Abstract
In skin, the basement membrane at the dermal-epidermal junction (DEJ-BM) is an important structure that tightly binds the epidermis to the dermis, and acts as a permeability barrier that controls exchange of macromolecules. Repair of the DEJ-BM during wound healing is important for restoration of skin functional properties after wounding. Here, we used a CO2 laser to perform partial thickness wounds in human volunteers, and directly compared wound repair in healthy young and aged individuals, focusing on the DEJ-BM. Our results show that the DEJ-BM is restored within four weeks after partial thickness wounds in young adults. We identified laminin-γ2 as preferred substrate for keratinocytes during reepithelialization of partial thickness human wounds. Laminin-γ2 is expressed continuously by migrating keratinocytes during reepithelialization, whereas collagen IV and collagen VII are deposited after wound closure. In contrast, our study shows that the DEJ-BM restoration following wounding is deficient in elderly individuals. Specifically, COL7A2 was barely increased during wound repair in aged skin and, as a result, the DEJ-BM in elderly skin was not restored and showed abnormal structure. Our data suggest that ameliorating the quality of the DEJ-BM restoration is a promising therapeutic approach to improve the quality of repaired skin in the elderly.
Keywords: Skin, Wound healing, Basement membrane, Laminin, Collagen, Wound repair
Introduction
The basement membrane (BM) at the dermal-epidermal junction (DEJ-BM) provides the interface between the dermis and epidermis. It consists of a highly organized assembly of glycoproteins and proteoglycans, and exerts several important functions: it tightly binds the epidermis to the dermis and provides resistance to shearing, it acts as a permeability barrier that controls exchange of macromolecules between epithelial and stromal compartments of the skin, and it determines the polarity of the epidermis thereby controlling cell organization and differentiation (Ryan et al. 1996; Ko and Marinkovich 2010; Breitkreutz et al. 2013). The DEJ-BM is subdivided into three morphological layers based on their transmission electron microscopy (TEM) appearance: the lamina lucida at the superior aspect of the BM is an electron-clear zone located directly beneath basal keratinocytes’ plasma membranes. It contains anchoring filaments, i.e. threadlike structures that link keratinocyte hemidesmosome adhesion complexes to the lamina densa, the basement membrane layer below the lamina lucida. Type XVII collagen (also called bullous pemphigoid antigen 180, or BP180) is a major component of the anchoring filaments. The lamina densa, which owes its name to its electron dense aspect in TEM, is formed by laminin/collagen IV polymers. On its under-side, the DEJ-BM is connected to the dermis by a layer of anchoring fibrils, formed by loop structures of collagen VII that link laminin-332 in the lamina densa with collagen I and III fibrils in the dermis (Bruckner-Tuderman 1999; Ghohestani et al. 2001; Villone et al. 2008).
Collectively, the DEJ-BM is essential to the functional integrity of the skin. Inherited or acquired defects of BM-associated components result in severe or lethal blistering skin diseases (Bruckner-Tuderman 1999; Ghohestani et al. 2001). Thus, repair of the DEJ-BM during wound healing is important for restoration of skin functional properties after wounding. However, details regarding repair of the DEJ-BM during wound healing of human wounds remain incompletely understood.
Stanley and collaborators observed that BP antigen, but not laminin-and type IV collagen, was present in the wound bed during migration of the new epithelium following partial thickness wounds in Yorkshire pigs (Stanley et al. 1981). Clark et al. reported similar findings in guinea pig full thickness wounds, and observed that laminin-and collagen IV reappeared upon completion of wound reepithelialization (Clark et al. 1982). Interestingly, Fine et al. had opposite findings with partial thickness wounds in cynomolgus monkeys, in which they detected laminin-and type-IV collagen, but not BP antigen (Fine et al. 1987). In human palate (mucosa, not skin), migrating keratinocytes were found to express laminin-332 (formerly known as Kalinin (Burgeson et al. 1994) or Epiligrin (Carter et al. 1991)) but not collagens IV or VII (Larjava et al. 1993). In all, these data suggest that the sequence of events leading to DEJ-BM reconstruction differ according to the type of wound, species, or both. To our knowledge, no one has studied the ultrastructural and biochemical aspect of DEJ-BM restoration during skin wound repair in vivo.
To address lack of knowledge, we sought to investigate the restoration of the DEJ-BM after partial-thickness wounding in human skin in vivo. Partial-thickness wounds such as those triggered by a second-degree burn, abrasion, or pressure ulcers are thought to be clinically more relevant than full-thickness wounds, which are typically closed surgically. We generated partial-thickness wounds on the forearm skin of healthy human individuals with a carbon dioxide (CO2) laser. We have previously reported that CO2 laser wounding triggers a typical repair reaction with inflammatory, proliferative, and remodeling phases that can be readily studied (Orringer et al. 2004; Rittié et al. 2011, 2013b, 2016). Here, we report the previously undocumented sequence of events that leads to restoration of the DEJ-BM in young human adults. We show that laminin-γ2 is expressed continuously by migrating keratinocytes during reepithelialization and that collagens IV and VII are deposited after wound closure. We show TEM evidence of DEJ-BM restoration by week 4 post-wounding. We also report a deficiency of DEJ-BM restoration in elderly individuals. Our data suggest that laminin-γ2 may play a significant role for keratinocyte migration during reepithelialization, and provide a biological basis for the observed susceptibility of elderly skin to tearing, following wound repair.
