Abstract
Tsukushi (TSK) is a small signaling molecule which takes part in different developmental processes of multiple vertebrate organisms. The diverse activity of TSK depends on its ability to bind various intermediate molecules from different major signaling pathways. Interactions of TSK with BMP, FGF, TGF-β and Wnt pathways have already been confirmed. In this review, we will introduce the latest information regarding the involvement of TSK in developmental events. We suggest a fine tuning role for TSK in multiple signaling cascades. Also, we recommend further studies on the developmental role of TSK to fully reveal its potential.
Keywords: Extracellular signaling molecule, SLRP family, Tsukushi, Vertebrate development
Introduction
Small soluble molecules play major role for the maintenance of cell-cell communication in multicellular organisms. They carry vital information for the proper functioning of numerous biological processes in all phases of life - starting from early embryonic stages up to the apoptosis and all the growth phases and homeostasis in between. A successful communication between cells depends both on the production of signaling molecules from the sources and their reception at the target (Lim et al. 2015). Although there are many signaling pathways functioning at any given time, Wnt, FGF, TGF-β and Shh are considered the major pathways. It is quite striking that similar signaling pathways tend to govern the similar processes in different organisms, although some organisms have bias to the subtype of signaling molecules employed (Muller and Schier 2011; Muller et al. 2013). Numerous signaling molecules and their cell surface receptors are contained within the extracellular space, where specific binding between them regulates a specific pathway. There are also some molecules which can interact with multiple signaling pathways owing to their ability to bind multiple receptors. For example, Cerebrus can interact with Wnt, Nodal and BMP (Piccolo et al. 1999); Coco interacts with Wnt, Xnrs and BMP (Bell et al. 2003), while follistatin interacts with activin and BMP (Fainsod et al. 1997; Harrington et al. 2006). Although these interactions are confirmed by several studies, their implications in the biological system are not fully revealed yet.
In this review, we will discuss about Tsukushi (TSK) protein, a signaling molecule which belongs to small leucine-rich proteoglycan family (SLRP) subclass IV (Iozzo and Schaefer 2015). It was initially identified in the chick lens and the name ‘Tsukushi’ comes from its expression pattern in blastoderm-stage chick embryo which resembles a shoot of the horsetail plant (Equisetum), ‘Tsukushi’ in Japanese (Ohta et al. 2004). TSK homologues have also been identified in vertebrates, like Xenopus, zebrafish, mouse and human; but not in any invertebrates. Since its first report on 2004, several studies have indicated its role as extracellular signal mediator contributing to various developmental events through its interactions with multiple signaling pathways (Ohta 2014).
Previously reported functions of TSK
TSK was first reported in 2004, when Ohta et al., described chicken TSK (C-TSK) as a BMP antagonist which is involved in the induction of primitive streak and Hensen’s node (Ohta et al. 2004). Later it was reported that C-TSK has two isoforms, C-TSKA (originally reported form) and C-TSKB, which play vital roles for the specification of cell fates during early chick development (Ohta et al. 2006). C-TSKA, having stronger BMP inhibitory effect, functions mainly at the anterior terminal point of primitive streak. During pre-streak phase, BMP is antagonized at posterior epiblast region through inductive effect of C-TSKA in cooperation with Chordin. The primitive streak development is stimulated by the interactions of both C-TSKA and C-TSKB with Vg1. At primitive streak stages, C-TSKA antagonizes BMP activity in the anterior part of the streak and C-TSKB promotes node-inducing activity by interacting with Vg1. These studies confirmed the multiple direct binding of C-TSK with BMP4, BMP7, Chordin and Vg1 by immunoprecipitation assay (Ohta et al. 2004, 2006). In vivo formation of the ternary complex by C-TSK, BMP4 and Chordin was also confirmed using chemical linking experiment (Ohta et al. 2004).
Kuriyama et al. reported the role of TSK in Xenopus for the specification of neural crest through its interaction with BMP and Notch pathways (Kuriyama et al. 2006). The timing and distribution of Xenopus-TSK (X-TSK) expression at neural plate border closely resembles that of Xbmp4 and Xslug, a neural crest marker. X-TSK binds BMP4 and antagonizes BMP activity, an effect similar to that of C-TSK. Depletion of X-TSK reduces the expression of neural crest markers Sox9, Zic5 and Xslug, and impairs the neural crest formation. The X-TSK siRNA injection inhibited the expression of Hairy2A and Msx1, which identify the pre-neural crest region. Animal cap injection experiments confirmed that, X-TSK regulates the neural crest specification in cooperation with XWnt-8, which acts as a posteriorizing signal. X-TSK was also found to regulate Notch signaling pathway through its binding with X-delta-1 and thereby indirectly modulate BMP4 transcription. The combination of all these experiments indicates that, X-TSK has a unique role in the molecular network for neural crest specification - more complex than what its BMP antagonizing function alone can explain. This study confirmed the binding of X-TSK with BMP4 and X-delta-1.
