Abstract
A member of the lectin family, galectin-3 is a 250 amino-acid protein that contains a C-terminus carbohydrate recognition domain (CRD) that recognizes β-galactosides. Considered to have certain common properties associated with matricellular proteins, galectin-3 is expressed in the dermis and epidermis in healthy skin and is upregulated in skin healing, peaking at day 1 post wounding in mice. Galectin-3 has been implicated in several processes central to the wound healing response, specifically in the regulation of inflammation, macrophage polarization, angiogenesis, fibroblast to myofibroblast transition and re-epithelialization. However, it appears that many of the effects of Galectin-3 are highly tissue specific and context dependent. Genetic deletion of galectin-3 shows different effects in skin compared to lung, heart, and kidney remodeling. In this review, we will compare galectin-3 functions in these tissues. Furthermore, we will discuss, based on its identified regulation of cell processes, whether in an exogenous form, galectin-3 could represent a novel therapeutic for impaired skin healing.
Keywords: Galectin-3, Matricellular protein, Skin healing, Inflammation, Re-epithelialization
Introduction
Matricellular proteins are non-structural components of the extracellular matrix that become upregulated during wound healing and pathological processes (Midwood et al. 2004; Walker et al. 2015). During the wound healing process, they act spatially and temporally to control specific cell behaviours. Galectin-3, posited to possess certain properties of a matricellular protein through its localization in the extracellular matrix (ECM) (Ochieng et al. 2002; Melo et al. 2011), is also established to have several intracellular functions (Funasaka et al. 2014; Honig et al. 2015; Fritsch et al. 2016). Galectin-3 is implicated in several inflammatory and immunomodulatory processes, making it an ideal candidate for treatment of chronic skin wounds, where the lesions remain in a toxic pro-inflammatory state (Elliott et al. 2015). Galectin-3 has been shown to influence monocyte and macrophage migration (Sano et al. 2000), increase clearance of neutrophils (Karlsson et al. 2009), and regulate alternative macrophage polarization (MacKinnon et al. 2008), all processes that can contribute to modulating the inflammatory response. With demonstrated regulation of re-epithelialization (Cao et al. 2002; Saravanan et al. 2009; Panjwani 2014) and contrasting data on its modulation of myofibroblast differentiation (Okamura et al. 2011; Mackinnon et al. 2012; Walker et al. 2016), it could be hypothesized that the use of galectin-3 would provide an effective strategy in promoting healing by stimulating the proliferative phase of healing. The focus of this review is to discuss the role of galectin-3 in various models of wound healing and fibrosis, and whether galectin-3 could potentially be utilized for resolution of impaired skin healing.
Galectin-3 protein structure
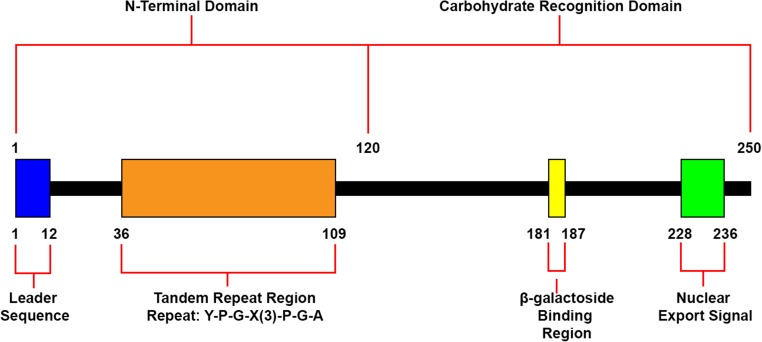
Galectin-3 is a protein consisting of 250 amino acids, separated into two distinct domains (Fig. 1) (Robertson et al. 1990). The carbohydrate recognition domain (CRD) of this protein accounts for approximately 130 amino acids and is globular in structure (Dumic et al. 2006). The CRD domain contains S-lectin motifs that provide the protein with the ability to bind β-galactosides, a property shared by all proteins in the Galectin family (Cherayil et al. 1990; Barondes 1994), as well as a nuclear export signal (Nakahara et al. 2006). In addition to its CRD, galectin-3 contains an amino terminal domain, which spans approximately 120 amino acids and contains a highly conserved tandem repeat rich in proline, glycine and tyrosine (Seetharaman et al. 1998; Dumic et al. 2006). The N-terminus contains a 12 amino acid leader sequence that is required for galectin-3 secretion (Dumic et al. 2006). Within this leader sequence serine can be phosphorylated, a process which significantly reduces binding to two of its ligands, laminin and mucin, and may act as an on/off switch for its ability to bind to sugars (Mazurek et al. 2000). The N-terminal domain also enables the formation of oligomers and is required for full biological function of the protein, including its role in modulating cell adhesion and inducing intracellular signalling (Seetharaman et al. 1998; Ahmad et al. 2004). Galectin-3 has been detected within cells, localized in the nucleus and cytoplasm, and has also been described outside of the cell, despite its lack of a known transmembrane domain and sequence (Frigeri and Liu 1992; Dumic et al. 2006). It has been found to interact with a variety of wound healing cell types including monocytes, macrophages, neutrophils, keratinocytes, and fibroblasts (Sano et al. 2000; Dvorankova et al. 2011; Liu et al. 2012).