Material and methods
Research subjects
All protocols were approved by the Institutional Review Board of the University of Michigan, and each human subject provided written informed consent prior to entering the study. Partial-thickness wounds were made on square areas (side ~5-mm) with a CO2 laser as previously described in details (Rittié et al. 2016). Wounds were gently rinsed with tap water and covered with semipermeable dressing (Tegaderm, 3 M, Minneapolis, MN, USA). Four mm-full thickness punch biopsies were taken under local anesthesia (1% lidocaine) from the center of wounds, or across the edge of the wound, at various time points. Skin samples were embedded in Tissue-Tek OCT compound (Miles Scientific Labs, Naperville, IL, USA), frozen in liquid nitrogen, and stored at −80 °C until processing.
Subjects were enrolled in the ‘young’ group (9 men, 4 women; 27 to 47 years of age —mean: 35.8, median: 35.0) and in the ‘aged’ group (3 men, 6 women; 75 to 92 years of age—mean: 81.9, median: 81.0). All subjects were in general good health, without chronic disease that could affect wound healing.
Reagents
All common laboratory reagents were purchased from Sigma Aldrich unless otherwise specified.
Gomori’s periodic acid Methenamine silver staining of basement membranes and reticular fibers
Seven-μm-thick frozen skin sections were fixed in 2% (w/v) paraformaldehyde for 20 min, rinsed in distilled water, and oxidized in 0.5% periodic acid solution for 15 min at room temperature. After three rinses in distilled water, sections were stained in methenamine silver solution [3% methenamine, 0.25% silver nitrate, 5% borax (all w/v) in distilled water] for 30 min to 1 h at 60 °C. After thorough rinses in distilled water, sections were incubated in 0.2% (w/v) gold chloride until toned (~ 1 min), rinsed in distilled water, and treated with 3% (w/v) sodium thiosulfate for 2 min. After abundant rinsing in tap water, sections were counter stained with nuclear fast red, dehydrated, cleared in xylene and coversliped with Permount™ mounting medium (Thermo Fisher Scientific, Waltham, MA). Basement membranes and reticular fibers appear black, nuclei are pink (Avalone 1968).
Immunohistochemistry (IHC)
IHC was performed as previously described (Rittié et al. 2008) on 7-μm-thick frozen skin sections fixed in 2% paraformaldehyde or ice-cold acetone, using the following primary antibodies: anti-laminin-γ2 (ab1207 at a:2000, Abcam, Cambridge, MA); anti-collagen IV and –collagen VII (MAB1910 and MAB1345, both at 1:500, EMD Millipore, Billerica, MA). Slides were mounted with Supermount (Biogenex, Fremont, CA) or with ProLong Gold medium and coverslips (Thermo Fisher Scientific). Imaging was performed with an Axioscope 2 (Carl Zeiss, Thornwood, NY) equipped with a Spot digital camera (Spot Imaging Solutions, Sterling Heights, MI). A microscope micrometer (Thermo Fisher Scientific) was used during imaging for image calibration.
Quantification methods
IHC staining density was measured with ImageJ 1.49v (http://imagej.nih.gov) on the isolated red staining channel, using percent area from pixel counts of the thresholded image. Staining outside the dermal–epidermal area, if any (e.g., around appendages) was excluded. Four-to-six 400X images encompassing the width of the biopsy were analyzed for each subject. Measurements were averaged among three subjects per age group.
Plastic embedding and transmission electron microscopy
Four-millimeter full-thickness skin biopsies were used for TEM and prepared as described in details (Rittié et al. 2016). TEM was performed on a Phillips CM-100 (Eindhoven, The Netherlands), accessed at the University of Michigan Microscopy and Image-Analysis Laboratory core facility.
Laser capture microdissection (LCM) and quantitative real-time RT-PCR (qPCR)
LCM was performed to isolate interfollicular epidermis and dermis (without hair follicles, sebaceous glands, or coiled part of sweat glands) from 14 μm-thick frozen sections as previously described (Rittié et al. 2006, 2007). Total RNA was extracted from microdissected tissue, used as template for preparing cDNA, which was in turn pre-amplified and quantified by qPCR as previously described (Rittié et al. 2009). Primer/Probe pairs were obtained from Applied Biosystems (ThermoFisher Scientific) as follows: COL4A2 (Hs01098873_m1), COL7A1 (Hs00164310_m1), and LAMC2 (Hs01043711_m1). Target gene levels were normalized to transcript levels of RPLP0 (or 36B4, housekeeping gene (Minner and Poumay 2009)) (primer/pair synthesized by Sigma, sequence in (Rittié et al. 2006)), measured in parallel. Results are presented as fold change in wounded vs. unwounded skin sample, or as “fold vs. 36B4” (calculated as 2^-(CTtarget – CT36B4), in arbitrary units).