In the early Xenopus embryo, X-TSK activates Xnr2 signaling and inhibits FGF and BMP signaling, to exert the combined effect of inducing endoderm formation while inhibiting ventrolateral mesoderm formation (Morris et al. 2007). During late blastula and gastrula stages, X-TSK expression is coherent with its stated function - as X-TSK is expressed in the ectoderm, dorsal mesoderm and endoderm, and not in the ventral mesoderm. Both loss-of-function and overexpression experiments confirmed that, X-TSK upregulates endoderm markers (Sox17a and GATA4) and dorsal mesoderm organizer Gsc; and downregulates pan-mesoderm marker Xbra. This study confirmed the binding of X-TSK with FGF8b and Xnr2 by pulldown assay.
TSK was also reported to have important role for the retinal and peripheral eye development. TSK exerts this effect by direct binding with Fzd4 inhibiting Wnt2b activity (Ohta et al. 2011). Wnt signaling has important role for the differentiation of peripheral eye structure and in the anterior parts of the eye Wnt signaling is mainly activated by Wnt2b (Cho and Cepko 2006). TSK binding to Fzd4 was confirmed by immunoprecipitation experiment. Withdrawal of TSK signal results abnormal expansion of ciliary body in mice. Although BMP signaling has important role for ciliary body formation, the effect of TSK was not via BMP inhibition, because the TGF-β signaling was not hampered (Ohta et al. 2011).
The role of TSK for the anterior commissure (AC) formation in mouse brain was reported by Ito et al. (2010). Commissural formations convey neuronal information from one side of the nervous system to the other and guidance signals are required for the proper direction of the neuronal projections. The area of AC axons in TSK knockout mice was drastically reduced compared to that of WT mice. Furthermore, in the knockout mice the anterior part of the AC was never found to cross the midline. Although no molecular mechanism was established in the study, the importance of TSK signal for the AC formation was obvious. In a later study, Hossain et al. reported the axon guidance activity of TSK through the inhibition of anterior olfactory neuronal and cortical axons (Hossain et al. 2013). In this study, TSK/draxin doubly heterozygous mice was used where draxin was previously reported as an axon guidance molecule (Islam et al. 2009). The doubly heterozygous mice showed severely affected commissure formation compared to both the single gene mutants. TSK showed inhibitory effect on neurite outgrowth during in vitro cortical explant culture which suggests an axon guidance activity. Although TSK and draxin effects seemed combinatorial, TSK does not bind draxin or draxin receptor, DCC - as revealed by immunoprecipitation assays. Rather, TSK was found to bind another axon guidance molecule netrin, which shares the common receptor as draxin, DCC.
TSK was also found to be involved in the regulation of mouse hair cycle. Starting from embryonic stage E12, TSK was found to be expressed in the hair bulb, bulge cells and sebaceous gland (Niimori et al. 2012). TSK expression was also found to merge with the hair follicular stem cells. In TSK knockout mice, the progression of the hair cycle was delayed and the expression of TGF-β1 and TGF-β2 was suppressed. This study confirmed that TSK binds directly to TGF-β1, and it is considered that TSK is involved in the regulation of hair cycle progression through TGF-β signaling. Wnt, BMP and Shh signals play essential role at different stages of hair cycle (Blanpain and Fuchs 2009). TSK knockout mice showed altered expression pattern of selected molecules (Wnt5a, BMP2, Gil1 and PTCH1) at different stages indicating disturbed output from all these pathways.
More recently, TSK has been found to be involved in some other developmental processes, like somitogenesis in chicken, and wound healing and bone formation in mice. Now we will discuss briefly about these new reports.
