Abstract
This article looks mostly at the steps that have led to the primary cilium finding its place in our understanding of cell biology, developmental biology, and medical syndromes due to its aberrations. It is a personal account that stresses, if nothing else, the value of the adage “stick to your guns”. My obsession with this organelle, following on from fascination with the centriole, has led to a whole career devoted to determining the nature and role of primary cilia in basic cell biology, which has proved much more important than had been appreciated for almost a century. They are heavily involved in very many aspects of cell physiology that have much wider implications with regard to human biology and probably throughout the animal kingdom. That aberrations, to the surprise of many researchers in their structure or functioning has led to their being implicated or perhaps deeply involved in an extraordinary range of medical conditions. This invitation allows me to raise crucial questions that need answers regarding the regulation of their genesis, their cache of both intracellular and extracellular signal, and their association with a multitude of development processes from embryo to adult status.
Keywords: Primary cilia, Centrioles, Developmental differentiation, Signalling
“Nothing a cell does is without significance.”
Introduction
It is always a pleasure to be invited to contribute to a journal, especially in this case on the 10th anniversary of Journal of Cell Communication and Signalling. One advantage is that it does not have to be scholarly paper, and in this case it will be one of reflection about a passion I have had since 1965 concerning the primary cilium. It is also an opportunity to express oneself with some of that emotion too often suppressed, especially in publications, of research scientists. Journals are full of serious scientific articles; any hint of humour is frowned upon and cartoons generally have to go to magazines as distinct from scholarly journals, in which you can be a frivolous. I intend to include a few things in this paper that should make you smile.
For a century the significance of a small cell organelle was never recognised. In the last 20 years it has become of such significance that it is now seen not only as a busy organelle communicating internally with other organelles (even mitochondria), but as the root cause in many cases of syndromes and disorders of the body. Its significance, however, had been repeatedly pointed out over decades by researchers who devoted much of their time studying this organelle. The record has been put to rights by the sterling efforts of Bloodgood (2009). The 10 main landmarks since the 1960s in the advancement of our understanding of these cilia have been recorded (Wheatley 2005). Poole et al. (1985) espoused their significance and putative role decades ago, but their words fell on deaf ears – others to whom the primary cilium remained for almost a century a vestigial or rudimentary structure of no particular relevance to the cells that possess them. Admittedly the work of Poole and his colleagues was speculative, but not wildly so, since it was quite clear it could have a mechanosensory function, if at that time nowhere else than in the kidney and cartilage. The 2 papers that later clinched this matter were Schwartz et al. (1997) and Praetorius et al. (2004), who showed that it was almost certainly mechanosensory; on being bent it sent signals into the cell that caused a (free) Ca++ transient, which had by then been established as a mediator of widespread activation of intracellular signalling pathways.
The primary cilium – its return to centre stage
My opening aphorism, indicating that such an organelle will (must) have some significance, was stimulated when I first came across a magnificent example of a primary cilium in a cell of the rat zona glomerulosa, where they had not been seen before. I was also surprise as they did not seem to be present in the zona fasciculate and zona reticularis of the adrenal. This exceptional example led to my first report on primary cilia along with my boss at that time (Currie and Wheatley 1966), just 4 years after the excellent work of Sorokin (1962, and later in 1968) - we are talking here about work done over half a century ago. When primary cilia were followed in the renal epithelial cells, they proved to be extraordinarily well developed and could reach up to 30 μmin length. Ironically these were the first ones seen by Zimmerman (1898) - how he got sufficient resolution in those days to see them remains a mystery - which makes it all the more puzzling why such a structure was largely ignored for many decades. But in celebrating the 10th anniversary of the JCCS, I cannot think of a more fitting example of the vital role (literally) of any other organelle than the primary cilium, now seen as literally and incomparably, the main antenna involved in intracellular and extracellular communication.