Data replication and statistical analysis
Qualitative immunostaining was assessed on 3 to 6 subjects per time point, with 2 to 4 technical replicates per samples. TEM images were obtained from 3 to 4 fields per section, 2 to 3 sections per sample, on 3 subjects per age group. Representative images are shown. RNA data were obtained from 2 averaged technical replicates per samples, on 3 to 6 subjects per age group. Data are expressed as mean ± standard error of the mean (SEM). Comparisons among groups were made with the Wilcoxon signed rank test, or unpaired Student’s t test. All p-values are two tailed, and considered significant when less than 0.05.
Results
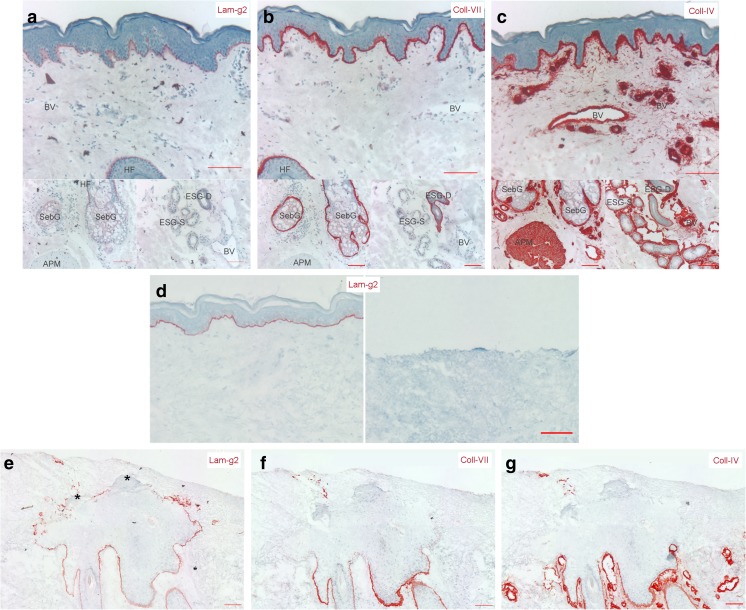
Our prior studies show that reepithelialization of partial-thickness human wounds originates from keratinocyte outgrowths produced by eccrine sweat glands and pilo-sebaceous units (hair follicles and associated sebaceous glands) underlying the wound (Rittié et al. 2013a, 2016). Here, we focused on three major protein components of the BM, laminin-γ2, collagen IV, and collagen VII, and asked whether (and how) the DEJ-BM was restored during repair of partial thickness wounds. In unwounded human skin, we observed that all three proteins are expressed at the DEJ-BM and around skin appendages (Fig. 1a-c). In addition, collagen IV was detected in arrector pili muscle and in all BMs surrounding blood vessels, as opposed to laminin-γ2 and collagen VII that were not expressed around blood vessels, in arrector pili muscle, or secretory portion of eccrine sweat glands. Moreover, laminin-γ2 was not detected around sebaceous glands. CO2 laser treatment of the skin as used in our experimental conditions ablates the entire epidermis, the DEJ-BM, and the superficial papillary dermis (Rittié et al. 2011, 2013a) (Fig. 1d). During wound repair, only laminin-γ2, but neither collagen IV nor collagen VII, was detected near keratinocytes forming the new epidermis (Fig. 1e-g). However, all three proteins were expressed around the base of hair follicles (below the wound level), as expected (Fig. 1e-g). Interestingly, laminin-γ2 was detected by keratinocytes at the edges of outgrowths, regardless of whether they were migrating alongside the dermis or the scab. In areas where the new epidermis started to differentiate, laminin-γ2 was no longer expressed (Fig. 1e).
Fig. 1.
Basement membrane protein expression and localization before and during wound healing in young adult human skin. Expression of collagen IV (a, d), collagen VII (b, e), and laminin-γ2 (c, f) proteins were assessed by immunohistochemistry in unwounded skin (a-c) and in appendage-derived outgrowths 7 days post-wounding (d-f). a-c) Baseline expression in intact skin. The three studied proteins are all expressed in the basement membrane of the dermal-epidermal