TSK facilitates wound healing by TGF-β mediated controlling of macrophage function
Wound at the surface skin can be caused by a range of physical impact. Although the appearance of damage can be different, such as incision, crush and burn, the in vivo molecular reactions regulating wound healing are common. The wound healing in the epidermis proceeds along inflammatory phase, proliferative phase and remodeling maturation (Bielefeld et al. 2013; Eming et al. 2014). At the onset, blood clotting factors such as platelets accumulate in the wounded part due to bleeding and hemostasis. These factors control bleeding and stabilize the wound by clot formation. Neutrophils and macrophages decontaminate the wound area by killing the bacteria and scavenging the foreign debris. Macrophages secrete various enzymes and cytokines including TGF-β, which induce the formation of granulation tissue (Ahamed et al. 2008). Granulation tissue is then fibrillated with collagen reconstruction, cell rearrangement and angiogenesis. In the remodeling process, granulation tissue undergoes contraction and deposits collagen to form scar tissue (Mungunsukh and Day 2013; Hsu et al. 2014).
TSK expression was observed in the macrophages of the wound site at 2 days post-wounding (dpw) and in the epidermis, neoepidermis and granulation tissue at 7 dpw (Niimori et al. 2014). TSK knockout mice showed excess inflammation at the wound site, evident by the excess levels of TGF-β1, IL-6 and Stat3. In vitro cell induction assay on NIH3T3 cells showed that TSK inhibits myofibroblast differentiation while TGF-β1 induces this process. These results suggest that TSK works as a modulator in the wound healing process via TGF-β inhibition and is involved in maintaining signal transduction via cytokine expression. A model for TSK function during wound healing is proposed in Fig. 1.
Fig. 1.
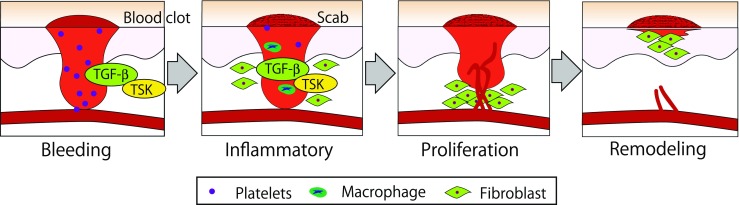
TSK is involved in wound healing. Cellular proliferation in wound skin and blood vessels are activated by cytokines, PDGF, TGF-β, FGF and EGF. TGF-β is secreted from platelets and macrophages and activate proliferation of fibroblasts and blood vessels. TSK binds TGF-β directly and modulate TGF-β activity
TSK depends on notch signaling and influences somitogenesis in chicken
Somitogenesis is a crucial event for all vertebrates that ultimately gives rise to vertebrae, skeletal muscles and some dermis. Somites, the unit paired block of paraxial mesoderm, evolve bilaterally and symmetrically along the antero-posterior body axis from the paraxial or presomitic mesoderm (PSM) (Maroto et al. 2012). Many molecular oscillators, segmentation clock and the gradients of signaling molecules play roles to develop the somites within the PSM (Aulehla and Pourquie 2006). Failure of coordination between these factors may result defective skeletal structure and other developmental disorders (Turnpenny 2008). The periodic wave of molecular oscillation driven by Notch, Wnt and FGF signaling pathways maintains the order of the somites formation (Gibb et al. 2010).
