Abstract
Cells behave in a variety of ways when they perceive changes in their microenvironment; the behavior of cells is guided by their coordinated interactions with growth factors, niche cells, and extracellular matrix (ECM). Modulation of the microenvironment affects the cell morphology and multiple gene expressions. Rho/Rho-associated coiled-coil-containing protein kinase (ROCK) signaling is one of the key regulators of cytoskeletal dynamics and actively and/or passively determines the cell fate, such as proliferation, migration, differentiation, and apoptosis, by reciprocal communication with the microenvironment. During periodontal wound healing, it is important to recruit the residential stem cells into the defect site for regeneration and homeostasis of the periodontal tissue. Periodontal ligament (PDL) cells contain a heterogeneous fibroblast population, including mesenchymal stem cells, and contribute to the reconstruction of tooth-supporting tissues. Therefore, bio-regeneration of PDL cells has been the ultimate goal of periodontal therapy for decades. Recent stem cell researches have shed light on intrinsic ECM properties, providing paradigm shifts in cell fate determination. This review focuses on the role of ROCK activity and the effects of Y-27632, a specific inhibitor of ROCK, in the modulation of ECM-microenvironment. Further, it presents the current understanding of how Rho/ROCK signaling affects the fate determination of stem cells, especially PDL cells. In addition, we have also discussed in detail the underlying mechanisms behind the reciprocal response to the microenvironment.
Keywords: Extracellular matrix, Microenvironment, Periodontal ligament cells, Rho-associated coiled-coil-containing protein kinase, Stem cell fate
Introduction
Periodontitis is an oral biofilm-induced chronic inflammation, leading to the loss of connective tissue around the tooth, and is one of the most prevalent infectious diseases worldwide (Pihlstrom et al. 2005). The tooth-supporting periodontium is a complex organ consisting of dental epithelial-mesenchymal tissues (Nanci and Bosshardt 2006) (Fig. 1). Homeostasis and regeneration of the periodontium is important for improving the oral health and systemic conditions (Tonetti et al. 2007). Regeneration of periodontium requires orchestration of several cell types including gingival epithelial cells, periodontal ligament cells (PDL cells), cementoblasts, and bone cells (See Glossary). The cell lineages of periodontium can construct the epithelial barrier, fibrous attachment between tooth root and bone with vascular and nerve supplies, and supporting bone (alveolar bone) surrounding tooth. The tissue healing patterns following periodontal therapy depend on the cell type that can dominantly migrate and proliferate into the wound sites to reconstruct the “periodontal defects” (Fig. 1). Moreover, the recruitment of residential tissue stem cells and subsequent differentiation is important to achieve the regeneration of the wound tissue (Melcher 1976; Aukhil 2000).
Fig. 1.
Regeneration and homeostasis of periodontium regulated by proliferation, migration, and differentiation of PDL cells. Periodontium mainly consists of gingival epithelial cells, periodontal ligament cells (PDL cells), cementoblasts, and bone cells (osteoblasts). PDL cells-mediated fibrous attachment (the bottom area) prevents the spread of inflammation into the depth. Furthermore, the perivascularly mediated vascular and nerve supplies are integrated with PDL fibers, which contribute to homeostasis of periodontium. In an ideal microenvironment, PDL cells can proliferate and migrate into the bone defect site and differentiate into cementoblasts and osteoblasts. Subsequently, PDL cells can regenerate cementum, bone, and the PDL connective tissue itself until they cure the bone defect
PDL cells contain a heterogeneous fibroblast population including osteogenic progenitor cells and mesenchymal stem cells (MSC) that can regenerate cementum, bone, and the PDL tissue itself (Seo et al. 2004) (Fig. 1). PDL cells-mediated fibrous attachment prevents inflammation from spreading across the epithelial-mesenchymal boundary. PDL cells adjacent to the cementum are derived from the ectomesenchymal cells of the dental papilla while those adjacent to the bone are derived from perivascular mesenchyme cells (See Glossary). Therefore, PDL cells have specialized properties that are different from those of the cells in other connective tissues. PDL cells have high alkaline phosphatase (ALP) activity, and they produce mineralized nodules and high quantities of various extracellular matrix (ECM) components, with a higher matrix turnover and remodeling rates than those in gingiva, skin, and bone (Beertsen et al. 1997). For the purpose of exogenous stemness, although transplantation of PDL cells can contribute to the reconstruction of periodontal tissue in vivo and in clinical case studies (Seo et al. 2004; Chen et al. 2016), there are yet many socio-economic hurdles to overcome. Therefore, it is important to modulate wound healing by recruiting residential PDL cells into the tissue defect for regeneration and homeostasis of periodontium.
All the categories of cell behavior, including cell migration, proliferation, and differentiation, are regulated as the cells can perceive the changes in the local biochemical and mechanical microenvironment, defined by coordinated interactions with growth factors, neighboring niche cells, and ECM (Bottaro et al. 2002; Discher et al. 2009) (Fig. 2). Progenitor and stem cells are highly sensitive to the intrinsic properties of their ECM (Engler et al. 2006; Reilly and Engler 2010). These cells tune their internal stiffness in compliance with the ECM by modulating actin polymerization and crosslinking, which are responsible for altering the cell morphology and gene expression during tissue remodeling (Mammoto and Ingber 2009). The mammalian Rho guanosine triphosphatases (Rho GTPases) are a family of 20 intracellular signaling molecules within the superfamily of Ras-related small GTP-binding proteins, which act as molecular switches cycling between an active GTP-bound state and an inactive GDP-bound state (Jaffe and Hall 2005). ECM-integrin adhesion and cytoskeletal structures contribute to the regulation of RhoA (hereafter referred as Rho) and Rho-associated coiled-coil-containing protein kinase (ROCK). Rho/ROCK signaling is one of the most extensively characterized Rho GTPase pathways in actin cytoskeletal dynamics (Ishizaki et al. 1996; Manneville and Hall 2002). Changes in cell shape, cytoskeleton, and matrix stiffness regulate osteogenic or adipogenic differentiation of MSC by modulating Rho/ROCK signaling (McBeath et al. 2004; Kilian et al. 2010). ROCK is also activated by several growth factors such as platelet-derived growth factor-BB (PDGF-BB) (Schmidt and Hall 1998; Schoenwaelder and Burridge 1999). The current understanding about Rho/ROCK signaling suggests that this signaling pathway is essential for directing cell fate determination in response to both mechanical and soluble biochemical factors (Fig. 2).
