 | Up | (Kalla Singh et al. 2010b) |
| BCL-xL | B-cell lymphoma-extra large | Up | (Kalla Singh et al. 2010b) |
| BIRC5 | Baculoviral inhibitor of apoptosis repeat containing 5 or survivin | Up | (Kalla Singh et al. 2010b) |
| IGF1R | Insulin-like growth factor 1 receptor | Up | (Kalla Singh et al. 2010b) |
| IGFR2 | Insulin-like growth factor 2 receptor | Down | (Kalla Singh et al. 2010a) |
| ESR1 | Estrogen receptor1 | Down | (Grunda et al. 2012; Stewart et al. 2013) |
| PGR | Progesterone receptor | Down | (Grunda et al. 2012) |
| ERBB2 | Receptor tyrosine-protein kinase erbB-2 | Down | (Grunda et al. 2012) |
| AURKB | Aurora kinase B | Up | (Lindner et al. 2013; Stewart et al. 2013) |
| CDCA5 | Cell Division Cycle Associated 5 | Up | (Lindner et al. 2013) |
| CENPM | Centromere Protein M | Up | (Lindner et al. 2013) |
| DDX11 | DEAD/H-Box Helicase 11 | Up | (Lindner et al. 2013) |
| MK767 | Merck and Kyorin 767 | Up | (Lindner et al. 2013) |
| BRCA1 | Breast cancer gene 1 | Down | (Tkocz et al. 2012) |
| GATA-3 | GATA Binding Protein 3 | Down | (Tkocz et al. 2012) |
| Resistin 1 | Adenylyl Cyclase-Associated Protein 1 | Up | (Stewart et al. 2013) |
| p73 | Tumor protein p73 | Up | (Stewart et al. 2013) |
| PLK | Polo like kinase | Up | (Stewart et al. 2013) |
| ADAMTS15 | ADAM metalloprotease with thrombospondin type 1 motif | Down | (Stewart et al. 2013) |
| IL-6 | Interleukin-6 | Up | (Deshmukh et al. 2015; Park and Kang 2013; Stewart et al. 2013) |
| IFN-γ | Interferon-gamma | Up | (Park and Kang 2013) |
| KIFC1 | Kinesin Family Member C1 | Up | (Ogden et al. 2017a) |
Besides clinicopathological factors, epigenetic alterations may also contribute to breast cancer risk related to racial/ethnic disparities (Wu et al. 2015). The frequency of promoter hypermethylation in genes like HIN-1, Cyclin D2, Twist, RAR-β, and RASSF1A from AA and EA patients was tested and a higher methylation frequency of these genes in AA women compared with EA women was reported (Mehrotra et al. 2004). Further insights regarding the contribution of epigenetic variances to racial/ethnic disparities in BC showed that CpG sites within gene bodies and intergenic regions were more frequently hypermethylated in AA women than EA women whereas promoter-related differentially-methylated CpG sites were more frequently hypermethylated in EA women (Song et al. 2015). Analysis of tumor suppressor gene promoter hypermethylation in breast tissue from AA and EA women showed that tumor suppressor p16 INK4 promoter hypermethylation was more often detected among EA women with family history of breast cancer. In contrast, BRCA1 promoter hypermethylation was more frequently observed among AA women with family history (Dumitrescu 2012). Furthermore, in a separate study, a significant difference in frequencies of DNA methylation was found between AA and EA (Adkins et al. 2011). Hence, it can be suggested that differences in the frequency of gene promoter methylation may influence the disease outcome of breast cancer among AA and EA women and may potentially provide early diagnostic markers and drug targets for these patients.
MicroRNAs (miRNAs), non-coding, small RNA molecules, have recently emerged as crucial regulators of BC (Rahman and Sakr 2012). In a pilot study, several differentially expressed miRNAs were observed between normal and BC women of both ethnic origins (Zhao et al. 2010). Thirty-one miRNAs were differentially regulated between normal and BC patients of EA origin, whereas in AA, 18 miRNAs differed between normal and BC. While this clearly demonstrates how important miRNAs are in BC, the fact that out of 31 and 18 differentially expressed genes, only two miRNAs (miR181a, miR-1304) were common between EA and AA indicates a major racial disparity involving miRNA expression in BC (Zhao et al. 2010). Interestingly, they also found that let-7d, which targets epithelial-mesenchymal transition, was down-regulated in AA women suffering from BC compared to healthy AA women, again emphasizing the role of miRNA in BC. Analysis of Single Nucleotide Polymorphism (SNPs) in miRNAs that are important in BC showed that allele frequencies of almost 90% SNPs were significantly influenced by ethnicity and there are multiple SNPs and combinations that increased the risk of ER-positive BC in EA more than in AA (Yao et al. 2013). Recently, a genome-wide miRNA profiling of TNBC tumor tissues from AA and EA was performed and 26 miRNAs were found to be differentially regulated (upregulated in AA) between the two populations (Sugita et al. 2016). At least 23 miRNAs identified were known to be involved in pathways crucial to cancer, namely, Neutrophin (most significantly affected), PI3K/AKT, MAPK and insulin pathways. The role of miRNAs in BC disparity was recently compiled in a review, where several miRNAs were shown to be differentially expressed in BC directly or indirectly (Evans-Knowell et al. 2017).