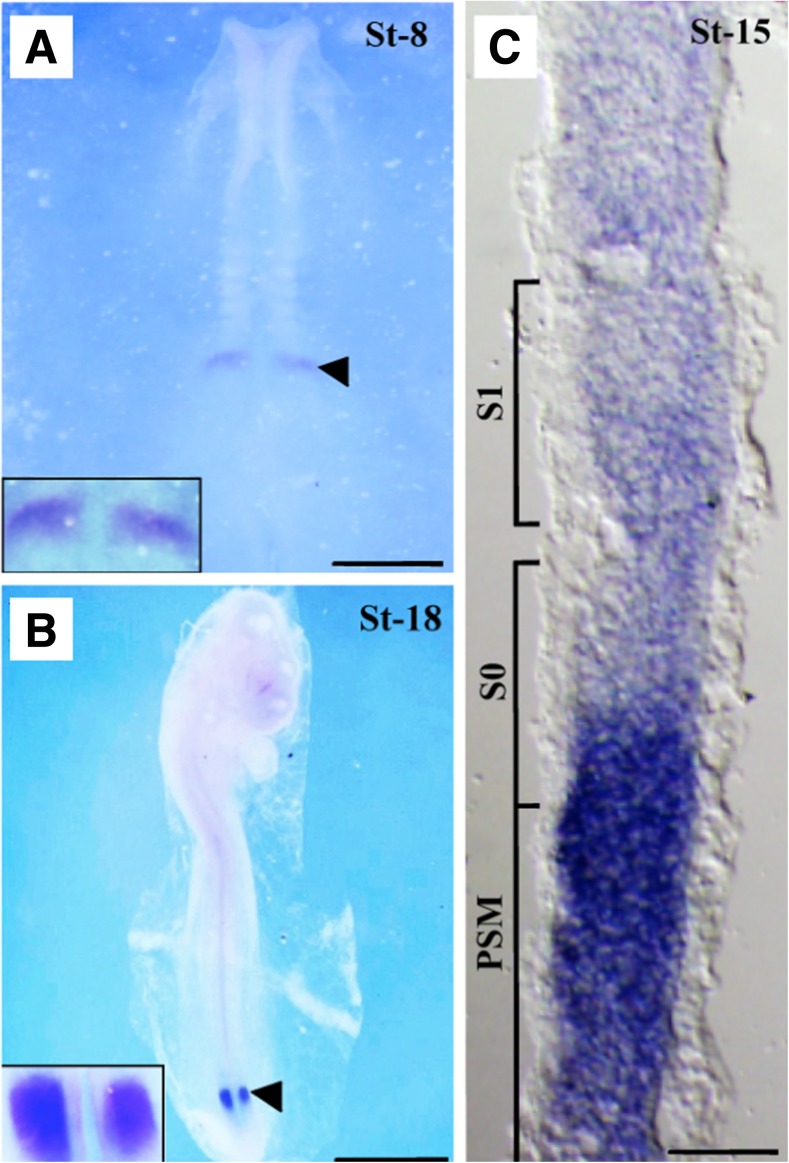
C-TSK can be detected as early as Hamburger and Hamilton stage 7 (HH7). During this stage C-TSK is detected in both newly formed first pair of somites located on either side of the neural tube (Acharjee et al. 2015). A gradient of the signals has been observed at HH15 where anterior unsegmented PSM shows the strongest. Caudal half of forming somite shows weak signal that subsequently increases along the unsegmented PSM and the newly formed somite shows gradient of relatively weaker signals (Fig. 2). In addition to the somites and PSM, C-TSK expression is extended in leg bud and wings at later developmental stages (HH18 and HH23). The comparative expression pattern analysis between C-TSK and c-hairy1, c-Notch1 and c-Delta1 demonstrate the oscillatory behavior of C-TSK during somitogenesis. c-hairy1, a Notch signaling pathway dependent molecule, is expressed in a gradient fashion during one cycle of somitogenesis. The gradient is an oscillation of strong signals from the caudal most part of PSM to weaker signals anteriorly. As the development of somites proceeds from the PSM, signal in the caudal most part of matured somites abolishes at the end. In comparison, strong expression of C-TSK is observed at the caudal end of the newly forming somites, which gradually decreases while developing into mature somites at the anterior part. c-Notch1 is expressesd weakly compared to C-TSK on the caudal region of newly formed somite and shows gradient of signals on the developing somite which strongly expanded towards the unsegmented PSM. At the end of a somite formation c-Notch1 has been expressed strongly to the anterior-posterior direction and extended beyond the PSM. In contrast C-TSK shows discrete patterns of signaling bands along the anterior-posterior direction. At the end of a complete cycle of somitogenesis C-TSK expression has not been seen at all in the newly formed somite. During a mature somite development both C-TSK and c-Delta1 are strongly expressed in the anterior PSM. However, c-Delta1 does not show any wavy expression pattern throughout the somitogenesis.
Fig. 2.
C-TSK expression patterns at different embryonic stages in the PSM. Whole mount in situ hybrdization at chicken embryonic age HH8 (a) and HH18 (b), showing C-TSK expression patterns (arrowheads) in the PSM. Box areas are magnified views of the PSM. (C) Sagittal section of HH15 PSM, showing weak expression of C-TSK in newly formed somite (S1) and gradually increased signal from the caudal half of the newly forming somite (S0) to the strongest at posterior end. Scale bars: 300 um in A, 400 um in B, 50 um in C
The pharmacological inhibition by DAPT at HH14 stage of unsegmented PSM indicates that Notch signaling is necessary for C-TSK. In situ hybridization of explant cultures shows the retention of C-TSK positive signal without somite formation and a signal gradient is observed as a downregulated express ion pattern towards the caudal end of each explant. From these analyses we conclude that, C-TSK expression is an intrinsic attribute of the unsegmented PSM and during somitogenensis, C-TSK acts as a molecular oscillator which is dependent on the Notch signaling pathway (Acharjee et al. 2015).