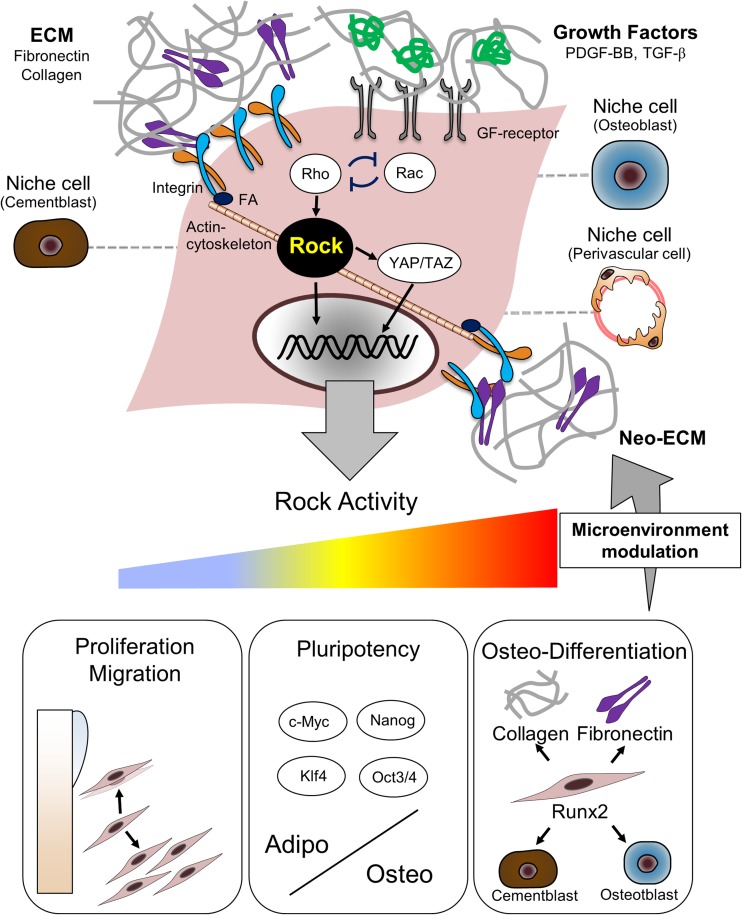
Fig. 2.
ECM-microenvironment and ROCK activity interactively regulate proliferation, migration, and differentiation-state of PDL cells. All cell behavior regulated by the local microenvironment and directed by coordinated interactions with growth factors, extracellular matrix (ECM), and niche cells. ECM-integrin adhesion and growth factors (GF) respectively mediate multiple intercellular signaling, such as Rho-GTPases via assembly of focal adhesion (FA) and actin cytoskeleton. Rho and Rac are mutually antagonistic (Bottaro et al. 2002; Lutolf and Hubbell 2005; Discher et al. 2009). YAP/TAZ activity requires Rho/ROCK signaling and cytoskeletal tension. ROCK activity plays a central role in modulating ECM-microenvironment, which strongly affects proliferation, migration, and differentiation-state of PDL cells. Inhibiting ROCK activity using Y-27632 induces cell proliferation and migration of PDL cells. Y-27632 also increases pluripotency with the expression of c-Myc, Nanog, Klf4, and Oct3/4. Inactivation of ROCK activity directs PDL cells to an adipogenic fate while the activation of ROCK activity directs them to an osteogenic fate. ROCK mediates osteogenic differentiation by interacting with Runx2 expression. The expression of collagen-I and fibronectin is also dependent on ROCK activity. The ECM-microenvironment may mediate osteogenic differentiation of PDL cells through a mechanism involving ROCK-dependent upregulation of ECM component expression, which subsequently initiates neo-ECM synthesis in an autocrine manner. Niche cells, such as cementoblasts, osteoblasts, and perivascular cells, may partly affect ROCK activity
This review highlights how Rho/ROCK signaling affects fate determination of stem cells, especially PDL cells, and the currently known underlying mechanisms of reciprocal PDL cells—microenvironment interactions. The inflammatory responses by Rho/ROCK signaling are also discussed from a different perspective for designing new therapeutic strategies.
Mechanotransduction
Mechanotransduction is the process by which cells transduce physical force-induced signals into biochemical responses and direct differential gene expression to facilitate adaptations in mechanical stress (Ingber 2003). Mechanical stress-induced alterations in cell shape and structure are vital for the control of cell growth, migration, differentiation, and apoptosis (Chicurel et al. 1998). Mechanical forces and matrix mechanical properties impact the cytoskeletal tension and regulate stem cell lineage commitment in part by Rho/ROCK signaling (Chen and Jacobs 2013; Ivanovska et al. 2015). Recent studies shed light on the striking roles of transcriptional regulators Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ), which are the downstream effectors of the Hippo pathway (Pan 2010), in mechanotransduction. YAP/TAZ activity requires Rho and tension of the actin cytoskeleton, but is independent of the Hippo cascade (Dupont et al. 2011; Wang et al. 2016). Our understanding of the entire process of mechanotransduction is yet preliminary, and further in vivo investigation is needed.
