Abstract
Hepatocyte exosomes (ExoHep) are proposed to mediate physiological or pathophysiological signaling in a variety of hepatic target cells. ExoHep were purified from the medium of primary mouse hepatocytes or AML12 cells and characterized as ~100 nm nanovesicles that were positive for proteins commonly found in exosomes (CD9, CD81, flotillin) or hepatocytes (asialoglycoprotein receptor). Ethanol treatment of hepatocytes caused increased ExoHep release and increased cellular mRNA expression of components involved in intracellular vesicle trafficking (Rab 5a,b,c, Rab 7a, Rab 27a,b) or exosome biogenesis via the ESCRT (HGS, Alix, STAM1, TSG101, VTA1, YKT6) or ceramide (nSmase2) pathways. RNA interference of HGS, Alix, TSG101 or nSmase 2 caused exosome production by normal or ethanol-treated hepatocytes to be reduced. In mice, in vivo administration of fluorescently-labeled ExoHep resulted in their accumulation in the liver and preferential localization to hepatic stellate cells (HSC) or hepatocytes, the latter of which showed enhanced ExoHep binding when isolated from fibrotic mice. In cell co-cultures, the intercellular transfer of RNA from hepatocytes to hepatocytes or HSC was blocked by the exosome inhibitor GW4869. ExoHep binding to HSC or hepatocytes occurred via mechanisms that involved heparin-like molecules and cellular integrin αv or β1 subunits , and resulted in a reversal of fibrosis-associated gene expression in HSC and of ethanol-induced damage in hepatocytes. These studies provide insight regarding the regulation and/or participation of exosome biogenesis or trafficking components in hepatocytes and show that ExoHep can mediate therapeutic changes in activated HSC or injured hepatocytes that occur downstream of heparin- or integrin-dependent binding interactions.
Keywords: Alcohol, Ceramide, ESCRT, Extracellular vesicle, Hepatic stellate cell, Liver
Introduction
Extracellular vesicles (EVs) are produced by many if not all cell types and consist of exosomes, microvesicles, and apoptotic bodies (Gyorgy et al. 2011; Raposo and Stoorvogel 2013). Recent research on exosomes and microsomes has established their central role in cell-cell communication and the possibility of exploiting components of their molecular cargo for disease diagnosis or treatment (Thery 2011; Stremersch et al. 2016; Maas et al. 2017). Exosomes and microvesicles contain a broad spectrum of microRNAs, mRNAs and proteins that are protected from extracellular degradation and which reflect the phenotypic status of their respective producer cells (Raposo and Stoorvogel 2013). Both types of EV are able to mediate cell-cell transfer of their respective molecular payloads, resulting in transcriptional or translational modifications in the recipient cells that cause them to undergo phenotopic and functional changes (Thery 2011). Despite these shared properties, EVs have distinct pathways of biogenesis in that microvesicles are formed by budding of the plasma membrane whereas exosomes arise by inward budding of multivesicular bodies (MVB) which then fuse with the plasma membrane causing their internal vesicles to be released as exosomes from the cell (Gyorgy et al. 2011; Raposo and Stoorvogel 2013). Exosomes are typically smaller (50 – 200 nm) than microvesicles (200-1000 nm) but additional features such as sedimentation characteristics or protein expression (e.g. tetrapanins such as CD81 which is considered exosome-specific (Kowal et al. 2016)) are essential for correct identification.
Exosome biogenesis involves pathways that are either dependent or independent on endosomal sorting complex required for transport (ESCRT) machinery. In the ESCRT-dependent pathway, at least 20 proteins are involved in the formation of complexes termed ESCRT-0, −I, −II, and -III that act with other accessory proteins to drive MVB formation by invagination and scission of endosomal membranes (Henne et al. 2011). Certain ESCRT components such as TSG101 (ESCRT-1) or Alix (an ESCRT accessory protein) are commonly found in exosomes themselves, consistent with them having originated via the ESCRT pathway. Combined suppression of key ESCRT subunits to deplete the ESCRT pathway does not completely block MVB formation suggesting that other mechanisms contribute to MVB (and exosome) production (Stuffers et al. 2009). One such ESCRT-independent pathway involves incorporation of ceramide into the endosomal membrane and the subsequent development of lipid rafts that favor inward budding of the membrane (Trajkovic et al. 2008). Ceramide is generated from sphingomyelin via the action of neutral sphingomyelinase 2 (nSmase2) and inhibition of this enzyme with the drug GW4869 or by gene knockdown has been shown to block exosome release or exosome-mediated signaling in some cell types (Chairoungdua et al. 2010; Kosaka et al. 2010; Li et al. 2013; Charrier et al. 2014; Chen et al. 2014; Lang et al. 2016). However, the roles played by the many components of the ESCRT-dependent and ceramide- dependent pathways are very complex and the relative contribution of each pathway is likely dependent on the individual cell type as well as contextual and environmental factors. Additionally, many small Rab GTPases play important roles in exosome production because they regulate intracellular trafficking of endosomes (from which MVB originate) as well as transport of vesicles to the plasma membrane and their subsequent release from the cell (Blanc and Vidal 2017). There is considerable cell- or context-specificity in the regulation of vesicle trafficking of Rabs, of which at least 30 different isoforms have been implicated in exosome biogenesis by virtue of their detection in exosomes themselves.
The production and action of EVs within the liver likely represents a central regulatory system that ensures its many functions are correctly orchestrated and that, on the other hand, may drive pathophysiological pathways during times of organ damage, infection, or disease (Maji et al. 2017; Szabo and Momen-Heravi 2017). In view of the central role of hepatocytes as mediators of many hepatic functions, EVs produced by this cell type have attracted attention for their role in liver physiology and pathology. Even so, fundamental questions still remain regarding mechanisms regulating hepatocyte EV production and understanding their target cell repertoire, receptors, and actions. In this study we describe the biogenic pathways involved in the production of hepatocyte exosomes (ExoHep) and the manner in which components of these pathways are regulated in response to toxic stimuli such as ethanol. We also show that in vivo administration of ExoHep results in their accumulation in the liver where they preferentially bind to hepatic stellate cells (HSC, a normally quiescent cell type that becomes activated and highly fibrogenic after liver injury) or hepatocytes, the latter of which show enhanced ExoHep binding during fibrotic injury. We further show that ExoHep binding to these target cells involves integrin-like and heparin-like interactions and results in attenuation of activation- and fibrogenesis-related gene expression in activated HSC and of ethanol-mediated cytotoxicity in hepatocytes.
Materials and methods
Experimental liver fibrosis
Animal protocols were approved by the Institutional Animal Care and Use Committee of Nationwide Children’s Hospital (Columbus, OH). Liver fibrosis was established in male Swiss Webster mice (6–8 wk) (n = 10) by i.p. administration of carbon tetrachloride (CCl4; 175 μl in 1325 μl corn oil /kg; Sigma-Aldrich, St Louis, MO) three times per week for 5 weeks. Control mice received i.p. corn oil (1500 μl corn oil /kg) alone. Control or CCl4 -treated mice received a single tail vein injection of 40 μg of PKH26- or PKH67-labeled hepatocyte exosomes (PKH26-ExoHep; PKH67-ExoHep; see below) and liver lobes were harvested four hours later either for fluorescence imaging using a Xenogen IVIS 200 instrument (PerkinElmer, Waltham, MA) or for isolation of the principal hepatic cell types.
Cell cultures
Livers from Swiss Webster mice were perfused and digested with pronase/collagenase I for isolation of HSC which were purified by Opti-prep buoyant density centrifugation as described (Chen et al. 2011). Alternatively, mouse livers were perfused and digested with collagenase I, homogenized, sieved, centrifuged and subjected to Percoll density centrifugation as described (Chen et al. 2016). Hepatocytes were collected in the pellet while cells at the 25/50% Percoll interface were stained with antibodies to CD45 (APC Fire750, 30-F11, Biolegend, San Diego, CA), CD31 (APC, 390, Biolegend), CD146 (PE, ME-9F1, Biolegend), F4/80 (APC, BM8, Biolegend), and CD11b (PECy7, M1/70, BD Pharmingen, San Diego, CA), allowing luminal sinusoidal endothelial cells (LSEC) and Kupffer cells (KC) to be separated by flourescence activated cell sorting with an Influx cell sorter (BD Biosciences, San Jose, CA). Purity of each cell subset was verified to be >95% (Chen et al. 2016). The various hepatic cell types from PKH67-ExoHep-injected mice were collected by using Thermo Electron Shandon cytospins (Thermo Fisher Scientific, Waltham, MA), stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI), and immediately analyzed by confocal microscopy for PKH67 and DAPI. HSC from control mice that underwent autonomous activation over the first 7–10 days of primary culture were maintained up to passage 6 in DMEM/F12 medium containing 10% fetal bovine serum (FBS), with a split ratio of 1:3 every 5 days. Hepatocytes were cultured in complete William E medium (Gibco, Billings, MT) containing 10% FBS or 1% FBS, the latter of which had been depleted of its instrinsic exosome components prior to use by serial step-wise centrifugation and ultra-centrifugation steps as described (Shelke et al. 2014). AML12 mouse hepatocytes (American Type Culture Collection, Manassas, VA) were cultured in DMEM/F12/10% FBS supplemented with insulin, transferrin, selenium and dexamethasone (Chen et al. 2015).