TSK regulates the chondrogenesis process during bone growth in mice
Vertebrate skeleton is mostly formed by endochondral ossification. This process starts with the condensation of mesenchymal progenitor cells which forms chondrocytes. The chondrocytes proliferate and mature to form hypertrophic chondrocytes, which direct mineralization and attract blood vessels, resulting in the ultimate replacement of chondrocytes with bone (Kronenberg 2003; Long and Ornitz 2013). The whole process is regulated by multiple signaling pathways, like FGFs, BMPs, Shh and Wnts (Kozhemyakina et al. 2015).
The role of TSK in bone growth has been recently reported by Yano et al. (2017). They showed that TSK is necessary for the skeletal development of mouse and the loss of TSK results decreased weight and short stature primarily because of reduced growth of long bones (Yano et al. 2017). Sagital sections of femurs showed TSK expression in almost all regions of bone from juvenile mice. However, the columnar array of chondrocytes was found to be disturbed in TSK knockout mice. The growth plate thickness was also significantly reduced in the knockout mice which is associated with the reduction of both proliferating and hypertrophic zones. The growth plate cells in knockout mice were found to exhibit lower expression of Sox9 and Runx2, while higher expression Col10a1 and Mmp13. Sox9 and Runx2 have major role in chondrogenesis, and Col10a1 and Mmp13 are mid-to-late chondrogenesis markers (Stricker et al. 2002; Zimmermann et al. 2008; Hattori et al. 2010). Altered expression pattern of these markers might accelerate the differentiation of chondrocytes to the late stages of endochondral ossification resulting in reduced number of total chondrocytes and premature ossification. The major signaling pathways for the regulation of these markers are Wnt, BMP, FGF, TGF-β and Notch (Zamurovic et al. 2004; Mirzamohammadi et al. 2016). Considering the ability of TSK to interact with all these pathways, TSK presumably interacts with one or more of these pathways to regulate chondrogenesis and later stages of endochondral ossification, and the withdrawal of TSK alters the signaling equilibrium to disturb the whole process. The effect of TSK on these marker expressions was also confirmed by TSK-siRNA transfected ATDC5 cell line - an in vitro model for studying chondrogenesis. Altogether these data suggest that TSK is necessary for the proper bone formation, and the absence of TSK compromise the development.
Conclusion
Several studies have confirmed the interactions of TSK with different signaling pathways, either by direct binding or via some intermediate molecules. These interactions mostly inhibit the signal outputs with a few instances where the outputs are enhanced. Developmental events in multiple model organisms have been reported to be affected by TSK. It is also noteworthy that the knockdown or overexpression of TSK is not lethal, which leads to the speculation that TSK has a fine tuning role for the maintenance of signal outputs from multiple pathways. We have summarized the TSK interactions in the development of different organisms in Table 1. TSK is a remarkable signaling protein considering its ability to interact with almost all the major signaling pathways. Since the combination of these signaling pathways govern many developmental events, we assume that TSK has a broader range of activity then what is revealed until now. We recommend further study on the involvement of TSK in different developmental processes to gain more insights and to find out the causes behind many developmental disorders.
Table 1.