It became apparent in the course of many years of research with Sam Bowser at the Wadsworth Institute in Albany NY that there was a ubiquity, along with a considerable diversity, of primary cilia (Wheatley 2005) in the cells of the mammalian (and most other animals) body. After creating an early website (for those days) listing cell types in which primary cilia had been found, it became apparent in the late 1980s that it was easier to catalogue tissues in which cells did not have primary cilia. Suspended cells may not be influenced by contact with a substratum that helps release many signals and factors. Blood borne cells, such as lymphocytes and macrophages that are suspended cells have no cilia, but the problem is whether their progenitors might have been ciliated. We might ask to what extent are embryo cells, e.g. in the blastula, gastrula or later stages. The same question might be posed regarding cells that are amassed in a similarly suspended state, i.e. in spheroids. Our findings were unequivocal when we compared a strain of BHK12 cells that were adherent as monolayers in cultures with another that had been slowly adapted over several months to grow in suspension, since the latter were unciliated, whereas >60% of confluent monolayer cells were well ciliated (Wheatley and MacPherson, unpublished data). Adhesion may be critical in the situation, since by far the biggest structure of a sensory nature in the cell is its membrane, just as our skin is our largest sensory organ. Distribution of charge on the cell surface might be quite different in spherical cells compared perhaps with a more polarized nature on adherent cells.
Although the cells of the inner zones of the adrenal cortex did not appear to have cilia, I would not be surprised if someone someday reports them. But much time has passed and it seems that cells such as hepatocytes or pancreatic endocrine cells have not yet been seen to have primary cilia.
The nearly ubiquitous nature of primary cilia
Indeed, we had thought that some tissues cell types were most unlikely to have them, e.g. neurons, the closest in the early days to this finding being reported by Dahl (1963) in the mouse adenohypophysis. Neurones and brain support cells were soon found to be ciliated, and in recent years they have been implicated in a remarkably wide range of mental disorders. It is now common knowledge that a considerable number of pathological conditions (40+ to date) are due to, or in some way involve, defective primary cilia.
Dahl’s images suggest that almost every cell probably had a primary cilium. It required careful and orderly preparation of ultrathin section for TEM to ensure that all cells of a tissue were ciliated. When ID5 became available, the problem was solved, and indicated that ciliation of population of cells in a tissue could reach almost 100%. However, this percentage could be much lower in some (~20% in a few cases). This phenomenology leads to a number of questions that are in some cases still on partly solved:
What decides whether a cell develops a primary cilium or not?
What prevents almost every cell type so far examined that has 9 + 2 cilia from also developing primary (9 + 0) cilia?
What controls whether a primary cilium is long and well developed, as in renal epithelial cell, compared with those that look small and stunted (up to 3 μm), as found in proliferating fibroblasts?
What influences the development of those cilia that are very different in structure and appearance from others (as seen in ref. 2)?
What factors inhibit ciliogenesis when cells of the same line are ciliated in monolayer but their suspension counterparts are non-ciliated.
Defects in primary cilia - the pathological sequela
A pathologist can often learn a lot about the behavioural significance of a normal bodily process by exploring what happens if it becomes deranged and dysfunctional. In the same way I had posed the question as to what the consequence would be in the kidney and other tissues if their primary cilia were defective or absent? It seems obvious that there would be some associated pathology, as explicitly set out in Wheatley (1995). In the late 1990s, I was approached about the biology of primary cilia after Doug Cole had discovered that lack of a gene for one of the ciliary proteins resulted in aciliogenesis Pazour et al. (2000). The primary cilium suddenly moved front stage where it seems to have remained. Today there could be as many as a thousand of people research the primary cilium and its normal or pathological functioning. In 15 years a massive amount of information on their structure, involvement in development, their relationship to other organelles (e.g. mitochondria), their significance in the centrosome and cell cycle regulation, and of course to their broad signalling capacity from their vantage points as antennae often sticking way out from cells, has been gleaned. In a short review (Wheatley 2008) I humorously compared a spherical cell of diameter 12 μm with a well-developed protruding primary cilium to a signalling mast sticking out from earth, which would have been 2000 miles high! It is little wonder that primary cilia can have so much influence within their immediate environment and beyond considering the plethora of incoming and outgoing communications it relays.