Identifying markers from peripheral blood is a quick and minimally-invasive method of analyzing any disease. Blood analysis in AA women affected by BC showed higher levels of inflammatory cytokines, namely, IL-6 and IF-gamma (Park and Kang 2013). AA patients also had higher expression of Resistin and IL-6 compared to EA patients (Deshmukh et al. 2015). It has been shown that Resistin caused IL-6 production and STAT-3 activation, thereby aiding BC cell proliferation, migration and invasion. STAT-3 was recently shown to be crucial in developing chemo-resistance in BC (Deshmukh et al. 2017; Marusyk et al. 2016). Resistin upregulation in AA tumors has also been reported earlier. (Stewart et al. 2013). In contrast to these observations, genomic profile and protein array studies in a small population of BC patients coming from various ethnic backgrounds did not find significant changes in gene or protein expression between those ethnic group, which included AA and EA women as well (Chavez-Macgregor et al. 2014). Recently, KIFC1 was shown to be expressed in higher levels in AA breast cancer compared to EA (Ogden et al. 2017a). KIFC1 was also observed to be required for cell migration in AA population, where as in EA, it did not appear to be rate limiting. Hence, KIFC1 may be used as a potential biomarker of poor BC prognosis in AA women. Biochemical composition of tumors has gained the attention of investigators recently. About 32% metabolites that differ between ER+ and TNBC from AA women have been identified (Kanaan et al. 2014; Keenan et al. 2015). TNBC tumors had a significantly high number of metabolites that play a role in energy metabolism, trans-methylation, and proliferation. Oncometabolite like 2-hydroxyglutarate and sarcosine were also found in higher levels in TNBC compared to ER+ tumors. It will be interesting to know if tumor metabolic profiles differ between AA and EA.
Thus, there exists a convincing amount of evidence that tumor microenvironment varies significantly between AA and EA BC patients and play a crucial role in cancer prognosis, survival and response to treatment.
Racial disparity in alcohol-induced breast cancer
While alcohol consumption has been added to the list of risk factors of BC, research to determine whether the pre-disposition is racially determined is still in its infancy. A study of the Carolina BC group found no significant link between alcohol consumption and BC in either AA or EA women (Kinney et al. 2000). Similarly, no significant correlation was seen between alcohol consumption and BC risk in AA women (Chandran et al. 2013). In fact, a marginal decrease in BC incidence was noted with an increased life time of alcohol consumption in AA women, especially those who started drinking below the age of 20. No racial difference in alcohol consumption and mammographic density between AA and EA was further reported (Quandt et al. 2015). However, a convincing number of studies found a link between racial background and alcohol-induced BC susceptibility. Earlier, a significant association between heavy alcohol consumption and BC in EA compared to AA was reported (Hiatt et al. 1988). Alcohol consumption was more strongly associated with ER+ breast cancer than ER-breast cancer (Nasca et al. 1994). Given the fact that ER+ breast cancer is more prevalent among EA women, this correlates with an earlier report (Hiatt et al. 2014). In contrast, a positive link between alcohol consumption and BC in various ethnic groups irrespective of ER/PR status has been found (Park et al. 2014). Alcohol consumption caused adverse effects on BC survival in AA women (McDonald et al. 2002). Even one alcoholic drink/week lead to a 2.7-fold higher risk of death in post-menopausal AA breast cancer patients compared to non-drinkers. Emphasizing the correlation between alcohol consumption and BC risk, it was observed that a ten-year increase of alcohol consumption leads to 54% increased a risk of BC in sub-Saharan African women (Qian et al. 2014). Recently, Carolina BC study showed that the association of ER-breast cancer and TNBC with alcohol consumption was much higher in AA women who had more than seven drinks/week, compared to EA women with similar alcohol consumption (Williams et al. 2016). A chronic exposure of non-tumorigenic epithelial cell line of EA origin MCF-7 and MCF-12A to alcohol leads to EMT (Epithelial Mesenchymal Transition) and oncogenic transformations (Gelfand et al. 2016; Gelfand et al. 2017). The role of alcohol consumption and cardio-protective effect in AA and EA men and women was studied and it was found that the mortality risk was reduced more in EA than in AA, suggesting that AA population are prone to alcohol-related health issues (Jackson et al. 2015). Overall, we observed somewhat contrasting reports regarding the racial disparity role of alcohol in contributing to BC risk in AA and EA. Inconsistency in reporting alcohol consumption, frequency and amount of drinking do make it difficult to come to a conclusive and comparable statement between various study reports. However, there is a strong indication that AA women are genetically more prone to health issues in general and more likely to be affected by alcohol-induced BC risk and poor survival post-BC detection, compared to their EA counterparts. There is clearly need to dig deeper towards exploring alcohol-related BC racial disparity.