The study to uncover the role of Rho/ROCK signaling in PDL cells began with the analysis of mechanotransduction because the homeostasis and regeneration of PDL cells are closely related to the mechanical changes arising because of occlusal forces (Beertsen et al. 1997; Pavlin and Gluhak-Heinrich 2001). It has been suggested that mechanical stress-mediated Rho/ROCK signaling is linked to the expression of a series of osteogenic molecules, such as activator protein-1, osteopontin, and receptor activator of nuclear factor-kappa B ligand, in PDL cells (Kletsas et al. 2002; Yamashiro et al. 2007; Wongkhantee et al. 2008; Hong et al. 2010; Pan et al. 2014). A better understanding of how PDL cells adapt to the mechanical stress may contribute to the analysis of their osteogenic lineage.
Pluripotency and proliferation
ROCK directly phosphorylates myosin light chain 2 (MLC2) (Amano et al. 1996), which leads to increased contractility of actin–myosin bundles because of an increase in myosin ATPase activity (Katoh et al. 2001). These processes are prevented by exposure of the cells to Y-27632, a pharmacological inhibitor of ROCK. Y-27632 is capable of reducing the ROCK activity with only minimal alterations in other kinase pathways (Uehata et al. 1997). In PDL cells, Y-27632 can decrease MLC2 phosphorylation and actin polymerization, which are accompanied by a marked reduction in F-actin formation (Yamamoto et al. 2014). Y-27632 is now commonly used to increase the cloning efficiency of pluripotent human embryonic stem cells (hES cells) by decreasing dissociation-induced apoptosis. Y-27632-treated hES cells produce many large colonies, showing positive signal for the undifferentiated-state markers, ALP, E-cadherin, POU class 5 homeobox 1 (POU5F1, Oct3/4), and stage-specific embryonic antigen 4 (SSEA4). Y-27632-treated hES cells also retain autonomous proliferation ability and enhanced pluripotency (Watanabe et al. 2007). In PDL cells, Y-27632 remarkably reduce the rate of appearance of ALP-positive colonies; however, the total number of colonies is not affected (Ugawa et al. 2017) and the size of Y-27632-treated colonies is larger than that of untreated colonies (Wang et al. 2017). Moreover, Y-27632 significantly increase the rate of cell proliferation and the expression of pluripotent markers, MYC proto-oncogene (c-Myc), Nanog homeobox (Nanog), Kruppel-like factor 4 (Klf4), and Oct3/4 (Wang et al. 2017). These findings indicate that ROCK inhibitor is capable of increasing cell proliferation and pluripotency of PDL-derived somatic stem cells.
Differentiation
Y-27632 is widely used to improve the reproducibility and efficiency of differentiation of hES cells and induced pluripotent stem cells (iPS cells) (Watanabe et al. 2007; Kurosawa 2012). The earlier studies have reported that Y-27632 can decrease cytoskeletal tension and contractility and can inhibit osteogenesis of MSC and osteoblasts (McBeath et al. 2004; Khatiwala et al. 2009; Kilian et al. 2010; Shih et al. 2011). In contrast, Y-27632 shows differentiation lineage specificity; Y-27632 can induce stem cells to differentiate into adipocytes, neuron cells, endothelial cells, and keratinocytes (McBeath et al. 2004; Pacary et al. 2006; Joo et al. 2012; Li et al. 2015). Although Y-27632 has been used as a multifunctional agent, the underlying molecular mechanism that determines inhibition or induction of stem cell differentiation remains unclear. Multiple pathways are involved in ROCK-mediated osteogenic differentiation. ROCK-dependent-YAP/TAZ activity has crucial roles in regulating differentiation by interacting with runt-related transcription factor 2 (Runx2) (Pan 2010; Dupont et al. 2011). YAP/TAZ has similar effects on Rho/ROCK in regulating MSC osteogenic differentiation; the activation of Rho/ROCK signaling or YAP/TAZ directs MSC toward an osteogenic fate (McBeath et al. 2004; Halder et al. 2012). The depletion of YAP/TAZ could also block osteogenic differentiation of MSC (Dupont et al. 2011).
In PDL cells, similar to MSC, F-actin and phosphorylated MLC2 were remarkably enhanced during osteogenic differentiation. The mineralization process was significantly inhibited by Y-27632 in a dose-dependent manner as shown by decreased ALP activity, calcified nodule formation, and expression of osteogenic genes such as ALP and Runx2 while Y-27632 induced adipogenesis in PDL cells. These reports indicate that ROCK dominantly regulates osteogenic differentiation of PDL cells (Yamamoto et al. 2014; Ugawa et al. 2017; Wang et al. 2017). Therefore, ROCK activity governs osteo-adipogenic transdifferentiation by modifying the actin cytoskeleton.
Microenvironment modulation
As stated above, Rho/ROCK signaling may be essential for directing cell fates in response to both mechanical and soluble biochemical factors. Y-27632 affects the expression of numerous genes that regulate actin cytoskeleton, ECM-integrin interaction, epithelial-mesenchymal transition (EMT), and cell cycle (Berenjeno and Bustelo 2008; Croze et al. 2014). Y-27632 treatment may cause an increase in cell-cell and cell-ECM interactions, which eventually modulate ECM-microenvironment (Kurosawa 2012). The ECM contains multiple domains that bind with several growth factors. This binding facilitates activation and spatiotemporally controlled release of the growth factors. These events in turn regulate growth factor-induced intracellular signaling (Hynes 2009; Martino et al. 2014). Therefore, modulation of ECM-microenvironment by ROCK is important for cell fate determination.