Hepatocyte exosome purification
Primary hepatocytes were cultured for 48 h in complete William E medium containing 1% exosome-depleted FBS while AML12 mouse hepatocytes were grown under serum-free conditions for 48 h after which the conditioned medium was subjected to two low-speed centrifugation steps (300×g for 15 min; 10,000×g for 30 min, 4 °C). The supernatant was filtered (0.22 μm; Merck, Darmstadt, Germany) and centrifuged at 10,000×g for 30 min at 4 °C from which the supernatant was the subjected to two sequential ultracentrifugation steps (100,000×g for 90 mins at 4 °C) in a T-70i fixed-angle rotor (Beckman Coulter, Brea, CA, US)(Chen et al. 2014; Chen and Brigstock 2016; Chen et al. 2016). This procedure ensures uniformity of purified vesicles in the resulting pellet and prevents contamination by larger microvesicles (400-1000 nm) or apoptotic bodies (1-5 μm) (Gyorgy et al. 2011). The pellet was resuspended in phosphate-buffered saline (PBS) and verified to contain exosomes (see below). For some experiments, AML12 exosomes were isolated using PureExo® kits (101Bio, Palo Alto, CA) according to the manufacturers’ directions (Chen et al. 2015). Exosomes from primary cultured hepatocytes or AML12 cells are hereafter termed “ExoHep”. In some cases, exosomes were also isolated from AML12 cells that had been exposed to 10 ng/ml tumor necrosis factor alpha (TNFα) for 48 h, the latter 24 h of which also included incubation in the presence of 50 mM ethanol. These exosomes were termed “ExoHep-TNFα/EtOH”.
Exosome characterization
ExoHep were diluted to 107–108 particles/ml in PBS for nanoparticle tracking analysis (NTA) using a Nanosight 300 (Malvern Instruments, Westborough, MA). Exosomes were also characterized by transmission electron microscopy (TEM) as described (Chen et al. 2014) or by Western blot analysis as described below. Fluorescence activated nanoparticle sorting of ExoHep with Cy3-labeled anti- asialoglycoprotein receptor 1 (ASPGR1; GeneTex, Irvine, CA) was accomplished using the small particle detection capability of the Influx cell sorter (see above) that had been calibrated using 50-1000 nm diameter latex beads (Sigma Aldrich). For organ localization and cell binding studies, ExoHep were labeled with the fluorescent lipophilic membrane dyes PKH26 (red) or PKH67 (green), according to the manufacturer’s specifications (Sigma-Aldrich) (Chen et al. 2014).
Exosome binding assays
PKH26-ExoHep were added (0-8 μg/ml) for up to 24 h to P4 mouse HSC or AML12 hepatocytes which were then washed in PBS and imaged using a confocal microscope (Zeiss, Obercochen, Germany) or lysed in lysis buffer (Boston Bioproducts, Ashland, MA) and measured at 590/540 nm using a Spectra Max® M2 microplate reader (VWR, Atlanta, GA), with normalization to total cellular protein to assess cell-associated PKH26 flourescence. In some experiments, target cells were co-incubated with 0–100 μg/ml RGD or RGE tripeptides (American Peptide, Sunnyvale CA), 0–100 μg/ml heparin or chondroitin sulfate (Sigma-Aldrich) or 0–100 μM ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich), or were pre-treated with 0–10 μg/ml rabbit anti-mouse integrin αvβ3 IgG (Bioss Inc., Woburn, MA), 0–10 μg/ml rat anti-mouse integrin α5β1 IgG (Millipore, Temecula, CA), or 0-10 μg/ml non-immune IgG (Santa Cruz Biotechnology, Dallas, TX). siRNA-mediated knockdown of specific integrin components was achieved as described below.
Effect of ExoHep on HSC or hepatocytes in vitro
P2–3 mouse HSC were seeded in 6-well plates for 24 h in DMEM / 10% FBS, cultured in serum-free medium for 12–24 h and treated for up to 24 h with 0–8 μg/ml ExoHep or ExoHep-TNFα/EtOH that had been isolated from primary hepatocytes. Expression of connective tissue growth factor (CCN2), alpha smooth muscle actin (αSMA) or collagen α1(I) mRNA was determined by quantitative real-time polymerase chain reaction (qRT-PCR; see below) and the presence of αSMA after a 48-h exposure to 10 ng/ml transforming growth factor beta (TGF-β) under serum-free conditions was determined by immunocytochemistry using anti-αSMA (1:1000; Dako Cytomation, Glostrup, Denmark). AML12 cells were incubated for 24 h in the presence or absence of 50 mM ethanol and/or 8 μg/ml ExoHep and metabolic activity was assessed by the ability of the cells to reduce the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to its formazan using a commercial kit (Thermo Fisher ).
Uptake of exosomal SYTORNA SelectTM into HSC or hepatocytes
AML12 cells were treated with 500 nM SYTO RNASelect™ Green Fluorescent Cell Stain (Thermo Fisher) for 1 h. Using a modification of our previously described co-culture system (Chen et al. 2014; Chen et al. 2015), the cells were then placed on one side of a 2-well μ-dish system (Ibidi, Martinsried, Germany) and the other chamber was seeded with PKH26-stained HSC or hepatocytes. Upon removal of the central barrier, cells were allowed to directly communicate for 24 h, after which recipient HSC or hepatocytes were evaluated for uptake of SYTO RNASelectTM by confocal microscopy. Some experiments were performed in the presence of 10 μM GW4869 (Sigma-Aldrich), an inhibitor of neutral sphingomyelinase2 (nSmase2) which is required for ceramide biosynthesis (Chairoungdua et al. 2010; Kosaka et al. 2010). In an alternative approach, SYTO-RNASelect™-loaded ExoHep were purified from the medium of SYTO-RNASelect™-treated AML12 cells and incubated (8 μg/ml) with AML12 cells or HSC for 12 h prior to evaluating SYTO RNASelectTM uptake into the target cells by confocal microscopy.
Exosome biogenic pathways
AML12 cells were incubated in serum-free medium for 12 h after which the medium was removed and replaced with serum-free medium containing 0-75 mM ethanol. After 24 h, exosomes in conditioned medium were purified as described above and characterized by NTA. The effects of ethanol on production of components of the ceramide-dependent or ESCRT-dependent pathways of exosome production were evaluated by qRT-PCR or Western blot as described below. The role of the ceramide-dependent pathway was established by treating target cells with 10 μM GW4869 or with nSmase2 small interfering RNA (siRNA), while the role of the ESCRT pathway was established using siRNA to Alix, HRS or TSG101.
siRNA-mediated knockdown of exosome biogenic pathway components or integrins
siRNA to target molecules or negative controls were purchased from Thermo Fisher or Biolegend. To avoid off-target effects, the siRNA preparations consisted of 3 target-specific 20–25 nt siRNAs. 105–106 AML12 cells were transfected with 100 nM siRNA by electroporation using a Nucleofector Kit (Lonza, Koln, Germany) and incubated for 12 h in medium containing 10% FBS which was then replaced with serum-free fresh medium. The effect of si-nSmase2, si-Alix, si-HRS or si-TSG101 on exosome release by the cells after 24 h under serum-free conditions or after treatment with 50 mM ethanol was established by NTA of conditioned medium. The effect of si-integrin αv or si-integrin β1 on exosome binding to HSC or hepatocytes was determined using PKH26-ExoHep binding assays. Knockdown was confirmed by qRT-PCR or Western blot analysis.
RNA extraction and qRT-PCR
Total RNA was extracted from AML12 cells or HSC cells using a microRNeasy Plus kit (Qiagen, Valencia, CA) and reverse-transcribed using a miScript II RT kit (Qiagen) according to the manufacturers’ protocols. Transcripts were evaluated by qRT-PCR using an Eppendorf Mastercycler System and SYBR Green Master Mix (Eppendorf, Hauppauge, NY). Primers are shown in Table 1. Each reaction was run in triplicate, and all samples were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Negative controls were a non-reverse transcriptase reaction and a non-sample reaction.
Table 1.
