Abstract
Endoplasmic reticulum (ER) stress is a key factor contributing to fibrotic disease. Although ER stress is a short-term adaptive response, with chronic stimulation, it can activate pathways leading to fibrosis. ER stress can induce TGF-β signaling, a central driver of extracellular matrix production in fibrosis. This review will discuss the role of an ER protein, calreticulin (CRT), which has both chaperone and calcium regulatory functions, in fibrosis. CRT expression is upregulated in multiple different fibrotic diseases. The roles of CRT in regulation of fibronectin extracellular matrix assembly, extracellular matrix transcription, and collagen secretion and processing into the extracellular matrix will be discussed. Evidence for the importance of CRT in ER calcium release and NFAT activation downstream of TGF-β signaling will be presented. Finally, we will summarize evidence from animal models in which CRT expression is genetically reduced or experimentally downregulated in targeted tissues of adult animals and discuss how these models define a key role for CRT in fibrotic diseases.
Keywords: Calreticulin, Endoplasmic reticulum stress, TGF-β, NFAT, Fibrosis
Link between ER stress and fibrosis
An increasing body of literature suggests a prominent role for endoplasmic reticulum (ER) stress in a variety of fibrotic diseases. The ER is the intracellular organelle that ensures proper folding and secretion of nascent proteins into functional forms and is crucial for cell survival (Malhotra and Kaufman 2007). Several factors, both physiological and pathological, can impair ER homeostasis and its capacity to properly fold proteins, resulting in ER stress. These conditions include hyperglycemia, nutrient deprivation, aging, viral infection, changes in redox and ionic conditions of the ER such as calcium depletion, gene mutations that alter the proper functioning of the ER, and environmental factors such as exposure to cigarette smoke (Kinnula et al. 2005; Kitamura 2008; Lindenmeyer et al. 2008; Pereira et al. 2006; Tanjore et al. 2013). In diabetes for instance, diabetic complications such as atherosclerosis and diabetic nephropathy have been linked to ER stress (Cybulsky 2010; Kurokawa et al. 2009; Lindenmeyer et al. 2008; Tabas 2010). ER stress is induced by multiple factors in the diabetic milieu such as hyperglycemia, oxidative stress, proteinuria and advanced glycation end products (Kitamura 2008; Lenna and Trojanowska 2012; Lindenmeyer et al. 2008; Tanjore et al. 2013).
The cell engages protective mechanisms, collectively referred to as the unfolded protein response (UPR), to restore and/or maintain homeostasis (Ron and Walter 2007). These adaptive mechanisms help the cell cope with ER stress. Prolonged ER stress conditions often result in cellular dysfunction and cell death when the protein load on the ER far exceeds its folding capacity. There are three major transmembrane proteins that act as stress sensors and govern UPR signaling pathways in mammals: inositol-requiring protein 1 (IRE1), protein kinase RNA (PKR)-like ER kinase (PERK) and activating transcription factor 6 (ATF6) (Haze et al. 1999; Ron and Walter 2007; Walter and Ron 2011). These proteins generally sense the abnormal conditions in the ER lumen and relay this information across the ER membrane to the nucleus. Under normal, unstressed homeostatic conditions, the three sensor proteins are maintained in an inactive state via binding to the molecular chaperone, GRP78 (Bertolotti et al. 2000). Under stressed conditions, there is an accumulation of unfolded proteins in the ER and GRP78 disassociates from the UPR protein sensors, IRE1, ATF6, and PERK, followed by activation of these UPR pathways which protect the cell from ER stress (Grootjans et al. 2016; Tanjore et al. 2013).
Over the past decade, evidence has accumulated to establish that ER stress is an underlying factor associated with fibrotic diseases (Lenna and Trojanowska 2012; Tanjore et al. 2013). The molecular mechanisms linking chronic ER stress and fibrosis are not completely understood and our current understanding suggests that ER stress facilitates fibrotic remodeling through multiple mechanisms, including activating pro-apoptotic signaling pathways, inducing epithelial to mesenchymal transition (EMT), and by enhancing production of the extracellular matrix (ECM) (Tanjore et al. 2013; Willis et al. 2005; Zhang et al. 2017). It is likely that the relative contribution of each mechanism will vary depending on the tissue, the microenvironmental milieu and the initial stimuli that induce ER stress.
Apoptosis is a well-characterized mechanism linking ER stress to fibrosis. When ER stress is severe and chronic, the UPR-mediated quality control measures to correct defective protein folding fail, resulting in the activation of several apoptotic pathways (Kim et al. 2008; Rutkowski and Kaufman 2007). ER stress-induced apoptosis has been demonstrated in models of pulmonary and renal fibrosis in which there is apoptotic death of key epithelial cell types in these organs (Chiang et al. 2011; Lawson et al. 2008; Tanjore et al. 2013). IRE1α, which is a major UPR signaling molecule that determines the fate of cells under stress conditions, promotes apoptosis by mediating activation of downstream stress kinases such as JNK and p38 MAPK (Ron and Hubbard 2008). Like IRE1α, sustained activation of PERK pathways also induces proapoptotic pathways. PERK phosphorylates the eukaryotic translation initiation factor eIF2α, resulting in increased expression of proapoptotic UPR target molecules, growth arrest, and DNA damage-inducible 34 (GADD34) and transcription factor C/EBP homologous protein (CHOP) via activating transcription factor 4, ATF4 (Ma et al. 2002). Fluctuations in ER Ca2+ have also been shown to play a role in ER stress-associated cell death by activating pro-apoptotic signaling pathways, mainly by Ca2+-mediated mitochondrial cell death (Rizzuto et al. 1998). ER membrane re-organization and the mitogen-activated protein kinase kinase kinase 1 (MEKK1) signaling pathway are other UPR-independent mechanisms that can induce apoptosis (Kang et al. 2012; Varadarajan et al. 2012).
