Abstract
Angiogenesis or neovascularization is a complex multi-step physiological process that occurs throughout life both in normal tissues and in disease. It is tightly regulated by the balance between pro-angiogenic and anti-angiogenic factors. The angiogenic switch has been identified as the key step during tumor progression in which the balance between pro-angiogenic and anti-angiogenic factors leans toward pro-angiogenic stimuli promoting the progression of tumors from dormancy to dysplasia and ultimately malignancy. This event can be described as either the outcome of a genetic event occurring in cancer cells themselves, or the positive and negative cross-talk between tumor-associated endothelial cells and other cellular components of the tumor microenvironment. In recent years, the mechanisms underlying the angiogenic switch have been extensively investigated in particular to identify therapeutic targets that can lead to development of effective therapies. In this review, we will discuss the current findings on the regulatory pathways in endothelial cells that are involved in the angiogenic switch with an emphasis on the role of anti-angiogenic protein, thrombospondin-1 (TSP-1) and pro-angiogenic factor, vascular endothelial growth factor (VEGF).
Keywords: thrombospondin-1, TSP-1, vascular endothelial growth factor, VEGF, dormancy, angiogenesis, cancer
Introduction
Angiogenesis is the formation of new capillaries from existing blood vessels. It is a key process in embryological development and the remodeling of adult tissue during wound healing, formation of the placenta, and mammary gland development and involution. Angiogenesis is also a hallmark of tumor growth and progression (Folkman 1975). It a multistep event that involves the sprouting, branching, splitting, and differential growth of vessels from the primary plexus of existing vessel into a circulation system (Carmeliet 2000). Angiogenesis is controlled by the interactions between tumor cells, endothelial cells, stromal fibroblasts, and infiltrating inflammatory cells in the tumor microenvironment with extracellular matrix proteins. It also requires a shift in the normal balance of pro-angiogenic and anti-angiogenic factors (Carmeliet 2005). The importance of angiogenesis in tumor progression from growth to metastasis to distant organs was initially proposed by Judah Folkman (Folkman 1971). His group demonstrated that isolated solid tumors with continuous arterial perfusion continued growing, while tumors with no perfusion grew up to a maximum size of 1–2 mm. Moreover, the cells within the tumor mass underwent necrosis and died. On the other hand, the outer cells remained alive, but there was no tumor growth or neovascularization. Similar to cells in normal tissues, tumor cells require nutrients to grow and stroma to support the tissue. However, tumors are very heterogeneous in the amount of stroma that they have. This variation has been demonstrated even within the composition of stroma and tumor cells from one area of tissue to another in a single tumor. These findings demonstrated the strong correlation between tumor growth and vasculature formation and prompted Folkman to present the idea that tumor growth was angiogenesis-dependent, and therefore, inhibition of this process could be considered therapeutic (Folkman 1971).
Dormant cancer cells can be localized either in primary tumors or micrometastases in distance organs and in some cases, are left in the minimal residual disease following treatment. Under certain conditions, these cells can re-enter the proliferative stage and result in recurrence of tumors. Tumor dormancy has long been considered a clinical challenge with lack of a defined marker and unknown mechanism and has been observed in many cancers including breast, prostate, and melanoma (Quail and Joyce 2013; Sosa et al. 2014). One major proposed mechanism leading to tumor dormancy is angiogenic dormancy (Almog 2010; Evans and Lin 2015; Indraccolo et al. 2006). Angiogenic dormancy refers to the period when the factors that inhibit endothelial cell proliferation and vessel sprouting predominate leading to oxygen and nutrient deprivation (Almog 2010; Naumov et al. 2006; Sosa et al. 2014). As a result, there is a blockage of tumor growth and cells remain in a quiescent state until there is an increase in pro-angiogenic factors as the result of interaction of tumor cells with their microenvironment as well as the activity of immune cells. This event initiates endothelial cell proliferation and recurrence of tumors that, in some cases, cannot be treated. The term angiogenic switch refers to the time when a tumor switches from a dormant state to one in which angiogenesis promotes tumor growth. By extension, it also refers to the change in phenotype that occurs in the endothelial cell in response to pro-angiogenic signaling molecules. In this review, we will concentrate on recent findings regarding possible mechanisms for the angiogenic switch on the cellular and molecular levels.
Molecular regulation of the angiogenic switch
Stimulation of angiogenesis by VEGFA
VEGFA is a member of the VEGF/PLGF family, which includes VEGFA, VEGFB, VEGFC, VEGFD and placental growth factor (PLGF). VEGF was first identified in the supernatant of rodent tumor cells as a vascular permeability factor (VPF) in 1983 by Senger et al., (Senger et al. 1983). These authors suggested that VPF is responsible for an increase in vessel permeability, which is considered a crucial step in angiogenesis associated with tumors and wounds (Senger et al. 1983; Senger et al. 1986). Ferrara and coworkers also identified this protein in the conditioned media from bovine pituitary follicular cells and demonstrated that it is a heparin-binding, heat and acid stable, cationic protein with a molecular weight of 45 kDa (Ferrara and Henzel 1989; Leung et al. 1989). Moreover, they suggested that this protein had a mitogenic effect on vascular endothelial cells and proposed the name vascular endothelial growth factor or VEGF (Ferrara and Henzel 1989, Leung et al. 1989).