During osteogenic differentiation of PDL cells, array analysis indicated that the expression of ECM-encoding genes such as fibronectin 1, collagen type I alpha 1, collagen type III alpha 1, and biglycan were markedly downregulated by Y-27632, and a significant decrease in the expression of fibronectin and collagen-I proteins was also observed. Moreover, exogenous fibronectin and collagen-I mediate osteogenic differentiation, with fibronectin showing a more pronounced effect, and the differentiation was almost completely blocked by Y-27632 (Yamamoto et al. 2014; Ugawa et al. 2017). These reports indicate that ECM-mediated differentiation is dependent on ROCK signaling and may represent an autocrine regulation via which periodontal ECM mediates osteogenic differentiation of the PDL cells through a mechanism involving ROCK-dependent upregulation of ECM component expression. Remodeling of the ECM architecture is crucial to initiate differentiation of PDL cells. Therefore, ROCK signaling has substantial roles in the establishment of ECM-microenvironment during osteogenic differentiation of PDL cells.
Migration
Cell migration is highly integrated and multistep process that contributes to tissue repair and regeneration. Cell migration involves spatiotemporal regulation by intricate reciprocal interaction between cells and their microenvironment (Ridley 2003). Rho family proteins play important roles in cell migration by regulating actin and adhesion organization and by controlling the formation of lamellipodia and filopodia. The polarity of migrating cells is based on the fact that Rho and Rac family small GTPase 1 (Rac1; hereafter referred as Rac) are mutually antagonistic (Rottner et al. 1999). A leader cell in collective cell migration showed the upregulation of Rac (Yamaguchi et al. 2015). Active Rac at the leading edge of cells would suppress Rho activity (Ridley 2003). Indeed, Rho inhibition induces migration of fibroblasts and MSC (Totsukawa et al. 2004; Jaganathan et al. 2007). In contrast, Y-27632 decreased stromal cell-derived factor 1 alpha (SDF-1α)-induced migration distance but maintained directionality in MSCs (Park et al. 2017), suggesting an interactive behavior with other components of microenvironment. In PDL cells, Y-27632 is capable of enhancing migration (Wang et al. 2017), probably by activating Rac signaling (our unpublished observation). Integrin-mediated cell adhesion to ECM provides essential signals for cell migration. A potent inducer of PDL cell migration, PDGF-BB, activates Rac signaling and several integrin isoforms in migrating PDL cells. The Rac inhibitor—NSC23766 and neutralizing antibody for integrin α5 can significantly inhibit PDL cell migration (our unpublished observation). Therefore, microenvironment, which involves coordinated interactions with PDGF-BB, and integrin α5-Rac/Rho signaling axis may be important for migration of PDL cells.
Apoptosis
ROCK is also recognized as a pro-apoptotic factor; caspase-3-mediated cleavage of ROCK induces myosin phosphorylation and apoptotic membrane blebbing (Coleman et al. 2001; Sebbagh et al. 2001), and Y-27632 can reduce apoptosis of dissociated hES cells (Watanabe et al. 2007). The ROCK-dependent actomyosin hyperactivation can cause apoptosis, which is triggered by the loss of E-cadherin-dependent intercellular contact and involves Rho activation and Rac inhibition (Ohgushi et al. 2010). In PDL cells, the effect of Y-27632 on apoptosis is not significant (Wang et al. 2017). Because constitutively active expression of Rho mutants (Rho-V14; a generous gift from Dr. Christopher S. Chen, Boston University) in PDL cells ceased proliferation in prolonged culture (our unpublished observation), there might be some effects of Rho/ROCK signaling on apoptosis. Y-27632 upregulated mRNA expression of transforming growth factor beta 1 (TGF-β1) in osteogenic differentiated PDL cells (Yamamoto et al. 2014). Rho/ROCK signaling belongs to TGF-β receptor signaling through Smad-independent pathways. Depending on the cell line, TGF-β can activate Rho/ROCK signaling, which is implicated in TGF-β-induced actin stress-fiber formation, proliferation/apoptosis, and EMT (Derynck and Zhang 2003). The physiological roles of TGF-β show tissue specificity; major role of TGF-β can be represented by inhibition of cell proliferation in epithelia but by induction in mesenchymal cells (Bhowmick et al. 2003; Wrighton et al. 2009). In PDL cells, TGF-β1-induced proliferation and cytoskeleton rearrangement were dependent on ROCK activity (Wang et al. 2014). Therefore, apoptotic effects of ROCK may have tissue specificity and environmental dependency, which are intricately regulated by crosstalk between ROCK and TGF-β signaling. ROCK-mediated ECM such as fibronectin may be necessary for effective activation of TGF-β (Hynes 2009). The mechanisms underlying the pro-apoptotic role of ROCK are not fully understood yet.
The healing patterns of periodontium depend on the dominant cell type in the tissue defect (Melcher 1976). Owing to the higher proliferative rate of gingival epithelial cells than that of PDL cells, it is critical to inhibit epithelial migration, allowing the tissue defects to be repopulated by PDL cells (Polimeni et al. 2006). Meanwhile, re-epithelialization must be completed as rapidly as possible to re-establish tissue integrity. TGF-β/Smad2 signaling inhibits the proliferation and migration of gingival epithelium. It also contributes to maintain tissue integrity by increasing epithelial adhesion and mesenchymal collagen content during wound healing (Tomikawa et al. 2012; Shimoe et al. 2014; Alotaibi et al. 2014; Hongo et al. 2016). However, long-term effects of Smad2 induce excess apoptosis of gingiva and alveolar bone loss (Fujita et al. 2012; Alotaibi et al. 2016). Therefore, during regeneration in the epithelial-mesenchymal boundary, repopulating PDL cells may release regulatory factors such as TGF-β to counteract the apical migration of epithelium in a spatiotemporally controlled manner.