Primers used for RT-PCR
| Gene (mouse) | GenBank accession number | Primers | Product size (bp) | |
|---|---|---|---|---|
| Sense | Anti-sense | |||
| CCN2 | NM_010217 | 5′ CACTCTGCCAGTGGAGTTCA 3′ | 5′ AAGATGTCATTGTCCCCAGG 3′ | 111 |
| Collagen α1(I) | NM_007742 | 5′ GCCCGAACCCCAAGGAAAAGAAGC 3’ | 5′ CTGGGAGGCCTCGGTGGACATTAG 3’ | 148 |
| αSMA | NM_007392 | 5’GGCTCTGGGCTCTGTAAGG3’ | 5’CTCTTGCTCTGGGCTTCATC3’ | 148 |
| Integrin αv | NM_008402 | 5′ ACATCACCTGGGGCATTCAG 3’ | 5′ GTGAACTTGGAGCGGACAGA3’ | 251 |
| Integrin β1 | NM_010578 | 5′ AATGTTTCAGTGCAGAGCC3’ | 5′ TTGGGATGATGTCGGGAC3’ | 254 |
| Rab5a | AB_232593 | 5′ TCTGCTGTTGGCAAATCAAG3’ | 5′ TCTCGCAAAGGATTCCTCAT3’ | 246 |
| Rab5b | AB_232594 | 5’GAGAGTCTGCAGTGGGGAAG 3’ | 5’CAGCAGTGTCCCAGATCTCA 3’ | 153 |
| Rab5c | AB_232595 | 5’CAAGCAGCCATTGTGGTCTA3’ | 5’GCTTCCTGAAACTCCACAGC3’ | 164 |
| Rab7a | NM_001293652 | 5’GGCCTTCTACAGAGGTGCAG3’ | 5’TCTTTGTGGCCACTTGTCTG3’ | 188 |
| Rab27a | AB_232594 | 5’TTCCTGCTTCTGTTCGACCT3’ | 5’GGCAGCACTGGTTTCAAAAT3’ | 213 |
| Rab27b | AB_232623 | 5’CAACTGCAGGCAAATGCTTA3’ | 5’GTGTCCGGAACCTGTGTCTT3’ | 245 |
| nSmase2 | AJ_250461 | 5’CACCAACACCTCCATCAGTG3’ | 5’GTCAGCCTGTTCTCCAGAGG3’ | 181 |
| HGS | NM_001159328 | 5′ AGCGCCTACATGTACCCAAC3’ | 5′ TCTGTGGAGGCTGTGAGATG3’ | 175 |
| Alix | NM_011052 | 5′ TACCTGAGCTGCTGCAAAGA3’ | 5′ GTAGCGCTCCTTCACCTGTC3’ | 221 |
| STAM1 | BC_055326 | 5′ GGGACTGTGGCTACCAAAAA3’ | 5′ ATTATCCTCAGCGGCTTCAA3’ | 189 |
| TSG101 | U_52945 | 5′ CCAGTGGTTATCCTGGCTGT3’ | 5′ ATCCGCCATCTCAGTTTGTC3’ | 181 |
| VTA1 | NM_025418 | 5′ CCAGCTGTTGATCCTGACCT3’ | 5′ AAACACTGGCTTCCGTGAAC3’ | 210 |
| YKT6 | NM_019661 | 5′ GAGGCGAGAAGCTTGATGAC3’ | 5′ AGCATCCCTCCATTCCTCTT3’ | 246 |
| GAPDH | NM_002046 | 5′ TGCACCACCAACTGCTTAGC 3’ | 5′ GGCATGGACTGTGGTCATGAG 3’ | 66 |
Western blot
Sodium dodecyl polyacrylamide gel electrophoresis analysis was performed on proteins (10 μg) extracted by detergent-solubilization of ExoHep or that were present in lysates of AML12 cells or HSC. The separated proteins were transferred to nitrocelluose membranes which were then incubated with antibodies to CD81, (1:400; ProSci, Poway, CA), CD9 (1:300; Lifespan Bioscience Inc., Seattle WA), flotillin-1 (1:500; Abcam, Cambridge, MA), anti-αSMA (Dako), integrin αvβ3 (Millipore, Burlington MA), integrin α5β1(Millipore), nSmase2 (Abcam), HGS (Thermo Fisher), Alix (Thermo Fisher), TSG101 (Thermo Fisher) or β-actin (Abcam). Blots were developed using enhanced chemoluminescence and signals were normalized relative to β-actin.
Statistical analysis
All experiments were performed at least three times with triplicate measurements, with data expressed as mean ± s.e.m. Fluorescence images were scanned and quantified using Image J software (NIH, Bethesda, MD). Data from qRT-PCR, NTA, and imaging were analyzed by student’s t-test using Sigma plot 11.0 software (SPSS Inc., Chicago, IL). P values <0.05 were considered statistically significant.
Results
Characterization of hepatocyte exosomes
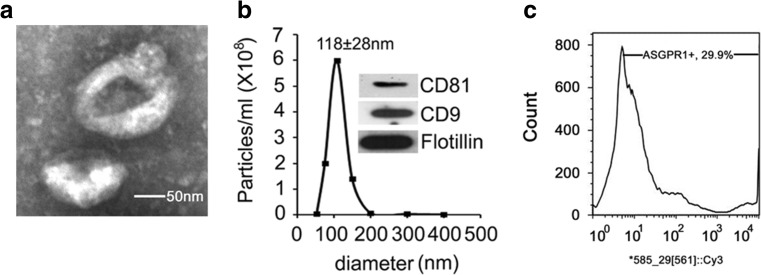
Extracellular vesicles from AML12 mouse hepatocytes were isolated from conditioned medium collected from cells that were maintained under serum-free conditions for up to 48 h. Processing of the medium using multiple sequential centrifugation steps or PureExo® kits resulted in the generation of samples that exhibited key properties of exosomes, including their appearance as ~100 nm cup-shaped vesicles as assessed by TEM (Fig. 1a), mean size of 118 ± 28 nm as determined by NTA (Fig. 1b) and the presence of CD81, CD9 and flotillin as assessed by Western blot (Fig. 1b, inset). Exosomes were also positive for ASGPR1, which is an abundant hepatocyte protein (Fig.1c). Similar data were obtained for exosomes from primary mouse hepatocytes (data not shown). Thus, the principal hepatocyte vesicles isolated for this study had properties consistent with their identity as exosomes and are hereafter termed “ExoHep”.
Fig. 1.