The primary cilium’s “base”
The duplication (reproduction) of centrioles (diplosomes) as cells move towards division is remarkable as it occurs in an orthogonal manner. Despite the belief for many years that the reproduction of centrioles assured binary fission, as Mazia (1978) had maintained for many years, this partially misguided me in publishing a similar book “The Centriole: a Central enigma in Biology” (Wheatley 1982), i.e. before the centrosome finally assumed this role. It had indeed been an enigma until its role was more firmly established as a “basal body” of a now fully recognised type of cilium of the (9 + 0) variety. Fortunately the whole of Chapter 6 had been entirely devoted to a review of the structure and significance of the primary cilium.
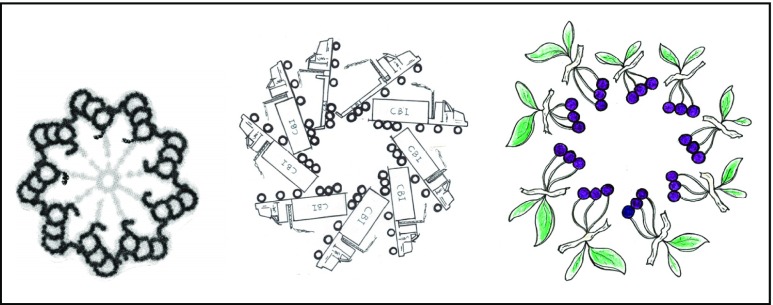
The centriole is remarkable as it the only organelle in the cell that has a very stable and aesthetically attractive structure, and one of extraordinary antiquity. My fascination in the centriole stayed with me in its quasi-symmetry, although my artistic temperament took over. The tubules in cross section conjured up many different ways in which you could represent them by everyday objects. Over 70 depictions of the centriole were rendered, of which 2 are illustrated in Fig. 1.
Fig. 1.
Cross-section of a primary cilium with two variations upon it (trucks and cherries)
The centrosome is the home from the primary cilia is extruded; they might move more towards the membrane, but most issue from this deeper site and might communicate more easily with nuclei. The relevance of the centrioles finally clicked because structurally they are exactly the same as the basal bodies of 9 + 2 cilia (Sorokin 1962). Its importance began to be understood only when the primary cilium finally got its full recognition. But the process by which these cilia are initiated and develop from their centriolar base is only slowly being elucidated.
We may soon learn what controls the development of the primary cilium, but it does not answer the underlying question of why (which should always be viewed with suspicion rather than how in science) certain cells never seem to produce cilia (as mentioned above). So many motor proteins are involved like steeplejacks in building a towering primary cilium, the genome involved now becoming well known as it can help in diagnosing situations leading to aciliogenesis and pathology. The number of reports in the complex process by which cilia are initiated and start to develop is growing almost daily (e.g. Agbu et al. 2017; Pitaval et al. 2017; Légaré et al. 2017; Bernabé-Rubio and Alonso 2017). At the risk of posing yet more questions, I would want to know more specifically what differences are there between ciliogenesis of the 9 + 0 and 9 + 2 types (Satir 2017 - who has had a foot in both camps for many years); it seems there is a dichotomy – it has to be one or the other, never (?) both.