All VEGF genes have seven exons that are highly conserved, with the exception of VEGFA, which has eight exons (Holmes and Zachary 2005). Alternative splicing of VEGFA between exons six and seven results in six VEGFA isoforms with 121, 145, 165, 183, 189, and 206 amino acids in human, and 120, 145, 164, 183, 189, and 206 amino acids in mouse (Holmes and Zachary 2005; Park et al. 1993). These isoforms are expressed by a variety of cells including tumor cells, macrophages, platelets, endothelial cells, keratinocytes, and fibroblasts. Because of its wide range of expression patterns and diversity in its isoforms, VEGFA is involved in many physiological functions not limited to wound healing, bone formation, and neovascularization (blood and lymphatic vessels) (Eichmann and Simons 2012). The VEGFs play a role in many diseases such as diabetic retinopathy, arthritis, neurodegenerative, various malignancies, and preeclampsia. Moreover, VEGFA164/165 is recognized as a potent mediator of vascular permeability, angiogenesis, lymphangiogenesis, and tumorigenesis (Dvorak 2002; Hoeben et al. 2004).
The importance of VEGFA in mediating angiogenesis processes is underscored by gene knockout studies. The findings showed that targeting only a single allele of the VEGFA gene in mice resulted in lethality between days 11 and 12 due to impaired embryonic vessel development (Ferrara et al. 1996; Holmes and Zachary 2005). VEGFA exerts its functions on endothelial cells by binding to two receptor tyrosine kinases, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/FLK1), with VEGFR-2 being considered the major receptor for pro-angiogenic signaling (Ellis and Hicklin 2008; Olsson et al. 2006; Rahimi 2006). Recent studies have shown that neuropilin-1, a non-tyrosine kinase transmembrane protein, functions as a co-receptor for VEGFA (Fantin et al. 2014; Gelfand et al. 2014). Binding of VEGFA to VEGFR-2 results in conformational changes that promote receptor dimerization and autophosphorylation of various tyrosine residues in its cytoplasmic domain. These phosphorylated tyrosines can recruit a variety of adaptor proteins including Shc, Grb2, c-Src, PLCγ, and TSad, to promote signal transduction pathways that induce proliferation and migration, nitric oxide release, survival, and vascular permeability (Kliche and Waltenberger 2001, Nagy et al. 2007, Olsson et al. 2006). All these findings have increased our knowledge of VEGFA and its signaling pathways in normal and pathological angiogenesis and provided promising opportunity for the development of new and more effective therapeutic approaches for the treatment of cancer and a variety of other diseases.
Inhibition of angiogenesis by TSP-1
Like most biological processes, angiogenesis is tightly regulated by positive and negative signal transduction pathways. TSP-1 was the first naturally-occurring protein to be identified as an endogenous inhibitor of angiogenesis (Good et al. 1990; Lawler and Detmar 2004; Taraboletti et al. 1990). Tumors grow more quickly and exhibit increased angiogenesis in TSP-1-null mice (Lawler et al. 2001; Rodriguez-Manzaneque et al. 2001). Consistent with this observation, over-expression of TSP-1 in transgenic mice reduces the angiogenic response that occurs during wound healing (Streit et al. 2000). The TSPs are a group of high molecular weight multi-domain glycoproteins consisting of five members (TSP-1, through −4 and cartilage oligomeric matrix protein or COMP). They can be divided into two subgroups based on their structure (Adams and Lawler 2004; Lawler and Lawler 2012; Stenina-Adognravi 2013; Stenina-Adognravi 2014). All TSPs have a highly conserved carboxyl-terminal region that consists of a variable number of EGF-like domains, seven TSP type 3 repeats, and a globular C-terminal domain (Fig. 1) (Adams and Lawler 2004). The amino-terminal domain is found in all members of TSP family except for COMP and has variable sequences among the TSP members. Other features of the TSP family are the presence of a von Willebrand Factor type C (VWC) domain and the three tandem type 1 repeats, designated 3TSR, that are only present in subgroup A consisting of TSP-1 and TSP-2 (Carlson et al. 2008). Because of multi-domain structure, TSPs can bind to multiple membrane receptors, which, in turn, can activate distinct signaling pathways eventually resulting in different cellular phenotypes and tissue specific effects (Adams and Lawler 2004; Carlson et al. 2008; Kosfeld et al. 1991; Mosher 1990; Roberts 1996).
Fig. 1.