Although there is a clear role for ER stress-induced alveolar epithelial cell apoptosis in lung fibrosis (Gunther et al. 2003; Kamp et al. 2013; Kuwano 2008; Plataki et al. 2005), ER stress is also associated with fibrotic sequelae in diseases in which apoptosis is not the primary initiating event, such as fibroproliferative vascular remodeling in diabetes (Baek et al. 2012; Tanaka et al. 2012). These data suggest that ER stress also directly impacts cellular responses of mesenchymal cells to injury. Several lines of evidence show that ER-stress induces EMT, along with recruitment and differentiation of fibrocytes (Tanjore et al. 2011; Willis et al. 2005; Wynn and Ramalingam 2012). An association between ER stress and cell plasticity was first confirmed in thyroid epithelial cells in which ER stress induced by tunicamycin was shown to trigger EMT (Ulianich et al. 2008). In pathological conditions, ER stress has also been demonstrated to induce EMT in lung, kidney, and liver cells (Tanjore et al. 2011; Willis et al. 2005). The pro-fibrotic cytokine, transforming growth factor-β (TGF-β), is a major driver of EMT and there is emerging evidence to show that components of the ER stress response can regulate TGF-β signaling (Sato et al. 2003; Willis and Borok 2007; Willis et al. 2005). For example, knockdown of the chaperone GRP78 reduces TGF-β or tunicamycin-stimulated collagen and α-smooth muscle actin production in human lung fibroblasts (Baek et al. 2012). In addition, the ER stress protein, 150-kDa oxygen-regulated protein (ORP150), mediates TGF-β-driven myofibroblast induction and collagen production in human lung fibroblasts (Tanaka et al. 2012). The mechanisms by which GRP78 and ORP150 mediate responses to TGF-β have not been addressed. Furthermore, data show that the ER chaperone and calcium regulatory protein, calreticulin (CRT), plays a significant role in fibrotic remodeling through multiple mechanisms, including regulation of EMT, cell apoptosis and migration, and via regulation of TGF-β-stimulated ECM production (Kypreou et al. 2008; Papp et al. 2007; Prakoura et al. 2013; Tanjore et al. 2011; Van Duyn Graham et al. 2010; Wu et al. 2017; Zimmerman et al. 2013). Interestingly, CRT mediates TGF-β-dependent transcriptional responses through its role as a regulator of calcium signaling, rather than through its chaperone/UPR function, suggesting a unique role for CRT in the ER stress response.
Due to the pivotal role that ER stress plays in the pathogenesis of fibrotic diseases, therapeutic approaches that target the ER and UPR are being explored. Chemical chaperones such as 4-phenylbutyrate (4-PBA) and tauroursodeoxycholic acid (TUDCA), which enhance the protein folding capacity and function of ER, help alleviate fibrosis of the liver, lung and heart (Ayala et al. 2012; Baek et al. 2012; Groenendyk et al. 2016; Omura et al. 2013; Wang et al. 2013). Liu et al. recently reported that 4-PBA ameliorates ER stress-induced renal tubular cell apoptosis and renal fibrosis by acting as an ER chaperone (Liu et al. 2016). Using a mouse model of heart failure with increased CRT expression, Groenendyk et al. recently showed that blocking activation of the transiently activated UPR pathway by TUDCA is sufficient to prevent cardiac fibrosis and improves prognosis (Groenendyk et al. 2016). Valproate, a histone deacetylation enzyme, was recently shown to attenuate renal injury in a rat model by relieving ER stress and decreasing renal cell apoptosis (Sun et al. 2016). Oleanolic acid and the antioxidant, N-acetylcysteine, were also recently demonstrated to ameliorate diabetic nephropathy by reducing oxidative stress and ER stress in a type 2 diabetic rat model (Lee et al. 2016).
Introduction to calreticulin
Calreticulin (CRT), (Calr), is a 46 kDa Ca2+-binding chaperone, which has multi-faceted roles in diverse cellular functions. CRT is composed of three distinct domains: a lectin-binding N domain, a proline-rich high affinity, low capacity calcium-binding P domain, and a C-terminal domain comprised of multiple acidic residues. The N and P domains of CRT combine to mediate the lectin binding and chaperone functions of CRT, whereas the C domain is responsible for its Ca2+ buffering functions through high capacity Ca2+ binding (Baksh and Michalak 1991; Kapoor et al. 2004; Leach et al. 2002). The C-terminal domain of CRT also contains the KDEL ER retention sequence (Smith and Koch 1989). CRT has multiple cellular functions, both in the context of its ER functions in the UPR and in regulating ER calcium storage and release, and also as a protein localized to the cell surface, where it plays critical roles in cell adhesion and migration, wound healing, apoptotic clearance of cells, and anti-tumor responses (Gold et al. 2010; Groenendyk et al. 2004). CRT protein expression is increased by conditions that induce ER stress, such as amino acid deprivation, ER Ca2+ depletion, oxidative stress, heat shock, high glucose, and also by factors associated with fibrotic disease (Conway et al. 1995; Heal and McGivan 1998; Jethmalani and Henle 1998; Kurokawa et al. 2009; Llewellyn et al. 1995; Nguyen et al. 1996; Wu et al. 2007).
Role of CRT in unfolded protein response/ER stress
In the ER, CRT acts as a molecular chaperone that ensures proper folding and functioning of proteins. CRT, together with its 90 kDa ER integral membrane protein homologue, calnexin, and ERp57 (ER protein of 57 kDa) constitute the CRT/calnexin cycle, which is involved in chaperoning of nascent polypeptides that are transported across the ER. CRT and calnexin constitute the ER lectin chaperones, which recognize and ensure that specific glycoproteins and polypeptides are properly folded (Hebert and Molinari 2007; Helenius and Aebi 2001; Saito et al. 1999; Spiro et al. 1996). This mechanism not only ensures that proteins are properly folded, but also prevents secretion and transport of misfolded proteins and prevents aggregation of proteins that can be detrimental to the cell. Prolonged interaction with calnexin, however, results in the proteins being targeted for degradation (Hebert and Molinari 2007).
Role of CRT in regulating release of calcium from ER and activation of downstream signaling (calcineurin/NFAT)
ER CRT has important roles in regulating intracellular Ca2+ homeostasis, acting as both a molecular sensor and a buffer of Ca2+ in the ER lumen. CRT was first identified as a Ca2+-binding protein of the muscle sarcoplasmic reticulum (Ostwald and MacLennan 1974). It later became clear that CRT is not just limited to muscle tissues, but is also found abundantly in non-muscle tissues as one of the major Ca2+-binding proteins of the ER (Michalak et al. 1992).
Ca2+ is an important signaling molecule that affects various cellular and developmental processes. The ER is the major Ca2+-storing organelle and it plays a pivotal role in maintaining cellular Ca2+ homeostasis through regulation of Ca2+ uptake and release. Inositol 1,4,5- triphosphate receptor and the ryanodine receptor control the release of Ca2+ from the ER lumen, while the SERCA pump restores Ca2+ stores in the ER (Michalak et al. 2009). There are a number of Ca2+-buffering proteins within the lumen of the ER that bind ER luminal Ca2+ and which are involved in maintaining ER function. CRT is an important ER Ca2+-buffering protein involved in regulating ER Ca2+ storage and release. CRT also regulates downstream cytosolic Ca2+-dependent signaling via calcineurin and the transcription factor, nuclear factor of activated T cells (NFAT) (Mesaeli et al. 1999; Michalak et al. 2009). Calcium activates calmodulin to activate the phosphatase calcineurin, which then dephosphorylates NFAT, permitting nuclear translocation of activated NFAT for transcriptional regulation (Crabtree 2001; Gooch et al. 2004; Groenendyk et al. 2004). CRT-deficient mouse embryonic fibroblasts (MEFs) have decreased ER Ca2+ stores and impaired agonist-induced Ca2+ release from the ER. Conversely, cells overexpressing CRT have increased intracellular stores of Ca2+ (Groenendyk et al. 2004; Mery et al. 1996). Impaired ER Ca2+ release in CRT-deficient cells results in defects in downstream Ca2+/calcineurin signaling with decreased NFAT nuclear translocation (Lynch et al. 2005; Mesaeli et al. 1999). CRT-deficient mice die during embryogenesis due to impaired cardiac development (Guo et al. 2002; Lynch et al. 2005; Mesaeli et al. 1999). CRT-deficient mice can be rescued from embryonic lethality if calcineurin is constitutively activated during development, indicating the importance of CRT’s role in regulating ER Ca2+ release and downstream calcineurin/NFAT signaling (Guo et al. 2002; Lynch and Michalak 2003; Mesaeli et al. 1999).