Racial disparity in smoking-induced breast cancer
Cigarette smoking has been linked to BC due to its carcinogenic ingredients. Nicotine, one of the active ingredient in cigarettes, has been shown to be angiogenic (Heeschen et al. 2002). Further, nicotine induces cell proliferation in MCF-7 and MDA-MB 468 breast cancer cell lines and also increases epithelial to mesenchymal transition and invasion (Dasgupta et al. 2009). It is noteworthy that MCF-7 cells are derived from EA woman, whereas MDA-MB-468 from AA woman. A murine model for breast cancer metastasis showed that cigarette smoke increases lung metastases (Murin et al. 2004). Thus, cigarette smoke causes all the hallmark attributes of cancer, including proliferation, invasion, angiogenesis, metastases and EMT, thereby demanding a continued investigation. BC mortality was seen to be higher in women who smoked before diagnosis, whereas women who never smoked were less likely to die of BC (Xue et al. 2011). Women who continued smoking post diagnosis and during treatment had higher mortality rate compared to, women who quit smoking after BC diagnosis (Braithwaite et al. 2012; Passarelli et al. 2016). Consequently, smoking accelerates the detrimental effects of BC and also intervenes with the efficacy of treatment, thereby reducing the survival span of patients (Izano et al. 2015; Rosenberg et al. 2013). Smoking, when combined with alcohol consumption was more detrimental to BC patients and may even lead to a second primary cancer (Knight et al. 2017).
While reports on racial disparity in smoking-induced BC is scarce, a recent study showed that smoking reduces the life-span of AA women who suffer from BC, whereas the effect was not as severe in EA. Interestingly, it was further seen that cigarette smoke’s anti-estrogenic nature might be responsible for this disparity (Parada et al. 2017). Earlier too, the effect of smoking on BC was shown to be associated with menopause and hormone status, where it was seen that, the smoking affected post-menopausal AA breast cancer patients more than pre-menopausal patients. Further, in post-menopausal AA women, the effect of smoking was more pronounced in ER+ cancer, than in ER- and the determinantal effect of smoking increased with the duration of smoking. Surprisingly, in pre-menopausal AA women, smoking was found to reduce the risk of BC and no significant difference was seen between ER+, ER- or TNBC (Park et al. 2016). It is known that AA women have higher levels of estrogen during the menstrual cycle than EA women. Thus, the anti-estrogenic effect of nicotine lowers the estrogen, thereby reducing the risk of BC in pre-menopausal AA women. However, comparing several studies on smoking and BC, the “protective” effect of smoking on BC risk in younger AA does not appear to be helpful. In fact, evidence support that in young women, smoking increases the likelihood of ER+ breast cancer. However, no association was found between smoking and TNBC (Kawai et al. 2014). Another study showed that women who smoked were more prone to luminal type (ER+) BC, than basal type (TNBC) (Butler et al. 2016). They too found that a higher percentage of AA women were affected by smoking related BC compared to EA. Thus, smoking is more harmful to AA women, especially those suffering from BC. As per Center for Disease Control and Prevention facts for 2015, the percentage of adult AA and EA population who indulge in smoking is similar. However, AA smokers who quit smoking, have been shown to have smoked much longer than EA (Jones et al. 2016). In conclusion, it is evident that disparity exists between AA and EA women regarding effect of smoking on BC. Avoiding smoking will be beneficial to women, especially AA women who are undergoing BC treatment.
Conclusion and future perspective
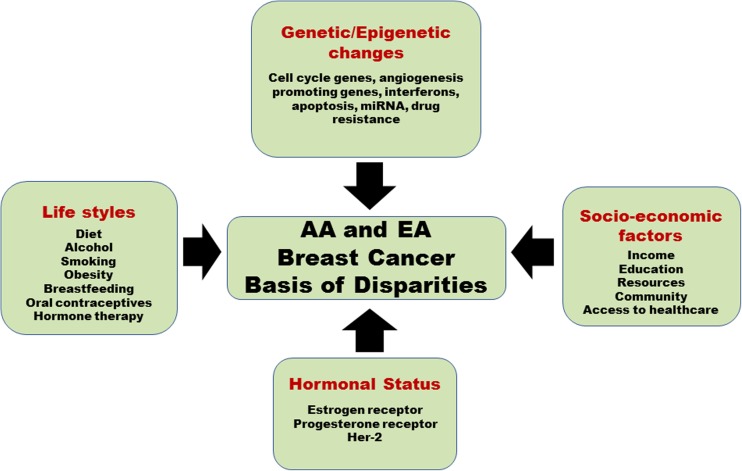
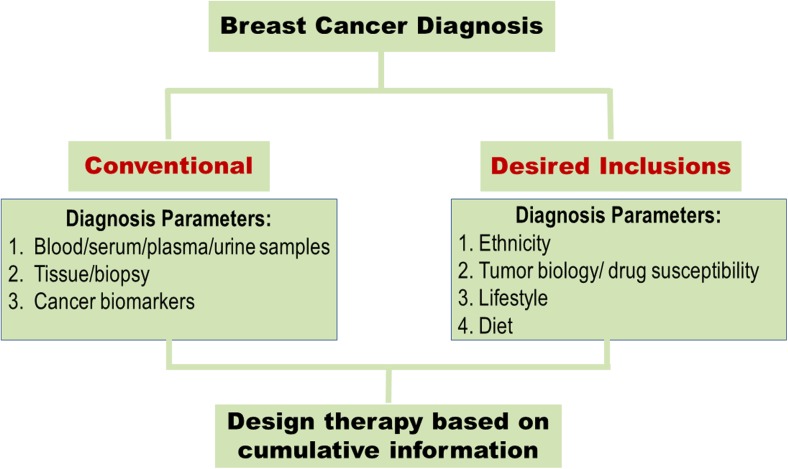
Breast cancer onset, prognosis and treatment exhibit a high racial disparity between AA and EA women. There is a significant amount of data supporting the biological basis of racial disparity which originates at genetic/epigenetic, hormonal, tumor biology level and includes diet and lifestyle as well (Fig. 2). The current situation warrants that BC diagnosis and treatment include as much race-based biology as possible to ameliorate the role of disparity in a poor survival of BC patients (Fig. 3). The racial disparity that exists between AA and EA women suffering from BC is an issue that cannot be ignored. The studies that have been summarized in this review confirm that racial disparity plays a leading role in poor prognosis, lower