junction. In contrast, their expression around blood vessels (BV), hair follicles (HFs), sebaceous glands (SebGs), arrector pili muscle (APM), eccrine sweat gland secretory portion (ESG-S) and ducts (ESG-D) differs: laminin-γ2 is absent around all appendages, collagen-VII expression is restricted to secretory portion of ESGs, SebGs, and HFs, while collagen VII is highly expressed around all appendages and BVs. d) Typical aspect of a partial thickness wound before (left) and 24 h post-CO2 laser (right). Collagen VII staining is used to locate the basement membrane for reference. e-g) Basement membrane protein expression during wound repair in young adult human skin. The three studied proteins are visible around hair follicles (untouched by the superficial wound, internal staining control). Collagen IV (d) and collagen VII (e) are not produced by keratinocytes forming the outgrowth. In contrast, laminin-γ2 (f) is produced by keratinocytes edging the outgrowth, regardless of whether they are migrating alongside the dermis or the scab. Lamininγ2 was no longer expressed where the new epidermis started to differentiate (asterisks). Scale bars = 100 μm
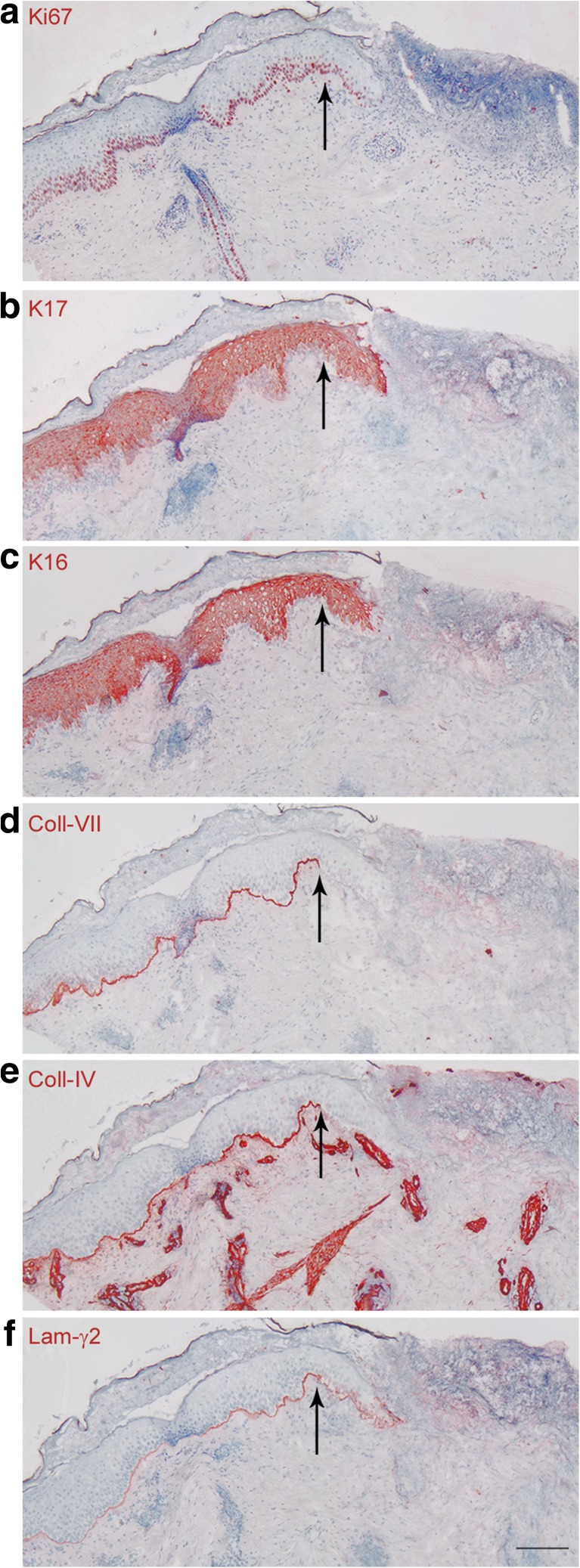
Keratinocytes at the wound edge also participate in formation of the new epidermis, although to a much lesser extent than from appendages, in human skin (Rittié et al. 2013a). In pigs, reepithelization from wound edges is estimated to represent only 10% of the new epidermis (Winter 1972). We nevertheless asked whether DEJ-BM protein expression pattern at this location differ from that seen in appendage-derived outgrowths. The formation of the epidermal tongue (process reviewed in (Rittié 2016)) is accompanied by a keratinocyte “activation” that involves cytoskeletal reorganization through production of specific keratin proteins [i.e. keratins 6, 16, and 17 (Coulombe 1997, Freedberg et al. 2001)]. In partial thickness wounds, we observed that wound healing keratins were expressed at least 2 mm into the adjacent normal epidermis (Fig. 2b-c). Partial thickness wounding increases mitotic activity in basal keratinocytes of appendage-derived outgrowths (Rittié et al. 2013a, 2016), and this phenomenon also extends into the adjacent normal skin (Fig. 2a). These observations confirm those previously described in humans after tape stripping (Williams and Hunter 1957). Remarkably, keratinocytes forming the epidermal tongue at the wound edge expressed similar DEJ-BM proteins as keratinocytes from appendage-derived outgrowths: they did not express collagen IV or collagen VII, but the outmost layer of the epidermal tongue was surrounded by a thin layer of laminin-γ2 (Fig. 2d-f). Taken together, these results suggest that migrating keratinocytes utilize laminin-γ2 as a substrate much more than collagen IV or collagen VII.
Fig. 2.