Characterization of ExoHep. EVs purified from AML12 conditioned medium were subjected to (a) TEM (scale bar: 50 nm), (b) NTA (the inset is a Western blot for exosomal proteins), or (c) fluorescence-activated nanoparticle sorting using Cy3 anti-ASGPR1
Effect of ethanol on production of exosome biogenic components and exosome secretion by hepatocytes
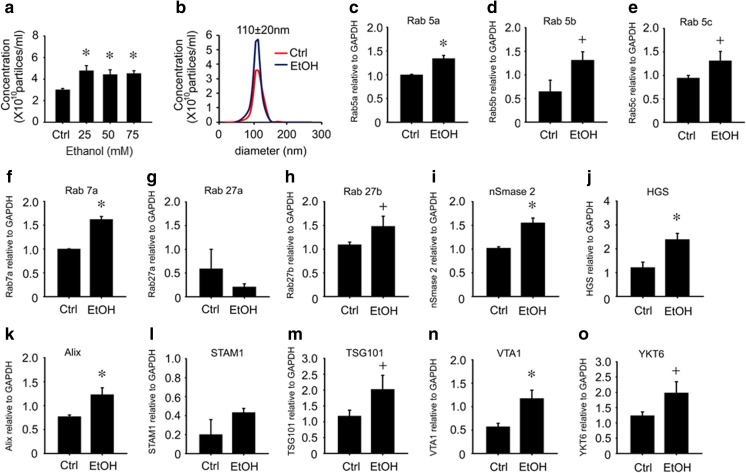
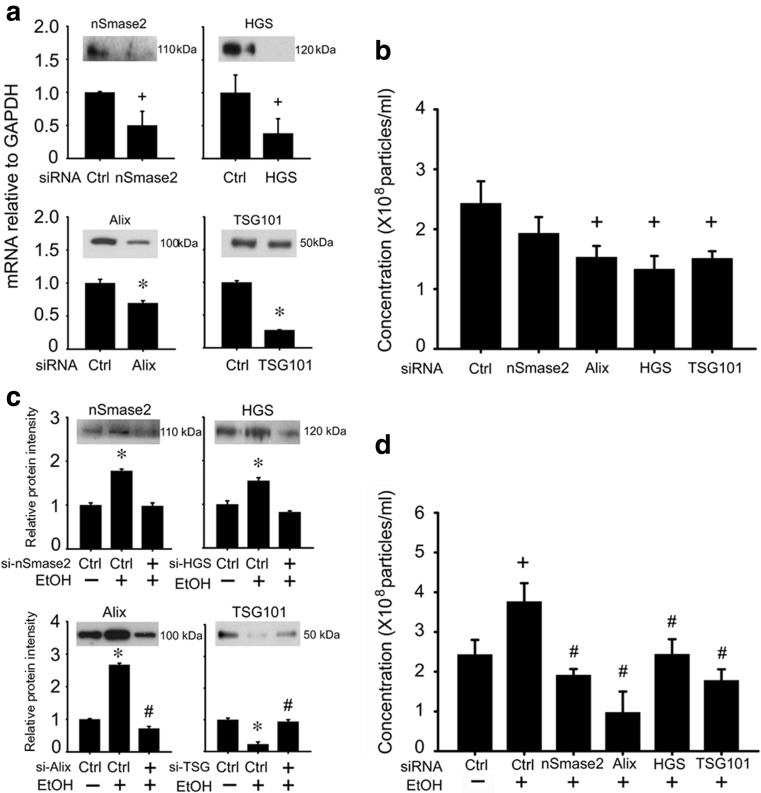
AML12 cells were placed in serum-free medium for 12 h, and the medium was then replaced with serum-free medium containing 0-75 mM ethanol for the next 24 h. NTA of the conditioned medium showed that ethanol increased the concentration of exosomes by about 1.5-fold, but did not change their mean size or size-range (Fig. 2a,b). Quantitative RT-PCR of vesicle trafficking Rab GTPases (Rab 5a,b,c, Rab 7a, Rab 27a,b) or of components of the ceramide (nSmase2) or ESCRT (HGS, Alix, STAM1, TSG101, VTA1, YKT6) exosome biogenesis pathways showed that the expression of all mRNAs were significantly enhanced by ethanol except STAM1 (which showed an upward but non-significant response to ethanol) and Rab 27a (which was decreased by ethanol) (Fig. 2c-o). To verify that some of these components were functionally involved in ExoHep production under basal conditions or in response to ethanol, an siRNA approach was adopted to block specific components that are known to play a key role in other cell types (Baietti et al. 2012; Colombo et al. 2013). Under basal conditions, siRNA to nSmase2, HGS, or Alix reduced expression of their respective targets in AML12 hepatocytes, resulting in reduced mRNA and/or protein expression (Fig. 3a) as well as reduced ExoHep production as assessed by NTA, except in the case of si-nSmase2 which caused a downward trend in ExoHep frequency but this was not significant (Fig. 3b). In response to ethanol, protein levels of nSmase2, HGS or Alix were increased (Fig. 3c), consistent with their corresponding mRNA levels (Fig. 2i-k) and treatment with individual siRNAs caused their ethanol-stimulated protein levels to be attenuated (Fig. 3c) along with suppressed exosome production (Fig. 3d). Whereas si-TSG101 also reduced basal or ethanol-stimulated exosome production (Fig. 3b,d), the underlying TSG101 expression pattern appeared discordant. For example, TSG101 mRNA levels were stimulated by ethanol (Fig. 2m) whereas TSG101 protein levels were suppressed (Fig. 3c). Additionally si-TSG101 transfection resulted, first, in substantially suppressed TSG101 mRNA levels but only slight diminution of TSG101 protein levels under basal conditions (Fig. 3a) and, second, a restoration of TSG101 protein levels that had been suppressed by ethanol (Fig. 3d).
Fig. 2.
Expression of exosome biogenic and trafficking components. NTA of exosomes secreted by AML12 cells, showing effect of (a) 0-75 mM ethanol on ExoHep concentration or (b) 50 mM ethanol on ExoHep concentration, with no change in mean size (110 nm ± 20 nm). (c-o) qRT-PCR for the indicated mRNAs for exosome trafficking or biogenesis components in AML12 cells, with or without 24-h treatment of the cells with 50 mM ethanol (n = 9, + p < 0.05 vs. Ctrl; * p < 0.001 vs. Ctrl, student’s test)
Fig. 3.
Role of components of the ceramide or ESCRT pathways in exosome production in AML12 cells. a AML12 cells were transfected for 24 h with siRNA for nSmase2, HGS, Alix or TSG101 and were analyzed for expression of each target by qRT-PCR (n = 9, * p < 0.001 vs. ctrl siRNA, + p < 0.05 vs. ctrl siRNA, student’s test). The insets show Western blots for the corresponding proteins using anti-nSmase2, anti-HGS, anti-Alix or anti TSG101 for which β-actin was used a loading control (not shown); data are representative of 3 individual experiments. b NTA of exosomes from a (n = 3, + p < 0.05 vs. ctrl siRNA student’s test). c Western blot detection of nSmase2, HGS, Alix or TSG101 in AML12 cells that were treated with or without 50 mM ethanol for 24 h and that had been transfected with the indicated siRNAs. β-actin was used as loading control (not shown). Data shown are images of representative blots of 3 individual experiments (upper panels) or quantified densitometric scans (lower panels) (n = 3, *p < 0.001 vs. ctrl siRNA without ethanol; #p < 0.05 vs. ctrl siRNA with ethanol, student’s test). d NTA of exosomes from c. (n = 3, + p < 0.05 vs. ctrl siRNA without ethanol; #p < 0.05 vs ctrl siRNA with ethanol, student’s test). “Ctrl” represents cells treated with a scramble siRNA sequence
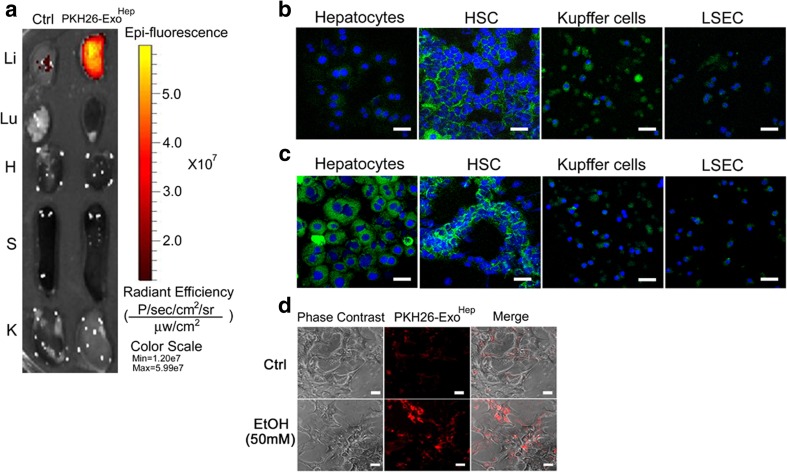
To determine targets of ExoHep binding in vivo, 40 μg PKH26-ExoHep or PKH67-ExoHep were injected i.v. into Swiss Webster mice. After four hours, the major organs were harvested and directly imaged with the result that the fluorescent signal was localized exclusively to the liver (Fig. 4). Isolation and confocal imaging of the main hepatic cell types revealed PKH67 fluorescence to be principally associated with HSC (quiescent) with weaker but appreciable staining of the hepatocytes as well (Fig. 4b). Weak staining was observed for LSECs or KCs. Next, we determined the effect of in vivo liver injury on PKH67-ExoHep distribution among these cells types. Exposure of mice to repeated i.p. injections of CCl4 for 5 weeks, which causes chronic liver injury and hepatic fibrosis, resulted in substantially increased levels of PKH67-ExoHep binding to hepatocytes as well as appreciable binding to HSC (activated; see below). Binding of PKH67-ExoHep to KC or LSEC was relatively weak and comparable to non-injured animals (Fig. 4c) Thus in control or fibrotic animals, the principal targets of ExoHep are HSC (quiescent or activated) or hepatocytes, the latter of which show enhanced ExoHep binding after exposure to injury. Similarly, binding of PKH26-ExoHep to hepatocytes in vitro was increased after the target cells had been exposed to ethanol (Fig. 4d).
Fig. 4.