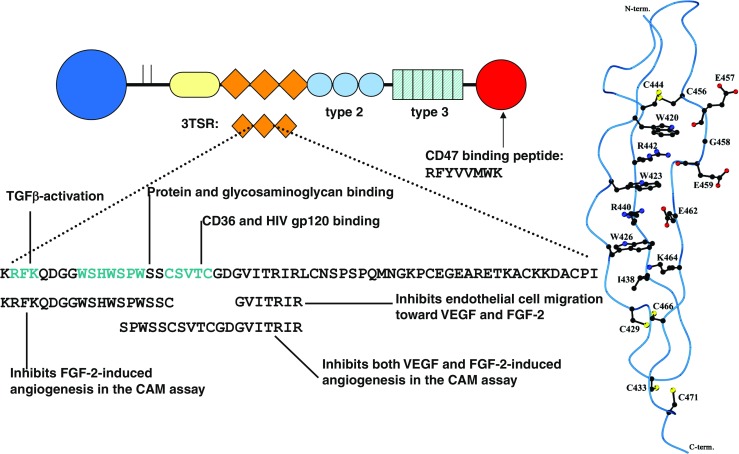
The structure of TSP-1. The various domains of TSP-1 are shown and the sequence of the second TSR is written out. The sequence of the peptides that have been shown to inhibit angiogenesis in various models are listed below the sequence of the TSR. The fact that non-overlapping sequences are active suggests that the protein folds to bring these sites into proximity. A schematic diagram of the structure of the second TSR is shown at the right
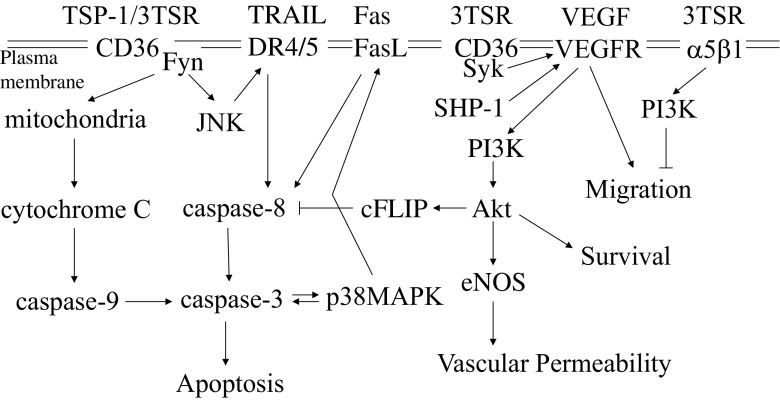
TSP-1 was first detected as a 420 kDa homotrimeric glycoprotein released from the α-granules of platelets in response to stimulation with thrombin (Baenziger et al. 1971; Lawler et al. 1978). To date, as many as 12 cellular receptors have been identified that bind to TSP-1 in a cell type-dependent manner and regulate its function in a variety of physiological and pathological processes (Adams and Lawler 2004; Kazerounian et al. 2008; Lawler and Lawler 2012; Lopez-Dee et al. 2012; Roberts et al. 2012; Sweetwyne and Murphy-Ullrich 2012). However, the majority of attention has been toward the role of TSP-1 in mediating anti-angiogenic events. This function is mainly meditated through the TSRs, which bind to the CLESH domain of CD36 and to β1 integrins. The importance of the anti-angiogenic activity of the TSRs is underscored by the fact that other proteins that contain TSRs also have anti-angiogenic activity (Gaustad et al. 2016). Engagement of α5β1 integrin by 3TSR inhibits endothelial cell migration in a PI3K-dependent manner (Short et al. 2005) (Fig. 2). The carboxyl-terminal globular domain of TSP-1 interacts with CD47 (Roberts 1996; Roberts et al. 2012). Upon binding to some of these receptors on the surface of endothelial cells, TSP-1 promotes apoptosis and inhibits nitric oxide signaling (Febbraio et al. 2001; Febbraio and Silverstein 2007; Ren et al. 2009; Roberts et al. 2012; Silverstein and Febbraio 2009). TSP-1 also suppresses angiogenesis by (1) inhibiting the activation of MMP9 and thus the subsequent release of VEGFA from the extracellular matrix, and (2) binding directly to VEGFA and facilitating its clearance from the extracellular space (Greenaway et al. 2007; Gupta et al. 1999; Rodriguez-Manzaneque et al. 2001). Therefore, the fact that TSP-1 antagonizes the pro-angiogenic signals elicited by VEGFA and initiates anti-angiogenic signals makes it a favorable target for developing novel therapeutic strategies for a variety of diseases including cancer. These strategies include direct delivery of TSP-1 mimetics, cell-based delivery of TSP-1 or systemic up-regulation of TSP-1 (Lawler and Lawler 2012). Since TSP-2 is similar to TSP-1, some groups have focused on developing portions of TSP-2 to inhibit angiogenesis (Koch et al. 2011).
Fig. 2.