Expression of basement membrane proteins at the wound edge of young adult human skin. Skin samples were collected across the wound edge 4 days post-wounding and consecutive sections were immunostained in that order: a) Ki-67, b) K17, c) K16, d) collagen IV, e) collagen VII, and f) laminin-γ2. Black arrows designate the junction between unwounded (left) and wounded (right) skin. Scale bar = 100 μm
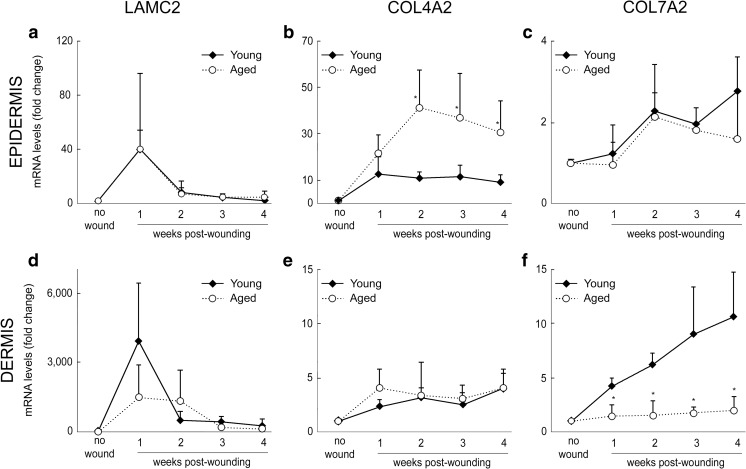
Next, we separated epidermis and dermis, by laser capture microdissection, to assess their relative contributions to expression of laminin-γ2, collagen VII, and collagen VII transcripts. Consistent with our immunohistochemistry (IHC) results, in unwounded skin, laminin-γ2 and collagen VII transcripts (LAMC2 and COL7A2 genes, respectively) were primarily detected in the epidermis, whereas collagen IV (COL4A2) was primarily detected in the dermis (presumably produced by appendages and blood vessels) (Fig. 3a). Upon wounding, peak production of laminin-γ2 transcripts occurred at week 1 post-wounding, primarily in the epidermis, expression tapered down at week 2 (after wound closure) and thereafter (Fig. 3). In contrast, both COL7A2 and COL4A2 transcript levels were moderately increased and steady during four weeks post-wounding, with COL7A2 greatest rise detected in the dermis, and COL4A2 greater in the epidermis. These results further support our conclusion from IHC results, i.e. keratinocytes closing the wound during the first week of repair utilize primarily laminin-γ2 as substrate, while collagen IV and collagen VII, other constituents of the DEJ-BM, are produced after wound closure.
Fig. 3.
Basement membrane protein expression in laser-capture microdissected epidermis and dermis before and during wound healing in young adult human skin. a Unwounded skin baseline levels of COL4A2, COL7A2, and LAMC2 transcripts in epidermis (light gray) and dermis (dark gray) separated by laser capture microdissection. *: p < .05 vs. Epidermis (Wilcoxon signed rank test). b-d Normalized transcript levels in epidermis following wounding (expressed in fold vs. 36B4 (housekeeping gene)) for COL4A2 (b), COL7A2 (c) and LAMC2 (d). N = 3–6 sample per time point. *: p < .05 vs. Epidermis (paired t-test)
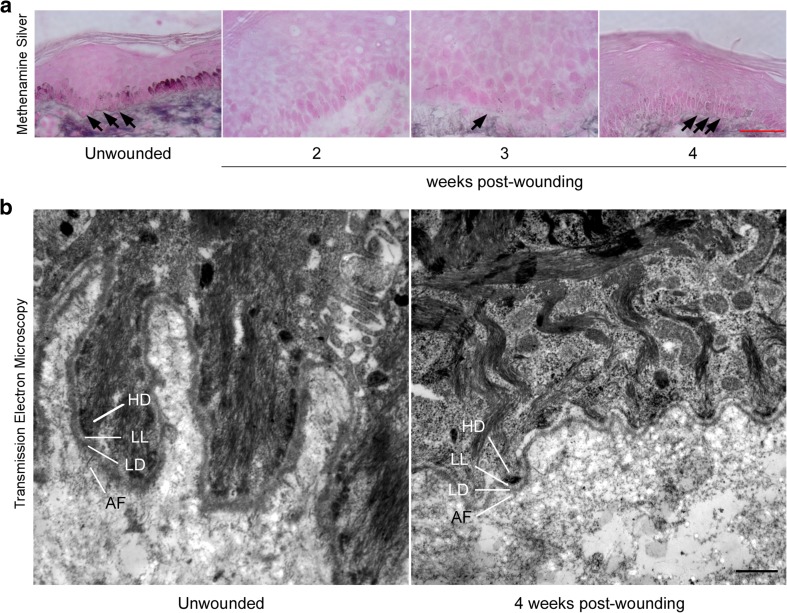
To assess whether laminin-γ2, collagen IV and collagen VII were assembled to form a functional DEJ-BM, we stained sections of skin, which were obtained at various time points after wounding, with methenamine silver to highlight basement membranes and reticular fibers (Avalone 1968). While the DEJ-BM was visible as a fine dark line at the DEJ in unwounded skin, it was completely absent two weeks post-wounding (Fig. 4a), although reepithelialization was complete, at this time point (Rittié et al. 2013a). At three weeks post wounding, the DEJ-BM appeared incomplete, while at four weeks post-wounding, the DEJ-BM appeared more continuous, indicating near complete restoration, at this time point. This conclusion was confirmed by TEM, which revealed that the DEJ-BM was composed of distinct hemidesmosomes, lamina lucida, lamina densa, and anchoring fibrils, four weeks post-wounding in young individuals (Fig. 4b).
Fig. 4.