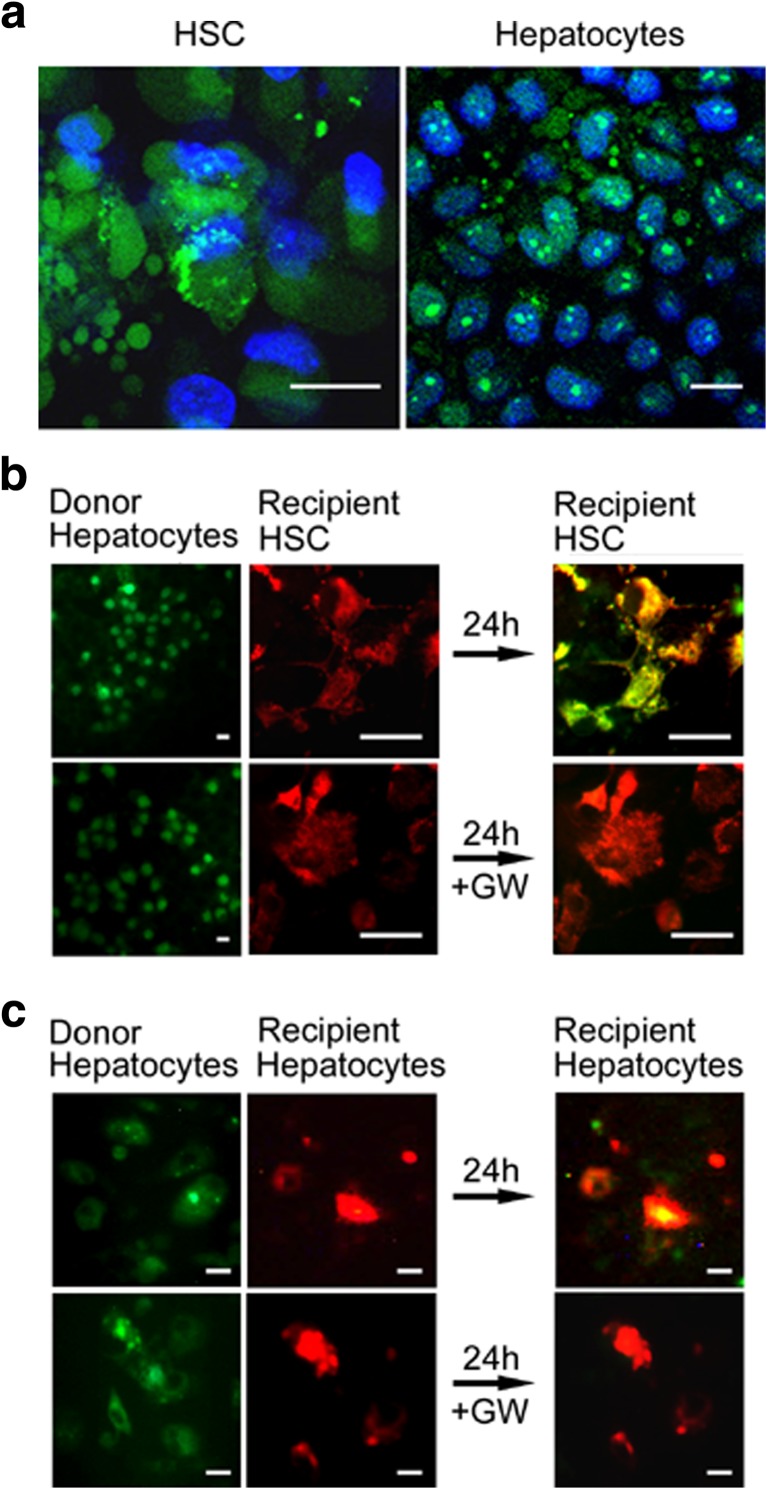
Identification of HSC and hepatocytes as in vivo targets of ExoHep. a 40 μg PKH26-ExoHep were injected i.v. into normal Swiss Webster mice and 4 h later the major organs were removed and imaged for the presence of PKH26 fluorescence which accumulated principally in the liver (Li), with no appreciable accumulation in the lung (Lu), heart (H), spleen (S) or kidney (K). “Ctrl” shows liver from a mouse that was not injected with exosomes. Red/Yellow is PKH26. Data are representative of 3 individual experiments. b Confocal microscopy of cytospin preparations of purified DAPI-stained hepatocytes, HSC, KC or LSECs from control mice that were injected with PKH67-ExoHep essentially as shown in a. Green is PKH67; Blue is DAPI. In the case of KC, some PKH67 foci were not associated with DAPI and the cellular nature of this signal was thus not confirmed. c Same as b except cells were recovered from mice that had received chronic CCl4 administration for 5 weeks to induce liver fibrosis. Green is PKH67; Blue is DAPI. Cells from b and c were harvested on the same day to allow direct comparison of PKH67-ExoHep binding to each cell type from each group of mice (n = 5). Data shown are representative of 3 individual experiments. d Binding of PKH26-ExoHep (8 μg/ml) after 24 h incubation with Day 1 primary mouse hepatocytes cultured in the presence or absence of 50 mM ethanol. Scale bars: 50 μm
Two approaches were used to demonstrate that the binding of ExoHep to their target cells resulted in cellular uptake of exosomal cargo. First, incubation of SYTO RNASelectTM-containing exosomes (isolated from conditioned medium of SYTO RNASelectTM-treated hepatocytes) with HSC or hepatocytes resulted in accumulation of a green fluorescent signal in each cell type (Fig. 5a). Second, using a customized 2-well μ-dish co-culture system (Chen et al. 2014; Chen et al. 2015; Chen et al. 2016), we documented transfer of SYTO RNASelectTM from hepatocytes to HSC (Fig. 5b) or to other hepatocytes (Fig. 5c). This effect was blocked by GW4869 (Fig. 5b,c), thus implicating the involvement of exosomes that were produced in a ceramide-dependent manner and demonstrating that uptake of exosomal RNA by HSC or hepatocytes occurs at intrinsic levels of production and release of exosomes by donor hepatocytes. These results collectively demonstrated delivery of the RNA payload in ExoHep to both HSC and hepatocytes.
Fig. 5.
ExoHep mediate transfer of RNA into HSC or hepatocytes. a Uptake into recipient HSC or AML12 cells of SYTO RNASelect™ (green) after 24-h incubation of the cells with SYTO RNASelect™-loaded ExoHep. Blue is DAPI, green is RNA. Control cells not incubated with exosomes did not stain green (data not shown). b AML12 exosome donor cells in which RNA was stained with SYTO RNASelect™ (green) were co-cultured in 2-well μ-dish devices with PKH26-stained mouse HSC (red) for 24 h. The green/yellow color (far right, upper panel) demonstrates uptake into recipient HSC of AML12 cell-derived RNA. Incubation with 10 μM GW4869 (“GW’) prevented RNA uptake from AML12 cells into HSC (far right, lower panel). c Same as b except recipient cells were PKH26-stained AML12 cells. Scale bars: 20 μm
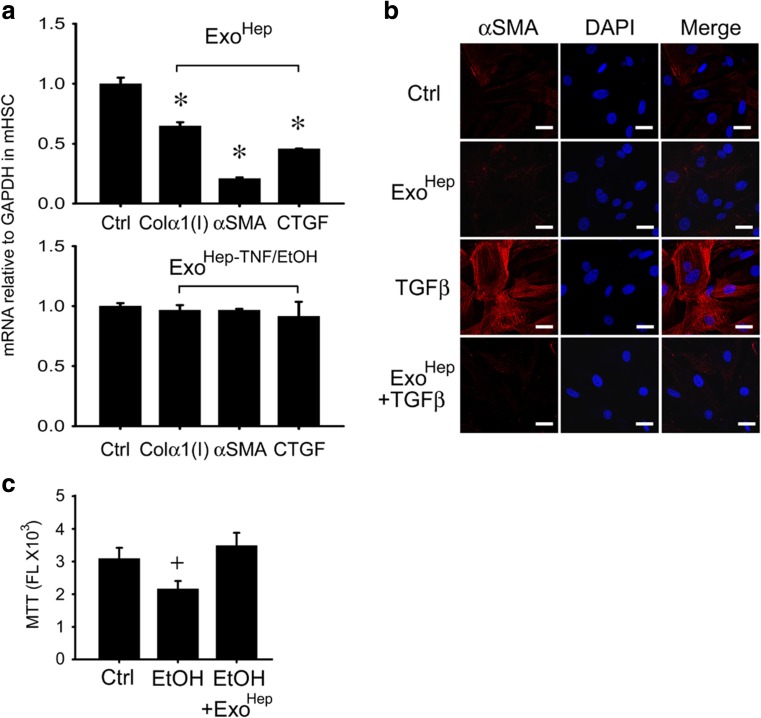
During chronic injury in the liver, HSC undergo a process of activation whereby they transition from quiescent fibroblastic cells to proliferative myofibroblasts that synthesize large quantities of contractile proteins (e.g. αSMA) or fibrogenic proteins (e.g. CCN2), leading to their overt production and deposition of insoluble fibrotic material (e.g. collagen) in the interstitial spaces. We were thus interested if the interaction between ExoHep and activated HSC resulted in a regulation of HSC gene expression. Treatment of activated HSC with ExoHep from primary hepatocytes or AML12 cells caused diminished expression of αSMA, CCN2 or collagen α1(I) assessed by qRT-PCR (Fig. 6a and data not shown) as well as decreased production of αSMA in response to TGF-β assessed by immunocytochemistry (Fig. 6b). However, suppressed gene expression was not evident when activated HSC were treated with ExoHep-TNFα/EtoH (Fig. 6a). Furthermore, ethanol-induced cytotoxicity in hepatocytes was reversed in the presence of ExoHep as assessed by MTT assay (Fig. 6c). Thus, exosomes from control non-damaged hepatocytes reverse the fibrosis-associated phenotype in HSC as well as toxin-mediated damage in hepatocytes.