Schematic representation of the anti- and pro-angiogenic signaling pathways involved in response to TSP-1 and VEGFA
Since TSP-1 is a large multifunctional protein, several groups have used a reductionist approach with synthetic peptides to identify active sites within the molecule that are highly specific and more readily synthesized (Fig. 1). Active peptides for engagement of CD36 have been identified in the TSRs (Dawson et al. 1999; Iruela-Arispe et al. 1999). Abbott Laboratories took a derivative of the GVITRIR peptide, designated ABT510, to the clinic for the treatment of cancer (Westphal 2004). Whereas this peptide was very well tolerated in Phase I trials, it did not have sufficient activity as a single agent in Phase II trials to warrant further development (Ebbinghaus et al. 2007). The TSRs fold into a three-stranded unique protein fold in a way that indicates that multiple sequences may be involved in CD36 binding (Fig. 1) (Tan et al. 2002). The tryptophan residues (W420, W423, and W426) from the first strand form cation-π bonds with arginines (R440 and R442) on the second strand. The structure is further stabilized by interchain hydrogen bonds and three disulfide bonds. As a result, it may be very difficult to capture the full-anti-angiogenic activity of the 3TSRs with a short peptide. Therefore, other groups have used a recombinant version of the complete 3TSR sequence in in vitro experiments and preclinical mouse models of cancer (Zhang et al. 2005a, b).
The TSRs of TSP-1 also bind to CD148, a transmembrane protein tyrosine phosphatase that is expressed in endothelial cells (Takahashi et al. 2016). Peptides based on the TSR sequence increase the activity of CD148 toward two of its substrates, EGFR and Erk1/2. These peptides inhibit endothelial cell proliferation and angiogenesis.
TSP-1 also inhibits angiogenesis through the suppression of cell cycle progression through a CD36-independent mechanism (Oganesian et al. 2008; Yamauchi et al. 2007). This effect is mediated by very low density lipoprotein receptor, Akt and MAPK in small vessel (Oganesian et al. 2008). By contrast, p21 and p53 have been implicated as mediators of this effect in large vessel endothelial cells (Yamauchi et al. 2007).
TSP-1 has also been reported to inhibit angiogenesis that is induced by basic fibroblast growth factor (FGF-2) (Iruela-Arispe et al. 1999; Margosio et al. 2008). This effect appears to be mediated by two portions of the protein. Iruela-Arispe et al. (1999) found that peptides that include sequences from the TSRs inhibit angiogenesis in the chorioallantoic membrane (CAM) assay. By contrast, Margosio et al. (2008) found that sequences in the type 3 repeats bind FGF-2 in the presence of calcium and inhibit its ability to promote endothelial cell proliferation. Based on these data, Colombo et al. (2010) identified a non-peptidic small molecule called sm27 that mimics the effect of TSP-1 on FGF-2. The sm27 molecule forms a ternary complex with FGF-2 and its receptor (FGFR1), and heparin sulfate proteoglycans to inhibit the action of down-stream signal transduction pathways (Pagano et al. 2012).
Not all of the domains of TSP-1 have been reported to inhibit angiogenesis. The N-terminal heparin-binding domain reportedly stimulates endothelial tube formation and survival (Ferrari do Outeiro-Bernstein et al. 2002). Whereas, the molecular basis for this effect remains to be determined, the proteoglycan syndican-4 appears to be involved (Ferrari do Outeiro-Bernstein et al. 2002). Since intact TSP-1 inhibits angiogenesis, the pro-angiogenic activity of the N-terminal domain is likely over-shadowed by the anti-angiogenic activity of other domains in the intact protein.
Integration of pro- and anti-angiogenic signaling
The behavior of endothelial cells is determined by the net input of concurrent signals from pro- and anti-angiogenic molecules. Endothelial cells are quiescent in healthy adult tissue where the presence of basal levels of TSP-1 that are present in the vessel wall probably serve to keep endothelial cells from initiating sprouting and capillary tube formation. Several studies have shown that TSP-1 activates apoptosis of endothelial cells through a pathway that involves CD36-mediated activation of the Src family kinase, Fyn (Fig. 2) (Jimenez et al. 2000; Ren et al. 2009). In the absence of TSP-1, Src is preferentially associated with CD36, but in the presence of TSP-1, Fyn is recruited to CD36 (Sun et al. 2009). Activation of Fyn in endothelial cells leads to phosphorylation of Jun N-terminal kinase (JNK), and up-regulation of FasL and death receptors (DR4 and DR5), which ultimately leads to activation of both caspase-8- and caspase-9-dependent cleavage of caspase-3 (Jimenez et al. 2000; Volpert et al. 2002; Ren et al. 2009).