In vivo expression
Two mammalian ROCK homologs have been identified, ROCK1 and ROCK2. ROCK proteins were initially reported to be ubiquitously expressed throughout embryogenesis and in adult tissues. ROCKs have also been demonstrated to have tissue-specific expressions; ROCK1, in comparison to ROCK2, is significantly expressed in the thymus and blood (Julian and Olson 2014). In bone marrow, ROCK1 is more abundantly expressed than ROCK2 (Fagerberg et al. 2014). ROCK1- and ROCK2-knockout mice have been found to have distinct phenotypes, suggesting that their biological roles and spatiotemporal expression patterns are certainly different (Shimizu et al. 2005; Thumkeo et al. 2005). Although Y-27632 is not isoform selective, it is widely used for biological studies and pharmaceutical therapies for various diseases (Duong-Quy et al. 2013; Mishra et al. 2013; Watzlawick et al. 2014; Pernis et al. 2016).
There have been a few reports on in vivo ROCK expression in periodontium. During tooth development, ROCK is expressed in the ameloblasts and involved in ameloblast differentiation and dental enamel development (See Glossary) (Otsu et al. 2011; Peng et al. 2011; Xue et al. 2013). During orthodontic tooth movement, the expression of ROCK has been identified in the tension side of periodontal ligament, suggesting that ROCK is involved in periodontal tissue remodeling during the movement (Meng et al. 2015). In the physiological condition of periodontium, phosphorylated MLC2, a direct effector of ROCK, is localized in the border region adjacent to the bone and the cementum (Yamamoto et al. 2014) where differentiated PDL cells are present with an increased expression of collagen-I and ALP activity (Rooker et al. 2010). These observations strongly suggest that increased ROCK activity and synergistic increase of ECM components play important roles during osteogenic differentiation of PDL cells (Fig. 2).
Inflammation
Rho/ROCK signaling also acts as an inflammatory regulator. In epithelial cells, inflammation disrupts tight junctions and induces hyperpermeability; these events not only promote bacterial invasion but also prolong inflammation. This entire process is generally dependent on Rho/ROCK signaling (Hirase et al. 2001; Adamson et al. 2002; Schaible et al. 2013). Certain functions of immune cells, such as neutrophil migration, neutrophil adhesion to epithelial cells, and T-cell immunity are also regulated by Rho/ROCK signaling (Saito et al. 2002; Yagi et al. 2006; Hasan et al. 2013). Interestingly, Rho/ROCK signaling-mediated extracellular environment may modulate pro-inflammatory (M1) vs. pro-healing (M2) activation (McWhorter et al. 2013). Moreover, Y-27632 significantly inhibits lipopolysaccharide-induced tissue failure and injured tissue damage in vivo. The expression of a series of inflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-1 beta, and monocyte chemoattractant protein 1, are reduced in the Y-27632-treated tissues (Segain et al. 2003; Meyer-Schwesinger et al. 2009; Poosti et al. 2012; Wang et al. 2015).
Although periodontitis is an oral biofilm-induced inflammatory disease, to the best of our knowledge, only few studies have investigated the regulation of periodontal inflammation by Rho/ROCK signaling and Y-27632 (Takemura et al. 2006; Kajiya et al. 2011; Lee et al. 2015). ROCK activity is important in regulation of inflammation-regeneration interplay, which would be valuable to establish a new strategy for treatment of periodontitis in future.
Conclusions
In the last decade, periodontal tissue engineering and regenerative capacity of PDL cells were extensively studied with emphasis on the effects of growth factors. Despite the presence of a biological rationale for the growth factors, the effect remains limited and unpredictable (Susin and Wikesjö 2013; Cochran et al. 2015). Use of growth factors in periodontal tissue engineering has certain limitations in terms of cost-effectiveness, biological stability, carcinogenicity, and spatiotemporal specificity (Bartold et al. 2000; Somerman 2011). Therefore, besides growth factors, other components of the microenvironment, especially ECM, may have synergistic and expanded effects on bioactivities of PDL cells.
As discussed in this review, Rho/ROCK signaling plays a central role in modulating ECM-microenvironment, which affects cell behavior, proliferation, migration, and differentiation-state of PDL cells, especially PDL-derived stem cells (Fig. 2). The ROCK inhibitor Y-27632 is generally capable of disassembling actin cytoskeleton and enhancing activities of stem cells. However, these effects exhibit cell specificity and environment dependency, which need to be extensively investigated in future. It is crucial that Rho and Rac should act as molecular switch between on and off state to maintain stem cells in control; such molecular switch should also prevent excessive apoptosis and neoplastic differentiation.
Recent studies have aimed to manipulate ECM-microenvironment for tissue regeneration by using either artificial ECM or self-produced ECM as a scaffold in the stem cell therapy (Gobaa et al. 2011; Martino et al. 2014; Takewaki et al. 2017; Di Maggio et al. 2017; Zhu et al. 2017). These studies have demonstrated strikingly improved regenerative efficiency and proposed promising approaches in clinical applications. It is intriguing how Rho/ROCK signaling is involved in the manipulation of ECM-microenvironment. Monitoring ROCK activity during wound healing could provide one of the lineage-specific clues in this phenomenon. Future studies will continue focusing on modulating the microenvironment to direct tissue regeneration.
Acknowledgements
We would like to thank colleagues of our research unit at Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences for helpful supports and valuable comments on this manuscript. We also thank Christopher S. Chen, Boston University, for kindly providing expression vectors of Rho/ROCK mutants. This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (20592429, 26463134) and Research Award from the Ryobi Teien Foundation.