The long and the short of it
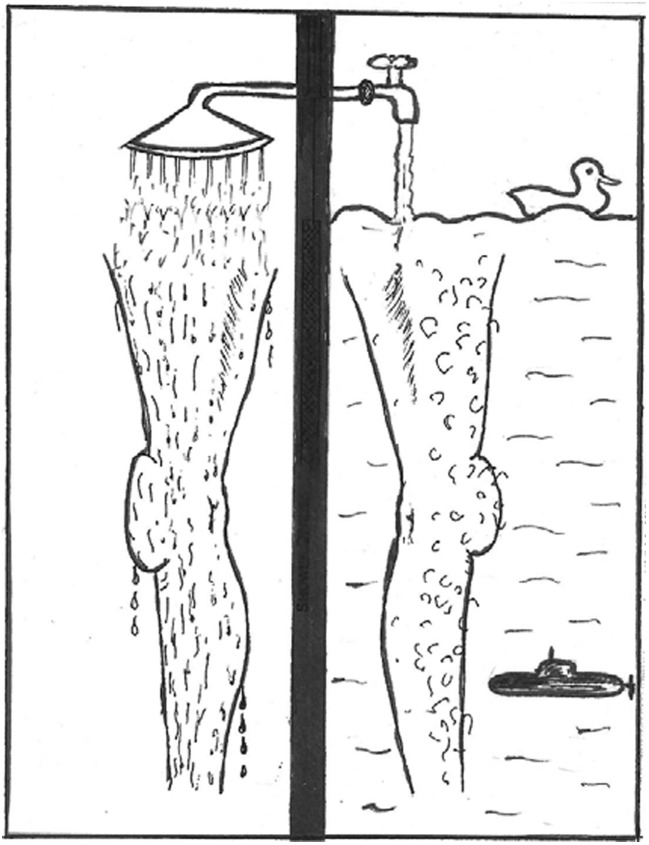
The answers to the above questions should give us a better understanding of question 3 of what in fact regulates how long a cilium will grow. As an aside, we found it difficult to measure the length of cilia when they are not straight or are tangled across one another in an SEM or fluorescent image. Sam Bowser and I devised a very simple to measure ciliary length, which was the same way as you would measure the length of hairs on your leg (Wheatley and Bowser 2000). A monolayer of cells on a slide placed at a slope of >45o was washed from the top with PBS. The cilia aligned downwards, clinging to the monolayer surface. It was so much easier and quicker to measure them after fixing and imaging (Fig. 2; science is not without its humour).
Fig. 2.
An analogy from which a simple technique was devised of straightening primary cilia in one plane to make them easier to measure
To return to the question of length regulation, it is without doubt that cilia can be long in certain cell types whereas others seldom grow them any more than 2–3 μm (Feilen et al.). It is unlikely that cells that proliferate rapidly will literally have time to extrude long cilia. However, even in the last year there have been several reports on factors influencing length (e.g. Sherpa et al. 2016; Gadadhar et al. 2017; Zhang et al. 2017).
This brings up the problem of ciliation through the cell cycle. Since cilia are resorbed during division, then it is unlikely that anything other than short cilia will be seen if proliferation is fast leaving little time to produce long ones. [Note that almost all cells become spherical when going through mitosis!] The association with the cell cycle is still under investigation. It is not accurate to consider that cilia tend to form as a cell moves towards the S-phase of the cycle, since in very early studies we found cilia developing in the very last stages of telophase, indicating that ciliogenesis can be quite a rapid process. However, we know that if cells move out of cycle and enter stationary phase (G0), many more of the population become ciliated (Wheatley et al. 1996; Goto et al. 2013), even if the cilia are quite short, as seen in normal fibroblast cultures. We also do not know if these more “stunted” cilia are less active as sensing and signalling organelles that those in cells where they are more prominent. Another issue that intrigues me in the context of progenitors is whether stem cells are as ciliated as some of their issue, the answer to which will be particular interesting (McMurray et al. 2013).
Cancer and ciliation
A particular concern throughout my career in which I have focused on the basic cell biology of cancer has always been the question of what distinguishes a malignant cell from a normal cell. The data from earlier days suggested that the more anaplastic a tissue became, the fewer primary cilia could be found, and most were also poorly developed and in disarray (Wheatley 1993). However, there have been quite a few equivocal papers on the subject within the last year, which begs the question of whether malignant cells do not respond properly to signals needed to initiate and sustain their development (Yang et al. 2017; Kobayashi and Itoh 2017).
Ciliopathies: the two types
We entered the era of the ciliopathies in the new millenium. However, there are 2 distinct (exclusively so) types of cilia, the 9 + 2 (motile) variety and the 9 + 0 (non-motile) primary cilium. Their development follows somewhat different courses, as so elegantly illustrated by Sorokin (1968).
Ciliopathies caused by loss of function of the motile variety have been known for decades, causing problems associated with oocyte movement, sperm motility, airway clearance, ventricular flow in the central nervous system, and so on. These conditions are referred to as primary ciliary dyskinesia, which is unfortunate because the word “primary” in this particular context refers in clinical usage to the main cause of such disorders. But primary ciliopathies might be adopted to connote disorders involving the primary cilia, although confusion will never be avoided, unless perhaps the former expression drops the word primary altogether (ciliary dyskinesia).