Aspect of the basement membrane at the dermal-epidermal junction before and during wound repair in young adult human skin. a Gomori’s periodic acid methenamine silver staining of basement membranes in human skin samples during wound healing. Basement membranes and reticular fibers stain silver and cell counter-staining is pink. Unwounded skin is shown as reference (left). Black arrows indicate location of the basement membrane at the dermal-epidermal junction (DEJ-BM). The DEJ-BM appears sporadic at the wound site three weeks post-wounding, and more continuous four weeks post-wounding. Scale bar = 50 μm. b Transmission electron microscopy images of skin samples taken prior to wounding (left) and four weeks post-wounding (right) in young adults. HD: hemidesmosome; LL: Lamina lucida; LD: lamina densa; AF: anchoring fibrils. Scale bar = 500 nm
The composition of the DEJ-BM was recently shown to be altered with aging, with reduction of laminin-γ2 (Rittié et al. 2016) and both collagens IV and VII (Langton et al. 2016) protein levels in aged vs young individuals. Thus, we asked whether the restoration of the DEJ-BM was altered with aging. We first assessed transcript levels in the epidermis and dermis from laser capture microdissected samples (Fig. 5). LAMC2 transcript levels were similar between young and aged skin during wound healing, both in the epidermis and dermis (Fig. 5a, d). In contrast, aged individuals tended to produce more COL4A2 in the epidermis between weeks 2 and 4 post wounding, while COL7A2 production was markedly reduced, in the dermis of elderly individuals. COL7A2 was barely increased during wound repair in aged skin (Fig. 5f).
Fig. 5.
Basement membrane protein gene expression in laser-capture microdissected epidermis and dermis of young and aged human skin during wound healing. mRNA levels of LAMC2 (a, d), COL7A2 (b, e), and COL4A2 (c, f) in epidermis (a-c) and dermis (d-f) following wounding (expressed in fold change vs. unwounded baseline). N = 3–6 per group. *: p < .05 vs. Young (unpaired t-test)
We next asked whether lack of induction of COL7A2 gene expression was an indicator of altered restoration of the DEJ-BM in aged skin. In non-wounded skin, staining with methenamine silver revealed a much thinner DEJ-BM in elderly skin (Fig. 6a), compared to younger adult skin (Fig. 4a). In aged skin, there was complete absence of detectable DEJ-BM at weeks 2 and 3 post wounding, as observed in younger adult skin (Fig. 4a). Interestingly, unlike younger skin, the DEJ-BM in elderly skin was not restored at 4 weeks post wounding. Methenamine silver staining revealed that the DEJ-BM was highly interrupted and very thin, if visible. TEM experiments confirmed the conclusion that the DEJ-BM is only partially restored, with multiple interruptions, at week 4 post-wounding (Fig. 6b). TEM also revealed abnormal structure of the DEJ-BM in elderly skin with re-duplication of the lamina densa in some location, as previously described (Amano 2009), and Altogether, these data indicate alterations of the restoration of the DEJ-BM after wounding of elderly skin.
Fig. 6.
Aspect of the basement membrane at the dermal-epidermal junction before and during wound repair in aged human skin. a Gomori’s periodic acid methenamine silver staining of basement membranes in elderly human skin samples during wound healing. Basement membranes and reticular fibers stain silver and cell counter-staining is pink. Unwounded skin is shown as reference (left). Black arrows indicate location of the basement membrane at the dermal-epidermal junction (DEJ-BM). The DEJ-BM appears very infrequently at the wound site four weeks post-wounding. Scale bar = 50 μm. b Transmission electron microscopy images of skin samples taken prior to wounding (left) and four weeks post-wounding (right) in elderly adults. HD: hemidesmosome; LL: Lamina lucida; LD: lamina densa; AF: anchoring fibrils. Scale bar = 500 nm
Discussion
Our study directly compared wound repair, in healthy young and aged individuals. Our results show that the DEJ-BM can be restored after partial thickness wounds in young adults, and that this restoration is impaired in skin of the elderly. DEJ-BM exerts the important role of tightly binding the epidermis to the dermis and providing resistance to the skin against friction and shearing forces. Hence, altered restoration of the DEJ-BM in wounded elderly skin is likely to cause further predisposition of elderly skin to tearing. Indeed, previous skin tears are risk factors for skin tearing in the elderly (Benbow 2017; Strazzieri-Pulido et al. 2017). We observed near complete lack of induction of collagen VII in the DEJ-BM during wound repair in elderly skin. This observation provides a potential biological mechanism that might explain the enhanced susceptibility of skin tearing in the elderly, and suggest that collagen VII might be a therapeutic target to increase the quality of wound repair in the elderly.
Our work identifies laminin-γ2 as preferred substrate for keratinocytes during reepithelialization of partial thickness human wounds, as previously described for laminin-α3 chain in incisional wounds (Nguyen et al. 2000). Laminin-γ2 is important for normal skin integrity as demonstrated in junctional epidermolysis bullosa patients, in whom genetic alteration of laminin-γ2 leads fragility of the skin and mucous membranes, and causes spontaneous blistering of the skin, even with little or no trauma [OMIM C0268374 and C0079683]. The role of laminin-γ2 in keratinocytes (alone or assembled in the laminin-332 (α3β2γ2, formerly laminin-5) heterotrimer) is highly context-dependent (Sugawara et al. 2008; Rousselle and Beck 2013). Cell culture experiments indicate that contact with collagen I significantly facilitate the deposition of laminin-332 by human keratinocytes. Keratinocytes adhere to laminin-332 (Gagnoux-Palacios et al. 1996; Dellambra et al. 1998), and expression of laminin-γ2 in keratinocytes triggers directional hypermobility (Natarajan et al. 2005). These data are consistent with our hypothesis that laminin-γ2 promotes migration of keratinocytes at the periphery of wound outgrowths in the early phases of the healing of human wounds. Consistently, cell migration is likely to be reduced once laminin-γ2 gets polymerized or keratinocytes modulate their integrin make-up in later stages of wound healing, or both.