Cell surface/extracellular CRT and wound healing
Interestingly, CRT has a number of extracellular functions, particularly in cell adhesion, migration and phagocytosis of apoptotic cells (Gold et al. 2010). Although it is not clear how CRT is transported from the ER to the cell surface (Tarr et al. 2010), CRT is found on the surface of many mammalian cells including fibroblasts, endothelial cells and platelets (Gardai et al. 2005; Goicoechea et al. 2000; Gray et al. 1995; Orr et al. 2003b). One prominent role of cell surface calreticulin is its involvement in focal adhesion disassembly and cell migration induced by heparin binding peptides from the N-terminal domain of thrombospondin 1 (TSP-1) (Goicoechea et al. 2000; Orr et al. 2003a). The TSP binding site in CRT that mediates TSP signaling is located in the N-terminus of CRT at amino acid positions 19–36 (Goicoechea et al. 2002). TSP-1 binding to CRT on the cell surface activates signaling cascades that trigger the activation of phosphoinositide-3 kinase (PI3K) and ERK as well as guanine nucleotide-binding protein to promote the cytoskeletal changes associated with focal adhesion disassembly (Murphy-Ullrich 2001; Orr et al. 2002; Orr et al. 2003b). Low-density lipoprotein (LDL)-receptor-related protein 1 (LRP1) has been identified as the co-receptor of CRT that mediates TSP-1-stimulated deadhesion and TSP-1 binding to CRT enhances CRT-LRP-1 association (Orr et al. 2003b; Yan et al. 2010).
TSP-1 signaling through the CRT-LRP1 receptor also protects cells from anoikis (apoptotic cell death due to loss of cell adhesion) and increases cell survival of fibroblasts under adhesion-independent conditions by down-regulating apoptotic signaling and stimulating pro-survival Akt signaling (Pallero et al. 2008). Recent studies also identified the CRT binding site of TSP1 as a mediator of collagen expression and deposition via a TGF-β-independent, Akt-dependent signaling pathway during tissue remodeling (Sweetwyne et al. 2010). Together these studies established a role for cell surface CRT in regulating cell adhesion and migration, cell survival, and collagen ECM production and showed that ligand binding to CRT can induce intracellular signaling through stimulation of CRT interactions with its transmembrane co-receptor LRP-1. Furthermore, these data suggest an important role for CRT and TSP-1 in tissue remodeling in response to injury.
Additional in vitro and animal studies provided further evidence for cells surface or exogenous CRT in mediating tissue responses to injury. Gray et al., were the first to show that cell surface CRT binds to the Bβ chain of fibrinogen, mediating its mitogenic activity (Gray et al. 1995). In wound healing studies using recombinant CRT, it was shown that topical application of CRT to porcine excisional wounds accelerates wound healing by increasing the rate of wound re-epithelialization and granulation tissue formation (Nanney et al. 2008). CRT-treated wounds showed a significant increase in TGF-β-3 in the dermis, which is thought to control wound healing by regulating migration of epidermal and dermal cells in injured skin (Bandyopadhyay et al. 2006). CRT-treated wounds also showed an increase in proliferating basal keratinocytes and cells of the neodermis. Consistent with these in vivo observations, in vitro studies also demonstrated that CRT stimulates proliferation and migration of cells that are crucial to both wound remodeling and resurfacing (Nanney et al. 2008). Exogenous CRT increases in vitro cell migration, proliferation, growth factor production, and the uptake of apoptotic cells by macrophages, functions shown to be lacking in chronic diabetic wound healing (Brem and Tomic-Canic 2007; Ochoa et al. 2007). Furthermore, topical application of CRT also improved the rate and quality of regenerative wound healing in diabetic animal models with reduced scarring and actual restoration of hair growth in these mice (Greives et al. 2012). Thus, CRT could have therapeutic potential for chronic non-healing wounds.
Calreticulin and regulation of ECM assembly and production
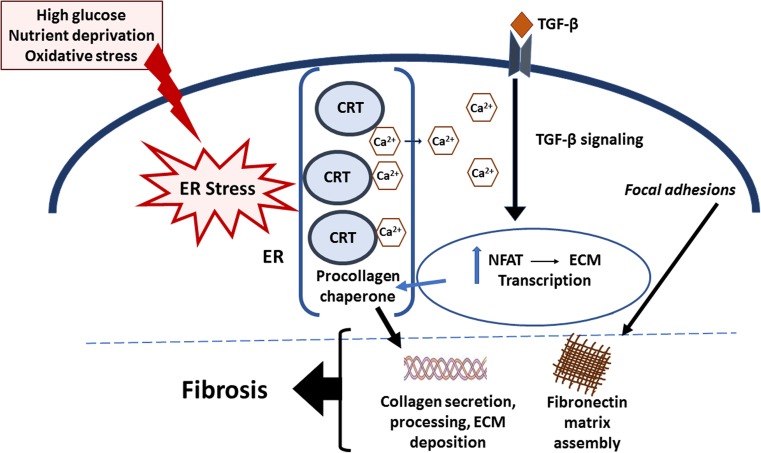
In addition to its roles at the cell surface in stimulating tissue remodeling, CRT in the ER compartment is emerging as an important factor in fibrotic remodeling. As will be discussed, intracellular CRT drives fibrotic responses through multiple mechanisms, including regulation of ECM production and assembly and TGF-β-induced ECM transcription and EMT (Fig. 1).
Fig. 1.
Model of CRT regulation of TGF-β-stimulated ECM production in fibrosis. Factors such as high glucose, nutrient deprivation, and oxidative stress trigger ER stress. ER stress increases CRT levels. In turn, CRT mediates Ca2+ release from the ER, resulting in an increase in cytosolic Ca2+, which stimulates increased calcineurin activity and NFAT dephosphorylation and nuclear translocation. Importantly, TGF-β stimulates increased cytosolic Ca2+ in a CRT-dependent manner: CRT is required for TGF-β stimulation of fibronectin and collagen I transcription through NFAT. In addition, ER CRT stimulates cell adhesion and fibronectin matrix assembly, and acts as a collagen chaperone assisting in collagen secretion and procollagen processing into the ECM. Furthermore, CRT is important for TGF-β stimulated EMT. These actions of CRT are thought to underlie its role in fibrogenesis
Regulation of fibronectin matrix assembly
It is well established that CRT regulates cellular processes that lead to ECM assembly and production. CRT’s role in Ca2+ regulation affects the expression of fibronectin in fibroblasts. CRT expression correlates with transcription and expression of fibronectin as well as cell adhesion to fibronectin and fibronectin matrix deposition (Papp et al. 2007; Papp et al. 2008; Szabo et al. 2007; Van Duyn Graham et al. 2010). Cell spreading and the formation of focal adhesions correlate with ER CRT expression (Fadel et al. 1999). Increased focal contact formation and a more robust stress fiber-containing actin cytoskeleton are observed in CRT-overexpressing cells. Cells that underexpress CRT on the other hand are poorly spread and have very few focal contacts and no stress fibers (Papp et al. 2007). These effects of CRT occur via a pathway that is modulated by c-Src, an important regulator of focal adhesion turnover. c-Src activity inversely correlates with CRT expression. c-Src activity increased in cells that underexpress CRT and importantly, the activity of c-Src was found to be sensitive to the release of Ca2+ from the ER (Papp et al. 2007). The use of thapsigargin to decrease ER Ca2+ levels resulted in decreased fibronectin protein expression and deposition in the ECM. Inhibition of c-Src also rescued the poorly adhesive phenotype of cells that underexpress CRT by forming more focal contacts and depositing a fibronectin matrix (Papp et al. 2007).