One of the first disorders attributed supposedly to aberration in the primary cilium was situs inversus totalis, from the experimental work on embryonic mice by Nonaka et al. (1998). This saga was highly perplexing for many years and can probably now be better understood as involving motile cilia rather than primary cilia. But it should always have been trenchantly obvious that blindness was due to aciliogenesis of rods and cones of the retina, unequivocally the most elaborate primary cilia structure (Sjöstrand 1953; Tokoyasu and Yamada 1959). The organ most likely to show malfunctioning as a result of primary aciliogenesisis was the kidney, and was strongly implicated in the disorder polycystic kidney disease (PCKD), briefly reviewed in Ong and Wheatley (2003). Because live births can ensue and life can continue with PCKD for some years, the finding nevertheless suggests that the primary cilium can be of importance during later development. If we fully explored this embryological aspect, it could probably be shown that primary cilium aciliogenesis, mutation or malfunctioning underlies much of inter-uterine death and congenital defects; Stiff et al. (2013), for example, showed that in the inherited disorder, the Meier-Gorlin syndrome, primary cilia developed poorly, and must be involved, along with other factors, in a condition that leads to stunted growth. The literature now abounds with hundreds of papers that implicate the primary cilium in many developmental aberrations, and by implication the reverse, i.e. that primary cilia are essential in many (most?) development processes, although this may be overstating the case (e.g. Youn and Han 2017; Hampl et al. 2017; Lepanto et al. 2016). Accurate development depends on signals passing from one cell type to another. The primary cilium may not account for a high percentage of the total number of signals, but in any such process a defect in one element can be enough to throw the whole process into disarray. Even so, a vast number of factors and signalling molecules, and the genes behind them, are now being identified (e.g. Moore and Jacobs 2017).
To search the literature for references on primary cilia in diseases and disorders is made more difficult because primary ciliary dyskinesia brings up far too many unwanted citations. There are hundreds of articles published annually on primary cilium involvement in pathology, although the rate is probably dropping as the range of possibilities narrows. What has been unexpected, at least as far as I was concerned, was the number of disorders or conditions that relate to disturbances in neurons and brain cells. Some of the milder conditions, such as dyslexia, seem to be traced to some altered state of primary cilia. Taking just one year’s reports, over 20 disorders of a wide ranging nature have implicated primary cilia involvement. These included retina and lens disorders, the motor neurone discoordination, mental retardation, the Bardet-Biedl syndrome(s), polycystic kidney disease and injuries, the Meckel-Gruber, Joubert, Lowe, Astrom, Birt-Hogg-Dube, and Meier-Gorlin syndromes, pre-invasive and invasive prostate, nephronophthisis, Jeune, Sensenbrenner, and Mainzer-Saldino chondrodysplasia, biliary atresia, ventricular septal defects, a number of cancers. If this list could be updated regularly, my estimate is that their number would be considerably more than a doubling by today (i.e. 2017), which is extraordinary considering the primary cilium had not been convincingly implicated possibly in no more than a couple of disorders before 1995.
Conclusion and - Quo vadis?
The primary cilium is an extraordinary cell organelle of considerable importance in so many functions of cells and tissues of the body; disturbance of its development often has quite disastrous consequences. The antiquity of the centriole structure throughout the whole of zoology from the most primitive cells in evolution is well known. That a towering edifice based on it should have been ignored for so long has now been rectified. The primary cilium has undoubtedly become one of the most investigated structures of the cell and is now truly centre stage. Many may think that we now know so much about the primary cilia that there will not be a great deal more to unravel, but like reflections in so many other previous discoveries, it soon becomes apparent that we have probably only scratched the surface of its full significance. In this regard, my essay herein has deliberately included many questions and issues that can be investigated in much greater depth, even if the growing gets tougher. “When the going gets tough, the tough get going.”