survival time and greater cancer-associated mortality seen in AA women. A comprehensive health care initiative started by Chicago city significantly reduced the disparity between AA and EA regarding BC survival and mortality, compared to nine other big cities across the country (Sighoko et al. 2017). While this was encouraging, the disparity that remained was still unacceptable. Thus, significant amount of data exist, which have confirmed that biological basis of racial disparity deserves attention and is fertile ground to harness information and use it to eliminate disparity and design better and race-specific BC screening and treatment modalities. An exciting proposal was recently described where genomes of 20,000 AA women with BC would be compared with genomes of EA and AA without BC (Printzcancer 2017). Besides, the availability of BC cell lines obtained from AA and EA population is a rich source of a biological specimen that could be utilized to generate a detailed disparity profile and possibly short-list race-specific druggable targets. A list of ATCC cell lines derived from AA and EA breast cancer patients has been compiled in Table 5.
Fig. 2.
A model depicting different factors which contribute to breast cancer disparities in African American and European American women
Fig. 3.
A pictorial representation showing how the current breast cancer diagnosis modality needs to be altered to include racial factors, tumor biology, lifestyle and diet and use this cumulative data to design appropriate treatment modality for breast cancer patients
Table 5.
A compilation of AA and EA origin BC cell lines available with ATCC
| Cell lines | Ethnicity | Characteristics | Catalogue # |
|---|---|---|---|
| MB 157 | AA | Epithelial carcinoma | ATCC® CRL-7721™ |
| HCC1806 | AA | Epithelial, squamous cell carcinoma | ATCC® CRL-2335™ |
| HCC1569 | AA | Epithelial, metaplastic carcinoma | ATCC® CRL-2330™ |
| ZR-75-30 | AA | Epithelial ductal carcinoma | ATCC® CRL-1504™ |
| HCC1008 | AA | Epithelial TNM stage IIA, grade 3, ductal carcinoma | ATCC® CRL-2320™ |
| HCC70 | AA | Epithelial, primary ductal carcinoma | ATCC® CRL-2315™ |
| HCC1500 | AA | Epithelial, primary ductal carcinoma | ATCC® CRL-2329™ |
| MDA-MB-157 | AA | Epithelial medullary carcinoma | ATCC® HTB-24™ |
| MDA-MB-468 | AA | Epithelial adenocarcinoma | ATCC® HTB-132™ |
| HCC2157 | AA | Epithelial, primary ductal carcinoma | ATCC® CRL-2340™ |
| MCF-7 | EA | Epithelial adenocarcinoma | ATCC® HTB-22™ |
| MCF-10A | EA | Epithelial fibrocystic disease | ATCC® CRL-10317™ |
| MCF-12A | EA | Non-tumorigenic luminal epithelial | ATCC® CRL-10782™ |
| MDA-MB-157 | EA | Epithelial medullary carcinoma | ATCC® HTB-24™ |
| ZR-75-1 | EA | Epithelial ductal carcinoma | ATCC® CRL-1500™ |
| SK-BR-3 | EA | Epithelial adenocarcinoma | ATCC® HTB-30™ |
| MDA-MB-361 | EA | Epithelial adenocarcinoma | ATCC® HTB-27™ |
| BT-474 | EA | Epithelial ductal carcinoma | ATCC® HTB-20™ |
| BT-20 | EA | Epithelial carcinoma | ATCC® HTB-19™ |
| BT-549 | EA | Epithelial ductal carcinoma | ATCC® HTB-122™ |
| BT-483 | EA | Epithelial ductal carcinoma | ATCC® HTB-121™ |
| HCC-1187 | EA | Epithelial, primary ductal carcinoma | ATCC® CRL-2322™ |
| HCC-38 | EA | Epithelial, primary ductal carcinoma | ATCC® CRL-2314™ |
| MDA-MB-231 | EA | Epithelial adenocarcinoma | ATCC® HTB-26™ |
| MDA-MB-435 | EA | Melanocyte ductal carcinoma | ATCC® HTB-129™ |
| MDA-MB-361 | EA | Epithelial adenocarcinoma | ATCC® HTB-27™ |
| HCC1937 | EA | Epithelial lymphoblast, primary ductal carcinoma | ATCC® CRL-2336™ |
| AU565 | EA | Epithelial adenocarcinoma | ATCC® CRL-2351™ |
| CRL-2327 | EA | Epithelial Stage IV, grade 4, adenocarcinoma | ATCC® CRL-2327™ |
| HCC1599 | EA | Epithelial lymphoblast Stage IIA, Grade 3, Primary Ductal Carcinoma | ATCC® CRL-2331™ |
An increased effort in studying biochemical metabolites and circulating markers that contribute to racial disparity is required, as it is not only convenient, minimally invasive, but also faster. Given the emerging role of alcohol and smoking in BC disparity, despite a lack of conclusive reports, it appears important to consider the lifestyle of BC patients and invest research efforts in delineating these effects in racial disparity. Drinking and smoking are modifiable risks, so the advantage of such research will be highly beneficial to BC patients undergoing treatment and to create awareness for women who want to prevent BC. A concerted effort in unveiling genetic/epigenetic basis of racial disparity is undoubtedly the way to ameliorate racial disparity and provide result-oriented BC treatment to AA women.