Regulation of collagen I trafficking, extracellular processing, and assembly into ECM
The synthesis and deposition of collagen are regulated at multiple levels, ranging from its transcription to degradation. Our laboratory demonstrated that CRT plays an important role in regulating multiple stages of collagen expression, processing and deposition into the ECM (Van Duyn Graham et al. 2010). We showed that CRT-deficient MEFs have reduced levels of fibrillar collagen I and III and decreased soluble collagen as compared to wild type MEFs. Accordingly, MEFs that overexpress CRT have increased collagen I RNA and protein expression. Levels of other ECM proteins, such as fibronectin, were similarly regulated by CRT expression. Both protein and transcript levels of collagens and fibronectin differed with CRT expression. Collagen expression was demonstrated to be regulated by ER Ca2+ levels and intracellular CRT, because treatment of cells with thapsigargan resulted in a decrease in collagen expression and exogenous recombinant CRT was unable to rescue CRT-deficient MEFs. This latter finding differs from the direct effects of topically applied CRT in wound healing and fibroblast migration studies and could reflect concentration dependent or cell type specific responses to exogenous CRT (Greives et al. 2012; Van Duyn Graham et al. 2010). CRT was also found to be crucial for trafficking of collagen through the ER: ER retention of collagen in CRT-deficient MEFS could be rescued by ascorbic acid treatment. Collagen and CRT were co-immunoprecipitated from wildtype cells, indicating that CRT is a chaperone for type I procollagen. Interestingly, these defects in collagen secretion in the absence of CRT occur in cells with normal levels of other collagen chaperones such as HSP47 and prolyl-4 hydroxylase. Processing of the C-terminal propeptide of type I procollagen was also reduced in the absence of CRT suggesting that perhaps other collagen processing enzymes are affected by the lack of CRT. CRT-deficient MEFs had reduced deposition of type I collagen into the ECM: this is likely due to deficient fibronectin matrix formation in the absence of CRT as discussed above, as it is well established that fibronectin matrix formation is required for subsequent collagen assembly into the ECM (Papp et al. 2007; Van Duyn Graham et al. 2010).
Regulation of TGF-β signaling through regulation of ER calcium/calcineurin/NFAT
TGF-β is a major trigger of ECM production, particularly in fibrotic diseases. TGF-β stimulation of downstream fibrotic events occurs primarily through Smad 2/3-dependent pathways (Jayaraman and Massague 2000; Roberts 1998; Wrana 2000). However, it is less well appreciated that TGF-β can trigger increased cytosolic Ca2+ accumulation (Alevizopoulos et al. 1997; Ishiyama et al. 1996; Junn et al. 2000; McGowan et al. 2002). It is well established that TGF-β can activate signaling downstream of increased cytosolic Ca2+, including activation of calcineurin and resultant NFAT dephosphorylation, which has been linked to increased fibronectin and collagen type I production in renal mesangial cells (Cobbs and Gooch 2007; Gooch et al. 2004).
CRT can regulate TGF-β-stimulated collagen production in different cell types including mouse embryonic and human lung fibroblasts, vascular smooth muscle cells, and renal tubular epithelial cells (Zimmerman et al. 2013, 2015). Our laboratory showed that CRT regulation of cytosolic Ca2+ and NFAT activity is critical for TGF-β stimulation of collagen type I and fibronectin transcripts (Zimmerman et al. 2013). A decrease in CRT expression, either in genetically-deficient MEFs or by siRNA-mediated knockdown, abrogates the responsiveness of cells to TGF-β stimulation even in the presence of active Smad 2/3 signaling. Interestingly, CRT-deficient MEFs failed to increase intracellular Ca2+ levels in response to TGF-β, suggesting that CRT plays a critical role in mediating TGF-β-dependent gene transcription through control of Ca2+ release from the ER into the cytosol, potentially impacting the calcineurin/NFAT pathway (Zimmerman et al. 2013). Consistent with this idea, TGF-β stimulation of NFAT nuclear translocation and reporter activity was impaired in CRT-deficient MEFs. Importantly, CRT was demonstrated to be essential for TGF-β stimulation of ECM under conditions of ER stress, since tunicamycin-induced ER stress was not sufficient to induce ECM production in TGF-β stimulated CRT-deficient MEFs (Zimmerman et al. 2013). This study shows that CRT is required for TGF-β-stimulated ECM production and identifies CRT-regulated Ca2+-dependent pathways as a mechanistic link between ER stress and fibrosis (Zimmerman et al. 2013).
Furthermore, recent work underscores the critical role CRT plays in TGF-β signaling in embryonic stem cell differentiation (Karimzadeh and Opas 2017). Using embryoid bodies derived from wild-type and CRT-null embryonic stem cells, they demonstrated that CRT promotes TGFβ-induced EMT via AKT/GSK3β during cardiac differentiation. Regulated Ca2+ signaling between CRT and calcineurin was shown to be critical for the TGF-β-mediated cadherin switch during EMT and the exit from pluripotency. A recent study also shows the importance of CRT-dependent Ca2+ regulation in TGF-β stimulated EMT of A549 lung epithelial cells (Wu et al. 2017).
Calreticulin is a factor in fibrotic disease through regulation of events downstream of TGF-β
There is accumulating evidence that CRT plays a critical role in fibrosis and that its expression is associated with fibrotic conditions such as renal, lung, and cardiac fibrosis, as well as, atherosclerosis (Gold et al. 2010; Groenendyk et al. 2016; Kurokawa et al. 2009; Kypreou et al. 2008; Prakoura et al. 2013). CRT overexpression induces EMT in Madin-Darby canine kidney (MDCK) cells via altered calcium homeostasis and regulation of the EMT transcription factor, Slug (Hayashida et al. 2006; Ihara et al. 2011). CRT overexpression in HK-2 cells downregulates epithelial markers and enhances fibronectin and collagen IV expression (Prakoura et al. 2013).