Fig. 6.
Effect of ExoHep on activated HSC or damaged hepatocytes. a qRT-PCR determination of mRNA for collagen α1(I), αSMA, or CCN2 in mouse P2–3 HSC treated for 24 h with or without 8 μg/ml ExoHep (upper panel) or ExoHep-TNFα/EtOH (lower panel). Exosomes were from primary hepatocyte cultures. (n = 9, *p < 0.001 vs. ctrl, student’s t-test). b Immunocytochemical detection of αSMA in mouse P3 HSC that were treated under serum-free conditions with 10 ng/ml TGF-β for 48 h, the last 24 h of which also included co-incubation with 10 μg/ml ExoHep from AML12 cells. Blue is DAPI, red is αSMA. Data shown are representative of 3 individual experiments. c MTT assay for primary mouse hepatocytes treated for 24 h with 50 mM ethanol in the presence or absence of 10 μg/ml ExoHep from AML12 cells (n = 9, +p < 0.05 vs. ctrl, student’s t test). Scale bars: 10 μm
Role of integrin-like and heparin-like binding between ExoHep and target HSC or hepatocytes
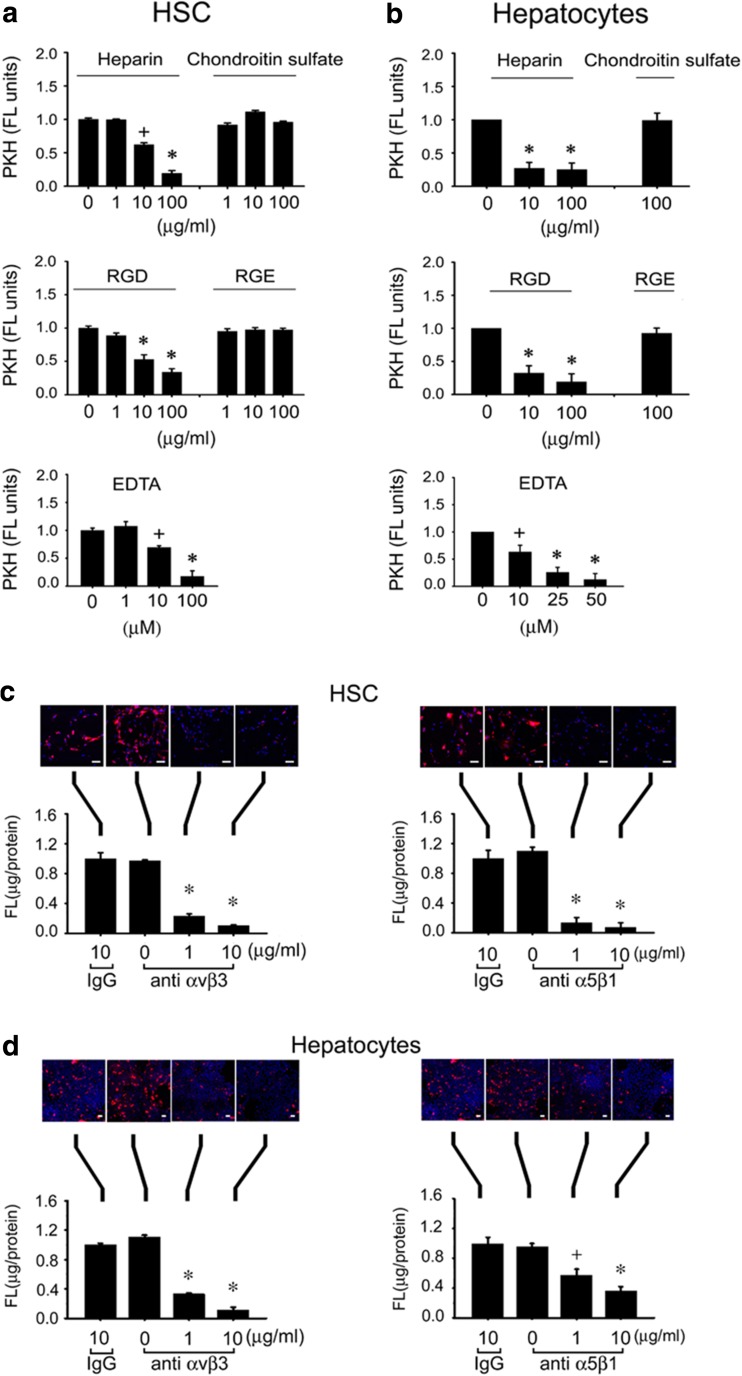
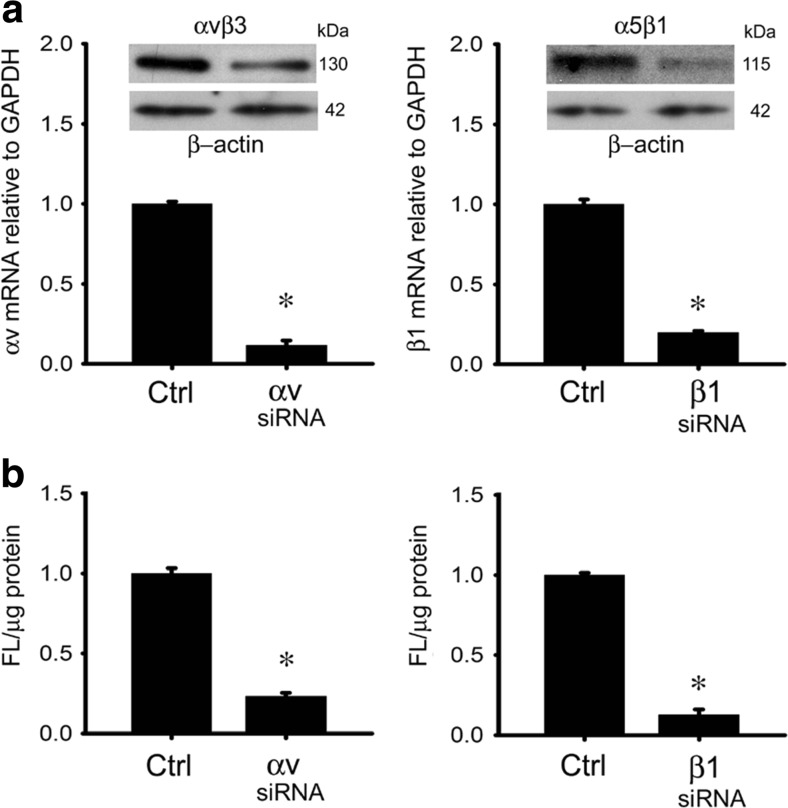
Binding of PKH26-ExoHep to HSC or hepatocytes was dose-dependently blocked by heparin, RGD, or EDTA, but not by comparable concentrations of chondtroin sulfate or RGE (Fig. 7a). The RGD- and EDTA-sensitivity suggested the possible involvement of integrins in the binding interactions between ExoHep and each type of target cell. Since we have previously shown that integrins αvβ3 and α5β1 expressed by HSC are important for engaging HSC-derived integrins (Chen and Brigstock 2016), we investigated these integrins in the context of ExoHep binding. Accordingly, binding of PKH26-ExoHep to either HSC or hepatocytes was inhibited when the assay was performed after pre-incubation of the target cells with 1–10 μg/ml anti-integrin αvβ3 or anti-integrin α5β1 (Fig. 7c,d). Whereas 10 μg/ml control IgG had no effect on PKH26-ExoHep binding, the same concentration of anti-integrin antibodies caused a 95% decrease in PKH26-ExoHep binding to HSC (Fig. 7c) and a 60% decrease in PKH26-ExoHep binding to hepatocytes (Fig. 7d). As we have successfully used an si-RNA approach to block exosome binding to HSC (Chen and Brigstock 2016), we next adopted this strategy for verifying the integrin binding partners for ExoHep on hepatocytes. Treatment of hepatocytes with si-RNAs to the integrin αv or β1 chains resulted in decreased expression of their respective mRNA target and downstream production of, respectively, integrin αvβ3- or α5β1-immunoreactive proteins (Fig. 8a). Each integrin knockdown was associated with a 90–90% decrease in PKH26-ExoHep binding to the cells (Fig. 8b).
Fig. 7.