A recent study suggests that abnormal functioning of the DEJ-BM in hypertrophic scars reduces the attachment of basal keratinocyte progenitor cells to their surrounding microenvironment (Yang et al. 2016). The authors concluded that this phenomenon was responsible for a proliferative phenotype in basal keratinocytes of hypertrophic scars. In light of our study, it seems that abnormal deposition of the DEJ-BM is not sufficient to trigger a hyper-proliferative phenotype, as we found no evidence of keratinocyte hyperproliferation during the healing of elderly vs. younger skin (Rittié et al. 2016). Thus, it is likely that different keratinocyte phenotypes observed in elderly skin and hypertrophic scars are influenced by factors other than abnormal DEJ-BM deposition, such as individual components of the immature DEJ-BM or paracrine factors from dermal or immune cells. Additional research will be needed to answer this question.
In summary, we report herein the previously undocumented sequence of events that leads to restoration of the DEJ-BM in young human adults. Laminin-γ2 is expressed continuously by migrating keratinocytes during reepithelialization whereas collagens IV and VII are deposited after wound closure. As a result, the restoration of the DEJ-BM is evident by week 4 post-wounding in the skin of young human adults. In contrast, our studies show that the DEJ-BM restoration following wounding is deficient in elderly individuals. Our data suggest that ameliorating the quality of the DEJ-BM restoration is a promising therapeutic approach to improve the quality of repaired skin in the elderly.
Acknowledgements
We thank Suzan Rehbine, LPN, for her help with volunteer recruitment and tissue collection, Dorothy Sorenson (Microscopy and Image-analysis Laboratory) and for her help with operating the electron microscope. This research was supported by the University of Michigan Dermatology Department research fund, and K01 grant AR059678 from the NIH/NIAMS (to LR). The Microscopy and Image-analysis Laboratory is a multiuser imaging facility supported by the University of Michigan.
Abbreviations
- AF
Anchoring fibrils
- BM
Basement membrane
- CO2
Carbon dioxide
- DEJ-BM
Dermal-epidermal junction basement membrane
- HD
Hemidesmosome
- TEM
Transmission electron microscopy
- IHC
Immunohistochemistry
- LCM
Laser capture microdissection
- LD
Lamina densa
- LL
Lamina lucida
- qPCR
Quantitative real-time RT-PCR
- SEM
Standard error of the mean
References
- Amano S. Possible involvement of basement membrane damage in skin photoaging. J Investig Dermatol Symp Proc. 2009;14:2–7. doi: 10.1038/jidsymp.2009.5. [DOI] [PubMed] [Google Scholar]
- Avalone FG. Jones' method for kidney. In: Luna LG, editor. Manual of histologic staining methods of the armed forces institute of pathology. Blakiston division. New York: McGraw-Hill; 1968. pp. 97–99. [Google Scholar]
- Benbow M. Assessment, prevention and management of skin tears. Nurs Older People. 2017;29:31–39. doi: 10.7748/nop.2017.e904. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Koxholt I, Thiemann K, Nischt R (2013) Skin basement membrane: the foundation of epidermal integrity--bm functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res Int 2013:179784 [DOI] [PMC free article] [PubMed]
- Bruckner-Tuderman L. Hereditary skin diseases of anchoring fibrils. J Dermatol Sci. 1999;20:122–133. doi: 10.1016/S0923-1811(99)00018-3. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053X(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-D. [DOI] [PubMed] [Google Scholar]
- Clark RA, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Investig Dermatol. 1982;79:264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Dellambra E, Vailly J, Pellegrini G, Bondanza S, Golisano O, Macchia C, Zambruno G, Meneguzzi G, De Luca M. Corrective transduction of human epidermal stem cells in laminin-5-dependent junctional epidermolysis bullosa. Hum Gene Ther. 1998;9:1359–1370. doi: 10.1089/hum.1998.9.9-1359. [DOI] [PubMed] [Google Scholar]
- Fine JD, Redmar DA, Goodman AL. Sequence of reconstitution of seven basement-membrane components following split-thickness wound induction in primate skin. Arch Dermatol. 1987;123:1174–1178. doi: 10.1001/archderm.1987.01660330085015. [DOI] [PubMed] [Google Scholar]
- Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Investig Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Vailly J, Durand-Clement M, Wagner E, Ortonne JP, Meneguzzi G. Functional re-expression of laminin-5 in laminin-gamma2-deficient human keratinocytes modifies cell morphology, motility, and adhesion. J Biol Chem. 1996;271:18437–18444. doi: 10.1074/jbc.271.31.18437. [DOI] [PubMed] [Google Scholar]
- Ghohestani RF, Li K, Rousselle P, Uitto J. Molecular organization of the cutaneous basement membrane zone. Clin Dermatol. 2001;19:551–562. doi: 10.1016/S0738-081X(00)00175-9. [DOI] [PubMed] [Google Scholar]