Involvement of TSK in various developmental processes
| Effects on development | Model organism | Signaling pathway | Interacting molecule | Mode of interaction | Reference |
|---|---|---|---|---|---|
| Restriction of BMP2/4 signal spreading to induce the formation of primitive streak and Hensen’s node | Chicken | BMP ↓ | BMP4/7, Chordin | Direct binding. Ternary complex formation. | Ohta et al. (2004) |
| C-TSKA and C-TSKB induce primitive streak formation, C-TSKB induces node formation | Chicken | Vg1 ↑ | Vg1 | Direct binding | Ohta et al. (2006) |
| Inhibition of BMP activity in dorsal ectoderm and specification of neural plate formation | Xenopus | BMP ↓ | BMP4 | Direct binding | Kuriyama et al. (2006) |
| Transcriptional inhibition of BMP4 at the neural plate border | Xenopus | Notch ↑ | X-delta-1 | Direct binding | Kuriyama et al. (2006) |
| Induction of dorsal mesoderm and endoderm formation | Xenopus | BMP ↓ | BMP | Direct binding | Morris et al. (2007) |
| Inhibition of Xbra expression to distinguish endoderm and mesoderm-specific gene expression | Xenopus | FGF ↓ | FGF8b | Direct binding | Morris et al. (2007) |
| Induction of dorsal mesoderm and endoderm formation | Xenopus | Xnr2 ↑ | Xnr2 | Direct binding | Morris et al. (2007) |
| Anterior commissure formation and axon guidance activity in mouse brain | Mouse | Unidentified | Unidentified | Unidentified | Ito et al. (2010) |
| Regulation of retinal and peripheral eye development | Chicken, Mouse | Wnt ↓ | Fzd4 | Direct binding | Ohta et al. (2011) |
| Induction of the anagen phase of hair cycle | Mouse | TGF-β ↑ | TGF-β1 | Direct binding | Niimori et al. (2012) |
| Axon guidance activity for AON and cortical neurons | Mouse | Unidentified | Unidentified | Unidentified | Hossain et al. (2013) |
| Regulation of macrophage function during wound healing | Mouse | TGF-β ↓ | Unidentified | Unidentified | Niimori et al. (2014) |
| Somite formation in chicken | Chicken | Unidentified | Unidentified | Unidentified | Acharjee et al. (2015) |
| Regulation of bone growth by affecting chondrogenesis and later stages of endochondral ossification | Mouse | Unidentified | Unidentified | Unidentified | Yano et al. (2017) |
Acknowledgements
The authors thank Mitsue Kumamaru and Megumi Takiguchi.
References
- Acharjee UK, Gejima R, Felemban Athary Abdulhaleem M, Riyadh MA, Tanaka H, Ohta K. Tsukushi expression is dependent on notch signaling and oscillated in the presomitic mesoderm during chick somitogenesis. Biochem Biophys Res Commun. 2015;465:625–630. doi: 10.1016/j.bbrc.2015.08.074. [DOI] [PubMed] [Google Scholar]
- Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-beta1. Blood. 2008;112:3650–3660. doi: 10.1182/blood-2008-04-151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulehla A, Pourquie O. On periodicity and directionality of somitogenesis. Anat Embryol. 2006;211(Suppl 1):3–8. doi: 10.1007/s00429-006-0124-y. [DOI] [PubMed] [Google Scholar]
- Bell E, Munoz-Sanjuan I, Altmann CR, Vonica A, Brivanlou AH. Cell fate specification and competence by coco, a maternal BMP, TGF-β and Wnt inhibitor. Development. 2003;130:1381–1389. doi: 10.1242/dev.00344. [DOI] [PubMed] [Google Scholar]
- Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70:2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/S0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Gibb S, Maroto M, Dale JK. The segmentation clock mechanism moves up a notch. Trends Cell Biol. 2010;20:593–600. doi: 10.1016/j.tcb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AE, Morris-Triggs SA, Ruotolo BT, Robinson CV, Ohnuma S, Hyvonen M. Structural basis for the inhibition of activin signalling by follistatin. EMBO J. 2006;25:1035–1045. doi: 10.1038/sj.emboj.7601000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bosl MR, Hess A, Surmann-Schmitt C, von der Mark H, et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010;137:901–911. doi: 10.1242/dev.045203. [DOI] [PubMed] [Google Scholar]
- Hossain M, Ahmed G, Naser IB, Shinmyo Y, Ito A, Riyadh MA, Felemban A, Song X, Ohta K, Tanaka H. The combinatorial guidance activities of draxin and Tsukushi are essential for forebrain commissure formation. Dev Biol. 2013;374:58–70. doi: 10.1016/j.ydbio.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SM, Shinmyo Y, Okafuji T, Su Y, Naser IB, Ahmed G, Zhang S, Chen S, Ohta K, Kiyonari H, et al. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science. 2009;323:388–393. doi: 10.1126/science.1165187. [DOI] [PubMed] [Google Scholar]