Attenuation of ExoHep binding to hepatocytes or HSC by heparin, RGD tripeptide, EDTA, anti- integrin αvβ3, or anti-integrin α5β1. PKH26-ExoHep were added (8 μg/ml) to (a) P4 HSC or (b) hepatocytes for 24 h in the presence of the indicated concentrations of heparin, chondroitin sulfate, RGD, RGE, or EDTA, prior to measurement of cell-associated fluorescence by spectrophometric quantification of PKH26 in cell lysates (*p < 0.001 vs. “0”, +p < 0.05 vs. “0”, student’s t-test). (c) P4 HSC or (d) hepatocytes were pre-treated for 1 h with 0–10 μg/ml anti-integrin αvβ3, 0–10 μg/ml anti-integrin α5β1, or 10 μg/ml non-immune IgG after which the cells were extensively rinsed and then incubated for 24 h with 8 μg/ml PKH26 ExoHep . Cell-associated fluorescence was determined by confocal microscopy (upper panel) or spectrophotometric quantification of cell lysates (lower panel). Data are from triplicate determinations from 3 or 4 individual experiments. (*p < 0.001 vs. “0”; +p < 0.05 vs. “0”, student’s t-test). Scale bars: 20 μm
Fig. 8.
Attenuation of ExoHep binding to hepatocytes by siRNAs to integrin αv or integrin β1. a qRT-PCR for integrin αv mRNA in control or si-integrin αv-transfected AML12 cells (left panel) (inset: Western blot using anti-αvβ3 or anti-β-actin) or qRT-PCR for integrin β1 mRNA in control or si-integrin β1-transfected AML 12 cells (right panel) (inset: Western blot using anti-integrin α5β1 or anti-β-actin). b Effect of knockdown of integrin αv (left panel) or integrin β1 (right panel) in hepatocytes on the ability of the cells to bind PKH26-ExoHep (8 μg/ml) over 24 h, as determined by spectrophotometric quantification of fluorescence in cell lysates. “Ctrl” is cells treated with a scrambled siRNA sequence. (*p < 0.001 vs. ctrl., student’s t-test)
Discussion
Accumulating evidence has shown that EVs are produced by multiple cell types in the liver including hepatocytes, HSC, LSEC, and cholangiocytes (Maji et al. 2017; Szabo and Momen-Heravi 2017). While it is speculated that these EVs participate in hepatic cell-cell communication, direct evidence is lacking because tools and models are lacking that would allow individual populations of exosomes to be tracked in situ from the time they are released by one hepatic cell to the time they are taken up by and act in another. Despite this limitation, a growing body of evidence, mainly from in vitro studies, supports the concept that important signaling events in the liver are orchestrated by hepatic exosomes. In this study we focused on exosomes produced by hepatocytes given the central role of this cell type in liver function. It was previously reported that normal primary rat hepatocytes in culture produce exosomes (approx. 50-60 nm) that are positive for ASPGR (Conde-Vancells et al. 2008) or microparticles (100-1000 nm) that are positive for annexin V (Povero et al. 2013). Primary mouse hepatocytes were also shown to produce EVs that were identified as exosomes based on their size (50-200 nm) and expression of TSG101, CD63 and CD81 (Nojima et al. 2016), the latter of which is currently considered to be exosome-specific (Kowal et al. 2016). The EVs purified from mouse hepatocytes in the current study also had characteristics consistent with their identity as exosomes including their size (~100 nm), expression of CD81, and appearance as cup-like nanovesicles by TEM.
Our results show that exposure of hepatocytes to ethanol resulted an increased frequency of their released exosomes, consistent with previous data from Huh7.5 hepatocytes which, in response to ethanol, were reported to show increased exosome production and Rab 27b mRNA expression (Momen-Heravi et al. 2015). Messenger RNA expression of all Rab GTPases or component of the ceramide- or ESCRT-dependent pathways were either significantly increased by ethanol or at least showed an upward trend in expression (i.e. STAM1), with the exception of Rab 27a which was decreased by ethanol. Numerous studies in the EV field have established that exosome biogenesis and trafficking is very complex, involving many more components than those studied here and likely intersecting with other membrane biogenic and transport pathways. In a comprehensive attempt to define the role of individual ESCRT components in exosome release, RNA interference was previously employed to individually silence 23 different members of the ESCRT pathway in Hela cells, revealing that EV secretion was reduced by targeting HRS or STAM1 (ESCRT 0) or TSG101 (ESCRT1) or increased by targeting VPS4B or Alix (ESCRT accessory proteins), and that other EV features (size heterogeneity, composition) were variably altered (Colombo et al. 2013). Depletion of TSG101 or Alix resulted in reduced exosome production in MCF-7 cells (Baietti et al. 2012). In light of these prior studies, we focussed our attention on the role of these particular ESCRT components in exosome production by hepatocytes using a siRNA approach. Our data show that exosome release was antagonized by transfection of the cells with si-Alix, si-HGS, or si-TSG101 in control and/or ethanol-treated hepatocytes. This outcome was consistent with the si-RNA mediated diminished expression of each target when assessed at the mRNA or protein level, except for TSG101 which exhibited seemingly inconsistent mRNA or protein responses to ethanol or RNA interference. It remains to be seen whether this discordance is due to ethanol-induced changes in TSG101 protein modification that reduce its antibody recognition, whether the TSG101 protein is less stable in ethanol-treated cells, or if there is another explanation. Despite these uncertainties, the ability of si-TSG101 to nonethlesss diminish exosome production by the cells supports a role for TSG101 in exosome biogenesis, as does the finding that TSG101 is a constituent of hepatocyte exosomes (Nojima et al. 2016). In addition to the ESCRT pathway, the ceramide pathway was also implicated in contributing to ExoHep production as shown by its sensitivity to si-nSMase2 (especially in ethanol-treated cells) and by the ability of GW4869 to block intercellular uptake of hepatocyte RNA into recipient HSC or hepatocytes. While our data supports an overall role for the ceramide and ESCRT pathways in basal or ethanol-stimulated exosome production in hepatocytes, other ESCRT-independent pathways may also be involved, such as those in which tetraspanins regulate exosome biogenesis or release (Escola et al. 1998; Chairoungdua et al. 2010; Nazarenko et al. 2010; Hurwitz et al. 2017). Furthermore, these various pathways may not be strictly delineated and instead may have shared components and various levels of inter-dependency (Maas et al. 2017). That said, our data suggests that inhibition of exosome production and release by targeting exosome biogenic pathways with drugs such as GW4869 or by gene silencing may have therapeutic value in hepatocarcinoma and chronic liver disease in which the exosomal payload is proposed to drive pathogenesis, as discussed below. However these strategies would need to be deployed in a hepatocyte-specific manner to prevent off-target effects.
Most studies of hepatocyte exosomes to date have focused on their potential contribution to pathogenic outcomes in the liver including their roles in progression of hepatocarcinoma (Moris et al. 2017), intercellular transmission of hepatitis viruses (Tamai et al. 2012; Ramakrishnaiah et al. 2013; Nagashima et al. 2014) and T cell expansion or activation of macrophages or HSC following their delivery from damaged hepatocytes in models of hepatitis infection, alcoholic liver disease, non-alcoholic fatty liver disease, or non-alcoholic steatohepatitis (Momen-Heravi et al. 2015; Povero et al. 2015; Hirsova et al. 2016; Kakazu et al. 2016; Kouwaki et al. 2016; Verma et al. 2016; Lee et al. 2017). While few studies have addressed the biological properties of exosomes from normal hepatocytes, their proteomic analysis led to speculation of their possible involvement in numerous processes usually associated with hepatocytes themselves, including fat and carbohydrate metabolism and detoxification (Conde-Vancells et al. 2008). Our data further show that ExoHep actually contain a molecular payload that exerts anti-fibrogenic actions in activated HSC and promotes reversal of ethanolic cytotoxicity in hepatocytes. These findings highlight an important functional difference between exosomes from healthy hepatocytes as compared to exosomes from lipid-laden or CCl4-treated hepatocytes which have been shown to promote HSC activation and to exacerbate experimental liver fibrosis (Povero et al. 2015; Seo et al. 2016; Lee et al. 2017). Interestingly, the presence of sphingosine kinase 2 in mouse hepatocyte exosomes appears to contribute to their ability to drive hepatocyte proliferation in vitro or after acute (ischemia reperfusion) liver injury in vivo (Nojima et al. 2016). While we have yet to identify the molecular constituents in ExoHep that account for the beneficial outcomes in HSC or hepatocytes, our data suggest that the exosomal cargo produced by normal non-injured hepatocytes may be therapeutic for liver fibrosis. Furthermore, since ExoHep were also found to preferentially bind to quiescent HSC and non-injured hepatocytes in control animals, this may represent an innate regulatory mechanism that serves to prevent or resist hepatocellular damage or pathogenesis in otherwise healthy individuals. Although, KC or LSECs bound ExoHep to a lesser extent than hepatocytes or HSC, this does not rule out the possibility that ExoHep are nonetheless involved in functional regulation of these cell types. Moreover, the degree to which any given cell type of is a target of hepatocyte exosomes is likely reflective of the stage or type of liver disease and whether the exosomes are derived from hepatocytes that are healthy or damaged. For example, exosomes from alcohol- or lipid-treated hepatocytes have been reported to drive macrophage sensitization and inflammatory signaling (Momen-Heravi et al. 2015; Hirsova et al. 2016).