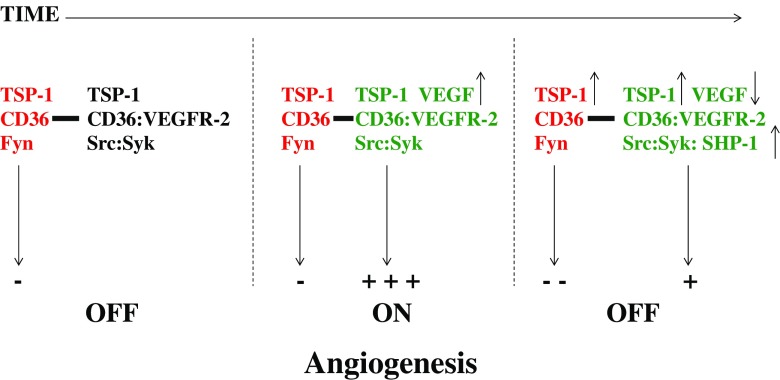
Interestingly, CD36, β1 integrins and VEGFR-2 form a complex in the plane of the endothelial cell plasma membrane (Kazerounian et al. 2011). The formation of this complex is dependent upon the presence of TSP-1 with markedly diminished association of VEGFR-2 with CD36 seen in TSP-1-null mice (Kazerounian et al. 2011). These complexes are probably included in CD36 nanoclusters that include multiple copies of CD36 and Fyn (Githaka et al. 2016). The presence of TSP-1 reportedly increases the size of these nanoclusters (Githaka et al. 2016). Primo and colleagues (Primo et al. 2005) reported that expression of CD36 in human umbilical vein endothelial cells (HUVECs), which don’t normally express CD36, mediates TSP-1-induced suppression of VEGFR-2 phosphorylation in response to VEGF. This activity of TSP-1 is lost when C464 of CD36 is mutated to serine. This mutation abolishes the ability of CD36 to associate with the β1 integrin subunit (Primo et al. 2005). These results showed for the first time that TSP-1 not only activated anti-angiogenic signal transduction, but also antagonized pro-angiogenic signaling induced by VEGF. The decreased phosphorylation of VEGFR-2 correlates with increased association of the phosphatase SHP-1 with the VEGFR-2 protein complex (Chu et al. 2013). The decrease in VEGFR-2 phosphorylation in turn results in a decrease in Akt phosphorylation, which promotes the activity of caspase-8 through modultion of cFLIP (Fig. 1) (Ren et al. 2009). Taken together, the data indicate that cross-talk between pro- and anti-angiogenic signaling occurs not only in a membrane protein complex that includes CD36, VEGFR-2 and β1 integrins, but also in the downstream signaling pathways of these receptor complexes. The changes in the composition of these protein complexes over time regulates the initial steps in angiogenesis. In the resting state, when VEGF is not present, basal levels of TSP-1 inhibit angiogenesis (Fig. 3). Note that we do not know what percentages of CD36 and VEGFR-2 are associated with each other, however, in the absence of VEGFA, we expect the VEGFR-2 signaling pathway to be silent. Expression of VEGFA leads to a very strong positive signal for induction of angiogenesis that shifts the angiogenic signaling balance to the “on” state. This effect can be seen in normal tissue such as the mammary gland or the hair follicle of the mouse where VEGFA levels initially increase to induce angiogenesis. The basal levels of TSP-1 appear to promote the initial response to VEGF by recruiting spleen tyrosine kinase (Syk) to the receptor complex (Kazerounian et al. 2011). We have found that Syk, which is a target of Src family kinases, is capable of phosphorylating VEGFR-2 on tyrosine 1175. This phosphorylation event is critical to VEGFR-2 function in that mutation of tyrosine 1175 results in an embryonic lethal phenotype (Sakurai et al. 2005). Over time, the VEGFA levels decrease and TSP-1 levels increase in normal adult tissue (Yano et al. 2003). The increase in TSP-1 levels leads to an increased activation of the CD36-mediated inhibitory pathway and recruitment of SHP-1 to VEGFR-2. These changes return the angiogenic state to the “off” position. Consistent with this model, angiogenic vessels are slow to regress in the hair follicles of TSP-1-null mice as compared to their wild-type counterparts (Yano et al. 2003). Similarly, TSP-1 levels increase during the later stages of angiogenesis induced by histamine and serotonin to promote vessel regression (Qin et al. 2013). The process of normal termination of angiogenesis is disrupted in cancer because the levels of VEGF remain high.
Fig. 3.
Temporal regulation of the pathways that are regulated by VEGFA and TSP-1
VEGF treatment of endothelial cells results in activation of eNOS and the production of nitric oxide, which promotes angiogenesis (Simons et al. 2016). The carboxyl-terminal domain of TSP-1 binds to CD47 and inhibits two downstream targets on nitric oxide, soluble guanylate cyclase and cGMP-dependent protein kinase (Kaur and Roberts 2011; Roberts et al. 2012). In contrast to its effect on CD36 and VEGFR-2 where TSP-1 promotes association of the two proteins, TSP-1 stimulates the dissociation of CD47 from VEGFR-2 (Kaur et al. 2010). Thus, TSP-1 decreases the association of VEGFR-2 with CD47 and increases its association with CD36. Whether or not the removal of CD47 affects the internalization and/or signaling activity of VEGFR-2 remains to be determined.