Acknowledgments
We would like to thank other Cancer Research Unit members for valuable suggestions and helpful comments on this manuscript. This work was supported by Merit Review Grant from the Department of Veterans Affairs (S.K.B. and S.B.)
Author contributions
VG and IH performed the literature search and wrote the initial draft of the manuscript, Jinia Chakraborty and SG helped revise the draft. Sushanta K Banerjee and Snigdha Banerjee revised it critically for important intellectual content.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Contributor Information
Snigdha Banerjee, Phone: (816) 861-4700 x 57120, Email: sbanerjee@kumc.edu.
Sushanta K. Banerjee, Phone: (816) 861-4700 x 57057, Email: sbanerjee2@kumc.edu
References
- American Cancer Society: Cancer facts and figures (2017) American Cancer Society Inc. Atlanta. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-factsand-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed 10 Nov 2017
- Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multiethnic cohort in the southeast. Cancer. 2012;118:2693–2699. doi: 10.1002/cncr.26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins RM, Krushkal J, Tylavsky FA, et al. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Res A Clin Mol Teratol. 2011;91:728–736. doi: 10.1002/bdra.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirikia KC, Mills P, Bush J, et al. Higher population-based incidence rates of triple-negative breast cancer among young African-American women : implications for breast cancer screening recommendations. Cancer. 2011;117:2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SK, Banerjee S. CCN5/WISP-2: a micromanager of breast cancer progression. J Cell Commun Signal. 2012;6:63–71. doi: 10.1007/s12079-012-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Dhar G, Haque I, et al. CCN5/WISP-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res. 2008;68:7606–7612. doi: 10.1158/0008-5472.CAN-08-1461. [DOI] [PubMed] [Google Scholar]
- Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite D, Izano M, Moore DH, et al. Smoking and survival after breast cancer diagnosis: a prospective observational study and systematic review. Breast Cancer Res Treat. 2012;136:521–533. doi: 10.1007/s10549-012-2276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Tsodikov A, Bauer KR, et al. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California Cancer Registry, 1999-2004. Cancer. 2008;112:737–747. doi: 10.1002/cncr.23243. [DOI] [PubMed] [Google Scholar]
- Butler EN, Tse CK, Bell ME, et al. Active smoking and risk of luminal and basal-like breast cancer subtypes in the Carolina breast cancer study. Cancer Causes Control : CCC. 2016;27:775–786. doi: 10.1007/s10552-016-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejalvo JM, Martinez de Duenas E, Galvan P, et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res. 2017;77:2213–2221. doi: 10.1158/0008-5472.CAN-16-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran U, Zirpoli G, Ciupak G, et al. Does alcohol increase breast cancer risk in African-American women? Findings from a case-control study. Br J Cancer. 2013;109:1945–1953. doi: 10.1038/bjc.2013.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Macgregor M, Liu S, De Melo-Gagliato D, et al. Differences in gene and protein expression and the effects of race/ethnicity on breast cancer subtypes. Cancer Epidemiol Biomark Prev. 2014;23:316–323. doi: 10.1158/1055-9965.EPI-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CA, Keegan TH, Yang J, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104:1094–1101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JE, Butler WM. Racial disparities in female breast cancer in South Carolina: clinical evidence for a biological basis. Breast Cancer Res Treat. 2004;88:161–176. doi: 10.1007/s10549-004-0592-9. [DOI] [PubMed] [Google Scholar]
- Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- Das A, Dhar K, Maity G, et al. Deficiency of CCN5/WISP-2-driven program in breast cancer promotes cancer epithelial cells to mesenchymal stem cells and breast cancer growth. Sci Rep. 2017;7:1220. doi: 10.1038/s41598-017-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P, Rizwani W, Pillai S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- Deshmukh SK, Srivastava SK, Bhardwaj A, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6:11231–11241. doi: 10.18632/oncotarget.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SK, Srivastava SK, Zubair H, et al. Resistin potentiates chemoresistance and stemness of breast cancer cells: implications for racially disparate therapeutic outcomes. Cancer Lett. 2017;396:21–29. doi: 10.1016/j.canlet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias K, Dvorkin-Gheva A, Hallett RM, et al. Claudin-low breast cancer; clinical & pathological characteristics. PLoS One. 2017;12:e0168669. doi: 10.1371/journal.pone.0168669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze EC, Sistrunk C, Miranda-Carboni G, et al. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu RG. Epigenetic markers of early tumor development. Methods Mol Biol. 2012;863:3–14. doi: 10.1007/978-1-61779-612-8_1. [DOI] [PubMed] [Google Scholar]