Abbreviations
- ALP
Alkaline phosphatase
- c-Myc
MYC proto-oncogene
- ECM
Extracellular matrix
- hES cells
Human embryonic stem cells
- Klf4
Kruppel like factor 4
- MLC2
Myosin light chain 2
- MSC
Mesenchymal stem cells
- Nanog
Nanog homeobox
- Oct3/4 (POU5F1)
POU class 5 homeobox 1
- PDGF-BB
Platelet-derived growth factor-BB
- PDL cells
Periodontal ligament cells
- Rac1
Rac family small GTPase 1
- Rho GTPase
Rho guanosine triphosphatase
- ROCK
Rho-associated coiled-coil containing protein kinase
- Runx2
Runt-related transcription factor 2
- TGF-β
Transforming growth factor beta
- YAP/TAZ
Yes-associated protein/transcriptional coactivator with PDZ-binding motif
Glossary
- Periodontal ligament
Connective tissue that surrounds the tooth root and connects to the alveolar bone
- Gingiva
The part of the oral mucosa that covers the alveolar bone processes and surrounds the necks of the teeth
- Cementum
Calcified mesenchymal tissue that forms the outer covering of the tooth root and provides attachment to periodontal ligaments
- Cementoblasts
Continuous cell layer that forms cementum on the root surface and interposes between bundles of PDL fibers
- Dental papilla
The ball of condensed ectomesenchymal cells that forms the dental pulp and dentin, the substance beneath the tooth enamel and the cementum
- Ectomesenchymal cells
A group of cranial neural crest-derived mesenchymal cells
- Perivascular cells
Cells migrate into periodontal ligament from the bone marrow by way of vascular channels, which occupy a perivascular location
- Enamel
Hard, acellular, inorganic, inert tissue that covers the tooth crown and mainly consists of hydroxyapatite crystallite
- Ameloblast
Enamel-forming cells that secret organic matrix proteins and present only during tooth development
Compliance with ethical standards
Our data described in this review were obtained using the protocol (no. 975, 2070), approved by the Research Ethics Committee for Human Genome/Gene Analysis Research in Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences.
Conflict of interest
All authors have no conflict of interest regarding this paper.
References
- Adamson RH, Curry FE, Adamson G et al (2002) Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 539:295–308. 10.1113/jphysiol.2001.013117 [DOI] [PMC free article] [PubMed]
- Alotaibi MK, Kitase Y, Shuler CF. Smad2 overexpression reduces the proliferation of the junctional epithelium. J Dent Res. 2014;93:898–903. doi: 10.1177/0022034514543016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi MK, Kitase Y, Shuler CF. Smad2 overexpression induces alveolar bone loss and up regulates TNF-α, and RANKL. Arch Oral Biol. 2016;71:38–45. doi: 10.1016/j.archoralbio.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by rho-associated kinase (rho- kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Aukhil I (2000) Biology of wound healing. Periodontol 2000 22:44–50. 10.1034/j.1600-0757.2000.2220104.x [DOI] [PubMed]
- Bartold P, Mcculloch CAG, Narayanan A, Pitaru S (2000) Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000 24:253–269. 10.1034/j.1600-0757.2000.2240113.x [DOI] [PubMed]
- Beertsen W, Mcculloch CAG, Sodek J (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 13:20–40. 10.1111/j.1600-0757.1997.tb00094.x [DOI] [PubMed]
- Berenjeno IM, Bustelo XR. Identification of the Rock-dependent transcriptome in rodent fibroblasts. Clin Transl Oncol. 2008;10:726–738. doi: 10.1007/s12094-008-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Aakre M, et al. TGF-beta-induced RhoA and p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc Natl Acad Sci U S A. 2003;100:15548–15553. doi: 10.1073/pnas.2536483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro DP, Liebmann-Vinson A, Heidaran MA. Molecular signaling in bioengineered tissue microenvironments. Ann N Y Acad Sci. 2002;961:143–153. doi: 10.1111/j.1749-6632.2002.tb03068.x. [DOI] [PubMed] [Google Scholar]
- Chen JC, Jacobs CR. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther. 2013;4:107. doi: 10.1186/scrt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Gao L, Tian B, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/S0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Cobb CM, Bashutski JD, et al. Emerging regenerative approaches for periodontal reconstruction: a consensus report from the AAP regeneration workshop. J Periodontol. 2015;86:S153–S156. doi: 10.1902/jop.2015.140381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Croze RH, Buchholz DE, Radeke MJ, et al. ROCK inhibition extends passage of pluripotent stem cell-derived retinal pigmented epithelium. Stem Cells Transl Med. 2014;3:1066–1078. doi: 10.5966/sctm.2014-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Di Maggio N, Martella E, Frismantiene A, et al. Extracellular matrix and α5β1 integrin signaling control the maintenance of bone formation capacity by human adipose-derived stromal cells. Sci Rep. 2017;7:44398. doi: 10.1038/srep44398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong-Quy S, Bei Y, Liu Z, Dinh-Xuan AT. Role of rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol Ther. 2013;137:352–364. doi: 10.1016/j.pharmthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Alotaibi M, Kitase Y, et al. Smad2 is involved in the apoptosis of murine gingival junctional epithelium associated with inhibition of Bcl-2. Arch Oral Biol. 2012;57:1567–1573. doi: 10.1016/j.archoralbio.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Gobaa S, Hoehnel S, Roccio M, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Hasan Z, Palani K, Zhang S, et al. Rho kinase regulates induction of T-cell immune dysfunction in abdominal sepsis. Infect Immun. 2013;81:2499–2506. doi: 10.1128/IAI.00126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T, Kawashima S, Wong EYM, et al. Regulation of tight junction permeability and Occludin phosphorylation by RhoA-p160ROCK-dependent and -independent mechanisms. J Biol Chem. 2001;276:10423–10431. doi: 10.1074/jbc.M007136200. [DOI] [PubMed] [Google Scholar]