Development of a recombinant version of the TSRs as a therapeutic
Since angiogenesis is regulated by pro- and anti-angiogenic signals, one would predict that it is possible to inhibit angiogenesis by antagonizing the pro-angiogenic side of the balance or by promoting that inhibitory side. The vast majority of drug development to date has focused on antagonizing the pro-angiogenic pathway with antibodies and small molecules that block the VEGFA/VEGFR-2 pathway. However, it is also possible to amplify the anti-angiogenic side of the balance. The recombinant protein 3TSR is a potent inhibitor of skin, colon, and pancreatic cancer (Zhang and Lawler 2007). Its activity is comparable to that of gemcitabine, a first line therapy for pancreatic cancer (Zhang et al. 2005a, b). The effect of 3TSR on these cancers is mediated by inhibition of angiogenesis with no direct effects on the tumor cells. By contrast, 3TSR directly inhibits the growth of ovarian cancer cells and this effect is mediated, at least in part, by CD36, which is expressed on ovarian cancer cells (Russell et al. 2015; Wang et al. 2016). Treatment of ovarian tumor-bearing mice with 3TSR markedly reduces the size of the primary tumor, the number of metastases and the quantity of ascites production. In addition, the 3TSR-treated mice display a significant increase in survival and vascular normalization (Russell et al. 2015). Much of the tumor vasculature is tortuous and chaotic, with little pericyte coverage and poor perfusion (De Bock et al. 2011). Anti-angiogenic therapy specifically targets these vessels leading to a more normal appearance and function, a process referred to as “vascular normalization” (Carmeliet and Jain 2011). An initial increase in perfusion is thus observed when anti-angiogenic therapy is initiated. 3TSR induces vascular normalization in an ovarian cancer model and consequently leads to increased delivery of chemotherapeutics (Russell et al. 2015). The combination of 3TSR with chemotherapy results in further decreases in tumor size and a marked increase in survival as compared to either monotherapy. The increase in survival seen with 3TSR as a single agent is greater than that seen with chemotherapy alone (Russell et al. 2015). Thus, 3TSR is a particularly effective inhibitor of ovarian cancer because it is able to concurrently target endothelial cells and ovarian cancer cells through CD36, which is expressed by both cell types. An up-regulation of SHP-1 recruitment to CD36 is also seen in both cell types.
Chemotherapeutics can be delivered by the traditional maximum tolerated dose regime or by metronomic dosing, which is more frequent, continuous, treatments with low doses. Metronomic chemotherapy reportedly inhibits angiogenesis by increasing Fas expression on endothelial cells and increasing circulating levels of TSP-1 (Bocci et al. 2003; Quesada et al. 2005). In addition, metronomic chemotherapy decreases VEGFA secretion and depletes regulatory T cells (Mpekris et al. 2017). The combination of 3TSR with metronomic chemotherapy is more effective in a murine model of ovarian cancer than the combination of 3TSR with chemotherapy delivered as a maximum tolerated dose (Russell et al. 2015).
Conclusions
Angiogenesis is a carefully-regulated process that relies on the integration of positive and negative signals at the plasma membrane of endothelial cells. The anti-angiogenic protein TSP-1 appears to have evolved with the ability to antagonize the pro-angiogenic function of VEGFA in a number of ways, including (1) inhibition of mobilization from the extracellular matrix, (2) increased clearance from the extracellular space, and (3) suppression of VEGFR-2 phosphorylation. Thus, TSP-1 and 3TSR may be uniquely well-suited to inhibit VEGF-induced angiogenesis.
The biochemical data indicate that the membrane proteins that function as receptors are organized into multiprotein complexes that facilitate integration of signal transduction. TSP-1 affects the size and composition of these complexes. The molecular interactions between TSP-1, CD36, Fyn, β1 integrins, CD47 and VEGFR-2 determine the function and temporal regulation of these complexes, and integrate pro-and anti-angiogenic signal transduction to determine endothelial cell behavior. A complete understanding of the biochemistry of theses protein-protein interactions will reveal novel therapeutic opportunities.
Strategies for regulating angiogenesis are showing promise in the clinic for treatment of various diseases including age-related macular degeneration and cancer. In addition, 3TSR can prevent the formation of cerebral cavernous malformations in the brain (Lopez-Ramirez et al. 2017). Anti-angiogenic therapy targets an essential step in tumor progression and can significantly improve the delivery of other anti-cancer modalities such as chemotherapy and immunotherapy.
Acknowledgements
We would like to acknowledge the assistance of Sami Lawler in editing the manuscript. This work was supported by a CAO Pilot grant from the Beth Israel Deaconess Medical Center.