Several models have been used to demonstrate the important role CRT plays in the pathological events that lead to fibrosis. Charonis and his colleagues were the first to show that CRT is involved in the fibrotic process. CRT was identified to be one of the proteins that is consistently up-regulated in the early stages of fibrosis in an in vivo model of lung fibrosis and in cultured human proximal tubule epithelial cells (Kypreou et al. 2008). In this study, TGF-β1 was found to be an upstream regulator of CRT overexpression (Kypreou et al. 2008). Using both in vitro and in vivo approaches to study the role of CRT in the development and progression of tubulointerstitial fibrosis, Prakoura et al. showed that CRT overexpression induced the acquisition of a pro-fibrotic phenotype in tubular epithelial cells (Prakoura et al. 2013). These cells acquired a more mesenchymal phenotype, indicative of the early phase of EMT, exhibited aberrant ECM production, and showed increased ER stress and apoptosis. Consistent with these observations, a decrease in CRT expression suppressed the development of tubulointerstitial fibrosis in a model of unilateral ureteric obstruction (UUO) in CRT heterozygous mice. Collagen accumulation was significantly reduced in the kidneys of the CRT heterozygous mice. There was a decrease in TGF-β1 and the transcripts of the profibrotic genes Col1a1, Col3a1, Col4a1 and fibronectin in the heterozygous mice, indicating that decreased CRT expression affects ECM deposition during the progression of fibrosis (Prakoura et al. 2013). These findings show that CRT is a major player in the molecular mechanisms that drive the progression of renal fibrosis.
Recent studies by our laboratory established an important role for CRT in mediating vascular responses to injury in a mouse carotid artery ligation model of acute vascular injury through the regulation of neointima formation and collagen deposition. TGF-β is known to be important for the fibroproliferative changes in vascular remodeling in response to vascular injury (Kanzaki et al. 1995; Majesky et al. 1991; Nabel et al. 1993). Knockdown of CRT in vascular smooth muscle cells isolated from Calr-floxed mice using cre-recombinase plasmid attenuates type I collagen production in TGF-β stimulated cells (Zimmerman et al. 2015). The NFAT inhibitor, 11R–VIVIT, also attenuates TGF-β stimulation of collagen, similar to findings in the MEFs. Following acute vascular injury induced by carotid artery ligation, we observed an increase in immunostaining for CRT in the neointimal regions and increased collagen deposition. To assess the role of CRT in neointima formation, we took advantage of newly available calr floxed mice to perform tissue-specific knockdown of CRT (Tokuhiro et al. 2015). Using ultrasound targeted to the carotid artery, we induced local sonoporation of Cre-recombinase-IRES-GFP plasmid with microbubbles to the carotid arteries of CRT-floxed mice to specifically knock down CRT expression in this tissue. As calr-deficient mice are embryonic lethal, it had not been possible previously to address the in vivo role of conditional calr knockdown in disease models in adult animals. Reduced CRT expression in the carotid artery significantly attenuated neointima formation and collagen deposition following carotid artery ligation (Zimmerman et al. 2015). In contrast, CRT knockdown had no demonstrable effect on vascular smooth muscle cell proliferation. This study identifies CRT as an important mediator of fibrotic vascular remodeling. Similar studies are ongoing in models of diabetic renal disease, another fibrotic condition in which TGF-β plays a critical role in fibrotic remodeling.
Concluding thoughts
Cellular stress response is an essential adaptive feature of normal cell physiology and is necessary to maintain cellular homeostasis. It has recently become well appreciated that ER stress-induced cellular dysfunction is directly involved in promoting fibrotic and vascular fibroproliferative diseases (Khan et al. 2009; Korfei et al. 2008; Kurokawa et al. 2009; Lawson et al. 2011; Tanjore et al. 2011).
Several studies have documented an association between ER stress and CRT expression, as well as, the pro-fibrotic effects of CRT in different disease models. CRT affects ECM production and deposition and also cellular responses to the ECM. Accumulating evidence implicates CRT as a key player in the pathological processes leading to fibrosis, making CRT an attractive target for therapeutic intervention.
It is important to note that moderate alterations of CRT have a significant impact on the ability of TGF-β to induce ECM gene expression. Partial knockdown of CRT in VSMCs or Thy 1 (−) rat lung fibroblasts significantly inhibits the ability of TGF-β to stimulate ECM, consistent with observations that mice heterozygous for CRT were protected from fibrosis (Prakoura et al. 2013). Although moderate downregulation of CRT in VSMCs or Thy-1 (−) rat lung fibroblasts impaired TGF-β stimulated ECM, reduced CRT expression in L-fibroblasts had little to no impact on TGF-β stimulated ECM (Zimmerman et al. 2013). This indicates that sensitivity to CRT regulated ECM production might vary with cell type. Similarly, Greives et al. observed differences in sensitivity to exogenous CRT between fibroblasts derived from diabetic and non-diabetic mice (Greives et al. 2012). The underlying reasons for differential cellular responsiveness to CRT remain to be determined.
Data clearly show that topical application of recombinant CRT to wounds accelerates scarless wound healing (Greives et al. 2012; Nanney et al. 2008). Given the pro-fibrotic effects of CRT regulation of cellular signaling from the ER compartment, the observation that topical application of CRT promoted non-fibrotic ECM was unexpected. The mechanisms underlying these different effects of CRT on ECM remodeling are not clear. It is possible that topical CRT is acting primarily through the TGF-β3 isoform, which is known to have anti-fibrotic effects rather than through enhancing pro-fibrotic TGF-β1 signaling (Karamichos et al. 2014; Nanney et al. 2008). Alternately, exogenous CRT might be promoting scarless healing by stimulating clearance of apoptotic cells to reduce inflammation (Gardai et al. 2005; Gold et al. 2006). Also of note is the fact that in our studies with CRT-deficient MEFS, cells were only exposed to exogenous CRT and presumably still lacked intracellular/ER CRT, whereas in the wound healing studies, animals did not have any genetic calr deficiencies (Leslie I. Gold, New York University School of Medicine, personal communication) (Greives et al. 2012; Nanney et al. 2008; Van Duyn Graham et al. 2010).
Taken together, this review highlights the important role CRT plays in ER stress-induced fibrosis. Due to its impact on multiple processes in the pathogenesis of fibrosis, targeting CRT could be an effective therapeutic approach for preventing the morbidity and mortality associated with fibrotic diseases. Given the varied and important functions of CRT, however, it will be important to find targeted therapeutic strategies to attenuate CRT’s pro-fibrotic actions. One example might be to target its downstream effectors such as NFAT. In fact, an NFAT inhibitor has been used in animal models of diabetic nephropathy (Zhang et al. 2013). Further studies aimed at more completely understanding the mechanisms by which CRT mediates ER stress-induced fibrosis will be crucial for identification of targeted approaches to attenuate the pro-fibrotic effects of CRT.
Acknowledgements
Original research presented in this review was supported by grants from the American Heart Association (12IRG9160008) and from the Department of Defense (W81XWH-14-1-0203) to JEMU and by NIH T32 HL007918 and T32 AI007051 to KAZ.