A number of previous studies have shown that interactions between exosomes and their target cells involve integrins that are located either on the surface of the exosome (Thery et al. 1999; Rieu et al. 2000; Thery et al. 2001; Wubbolts et al. 2003; Clayton et al. 2004; Janowska-Wieczorek et al. 2005; Fedele et al. 2015; Hoshino et al. 2015; Kawakami et al. 2015) or of the target cell (Segura et al. 2007; Nolte-'t Hoen et al. 2009). The reduction in binding of PKH26-ExoHep caused by treatment of the target HSC or hepatocytes with anti-integrin antibodies and/or by transfection with integrin siRNAs provided evidence that the integrins involved were localized on the target cell rather than on ExoHep. In the study here, we showed that ExoHep binding to HSC or hepatocytes was dependent on the presence of integrin αv or β1 subunits in the target cells, consistent with the RGD- and EDTA-sensitivity of ExoHep binding. Our findings are distinct from an earlier report in which a vanin-dependent mechanism was shown to be involved in the uptake by LSECs of microparticles (100-1000 nm) produced by fat-laden HepG2 hepatocarcimoa cells (Povero et al. 2013). Previous studies have shown that HSC utilize cell surface integrins αvβ3 and α5β1 to bind to HSC-derived exosomes (Chen and Brigstock 2016) or integrin β1 to bind to exosomes produced by LSECs (Wang et al. 2015). Thus different exosome populations in the liver utilize similar mechanisms of binding to the same target cells suggesting that they may compete with one another for cellular binding and uptake and delivery of their respective payloads. Whereas we previously showed that HSC-derived exosomes do not strongly associate with hepatocytes (Chen and Brigstock 2016), the current and previous studies (Nojima et al. 2016) identified hepatocytes themselves as targets of hepatocyte exosomes in both acute and chronic injury models. While our data show that the expression of integrins αv and β1 by target hepatocytes are important for their binding to ExoHep, the identity of their corresponding ligands on the exosome surface must await further study. We also showed that ExoHep binding to HSC or hepatocytes is displaced by heparin but not chondroitin sulfate, suggesting that the binding interactions are also dependent on heparin-like molecules on the surface of the exosome or target cells. While the precise role played by heparin-like molecules and the identity of their cognate ligands requires additional study, recent findings have shown that cellular heparan sulfate proteoglycans are also involved in the binding of HSC-derived exosomes to HSC (Chen and Brigstock 2016) or of U-87 glioblastoma cell-derived exosomes to U-87 or Chinese Hamster Ovary cells (Christianson et al. 2013).
In summary, the release of exosomes by hepatocytes is stimulated by ethanol and is correlated with ethanol-induced changes in expression of Rabs as well as components of the ceramide- or ESCRT-dependent biogenesis pathways, the latter two of which functionally involve, respectively, nSmase 2 or HRS, Alix, and/or TSG101. ExoHep preferentially bind to HSC or hepatocytes via mechanisms that involve heparin-like molecules and cellular integrins αvβ3 or α5β1 resulting in a reversal of activation and fibrogenic gene expression in activated HSC and of ethanol-induced damage in hepatocytes.
Acknowledgements
This work was supported by NIH grants R01AA021276, R21AA023626, and R21AA025974 awarded to D.R.B. and by pilot funding to D.R.B from NIH grant P50AA024333 in support of the Northern Ohio Alcohol Center (Principal Investigator, Laura Nagy, PhD). We thank David Dunaway and Victoria Velazquez for assistance with cell sorting and NTA, and Cindy McAllister for help with TEM.
Abbreviations
- αSMA
Alpha smooth muscle actin
- ASGPR1
Asialoglycoprotein receptor 1
- CCl4
Carbon tetrachloride
- CCN2
Connective tissue growth factor
- DAPI
4′,6-Diamidine-2′-phenylindole dihydrochloride
- EDTA
Ethylenediaminetetraacetic acid
- ESCRT
Endosomal sorting complex required for transport
- ExoHep
Exosomes from normal hepatocytes
- ExoHep-TNFα/EtOH
Exosomes from TNFα-primed ethanol-treated hepatocytes
- EV
Extracellular vesicle
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HSC
Hepatic stellate cell
- KC
Kupffer cell
- LSEC
Luminal sinusoidal endothelial cells
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MVB
Multivesicular bodies
- nSmase 2
Neutral sphingomyelinase 2
- NTA
Nanoparticle tracking analysis
- PBS
Phosphate-buffered saline
- qRT-PCR
Quantitative real time polymerase chain reaction
- siRNA
Small interfering RNA
- TEM
Transmission electron microscopy
- TGF-β
Transforming growth factor beta
- TNFα
Tumor necrosis factor alpha
References
- Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Blanc L, Vidal M (2017) New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases:1–12. 10.1080/21541248.2016.1264352 [DOI] [PMC free article] [PubMed]
- Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, Brigstock DR. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Brigstock DR. Integrins and heparan sulfate proteoglycans on hepatic stellate cells (HSC) are novel receptors for HSC-derived exosomes. FEBS Lett. 2016;590:4263–4274. doi: 10.1002/1873-3468.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Charrier AL, Leask A, French SW, Brigstock DR. Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor. J Hepatol. 2011;55:399–406. doi: 10.1016/j.jhep.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Charrier A, Zhou Y, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen R, Kemper S, Charrier A, Brigstock DR. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015;309:G491–G499. doi: 10.1152/ajpgi.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen R, Velazquez VM, Brigstock DR. Fibrogenic signaling is suppressed in hepatic stellate cells through targeting of connective tissue growth factor (CCN2) by cellular or Exosomal MicroRNA-199a-5p. Am J Pathol. 2016;186:2921–2933. doi: 10.1016/j.ajpath.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290:4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Hirsova, P., S. H. Ibrahim, A. Krishnan, et al. (2016) Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology [DOI] [PMC free article] [PubMed]
- Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz SN, Nkosi D, Conlon MM, York SB, Liu X, Tremblay DC, Meckes DG Jr (2017) CD63 regulates Epstein-Barr virus LMP1 Exosomal packaging, enhancement of vesicle production, and noncanonical NF-kappaB signaling. J Virol 91. 10.1128/JVI.02251-16 [DOI] [PMC free article] [PubMed]
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Fujita Y, Kato T, et al. Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int J Oncol. 2015;47:384–390. doi: 10.3892/ijo.2015.3011. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwaki T, Fukushima Y, Daito T, et al. Extracellular vesicles including Exosomes regulate innate immune responses to hepatitis B virus infection. Front Immunol. 2016;7:335. doi: 10.3389/fimmu.2016.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JK, Young RF, Ashraf H, Canty JM., Jr Inhibiting extracellular vesicle release from human Cardiosphere derived cells with Lentiviral knockdown of nSMase2 differentially effects proliferation and apoptosis in Cardiomyocytes, fibroblasts and endothelial cells in vitro. PLoS One. 2016;11:e0165926. doi: 10.1371/journal.pone.0165926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim SY, Ko E, et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710. doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu K, Liu Y, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- Maas SL, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji S, Matsuda A, Yan IK, Parasramka M, Patel T. Extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2017;312:G194–G200. doi: 10.1152/ajpgi.00216.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris D, Beal EW, Chakedis J, et al. Role of exosomes in treatment of hepatocellular carcinoma. Surg Oncol. 2017;26:219–228. doi: 10.1016/j.suronc.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Nagashima S, Jirintai S, Takahashi M, et al. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol. 2014;95:2166–2175. doi: 10.1099/vir.0.066910-0. [DOI] [PubMed] [Google Scholar]
- Nazarenko I, Rana S, Baumann A, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- Nojima H, Freeman CM, Schuster RM, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- Povero D, Eguchi A, Niesman IR, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povero D, Panera N, Eguchi A, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol. 2015;1:646–663.e644. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnaiah V, Thumann C, Fofana I, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267:583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- Seo W, Eun HS, Kim SY, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology. 2016;64:616–631. doi: 10.1002/hep.28644. [DOI] [PubMed] [Google Scholar]
- Shelke GV, Lasser C, Gho YS, Lotvall J (2014) Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles 3. 10.3402/jev.v3.24783 [DOI] [PMC free article] [PubMed]
- Stremersch S, De Smedt SC, Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016;244:167–183. doi: 10.1016/j.jconrel.2016.07.054. [DOI] [PubMed] [Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Shiina M, Tanaka N, et al. Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway. Virology. 2012;422:377–385. doi: 10.1016/j.virol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Verma VK, Li H, Wang R, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ding Q, Yaqoob U, et al. Exosome adherence and internalization by hepatic stellate cells triggers Sphingosine 1-phosphate-dependent migration. J Biol Chem. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]