- Enewold L, Zhou J, McGlynn KA, et al. Racial variation in breast cancer treatment among Department of Defense beneficiaries. Cancer. 2012;118:812–820. doi: 10.1002/cncr.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Knowell A, LaRue AC, Findlay VJ. MicroRNAs and their impact on breast cancer, the tumor microenvironment, and disparities. Adv Cancer Res. 2017;133:51–76. doi: 10.1016/bs.acr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque MU, Paul R, Ricks-Santi L, et al. Analyzing the Association of Polymorphisms in the CRYBB2 gene with prostate cancer risk in African Americans. Anticancer Res. 2015;35:2565–2570. [PMC free article] [PubMed] [Google Scholar]
- Field LA, Love B, Deyarmin B, et al. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118:1334–1344. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- Gelfand R, Vernet D, Bruhn K, et al. Long-term exposure of MCF-12A normal human breast epithelial cells to ethanol induces epithelial mesenchymal transition and oncogenic features. Int J Oncol. 2016;48:2399–2414. doi: 10.3892/ijo.2016.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R, Vernet D, Bruhn KW, et al. Long-term exposure of MCF-7 breast cancer cells to ethanol stimulates oncogenic features. Int J Oncol. 2017;50:49–65. doi: 10.3892/ijo.2016.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunda JM, Steg AD, He Q, et al. Differential expression of breast cancer-associated genes between stage- and age-matched tumor specimens from African- and Caucasian-American women diagnosed with breast cancer. BMC Res Notes. 2012;5:248. doi: 10.1186/1756-0500-5-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque I, Banerjee S, Mehta S, et al. Cysteine-rich 61-connective tissue growth factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1-induced signaling protein-2 (WISP-2) regulates microRNA-10b via hypoxia-inducible factor-1alpha-TWIST signaling networks in human breast cancer cells. J Biol Chem. 2011;286:43475–43485. doi: 10.1074/jbc.M111.284158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque I, Banerjee S, De A, et al. CCN5/WISP-2 promotes growth arrest of triple-negative breast cancer cells through accumulation and trafficking of p27(Kip1) via Skp2 and FOXO3a regulation. Oncogene. 2015;34:3152–3163. doi: 10.1038/onc.2014.250. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Weis M, Aicher A, et al. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–536. doi: 10.1172/JCI0214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt RA, Klatsky AL, Armstrong MA. Alcohol consumption and the risk of breast cancer in a prepaid health plan. Cancer Res. 1988;48:2284–2287. [PubMed] [Google Scholar]
- Hiatt RA, Porco TC, Liu F, et al. A multilevel model of postmenopausal breast cancer incidence. Cancer Epidemiol Biomarkers Prev: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:2078–2092. doi: 10.1158/1055-9965.EPI-14-0403. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2012. Bethesda: National Cancer Institute; 2015. [Google Scholar]
- Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- Izano M, Satariano WA, Hiatt RA, et al. Smoking and mortality after breast cancer diagnosis: the health and functioning in women study. Cancer Med. 2015;4:315–324. doi: 10.1002/cam4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Hu FB, Kawachi I, et al. Black-white differences in the relationship between alcohol drinking patterns and mortality among US men and women. Am J Public Health. 2015;105(Suppl 3):S534–S543. doi: 10.2105/AJPH.2015.302615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.R., Joshu, C.E., Navas-Acien, A., et al. (2016). Racial/ethnic differences in duration of smoking among former smokers in the National Health and nutrition examination surveys (NHANES). Nicotine Tob Res. 10.1093/ntr/ntw326 [DOI] [PMC free article] [PubMed]
- Kalla Singh S, Tan QW, Brito C, et al. Insulin-like growth factors I and II receptors in the breast cancer survival disparity among African-American women. Growth Hormon IGF Res. 2010;20:245–254. doi: 10.1016/j.ghir.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla Singh S, Tan QW, Brito C, et al. Differential insulin-like growth factor II (IGF-II) expression: a potential role for breast cancer survival disparity. Growth Hormon IGF Res. 2010;20:162–170. doi: 10.1016/j.ghir.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan YM, Sampey BP, Beyene D, et al. Metabolic profile of triple-negative breast cancer in African-American women reveals potential biomarkers of aggressive disease. Cancer Genomics Proteomics. 2014;11:279–294. [PubMed] [Google Scholar]
- Katayama A, Handa T, Komatsu K et al (2017) Expression patterns of claudins in patients with triple-negative breast cancer are associated with nodal metastasis and worse outcome. Pathol Int. 10.1111/pin.12560 [DOI] [PubMed]
- Kaushik V, Azad N, Yakisich JS, et al. Antitumor effects of naturally occurring cardiac glycosides convallatoxin and peruvoside on human ER+ and triple-negative breast cancers. Cell Death Dis. 2017;3:17009. doi: 10.1038/cddiscovery.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Malone KE, Tang MT, et al. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer. 2014;120:1026–1034. doi: 10.1002/cncr.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T, Moy B, Mroz EA, et al. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the Association of Racial Differences with Tumor Recurrence. J Clin Oncol. 2015;33:3621–3627. doi: 10.1200/JCO.2015.62.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killelea BK, Yang VQ, Wang SY, et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol. 2015;33:4267–4276. doi: 10.1200/JCO.2015.63.7801. [DOI] [PubMed] [Google Scholar]