Fig. 1.
Domains and structures of recombinant human galectin-3. Human recombinant galectin-3 is a protein consisting of 250 amino acids. It features a 120 amino acid N-terminal region that contains a leader sequence and a tandem repeat region rich in proline, glycine and arginine. It also comprises of a CRD containing a β-galactoside binding region and a sequence required for nuclear export
Role in inflammation
Galectin-3 has been demonstrated to influence a variety of processes associated with inflammation through its interaction with various cell types including neutrophils, monocytes, and macrophages (Yamaoka et al. 1995; Rabinovich et al. 2002; Karlsson et al. 2009; Danella Polli et al. 2013). In the initial stages of inflammation, neutrophils are recruited to the wound to eliminate foreign particles and bacteria. In vitro studies have shown that recombinant human galectin-3 can activate neutrophils in a dose-dependent manner, through a process involving its CRD (Yamaoka et al. 1995). A study investigating NADPH oxidase activity revealed that galectin-3 activated exudate neutrophils, with increased activity corresponding to increased surface-bound protein, while activity was unaltered in peripheral neutrophils (Karlsson et al. 1998). In addition to neutrophil activation, galectin-3 has been shown to facilitate neutrophil adhesion to laminin in vitro and has been implicated in the recruitment of neutrophils in a murine model of cutaneous infection (Kuwabara and Liu 1996; Bhaumik et al. 2013).
The inflammatory phase of healing also involves the recruitment of monocytes to the wound, which differentiate into macrophages of varying phenotypes that play distinct roles in inflammatory processes (Brancato and Albina 2011). Galectin-3 induces monocyte migration in vitro, stimulating chemotaxis at high concentrations and chemokinesis at lower concentrations. A migratory effect from galectin-3 is also observed in macrophages (Sano et al. 2000). Migration in both monocytes and macrophages is increased in the presence of fibronectin, suggesting that galectin-3 may mediate linkage of these cells to fibronectin (Danella Polli et al. 2013). One role of the macrophage in inflammation is to rid the wound of neutrophils, ingesting them and inducing their apoptosis (Brancato and Albina 2011). In vitro studies suggest that galectin-3 can influence this process as the addition of exogenous galectin-3 increases apoptotic neutrophil uptake in macrophages. It has also been postulated that galectin-3 acts as an opsonin, linking the phagocytic macrophages to the neutrophils (Karlsson et al. 2009).