- Hong SY, Jeon YM, Lee HJ, et al. Activation of RhoA and FAK induces ERK-mediated osteopontin expression in mechanical force-subjected periodontal ligament fibroblasts. Mol Cell Biochem. 2010;335:263–272. doi: 10.1007/s11010-009-0276-1. [DOI] [PubMed] [Google Scholar]
- Hongo S, Yamamoto T, Yamashiro K, et al. Smad2 overexpression enhances adhesion of gingival epithelial cells. Arch Oral Biol. 2016;71:46–53. doi: 10.1016/j.archoralbio.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ivanovska IL, Shin J, Swift J, Discher DE. Stem cell mechanobiology : diverse lessons from bone marrow. Trends Cell Biol. 2015;25:523–532. doi: 10.1016/j.tcb.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jaganathan BG, Ruester B, Dressel L, et al. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells. 2007;25:1966–1974. doi: 10.1634/stemcells.2007-0167. [DOI] [PubMed] [Google Scholar]
- Joo HJ, Choi DK, Lim JS, et al. ROCK suppression promotes differentiation and expansion of endothelial cells from embryonic stem cell-derived Flk1+ mesodermal precursor cells. Blood. 2012;120:2733–2744. doi: 10.1182/blood-2012-04-421610. [DOI] [PubMed] [Google Scholar]
- Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK) Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya M, Komatsuzawa H, Papantonakis A, et al. Aggregatibacter actinomycetemcomitans Omp29 is associated with bacterial entry to gingival epithelial cells by F-actin rearrangement. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, et al. Rho-kinase-mediated contraction of isolated stress fibers. J Cell Biol. 2001;152:569–583. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009;24:886–898. doi: 10.1359/jbmr.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletsas D, Basdra EK, Papavassiliou AG. Effect of protein kinase inhibitors on the stretch-elicited c-Fos and c-Jun up-regulation in human PDL osteoblast-like cells. J Cell Physiol. 2002;190:313–321. doi: 10.1002/jcp.10052. [DOI] [PubMed] [Google Scholar]
- Kurosawa H. Application of rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng. 2012;114:577–581. doi: 10.1016/j.jbiosc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Lee HK, Ji S, Park SJ, et al. Odontogenic ameloblast-associated protein (ODAM) mediates junctional epithelium attachment to teeth via integrin-odam-rho guanine nucleotide exchange factor 5 (ARHGEF5)-RhoA signaling. J Biol Chem. 2015;290:14740–14753. doi: 10.1074/jbc.M115.648022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Han S, Wang X, et al. Rho kinase inhibitor Y-27632 promotes the differentiation of human bone marrow mesenchymal stem cells into keratinocyte-like cells in xeno-free conditioned medium. Stem Cell Res Ther. 2015;6:17. doi: 10.1186/s13287-015-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Manneville SE, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Güç E, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McWhorter FY, Wang T, Nguyen P, et al. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- Meng R, Song M, Pan J. Rho is involved in periodontal tissue remodelling with experimental tooth movement in rats. Arch Oral Biol. 2015;60:923–931. doi: 10.1016/j.archoralbio.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Meyer-Schwesinger C, Dehde S, von Ruffer C, et al. Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-kappaB p65 signaling. Am J Physiol Renal Physiol. 2009;296:F1088–F1099. doi: 10.1152/ajprenal.90746.2008. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Alokam R, Sriram D, Yogeeswari P. Potential role of rho kinase inhibitors in combating diabetes-related complications including diabetic neuropathy--a review. Curr Diabetes Rev. 2013;9:249–266. doi: 10.2174/1573399811309030006. [DOI] [PubMed] [Google Scholar]
- Nanci A, Bosshardt D. Structure od periodontal tissues in health and disease. Periodontol. 2006;40:11–28. doi: 10.1111/j.1600-0757.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Ohgushi M, Matsumura M, Eiraku M, et al. Article molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Stem. Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Otsu K, Kishigami R, Fujiwara N, et al. Functional role of rho-kinase in ameloblast differentiation. J Cell Physiol. 2011;226:2527–2534. doi: 10.1002/jcp.22597. [DOI] [PubMed] [Google Scholar]
- Pacary E, Legros H, Valable S, et al. Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119:2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Wang T, Wang L et al (2014) Cyclic strain-induced cytoskeletal rearrangement of human periodontal ligament cells via the rho signaling pathway. PLoS One. 10.1371/journal.pone.0091580 [DOI] [PMC free article] [PubMed]
- Park S, Jang H, Jeong GS et al (2017) Directional and trans-endothelial migration of mesenchymal stem cell towards Sdf-1a gradient on a microfluidic device. PLoS One 12(9):e0184595. 10.1371/journal.pone.0184595. [DOI] [PMC free article] [PubMed]
- Pavlin D, Gluhak-Heinrich J (2001) Effect of mechanical loading on periodontal cells. Crit Rev Oral Biol Med 12:414–424. 12002823 [DOI] [PubMed]
- Peng L, Li Y, Shusterman K, et al. Wnt-RhoA signaling is involved in dental enamel development. Eur J Oral Sci. 2011;119:41–49. doi: 10.1111/j.1600-0722.2011.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis AB, Ricker E, Weng CH, et al. Rho kinases in autoimmune diseases. Annu Rev Med. 2016;67:355–374. doi: 10.1146/annurev-med-051914-022120. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366:1809–1820. 10.1016/S0140-6736(05)67728-8 [DOI] [PubMed]
- Polimeni G, Xiropaidis A V., Wikesjö UME (2006) Biology and principles of periodontal wound healing/regeneration. Periodontol 2000 41:30–47. 10.1111/j.1600-0757.2006.00157.x [DOI] [PubMed]