Acknowledgements
My thanks in particular to Bernard Perbal for inviting me to contribute, albeit rather tardily, to this anniversary issue of JCCS. It would not have been possible without the help from a band of devoted researchers I have had the privilege to work with for over more than 50 years. To name them all would be difficult, as it might be iniquitous to mention only a few. However, there were two points at which my research on primary cilia was bolstered during those times when as the French say “we were talking to the wind”. One was Tony Poole, who was perhaps the strongest advocate of the primary cilium, recognizing its importance from the start of his work on it, which has been thoroughly vindicated in the interim. The other most certainly was years of working with Sam Bowser in keeping the subject alive in the 1980s. Many facets of the basic biology of primary cilia were unfolded by our combined effort. Finally I would like to thank and congratulate everyone who has jumped on board in the last two decades; their combined effort has made unexpectedly huge waves in science and medicine. I must also apologise for using only representative references to recent reports on primary cilia to make some of my points. This article is not intended as a review, but rather a personal perspective of this remarkable organelle.
References
- Agbu SO, Liang Y, Liu A, Anderson KV (2017) The small GTDase RSG1 controls a final step in primary cilia initiation. J Cell Biol. 10.1083/jcb.201604048 [DOI] [PMC free article] [PubMed]
- Bernabé-Rubio M, Alonso MA. Routes and machinery of primary cilium biogenesis. Cell Mol Life Sci. 2017;74:30201–30206. doi: 10.1007/s00018-017-2570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood RA. From central to rudimentary to primary: the history of an under appreciated organelle whose time has come. The primary cilium. Methods Cell Biol. 2009;94:3–52. doi: 10.1016/S0091-679X(08)94001-2. [DOI] [PubMed] [Google Scholar]
- Currie AR, Wheatley DN. Cilia of a distinctive structure (9+0) in endocrine and other tissues. Postgrad Med J. 1966;42:403–408. doi: 10.1136/pgmj.42.489.403. [DOI] [Google Scholar]
- Dahl HA. Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat. 1963;60:369–386. doi: 10.1007/BF00336612. [DOI] [PubMed] [Google Scholar]
- Gadadhar S, Dadi H, Bodakuntla S, Schnitzler A, Bieche I, Ruscin F, Janke C. Tubulin glycylation controls primary cilia length. J Cell Biol. 2017;216:2701–2713. doi: 10.1083/jcb.201612050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Inoko A, Inagaki M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol Life Sci. 2013;70:3893–3905. doi: 10.1007/s00018-013-1302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl M, Cela P, Szabo-Rogers HL, Bosakova MK, Dosedelova H, Krejci P. Role of primary cilia in odontogenesis. J Dent Res. 2017;96:965–974. doi: 10.1177/0022034517713688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Itoh H. Loss of a primary cilium in PDAC. Cell Cycle. 2017;16:817–818. doi: 10.1080/15384101.2017.1304738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré S, Chabot C, Basik M. SPEN, a new player in primary cilium formation and cell migration in breast cancer. Breast Cancer Res. 2017;19:104. doi: 10.1186/s13058-017-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepanto P, Badano JL, Zolessi FR. Neuron’s little helper: The role of primary cilia in neurogenesis. Neurogenesis. 2016;3:e1248206. doi: 10.1080/23262133.2016.1253363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D (1978) Origin of twoness in cell reproduction. In: Dirksen et al. (eds) Cell Reproduction – In Honor of Dan Mazia, Academic press, N Y, pps 1-14
- McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;3:3545. doi: 10.1038/srep03545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Jacobs CR (2017) The primary cilium as a signalling nexus for growth plate function and subsequent skeletal development. J Orthop Res. 10.1002/jor.23732 [DOI] [PMC free article] [PubMed]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Ong AC, Wheatley DN. Polycystic kidney disease - the ciliary connection. Lancet. 2003;361:774–776. doi: 10.1016/S0140-6736(03)12662-1. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaval A, Senger F, Letort G, Gidrol X, Guyon L, Sillibourne J, Théry M (2017) Microtubule stabilization drives 3D centrosome migration to initiate ciliogenesis. J Cell Biol. 10.1083/jcb201610039. [DOI] [PMC free article] [PubMed]