Abbreviations
- ATF4
Activating transcription factor 4
- ATF6
Activating transcription factor 6
- CHOP
C/EBP homologous protein
- CRT
Calreticulin
- ECM
Extracellular matrix
- EMT
Epithelial to mesenchymal transition
- ER
Endoplasmic reticulum
- ERp57
Endoplasmic reticulum protein of 57 kDa
- GADD34
Growth arrest and DNA damage-inducible 34
- GRP78
Glucose regulated protein 78 kD
- HSP47
Heat shock protein 47 kD
- IRE1
Inositol-requiring protein 1
- JNK
c-jun N-terminal kinase
- LRP1
Low-density lipoprotein receptor-related protein 1
- MAPK
Mitogen-activated protein kinase
- MDCK
Madin-Darby canine kidney
- MEFs
Mouse embryonic fibroblasts
- MEKK1
Mitogen-activated protein kinase kinase kinase 1
- NFAT
Nuclear factor of activated T cells
- ORP150
150-kDa oxygen-regulated protein
- 4-PBA
4-phenylbutyrate
- PERK
Protein kinase RNA (PKR)-like ER kinase
- PI3K
Phosphoinositide-3 kinase
- Smad 2/3
Mothers against decapentaplegic homologs 2/3
- TGF-β
Transforming growth factor beta
- TSP-1
Thrombospondin 1
- TUDCA
Tauroursodeoxycholic acid
- UPR
Unfolded protein response
- UUO
Unilateral ureteric obstruction
Footnotes
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
References
- Alevizopoulos A, Dusserre Y, Ruegg U, Mermod N. Regulation of the transforming growth factor beta-responsive transcription factor CTF-1 by calcineurin and calcium/calmodulin-dependent protein kinase IV. J Biol Chem. 1997;272:23597–23605. doi: 10.1074/jbc.272.38.23597. [DOI] [PubMed] [Google Scholar]
- Ayala P, Montenegro J, Vivar R, Letelier A, Urroz PA, Copaja M, Pivet D, Humeres C, Troncoso R, Vicencio JM, Lavandero S, Diaz-Araya G. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol. 2012;92:97–104. doi: 10.1016/j.yexmp.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Baek HA, Kim do S, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46:731–739. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- Baksh S, Michalak M. Expression of calreticulin in Escherichia Coli and identification of its Ca2+ binding domains. J Biol Chem. 1991;266:21458–21465. [PubMed] [Google Scholar]
- Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT, Li W. A "traffic control" role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093–1105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CK, Hsu SP, Wu CT, Huang JW, Cheng HT, Chang YW, Hung KY, Wu KD, Liu SH. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol Med. 2011;17:1295–1305. doi: 10.2119/molmed.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs SL, Gooch JL. NFATc is required for TGFbeta-mediated transcriptional regulation of fibronectin. Biochem Biophys Res Commun. 2007;362:288–294. doi: 10.1016/j.bbrc.2007.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Ribeiro SP, Michalak M. Heat shock-sensitive expression of calreticulin. In vitro and in vivo up-regulation. J Biol Chem. 1995;270:17011–17016. doi: 10.1074/jbc.270.28.17011. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int. 2010;77:187–193. doi: 10.1038/ki.2009.389. [DOI] [PubMed] [Google Scholar]
- Fadel MP, Dziak E, Lo CM, Ferrier J, Mesaeli N, Michalak M, Opas M. Calreticulin affects focal contact-dependent but not close contact-dependent cell-substratum adhesion. J Biol Chem. 1999;274:15085–15094. doi: 10.1074/jbc.274.21.15085. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J Biol Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Pallero MA, Eggleton P, Michalak M, Murphy-Ullrich JE. The anti-adhesive activity of thrombospondin is mediated by the N-terminal domain of cell surface calreticulin. J Biol Chem. 2002;277:37219–37228. doi: 10.1074/jbc.M202200200. [DOI] [PubMed] [Google Scholar]
- Gold LI, Rahman M, Blechman KM, Greives MR, Churgin S, Michaels J, Callaghan MJ, Cardwell NL, Pollins AC, Michalak M, Siebert JW, Levine JP, Gurtner GC, Nanney LB, Galiano RD, Cadacio CL. Overview of the role for calreticulin in the enhancement of wound healing through multiple biological effects. J Investig Dermatol Symp Proc. 2006;11:57–65. doi: 10.1038/sj.jidsymp.5650011. [DOI] [PubMed] [Google Scholar]
- Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- Gray AJ, Park PW, Broekelmann TJ, Laurent GJ, Reeves JT, Stenmark KR, Mecham RP. The mitogenic effects of the B beta chain of fibrinogen are mediated through cell surface calreticulin. J Biol Chem. 1995;270:26602–26606. doi: 10.1074/jbc.270.44.26602. [DOI] [PubMed] [Google Scholar]
- Greives MR, Samra F, Pavlides SC, Blechman KM, Naylor SM, Woodrell CD, Cadacio C, Levine JP, Bancroft TA, Michalak M, Warren SM, Gold LI. Exogenous calreticulin improves diabetic wound healing. Wound Repair Regen. 2012;20:715–730. doi: 10.1111/j.1524-475X.2012.00822.x. [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Lynch J, Michalak M. Calreticulin, Ca2+, and calcineurin - signaling from the endoplasmic reticulum. Mol Cell. 2004;17:383–389. [PubMed] [Google Scholar]
- Groenendyk J, Lee D, Jung J, Dyck JR, Lopaschuk GD, Agellon LB, Michalak M. Inhibition of the unfolded protein response mechanism prevents cardiac fibrosis. PLoS One. 2016;11:e0159682. doi: 10.1371/journal.pone.0159682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, Grimminger F, Seeger W. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med. 2003;168:1358–1365. doi: 10.1164/rccm.2201082. [DOI] [PubMed] [Google Scholar]
- Guo L, Nakamura K, Lynch J, Opas M, Olson EN, Agellon LB, Michalak M. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem. 2002;277:50776–50779. doi: 10.1074/jbc.M209900200. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Urata Y, Muroi E, Kono T, Miyata Y, Nomata K, Kanetake H, Kondo T, Ihara Y. Calreticulin represses E-cadherin gene expression in Madin-Darby canine kidney cells via slug. J Biol Chem. 2006;281:32469–32484. doi: 10.1074/jbc.M607240200. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal R, McGivan J. Induction of calreticulin expression in response to amino acid deprivation in Chinese hamster ovary cells. Biochem J. 1998;329(Pt 2):389–394. doi: 10.1042/bj3290389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Inai Y, Ikezaki M. Alteration of integrin-dependent adhesion and signaling in EMT-like MDCK cells established through overexpression of calreticulin. J Cell Biochem. 2011;112:2518–2528. doi: 10.1002/jcb.23176. [DOI] [PubMed] [Google Scholar]
- Ishiyama N, Shibata H, Kanzaki M, Shiozaki S, Miyazaki J, Kobayashi I, Kojima I. Calcium as a second messenger of the action of transforming growth factor-beta on insulin secretion. Mol Cell Endocrinol. 1996;117:1–6. doi: 10.1016/0303-7207(95)03726-8. [DOI] [PubMed] [Google Scholar]
- Jayaraman L, Massague J. Distinct oligomeric states of SMAD proteins in the transforming growth factor-beta pathway. J Biol Chem. 2000;275:40710–40717. doi: 10.1074/jbc.M005799200. [DOI] [PubMed] [Google Scholar]