- Poosti F, Yazdani S, Dolman MEM, et al. Targeted inhibition of renal rho kinase reduces macrophage infiltration and lymphangiogenesis in acute renal allograft rejection. Eur J Pharmacol. 2012;694:111–119. doi: 10.1016/j.ejphar.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rooker SM, Liu B, Helms JA. Role of Wnt signaling in the biology of the periodontium. Dev Dyn. 2010;239:140–147. doi: 10.1002/dvdy.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/S0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Saito H, Minamiya Y, Saito S, Ogawa J (2002) Endothelial rho and rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol 72:829–836 [PubMed]
- Schaible B, McClean S, Selfridge A, et al. Hypoxia modulates infection of epithelial cells by Pseudomonas Aeruginosa. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0056491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/S0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoizé C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Segain JP, De la Blétière DR, Sauzeau V, et al. Rho kinase blockade prevents inflammation via nuclear factor κB inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124:1180–1187. doi: 10.1016/S0016-5085(03)00283-X. [DOI] [PubMed] [Google Scholar]
- Seo B-M, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shih YRV, Tseng KF, Lai HY, et al. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoe M, Yamamoto T, Shiomi N, et al. Overexpression of Smad2 inhibits proliferation of gingival epithelial cells. J Periodontal Res. 2014;49:290–298. doi: 10.1111/jre.12106. [DOI] [PubMed] [Google Scholar]
- Somerman M. Growth factors and periodontal engineering: where next? J Dent Res. 2011;90:7–8. doi: 10.1177/0022034510383144. [DOI] [PubMed] [Google Scholar]
- Susin C, Wikesjö UME. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol. 2013;62:232–242. doi: 10.1111/prd.12003. [DOI] [PubMed] [Google Scholar]
- Takemura A, Nakagawa I, Kawai S, et al. Inhibitory effects of tumor necrosis factor-alpha on migration of human periodontal ligament cells. J Periodontol. 2006;77:883–890. doi: 10.1902/jop.2006.050192. [DOI] [PubMed] [Google Scholar]
- Takewaki M, Kajiya M, Takeda K, et al (2017) MSC/ECM cellular complexes induce periodontal tissue regeneration. J Dent Res 2203451770877. 10.1177/0022034517708770 [DOI] [PubMed]
- Thumkeo D, Shimizu Y, Sakamoto S, et al. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- Tomikawa K, Yamamoto T, Shiomi N, et al. Smad2 decelerates re-epithelialization during gingival wound healing. J Dent Res. 2012;91:764–770. doi: 10.1177/0022034512451449. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Tonetti MS, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Wu Y, Sasaki Y, et al. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Yamamoto T, Kawamura M, et al. Rho-kinase regulates extracellular matrix-mediated osteogenic differentiation of periodontal ligament cells. Cell Biol Int. 2017;41:651–658. doi: 10.1002/cbin.10769. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang T, Song M, Pan J. Rho plays a key role in TGF-β1-induced proliferation and cytoskeleton rearrangement of human periodontal ligament cells. Arch Oral Biol. 2014;59:149–157. doi: 10.1016/j.archoralbio.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Wang C, Song S, Zhang Y, et al. Inhibition of the rho/rho kinase pathway prevents lipopolysaccharide-induced hyperalgesia and the release of TNF-α and IL-1β in the mouse spinal cord. Sci Rep. 2015;5:14553. doi: 10.1038/srep14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luo J-Y, Li B, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- Wang T, Kang W, Du L, Ge S (2017) Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J Cell Mol Med:1–13. 10.1111/jcmm.13222 [DOI] [PMC free article] [PubMed]
- Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Watzlawick R, Sena ES, Dirnagl U, et al. Effect and reporting bias of RhoA/ROCK-blockade intervention on locomotor recovery after spinal cord injury. JAMA Neurol. 2014;71:91. doi: 10.1001/jamaneurol.2013.4684. [DOI] [PubMed] [Google Scholar]
- Wongkhantee S, Yongchaitrakul T, Pavasant P. Mechanical stress induces osteopontin via ATP/P2Y1 in periodontal cells. J Dent Res. 2008;87:564–568. doi: 10.1177/154405910808700601. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Feng X-H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Li Y, Everett ET, et al. Ameloblasts require active RhoA to generate normal dental enamel. Eur J Oral Sci. 2013;121:293–302. doi: 10.1111/eos.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Otani H, Ando S, et al. Involvement of rho signaling in PAR2-mediated regulation of neutrophil adhesion to lung epithelial cells. Eur J Pharmacol. 2006;536:19–27. doi: 10.1016/j.ejphar.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Mizutani T, Kawabata K, Haga H. Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin β1 and PI3K. Sci Rep. 2015;5:7656. doi: 10.1038/srep07656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ugawa Y, Yamashiro K, et al. Osteogenic differentiation regulated by rho-kinase in periodontal ligament cells. Differentiation. 2014;88:33–41. doi: 10.1016/j.diff.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Yamashiro K, Myokai F, Hiratsuka K, et al. Oligonucleotide array analysis of cyclic tension-responsive genes in human periodontal ligament fibroblasts. Int J Biochem Cell Biol. 2007;39:910–921. doi: 10.1016/j.biocel.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Zhu B, Liu W, Liu Y, et al. Jawbone microenvironment promotes periodontium regeneration by regulating the function of periodontal ligament stem cells. Sci Rep. 2017;7:40088. doi: 10.1038/srep40088. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]