References
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139–146. doi: 10.1016/j.canlet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Baenziger NL, Brodie GN, Majerus PW. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971;68:240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122:1822–1832. doi: 10.1182/blood-2013-01-482315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Margosio B, Ragona L, Neves M, Bonifacio S, Annis DS, Stravalaci M, Tomaselli S, Giavazzi R, Rusnati M, et al. Non-peptidic thrombospondin-1 mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J Biol Chem. 2010;285:8733–8742. doi: 10.1074/jbc.M109.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Pearce SF, Schneider AJ, Silverstein RL, Henkin J, Bouck NP. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol. 1999;55:332–338. doi: 10.1124/mol.55.2.332. [DOI] [PubMed] [Google Scholar]
- De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, Humerickhouse RA, Qian J, Gordon GB, Figlin R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin Cancer Res. 2007;13:6689–6695. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- Evans EB, Lin SY. New insights into tumor dormancy: Targeting DNA repair pathways. World J Clin Oncol. 2015;6:80–88. doi: 10.5306/wjco.v6.i5.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Herzog B, Mahmoud M, Yamaji M, Plein A, Denti L, Ruhrberg C, Zachary I. Neuropilin 1 (NRP1) hypomorphism combined with defective VEGF-A binding reveals novel roles for NRP1 in developmental and pathological angiogenesis. Development. 2014;141:556–562. doi: 10.1242/dev.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Silverstein RL. CD36: Implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39(11):2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291X(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrari do Outeiro-Bernstein MA, Nunes SS, Andrade AC, Alves TR, Legrand C, Morandi V. A recombinant NH(2)-terminal heparin-binding domain of the adhesive glycoprotein, thrombospondin-1, promotes endothelial tube formation and cell survival: a possible role for syndecan-4 proteoglycan. Matrix Biol. 2002;21:311–324. doi: 10.1016/S0945-053X(02)00010-0. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: a possible control point in tumor growth. Ann Intern Med. 1975;82:96–100. doi: 10.7326/0003-4819-82-1-96. [DOI] [PubMed] [Google Scholar]
- Gaustad JV, Simonsen TG, Andersen LM, Rofstad EK. Properdistatin inhibits angiogenesis and improves vascular function in human melanoma xenografts with low thrombospondin-1 expression. Oncotarget. 2016;7:76806–76815. doi: 10.18632/oncotarget.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand MV, Hagan N, Tata A, Oh WJ, Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH, Gu C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. elife. 2014;3:e03720. doi: 10.7554/eLife.03720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githaka JM, Vega AR, Baird MA, Davidson MW, Jaqaman K, Touret N. Ligand-induced growth and compaction of CD36 nanoclusters enriched in Fyn induces Fyn signaling. J Cell Sci. 2016;129:4175–4189. doi: 10.1242/jcs.188946. [DOI] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210:807–818. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/A:1009018702832. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indraccolo S, Stievano L, Minuzzo S, Tosello V, Esposito G, Piovan E, Zamarchi R, Chieco-Bianchi L, Amadori A. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc Natl Acad Sci U S A. 2006;103:4216–4221. doi: 10.1073/pnas.0506200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.CIR.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Kaur S, Roberts DD. CD47 applies the brakes to angiogenesis via vascular endothelial growth factor receptor-2. Cell Cycle. 2011;10:10–12. doi: 10.4161/cc.10.1.14324. [DOI] [PubMed] [Google Scholar]
- Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerounian S, Yee KO, Lawler J. Thrombospondins: from structure to therapeutics : Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700–712. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerounian S, Duquette M, Reyes MA, Lawler JT, Song K, Perruzzi C, Primo L, Khosravi-Far R, Bussolino F, Rabinovitz I, Lawler J. Priming of the vascular endothelial growth factor signaling pathway by thrombospondin-1, CD36, and spleen tyrosine kinase. Blood. 2011;117:4658–4666. doi: 10.1182/blood-2010-09-305284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliche S, Waltenberger J. VEGF receptor signaling and endothelial function. IUBMB Life. 2001;52:61–66. doi: 10.1080/15216540252774784. [DOI] [PubMed] [Google Scholar]
- Koch M, Hussein F, Woeste A, Grundker C, Frontzek K, Emons G, Hawighorst T. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat. 2011;128:337–346. doi: 10.1007/s10549-010-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld MD, Pavlopoulos TV, Frazier WA. Cell attachment activity of the carboxyl-terminal domain of human thrombospondin expressed in Escherichia coli. J Biol Chem. 1991;266:24257–24259. [PubMed] [Google Scholar]
- Lawler J, Detmar M. Tumor progression: the effects of thrombospondin-1 and -2. Int J Biochem Cell Biol. 2004;36:1038–1045. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978;253:8609–8616. [PubMed] [Google Scholar]
- Lawler J, Miao WM, Duquette M, Bouck N, Bronson RT, Hynes RO. Thrombospondin-1 gene expression affects survival and tumor spectrum of p53-deficient mice. Am J Pathol. 2001;159:1949–1956. doi: 10.1016/S0002-9440(10)63042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Lopez-Dee ZP, Chittur SV, Patel B, Stanton R, Wakeley M, Lippert B, Menaker A, Eiche B, Terry R, Gutierrez LS. Thrombospondin-1 type 1 repeats in a model of inflammatory bowel disease: transcript profile and therapeutic effects. PLoS One. 2012;7:e34590. doi: 10.1371/journal.pone.0034590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ramirez MA, Fonseca G, Zeineddine HA, Girard R, Moore T, Pham A, Cao Y, Shenkar R, de Kreuk BJ, Lagarrigue F, et al (2017) Thrombospondin1 (TSP1) replacement prevents cerebral cavernous malformations. J Exp Med 214:3331–3346 (in press) [DOI] [PMC free article] [PubMed]