- Poole CA, Flint MH, Beaumont BW. Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 1985;5:175–193. doi: 10.1002/cm.970050302. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Praetorius J, Nielsen S, Frokiaer J, Spring KR. Beta1-integrins in the primary cilium of MDCK cells potentiate fibronectin-induced Ca2+ signaling. Am J Physiol Ren Physiol. 2004;287:F969–F978. doi: 10.1152/ajprenal.00096.2004. [DOI] [PubMed] [Google Scholar]
- Satir P. Cilia: before and after. Cilia 2017: 6, 6 (1) 10.1186/s13630-017-0046-8. [DOI] [PMC free article] [PubMed]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Phys. 1997;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Sherpa RT, Atkinson KF, Ferreira VP, Nauli SM. Rapamycin increases length and mechanosensory function pf primary cilia in renal epithelial and vascular endothelium cells. Int Educ Res J. 2016;2:91–97. [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand FS. The ultrastructure of the inner segments of the retinal rods of the guinea pig eye as revealed by electron microscopy. J Cell Comp Physiol. 1953;42:45–56. doi: 10.1002/jcp.1030420104. [DOI] [PubMed] [Google Scholar]
- Sorokin SP. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Sci. 1962;15:363–373. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin SP. Reconstruction of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- Stiff T, Alagoz M, Alcantara D, Outwin E, Brunner HG, Bongers EM, O'Driscoll M, Jeggo PA. Deficiency in origin licensing proteins impairs cilia formation: implications for the aetiology of Meier-Gorlin syndrome. PLoS Genet. 2013;9:e1003360. doi: 10.1371/journal.pgen.1003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyasu K, Yamada E. The fine structure of retina studied with the electron microscope.IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley DN (1982) The centriole: a central enigma of cell biology. Elsevier Biomedical, Amsterdam. ISBN 0-444-80359-9
- Wheatley DN. Oligocilia: incidence and significance in normal and pathological tissues. Ultrastructural Path. 1993;17:565–566. doi: 10.3109/01913129309027804. [DOI] [PubMed] [Google Scholar]
- Wheatley DN. Primary cilia in normal and pathological tissues. Pathobiology. 1995;63:222–238. doi: 10.1159/000163955. [DOI] [PubMed] [Google Scholar]
- Wheatley DN. Landmarks in the first hundred years of primary (9+0) cilium research. Cell Biol Int. 2005;29:333–339. doi: 10.1016/j.cellbi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wheatley DN. Nanobiology of the primary cilium--paradigm of a multifunctional nanomachine complex. Methods Cell Biol. 2008;90:139–156. doi: 10.1016/S0091-679X(08)00807-8. [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Bowser SS. Measurement and length control of primary cilia: analysis of monociliates and multiciliates in PtK1 cells. Biol Cell. 2000;92:573–583. doi: 10.1016/S0248-4900(00)01108-4. [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Wang A-M, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- Yang N, Leung EL, Liu C, Li L, Eguether T, Jun Yao XJ, Jones EC, Norris DA, Liu A, Clark RA, Roop DR, Pazour GL, Shroyer KR, Chen J. INTU is essential for oncogenic Hh signalling through regulating primary cilia formation in basal cell carcinoma. Oncogene. 2017;36:4997–5005. doi: 10.1038/onc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YH, Han YG (2017) Primary cilia in brain development and disease. Am J Pathol. 10.1016/j.ajpath.2017.08.031 [DOI] [PMC free article] [PubMed]
- Zhang J, Dalbay MT, Luo X, Vrij E, Barbieri D, Moroni L, de Bruijn JD, van Blitterswijk CA, Chapple JP, Knight MM, Yuan H. Topography of calcium phosphate ceramics regulates primary cilia length and TGF receptor recruitment associated with osteogenesis. Acta Biomater. 2017;57:487–497. doi: 10.1016/j.actbio.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman KW. Beiträge zur kenntniss cinigen drusen und epithelien. Arch mikrosk Anat EntwMech. 1898;52:552–576. doi: 10.1007/BF02975837. [DOI] [Google Scholar]