- Kinney AY, Millikan RC, Lin YH, et al. Alcohol consumption and breast cancer among black and white women in North Carolina (United States) Cancer Causes Control. 2000;11:345–357. doi: 10.1023/A:1008973709917. [DOI] [PubMed] [Google Scholar]
- Knight JA, Fan J, Malone KE, et al. Alcohol consumption and cigarette smoking in combination: a predictor of contralateral breast cancer risk in the WECARE study. Int J Cancer. 2017;141:916–924. doi: 10.1002/ijc.30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenaka IK, Martinez ME, Pennington RE, Jr, et al. Race and ethnicity and breast cancer outcomes in an underinsured population. J Natl Cancer Inst. 2010;102:1178–1187. doi: 10.1093/jnci/djq215. [DOI] [PubMed] [Google Scholar]
- Kurian AW, Fish K, Shema SJ, et al. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. doi: 10.1186/bcr2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117:3658–3669. doi: 10.1002/cncr.25961. [DOI] [PubMed] [Google Scholar]
- Lindner R, Sullivan C, Offor O, et al. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One. 2013;8:e71915. doi: 10.1371/journal.pone.0071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DN, Boersma BJ, Yi M, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L, Thames E, Kim J, et al. Increased sensitivity of African American triple negative breast cancer cells to nitric oxide-induced mitochondria-mediated apoptosis. BMC Cancer. 2016;16:559. doi: 10.1186/s12885-016-2547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Tabassum DP, Janiszewska M, et al. Spatial proximity to fibroblasts impacts molecular features and therapeutic sensitivity of breast cancer cells influencing clinical outcomes. Cancer Res. 2016;76:6495–6506. doi: 10.1158/0008-5472.CAN-16-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PA, Williams R, Dawkins F, et al. Breast cancer survival in African American women: is alcohol consumption a prognostic indicator? Cancer Causes Control. 2002;13:543–549. doi: 10.1023/A:1016337102256. [DOI] [PubMed] [Google Scholar]
- Mehrotra J, Ganpat MM, Kanaan Y, et al. Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin Cancer Res. 2004;10:2052–2057. doi: 10.1158/1078-0432.CCR-03-0514. [DOI] [PubMed] [Google Scholar]
- Murin S, Pinkerton KE, Hubbard NE, et al. The effect of cigarette smoke exposure on pulmonary metastatic disease in a murine model of metastatic breast cancer. Chest. 2004;125:1467–1471. doi: 10.1378/chest.125.4.1467. [DOI] [PubMed] [Google Scholar]
- Nahta R, Yu D, Hung MC, et al. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- Nasca PC, Liu S, Baptiste MS, et al. Alcohol consumption and breast cancer: estrogen receptor status and histology. Am J Epidemiol. 1994;140:980–988. doi: 10.1093/oxfordjournals.aje.a117205. [DOI] [PubMed] [Google Scholar]
- Newman LA, Griffith KA, Jatoi I, et al. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- O'Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina breast cancer study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden A, Garlapati C, Li XB, et al. Multi-institutional study of nuclear KIFC1 as a biomarker of poor prognosis in African American women with triple-negative breast cancer. Sci Rep. 2017;7:42289. doi: 10.1038/srep42289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden A, Rida PCG, Aneja R (2017b) Centrosome amplification: a suspect in breast cancer and racial disparities. Endocr Relat Cancer. 10.1530/ERC-17-0072 [DOI] [PMC free article] [PubMed]
- Onitilo AA, Engel JM, Greenlee RT, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2008.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada, H., Jr., Sun, X., Tse, C.K., et al. (2017). Active smoking and survival following breast cancer among African American and non-African American women in the Carolina breast cancer study. Cancer Causes Control : CCC. 10.1007/s10552-017-0923-x [DOI] [PMC free article] [PubMed]
- Park NJ, Kang DH. Inflammatory cytokine levels and breast cancer risk factors: racial differences of healthy Caucasian and African American women. Oncol Nurs Forum. 2013;40:490–500. doi: 10.1188/13.ONF.40-05AP. [DOI] [PubMed] [Google Scholar]
- Park SY, Kolonel LN, Lim U, et al. Alcohol consumption and breast cancer risk among women from five ethnic groups with light to moderate intakes: the multiethnic cohort study. Int J Cancer. 2014;134:1504–1510. doi: 10.1002/ijc.28476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Palmer JR, Rosenberg L, et al. A case-control analysis of smoking and breast cancer in African American women: findings from the AMBER consortium. Carcinogenesis. 2016;37:607–615. doi: 10.1093/carcin/bgw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette smoking before and after breast cancer diagnosis: mortality from breast cancer and smoking-related diseases. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2016;34:1315–1322. doi: 10.1200/JCO.2015.63.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2:a003244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100:2533–2542. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- Printzcancer C. Study to examine genetic factors behind increased risk of breast cancer in African Americans. Cancer. 2017;123:1083–1084. doi: 10.1002/cncr.30668. [DOI] [PubMed] [Google Scholar]