- Margosio B, Rusnati M, Bonezzi K, Cordes BL, Annis DS, Urbinati C, Giavazzi R, Presta M, Ribatti D, Mosher DF, Taraboletti G. Fibroblast growth factor-2 binding to the thrombospondin-1 type III repeats, a novel antiangiogenic domain. Int J Biochem Cell Biol. 2008;40:700–709. doi: 10.1016/j.biocel.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci U S A. 2017;114:1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J, Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, Bornstein P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol Biol Cell. 2008;19:563–571. doi: 10.1091/mbc.E07-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Pagano K, Torella R, Foglieni C, Bugatti A, Tomaselli S, Zetta L, Presta M, Rusnati M, Taraboletti G, Colombo G, Ragona L. Direct and allosteric inhibition of the FGF2/HSPGs/FGFR1 ternary complex formation by an antiangiogenic, thrombospondin-1-mimic small molecule. PLoS One. 2012;7:e36990. doi: 10.1371/journal.pone.0036990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primo L, Ferrandi C, Roca C, Marchio S, di Blasio L, Alessio M, Bussolino F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J. 2005;19:1713–1715. doi: 10.1096/fj.05-3697fje. [DOI] [PubMed] [Google Scholar]
- Qin L, Zhao D, Xu J, Ren X, Terwilliger EF, Parangi S, Lawler J, Dvorak HF, Zeng H. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood. 2013;121:2154–2164. doi: 10.1182/blood-2012-07-443903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada AJ, Nelius T, Yap R, Zaichuk TA, Alfranca A, Filleur S, Volpert OV, Redondo JM. In vivo upregulation of CD95 and CD95L causes synergistic inhibition of angiogenesis by TSP1 peptide and metronomic doxorubicin treatment. Cell Death Differ. 2005;12:649–658. doi: 10.1038/sj.cdd.4401615. [DOI] [PubMed] [Google Scholar]
- Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Song K, Parangi S, Jin T, Ye M, Humphreys R, Duquette M, Zhang X, Benhaga N, Lawler J, Khosravi-Far R. A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR. Cancer Res. 2009;69:3856–3865. doi: 10.1158/0008-5472.CAN-08-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J. 1996;10:1183–1191. doi: 10.1096/fasebj.10.10.8751720. [DOI] [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S, Duquette M, Liu J, Drapkin R, Lawler J, Petrik J. Combined therapy with thrombospondin-1 type I repeats (3TSR) and chemotherapy induces regression and significantly improves survival in a preclinical model of advanced stage epithelial ovarian cancer. FASEB J. 2015;29:576–588. doi: 10.1096/fj.14-261636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M (2009) CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal, 2:re3 [DOI] [PMC free article] [PubMed]
- Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenina-Adognravi O. Thrombospondins: old players, new games. Curr Opin Lipidol. 2013;24:401–409. doi: 10.1097/MOL.0b013e3283642912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenina-Adognravi O. Invoking the Power of Thrombospondins: Regulation of Thrombospondins Expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hopkins BD, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, Bornstein P, Lawler J, Benjamin LE. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am J Physiol Heart Circ Physiol. 2009;296:H1344–H1351. doi: 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Sumarriva K, Kim R, Jiang R, Brantley-Sieders DM, Chen J, Mernaugh RL, Takahashi T. Determination of the CD148-Interacting Region in Thrombospondin-1. PLoS One. 2016;11:e0154916. doi: 10.1371/journal.pone.0154916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Duquette M, Liu JH, Dong Y, Zhang R, Joachimiak A, Lawler J, Wang JH. Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol. 2002;159:373–382. doi: 10.1083/jcb.200206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8(4):349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- Wang S, Blois A, El Rayes T, Liu JF, Hirsch MS, Gravdal K, Palakurthi S, Bielenberg DR, Akslen LA, Drapkin R, et al. Development of a prosaposin-derived therapeutic cyclic peptide that targets ovarian cancer via the tumor microenvironment. Sci Transl Med. 2016;8:329ra334. doi: 10.1126/scitranslmed.aad5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal JR. Technology evaluation: ABT-510, Abbott. Curr Opin Mol Ther. 2004;6:451–457. [PubMed] [Google Scholar]
- Yamauchi M, Imajoh-Ohmi S, Shibuya M. Novel antiangiogenic pathway of thrombospondin-1 mediated by suppression of the cell cycle. Cancer Sci. 2007;98(9):1491–1497. doi: 10.1111/j.1349-7006.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Brown LF, Lawler J, Miyakawa T, Detmar M. Thrombospondin-1 plays a critical role in the induction of hair follicle involution and vascular regression during the catagen phase. J Invest Dermatol. 2003;120:14–19. doi: 10.1046/j.1523-1747.2003.12045.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lawler J. Thrombospondin-based antiangiogenic therapy. Microvasc Res. 2007;74:90–99. doi: 10.1016/j.mvr.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Galardi E, Duquette M, Delic M, Lawler J, Parangi S. Antiangiogenic treatment with the three thrombospondin-1 type 1 repeats recombinant protein in an orthotopic human pancreatic cancer model. Clin Cancer Res. 2005;11:2337–2344. doi: 10.1158/1078-0432.CCR-04-1900. [DOI] [PubMed] [Google Scholar]
- Zhang X, Galardi E, Duquette M, Lawler J, Parangi S. Antiangiogenic treatment with three thrombospondin-1 type 1 repeats versus gemcitabine in an orthotopic human pancreatic cancer model. Clin Cancer Res. 2005;11:5622–5630. doi: 10.1158/1078-0432.CCR-05-0459. [DOI] [PubMed] [Google Scholar]