- Jethmalani SM, Henle KJ. Calreticulin associates with stress proteins: implications for chaperone function during heat stress. J Cell Biochem. 1998;69:30–43. doi: 10.1002/(SICI)1097-4644(19980401)69:1<30::AID-JCB4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- Kamp DW, Liu G, Cheresh P, Kim SJ, Mueller A, Lam AP, Trejo H, Williams D, Tulasiram S, Baker M, Ridge K, Chandel NS, Beri R. Asbestos-induced alveolar epithelial cell apoptosis. The role of endoplasmic reticulum stress response. Am J Respir Cell Mol Biol. 2013;49:892–901. doi: 10.1165/rcmb.2013-0053OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Chung J, Ryoo HD. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nat Cell Biol. 2012;14:409–415. doi: 10.1038/ncb2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki T, Tamura K, Takahashi K, Saito Y, Akikusa B, Oohashi H, Kasayuki N, Ueda M, Morisaki N. In vivo effect of TGF- beta 1. Enhanced intimal thickening by administration of TGF- beta 1 in rabbit arteries injured with a balloon catheter. Arterioscler Thromb Vasc Biol. 1995;15:1951–1957. doi: 10.1161/01.ATV.15.11.1951. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Ellgaard L, Gopalakrishnapai J, Schirra C, Gemma E, Oscarson S, Helenius A, Surolia A. Mutational analysis provides molecular insight into the carbohydrate-binding region of calreticulin: pivotal roles of tyrosine-109 and aspartate-135 in carbohydrate recognition. Biochemistry. 2004;43:97–106. doi: 10.1021/bi0355286. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Hutcheon AE, Zieske JD. Reversal of fibrosis by TGF-beta3 in a 3D in vitro model. Exp Eye Res. 2014;124:31–36. doi: 10.1016/j.exer.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimzadeh F, Opas M. Calreticulin is required for TGF-beta-induced epithelial-to-mesenchymal transition during Cardiogenesis in mouse embryonic stem cells. Stem Cell Rep. 2017;8:1299–1311. doi: 10.1016/j.stemcr.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MI, Pichna BA, Shi Y, Bowes AJ, Werstuck GH. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid Redox Signal. 2009;11:2289–2298. doi: 10.1089/ars.2009.2569. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M. Endoplasmic reticulum stress in the kidney. Clin Exp Nephrol. 2008;12:317–325. doi: 10.1007/s10157-008-0060-7. [DOI] [PubMed] [Google Scholar]
- Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Hideshima M, Ishii Y, Kyuwa S, Yoshikawa Y. Aortic ER stress in streptozotocin-induced diabetes mellitus in APA hamsters. Exp Anim. 2009;58:113–121. doi: 10.1538/expanim.58.113. [DOI] [PubMed] [Google Scholar]
- Kuwano K. Involvement of epithelial cell apoptosis in interstitial lung diseases. Intern Med. 2008;47:345–353. doi: 10.2169/internalmedicine.47.0713. [DOI] [PubMed] [Google Scholar]
- Kypreou KP, Kavvadas P, Karamessinis P, Peroulis M, Alberti A, Sideras P, Psarras S, Capetanaki Y, Politis PK, Charonis AS. Altered expression of calreticulin during the development of fibrosis. Proteomics. 2008;8:2407–2419. doi: 10.1002/pmic.200700831. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Phys Lung Cell Mol Phys. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MR, Cohen-Doyle MF, Thomas DY, Williams DB. Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J Biol Chem. 2002;277:29686–29697. doi: 10.1074/jbc.M202405200. [DOI] [PubMed] [Google Scholar]
- Lee ES, Kim HM, Kang JS, Lee EY, Yadav D, Kwon MH, Kim YM, Kim HS, Chung CH. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol Dial Transplant. 2016;31:391–400. doi: 10.1093/ndt/gfv377. [DOI] [PubMed] [Google Scholar]
- Lenna S, Trojanowska M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol. 2012;24:663–668. doi: 10.1097/BOR.0b013e3283588dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlondorff D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol. 2008;19:2225–2236. doi: 10.1681/ASN.2007121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SH, Yang CC, Chan DC, Wu CT, Chen LP, Huang JW, Hung KY, Chiang CK. Chemical chaperon 4-phenylbutyrate protects against the endoplasmic reticulum stress-mediated renal fibrosis in vivo and in vitro. Oncotarget. 2016;7:22116–22127. doi: 10.18632/oncotarget.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn DH, Sheikh FN, Kendall JM, Campbell AK. Upregulation of calreticulin expression in HeLa cells by calcium-stress. Biochem Soc Trans. 1995;23:330S. doi: 10.1042/bst023330s. [DOI] [PubMed] [Google Scholar]
- Lynch J, Michalak M. Calreticulin is an upstream regulator of calcineurin. Biochem Biophys Res Commun. 2003;311:1173–1179. doi: 10.1016/j.bbrc.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Lynch J, Guo L, Gelebart P, Chilibeck K, Xu J, Molkentin JD, Agellon LB, Michalak M (2005) Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca(2+)-dependent signaling cascade. J Cell Biol 170:37–47 [DOI] [PMC free article] [PubMed]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/S0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan TA, Madesh M, Zhu Y, Wang L, Russo M, Deelman L, Henning R, Joseph S, Hajnoczky G, Sharma K. TGF-beta-induced ca(2+) influx involves the type III IP(3) receptor and regulates actin cytoskeleton. Am J Physiol Ren Physiol. 2002;282:F910–F920. doi: 10.1152/ajprenal.00252.2001. [DOI] [PubMed] [Google Scholar]
- Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Milner RE, Burns K, Opas M. Calreticulin. Biochem J. 1992;285(Pt 3):681–692. doi: 10.1042/bj2850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EG, Shum L, Pompili VJ, Yang ZY, San H, Shu HB, Liptay S, Gold L, Gordon D, Derynck R, et al. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci U S A. 1993;90:10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney LB, Woodrell CD, Greives MR, Cardwell NL, Pollins AC, Bancroft TA, Chesser A, Michalak M, Rahman M, Siebert JW, Gold LI. Calreticulin enhances porcine wound repair by diverse biological effects. Am J Pathol. 2008;173:610–630. doi: 10.2353/ajpath.2008.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TO, Capra JD, Sontheimer RD. Calreticulin is transcriptionally upregulated by heat shock, calcium and heavy metals. Mol Immunol. 1996;33:379–386. doi: 10.1016/0161-5890(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular. 2007;15:350–355. doi: 10.2310/6670.2007.00056. [DOI] [PubMed] [Google Scholar]
- Omura T, Asari M, Yamamoto J, Oka K, Hoshina C, Maseda C, Awaya T, Tasaki Y, Shiono H, Yonezawa A, Masuda S, Matsubara K, Shimizu K. Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem Biophys Res Commun. 2013;432:689–694. doi: 10.1016/j.bbrc.2013.01.131. [DOI] [PubMed] [Google Scholar]
- Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem. 2002;277:20453–20460. doi: 10.1074/jbc.M112091200. [DOI] [PubMed] [Google Scholar]
- Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostwald TJ, MacLennan DH. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem. 1974;249:974–979. [PubMed] [Google Scholar]
- Pallero MA, Elzie CA, Chen J, Mosher DF, Murphy-Ullrich JE. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J. 2008;22:3968–3979. doi: 10.1096/fj.07-104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp S, Fadel MP, Kim H, McCulloch CA, Opas M. Calreticulin affects fibronectin-based cell-substratum adhesion via the regulation of c-Src activity. J Biol Chem. 2007;282:16585–16598. doi: 10.1074/jbc.M701011200. [DOI] [PubMed] [Google Scholar]
- Papp S, Szabo E, Kim H, McCulloch CA, Opas M. Kinase-dependent adhesion to fibronectin: regulation by calreticulin. Exp Cell Res. 2008;314:1313–1326. doi: 10.1016/j.yexcr.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gomez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–274. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- Prakoura N, Politis PK, Ihara Y, Michalak M, Charonis AS. Epithelial calreticulin up-regulation promotes profibrotic responses and tubulointerstitial fibrosis development. Am J Pathol. 2013;183:1474–1487. doi: 10.1016/j.ajpath.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Roberts AB. Molecular and cell biology of TGF-beta. Miner Electrolyte Metab. 1998;24:111–119. doi: 10.1159/000057358. [DOI] [PubMed] [Google Scholar]
- Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI200319270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Koch GL. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO J. 1989;8:3581–3586. doi: 10.1002/j.1460-2075.1989.tb08530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG, Zhu Q, Bhoyroo V, Soling HD. Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver Golgi. J Biol Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC, Zhao DM, Li XN, Sun LK. Valproate attenuates diabetic nephropathy through inhibition of endoplasmic reticulum stressinduced apoptosis. Mol Med Rep. 2016;13:661–668. doi: 10.3892/mmr.2015.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetwyne MT, Pallero MA, Lu A, van Duyn Graham L, Murphy-Ullrich JE. The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am J Pathol. 2010;177:1710–1724. doi: 10.2353/ajpath.2010.090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Papp S, Opas M. Differential calreticulin expression affects focal contacts via the calmodulin/CaMK II pathway. J Cell Physiol. 2007;213:269–277. doi: 10.1002/jcp.21122. [DOI] [PubMed] [Google Scholar]
- Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Shirai A, Ito Y, Namba T, Tahara K, Yamakawa N, Mizushima T. Expression of 150-kDa oxygen-regulated protein (ORP150) stimulates bleomycin-induced pulmonary fibrosis and dysfunction in mice. Biochem Biophys Res Commun. 2012;425:818–824. doi: 10.1016/j.bbrc.2012.07.158. [DOI] [PubMed] [Google Scholar]
- Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, Blackwell TS. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2011;286:30972–30980. doi: 10.1074/jbc.M110.181164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta. 2013;1832:940–947. doi: 10.1016/j.bbadis.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr JM, Young PJ, Morse R, Shaw DJ, Haigh R, Petrov PG, Johnson SJ, Winyard PG, Eggleton P. A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol. 2010;401:799–812. doi: 10.1016/j.jmb.2010.06.064. [DOI] [PubMed] [Google Scholar]
- Tokuhiro K, Satouh Y, Nozawa K, Isotani A, Fujihara Y, Hirashima Y, Matsumura H, Takumi K, Miyano T, Okabe M, Benham AM, Ikawa M. Calreticulin is required for development of the cumulus oocyte complex and female fertility. Sci Rep. 2015;5:14254. doi: 10.1038/srep14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, Beguinot F, Consiglio E, Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121:477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- Van Duyn Graham L, Sweetwyne MT, Pallero MA, Murphy-Ullrich JE (2010) Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix. J Biol Chem 285:7067–7078 [DOI] [PMC free article] [PubMed]
- Varadarajan S, Bampton ET, Smalley JL, Tanaka K, Caves RE, Butterworth M, Wei J, Pellecchia M, Mitcheson J, Gant TW, Dinsdale D, Cohen GM. A novel cellular stress response characterised by a rapid reorganisation of membranes of the endoplasmic reticulum. Cell Death Differ. 2012;19:1896–1907. doi: 10.1038/cdd.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Chen X, Zhang C, Tao L, Zhang ZH, Liu XQ, Xu YB, Wang H, Li J, Xu DX. Phenylbutyric acid protects against carbon tetrachloride-induced hepatic fibrogenesis in mice. Toxicol Appl Pharmacol. 2013;266:307–316. doi: 10.1016/j.taap.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Nat Rev Mol Cell Biol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/S0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/S0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- Wu X, Liu X, Zhu X, Tang C. Hypoxic preconditioning induces delayed cardioprotection through p38 MAPK-mediated calreticulin upregulation. Shock. 2007;27:572–577. doi: 10.1097/01.shk.0000246901.58068.a8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Xu X, Ma L, Yi Q, Sun W, Tang L. Calreticulin regulates TGF-beta1-induced epithelial mesenchymal transition through modulating Smad signaling and calcium signaling. Int J Biochem Cell Biol. 2017;90:103–113. doi: 10.1016/j.biocel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Murphy-Ullrich JE, Song Y. Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry. 2010;49:3685–3694. doi: 10.1021/bi902067f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li R, Shi W, Liang X, Liu S, Ye Z, Yu C, Chen Y, Zhang B, Wang W, Lai Y, Ma J, Li Z, Tan X. NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. Br J Pharmacol. 2013;170:426–439. doi: 10.1111/bph.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Pandupuspitasari NS, Wu G, Xiang X, Gong Q, Xiong W, Wang CY, Yang P, Ren B. Endoplasmic reticulum stress, a new wrestler, in the pathogenesis of idiopathic pulmonary fibrosis. Am J Transl Res. 2017;9:722–735. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman KA, Graham LV, Pallero MA, Murphy-Ullrich JE. Calreticulin regulates transforming growth factor-beta-stimulated extracellular matrix production. J Biol Chem. 2013;288:14584–14598. doi: 10.1074/jbc.M112.447243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman KA, Xing D, Pallero MA, Lu A, Ikawa M, Black L, Hoyt KL, Kabarowski JH, Michalak M, Murphy-Ullrich JE. Calreticulin regulates Neointima formation and collagen deposition following carotid artery ligation. J Vasc Res. 2015;52:306–320. doi: 10.1159/000443884. [DOI] [PMC free article] [PubMed] [Google Scholar]