- Qian F, Ogundiran T, Hou N, et al. Alcohol consumption and breast cancer risk among women in three sub-Saharan African countries. PLoS One. 2014;9:e106908. doi: 10.1371/journal.pone.0106908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt Z, Flom JD, Tehranifar P, et al. The association of alcohol consumption with mammographic density in a multiethnic urban population. BMC Cancer. 2015;15:1094. doi: 10.1186/s12885-015-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman KM, Sakr WA. The therapeutic value of natural agents to treat miRNA targeted breast cancer in African-American and Caucasian-American women. Curr Drug Targets. 2012;13:1917–1925. doi: 10.2174/138945012804545461. [DOI] [PubMed] [Google Scholar]
- Rauscher GH, Silva A, Pauls H et al (2017) Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat. 10.1007/s10549-017-4166-z [DOI] [PMC free article] [PubMed]
- Rosenberg L, Boggs DA, Bethea TN, et al. A prospective study of smoking and breast cancer risk among African-American women. Cancer Causes Control : CCC. 2013;24:2207–2215. doi: 10.1007/s10552-013-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo HS, Brufsky AM, Ulcickas Yood M, et al. Racial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;141:461–470. doi: 10.1007/s10549-013-2697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Ghosh A, Banerjee S, et al. CCN5/WISP-2 restores ER- proportional, variant in normal and neoplastic breast cells and sensitizes triple negative breast cancer cells to tamoxifen. Oncogene. 2017;6:e340. doi: 10.1038/oncsis.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Sighoko D, Murphy AM, Irizarry B et al (2017) Changes in the racial disparity in breast cancer mortality in the ten US cities with the largest African American populations from 1999 to 2013: the reduction in breast cancer mortality disparity in Chicago. Cancer Causes Control. 10.1007/s10552-017-0878-y [DOI] [PMC free article] [PubMed]
- Song MA, Brasky TM, Marian C, et al. Racial differences in genome-wide methylation profiling and gene expression in breast tissues from healthy women. Epigenetics. 2015;10:1177–1187. doi: 10.1080/15592294.2015.1121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LA, Lash TL, Sobieraj JE, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA, Luks J, Roycik MD, et al. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS One. 2013;8:e82460. doi: 10.1371/journal.pone.0082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita B, Gill M, Mahajan A, et al. Differentially expressed miRNAs in triple negative breast cancer between African-American and non-Hispanic white women. Oncotarget. 2016;7:79274–79291. doi: 10.18632/oncotarget.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Bernard PS, Factor RE, et al. Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer Epidemiol Biomark Prev. 2014;23:714–724. doi: 10.1158/1055-9965.EPI-13-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E, Taube JM, Elwood H, et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29:249–258. doi: 10.1038/modpathol.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy JR, Deal AM, Anders CK, et al. Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat. 2015;150:667–674. doi: 10.1007/s10549-015-3350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkocz D, Crawford NT, Buckley NE, et al. BRCA1 and GATA3 corepress FOXC1 to inhibit the pathogenesis of basal-like breast cancers. Oncogene. 2012;31:3667–3678. doi: 10.1038/onc.2011.531. [DOI] [PubMed] [Google Scholar]
- Vranic S, Marchio C, Castellano I, et al. Immunohistochemical and molecular profiling of histologically defined apocrine carcinomas of the breast. Hum Pathol. 2015;46:1350–1359. doi: 10.1016/j.humpath.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33:2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LA, Olshan AF, Tse CK, et al. Alcohol intake and invasive breast cancer risk by molecular subtype and race in the Carolina breast cancer study. Cancer Causes Control. 2016;27:259–269. doi: 10.1007/s10552-015-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sarkissyan M, Vadgama JV. Epigenetics in breast and prostate cancer. Methods Mol Biol. 2015;1238:425–466. doi: 10.1007/978-1-4939-1804-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ye Y, Barcenas CH et al (2017) Personalized prognostic prediction models for breast cancer recurrence and survival incorporating multidimensional data. J Natl Cancer Inst 109. 10.1093/jnci/djw314 [DOI] [PMC free article] [PubMed]
- Xue F, Willett WC, Rosner BA, et al. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171:125–133. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Graham K, Shen J, et al. Genetic variants in microRNAs and breast cancer risk in African American and European American women. Breast Cancer Res Treat. 2013;141:447–459. doi: 10.1007/s10549-013-2698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Shen J, Medico L, et al. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS One. 2010;5:e13735. doi: 10.1371/journal.pone.0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]