- Ito A, Shinmyo Y, Abe T, Oshima N, Tanaka H, Ohta K. Tsukushi is required for anterior commissure formation in mouse brain. Biochem Biophys Res Commun. 2010;402:813–818. doi: 10.1016/j.bbrc.2010.10.127. [DOI] [PubMed] [Google Scholar]
- Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Lupo G, Ohta K, Ohnuma S, Harris WA, Tanaka H. Tsukushi controls ectodermal patterning and neural crest specification in Xenopus by direct regulation of BMP4 and X-delta-1 activity. Development. 2006;133:75–88. doi: 10.1242/dev.02178. [DOI] [PubMed] [Google Scholar]
- Lim W, Mayer B, Pawson T. Cell signaling: principles and mechanisms. New York: Garland Science; 2015. [Google Scholar]
- Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013;5:a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto M, Bone RA, Dale JK. Somitogenesis. Development. 2012;139:2453–2456. doi: 10.1242/dev.069310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzamohammadi F, Papaioannou G, Inloes JB, Rankin EB, Xie H, Schipani E, Orkin SH, Kobayashi T. Polycomb repressive complex 2 regulates skeletal growth by suppressing Wnt and TGF-β signalling. Nat Commun. 2016;7:12047. doi: 10.1038/ncomms12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Almeida AD, Tanaka H, Ohta K, Ohnuma S. Tsukushi modulates Xnr2, FGF and BMP signaling: regulation of Xenopus germ layer formation. PLoS One. 2007;2:e1004. doi: 10.1371/journal.pone.0001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Schier AF. Extracellular movement of signaling molecules. Dev Cell. 2011;21:145–158. doi: 10.1016/j.devcel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Yu SR, Brand M, Schier AF. Morphogen transport. Development. 2013;140:1621–1638. doi: 10.1242/dev.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungunsukh O, Day RM. Transforming growth factor-beta1 selectively inhibits hepatocyte growth factor expression via a micro-RNA-199-dependent posttranscriptional mechanism. Mol Biol Cell. 2013;24:2088–2097. doi: 10.1091/mbc.E13-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimori D, Kawano R, Felemban A, Niimori-Kita K, Tanaka H, Ihn H, Ohta K. Tsukushi controls the hair cycle by regulating TGF-β1 signaling. Dev Biol. 2012;372:81–87. doi: 10.1016/j.ydbio.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Niimori D, Kawano R, Niimori-Kita K, Ihn H, Ohta K. Tsukushi is involved in the wound healing by regulating the expression of cytokines and growth factors. J Cell Commun Signal. 2014;8:173–177. doi: 10.1007/s12079-014-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K. The role of Tsukushi as an extracellular signaling coordinator. In: Kondoh H, Kuroiwa A, editors. New principles in developmental processes. Japan: Springer; 2014. pp. 227–238. [Google Scholar]
- Ohta K, Lupo G, Kuriyama S, Keynes R, Holt CE, Harris WA, Tanaka H, Ohnuma SI. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell. 2004;7:347–358. doi: 10.1016/j.devcel.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Kuriyama S, Okafuji T, Gejima R, Ohnuma S, Tanaka H. Tsukushi cooperates with VG1 to induce primitive streak and Hensen's node formation in the chick embryo. Development. 2006;133:3777–3786. doi: 10.1242/dev.02579. [DOI] [PubMed] [Google Scholar]
- Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S, Nakagawa S, Tanaka H. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc Natl Acad Sci U S A. 2011;108:14962–14967. doi: 10.1073/pnas.1100513108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker S, Fundele R, Vortkamp A, Mundlos S. Role of Runx genes in chondrocyte differentiation. Dev Biol. 2002;245:95–108. doi: 10.1006/dbio.2002.0640. [DOI] [PubMed] [Google Scholar]
- Turnpenny PD. Defective somitogenesis and abnormal vertebral segmentation in man. Adv Exp Med Biol. 2008;638:164–189. doi: 10.1007/978-0-387-09606-3_9. [DOI] [PubMed] [Google Scholar]
- Yano K, Washio K, Tsumanuma Y, Yamato M, Ohta K, Okano T, Izumi Y. The role of Tsukushi (TSK), a small leucine-rich repeat proteoglycan, in bone growth. Regenerative Therapy. 2017;7:98–107. doi: 10.1016/j.reth.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamurovic N, Cappellen D, Rohner D, Susa M. Coordinated activation of notch, Wnt, and transforming growth factor-beta signaling pathways in bone morphogenic protein 2-induced osteogenesis. Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. J Biol Chem. 2004;279:37704–37715. doi: 10.1074/jbc.M403813200. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Boeuf S, Dickhut A, Boehmer S, Olek S, Richter W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis Rheum. 2008;58:2743–2753. doi: 10.1002/art.23736. [DOI] [PubMed] [Google Scholar]