- Ko MS, Marinkovich MP. Role of dermal-epidermal basement membrane zone in skin, cancer, and developmental disorders. Dermatol Clin. 2010;28:1–16. doi: 10.1016/j.det.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Langton AK, Halai P, Griffiths CE, Sherratt MJ, Watson RE. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech Ageing Dev. 2016;156:14–16. doi: 10.1016/j.mad.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Investig. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minner F, Poumay Y. Candidate housekeeping genes require evaluation before their selection for studies of human epidermal keratinocytes. J Investig Dermatol. 2009;129:770–773. doi: 10.1038/jid.2008.247. [DOI] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD, 2nd, Jones JC, Rheinwald JG. Co-expression of p16ink4a and laminin 5 by keratinocytes: a wound-healing response coupling hypermotility with growth arrest that goes awry during epithelial neoplastic progression. J Investig Dermatol Symp Proc. 2005;10:72–85. doi: 10.1111/j.1087-0024.2005.200415.x. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–562. doi: 10.1016/S0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Orringer JS, Kang S, Johnson TM, Karimipour DJ, Hamilton T, Hammerberg C, Voorhees JJ, Fisher GJ. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol. 2004;140:1326–1332. doi: 10.1001/archderm.140.11.1326. [DOI] [PubMed] [Google Scholar]
- Rittié L. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal. 2016;10:103–120. doi: 10.1007/s12079-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding egf and amphiregulin in human skin in vivo. J Investig Dermatol. 2006;126:732–739. doi: 10.1038/sj.jid.5700202. [DOI] [PubMed] [Google Scholar]
- Rittié L, Kansra S, Stoll SW, Li Y, Gudjonsson JE, Shao Y, Michael LE, Fisher GJ, Johnson TM, Elder JT. Differential erbb1 signaling in squamous cell versus basal cell carcinoma of the skin. Am J Pathol. 2007;170:2089–2099. doi: 10.2353/ajpath.2007.060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Kang S, Voorhees JJ, Fisher GJ. Induction of collagen by estradiol: difference between sun-protected and photodamaged human skin in vivo. Arch Dermatol. 2008;144:1129–1140. doi: 10.1001/archderm.144.9.1129. [DOI] [PubMed] [Google Scholar]
- Rittié L, Stoll SW, Kang S, Voorhees JJ, Fisher GJ. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738–751. doi: 10.1111/j.1474-9726.2009.00526.x. [DOI] [PubMed] [Google Scholar]
- Rittié L, Perbal B, Castellot JJ, Jr, Orringer JS, Voorhees JJ, Fisher GJ. Spatial-temporal modulation of ccn proteins during wound healing in human skin in vivo. J Cell Commun Signal. 2011;5:69–80. doi: 10.1007/s12079-010-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;182:163–171. doi: 10.1016/j.ajpath.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Orringer JS, Sachs DL, Voorhees JJ, Fisher GJ. Eccrine sweat gland-derived keratinocytes rapidly express epidermal differentiation markers during repair of human wounds. J Investig Dermatol. 2013;133:S251–S251. [Google Scholar]
- Rittié L, Farr EA, Orringer JS, Voorhees JJ, Fisher GJ. Reduced cell cohesiveness of outgrowths from eccrine sweat glands delays wound closure in elderly skin. Aging Cell. 2016;15:842–852. doi: 10.1111/acel.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Beck K. Laminin 332 processing impacts cellular behavior. Cell adhesion and. Migration. 2013;7:122–134. doi: 10.4161/cam.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Christiano AM, Engvall E, Wewer UM, Miner JH, Sanes JR, Burgeson RE. The functions of laminins: lessons from in vivo studies. Matrix Biol. 1996;15:369–381. doi: 10.1016/S0945-053X(96)90157-2. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Alvarez OM, Bere EW, Jr, Eaglstein WH, Katz SI. Detection of basement membrane zone antigens during epidermal wound healing in pigs. J Investig Dermatol. 1981;77:240–243. doi: 10.1111/1523-1747.ep12480082. [DOI] [PubMed] [Google Scholar]
- Strazzieri-Pulido KC, Peres GR, Campanili TC, de Gouveia Santos VL. Incidence of skin tears and risk factors: a systematic literature review. Journal of wound, Ostomy and continence. Nursing. 2017;44:29–33. doi: 10.1097/WON.0000000000000288. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and -511 in skin. Exp Dermatol. 2008;17:473–480. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen vii tightly binds to banded collagen fibrils. J Biol Chem. 2008;283:24506–24513. doi: 10.1074/jbc.M802415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MG, Hunter R. Studies on epidermal regeneration by means of the strip method. J Investig Dermatol. 1957;29:407–413. doi: 10.1038/jid.1957.116. [DOI] [PubMed] [Google Scholar]
- Winter GD. Epidermal regeneration studied in the domestic pig. In: Maibach HI, Rovee DT, editors. Epidermal wound healing. Chicago: Year Book Medical Publishers; 1972. pp. 71–112. [Google Scholar]
- Yang S, Sun Y, Geng Z, Ma K, Sun X, Fu X. Abnormalities in the basement membrane structure promote basal keratinocytes in the epidermis of hypertrophic scars to adopt a proliferative phenotype. Int J Mol Med. 2016;37:1263–1273. doi: 10.3892/ijmm.2016.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]