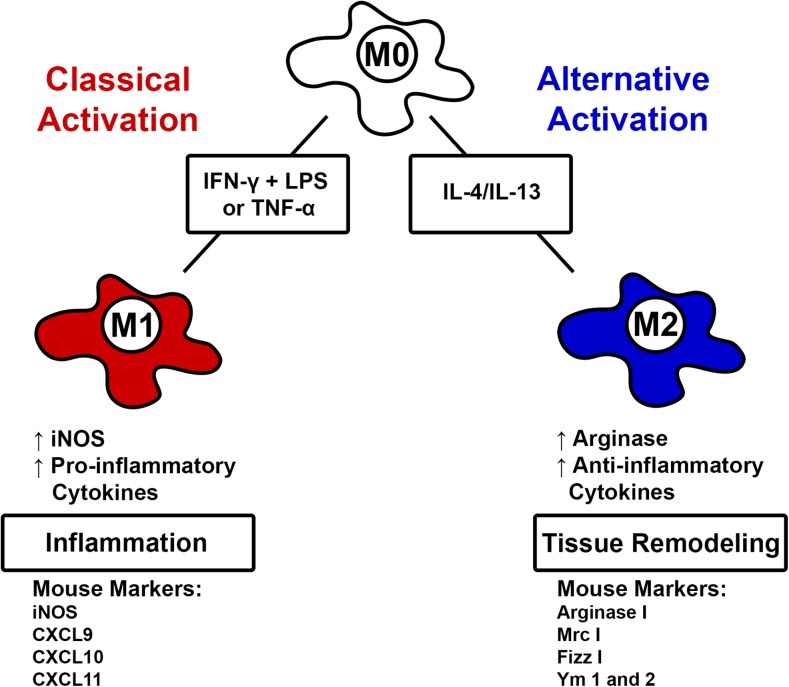
Galectin-3 has been linked to increasing the ratio of M2 alternatively activated macrophages to M1 classically activated macrophages (MacKinnon et al. 2008). These M2 macrophages are anti-inflammatory and promote tissue repair, whereas their M1 counterparts are pro-inflammatory. A summary of macrophage activation and polarization is shown in Fig. 2. MacKinnon et al. investigated the effect of galectin-3 on macrophage activation in bone marrow derived macrophages in vitro and in resident lung and recruited peritoneal macrophages in vivo (MacKinnon et al. 2008). Bone marrow derived macrophages from galectin-3 knockout and wild-type mice showed similar release of cytokines TNFα and interleukin-6 (IL-6) in response to stimulation with lipopolysaccharide (LPS) or interferon-γ (IFNγ). Interestingly, in all macrophages derived from galectin-3 deficient mice, IL-4/IL-13-induced M2 macrophage polarization was inhibited, suggesting that galectin-3 is involved in the regulation of alternative macrophage activation (MacKinnon et al. 2008). Moreover, this research showed following treatment with IL-4, the knockout macrophages showed significantly lower mRNA levels of the mouse M2 macrophage markers, mannose receptor, arginase I, FIZZ-1 and Ym-1. Thus, galectin-3 appears to be an extremely important modulator of both pro- and anti-inflammatory cellular processes.
Fig. 2.
Macrophage activation and polarization. Monocytes can undergo classical activation in the presence of interferon gamma (IFN-γ) and lipopolysaccharides (LPS) or TNF-α into M1-polarized macrophages, which are associated with inflammation. M1 macrophages produce inducible nitric oxide synthase (iNOS) as well as pro-inflammatory cytokines. In mice, markers of M1 macrophages include iNOS, chemokine ligand 9 (CXCL9), CXCL 10, and CXCL11. Monocytes can undergo alternative activation through stimulation with IL-4 or IL-13 into M2-polarized macrophages. M2 are associated with tissue remodeling and secrete arginase I and anti-inflammatory cytokines. M2 markers in mice include arginase I, Mrc I, Fizz I, Ym1, and Ym 2
Role in angiogenesis
Galectin-3 has been shown to induce angiogenesis both in vitro and in vivo. In vitro, the protein stimulated capillary tube formation of human umbilical cord endothelial cells grown on a matrigel, while in vivo, a galectin-3-loaded matrigel was able to induce angiogenesis in nude mice. Both processes relied on its CRD (Nangia-Makker et al. 2000). Markowska et al. later proposed that galectin-3 modulated VEGF and bFGFmediated angiogenesis by activating focal adhesion kinase-mediated signalling pathways that modulate endothelial cell migration during this process (Markowska et al. 2010). The protein has also been linked to angiogenesis and the migration of endothelial cells through integrin-linked kinase signalling (Vemuganti et al. 2013). Galectin-3 was also shown to bind to vascular endothelial growth factor receptor 2 (VEGFR2), promoting its phosphorylation and preventing its internalization, leading to an increased angiogenic response of human umbilical cord endothelial cells to VEGFA in vitro (Markowska et al. 2011).
In combination with galectin-1, galectin-3 can activate and prevent the internalization of VEGFR1, another process that enhances angiogenesis (Vemuganti et al. 2013). Despite these findings, we have recently demonstrated that during excisional wound repair in skin, galectin-3 deficient mice show no difference in vascular density or expression of angiogenic markers relative to wild-type mice (Walker et al. 2016). These conflicting findings indicate that the role of galectin-3 in angiogenesis is likely tissue and context-dependant (Walker et al. 2015).
Role in re-epithelialization
The first association of galectin-3 with re-epithelialization came from Kasper and Hughes who noted the surface expression of galectin-3 in Type I and II alveolar epithelial cells in a model of irradiation-induced lung inflammation and repair (Kasper and Hughes 1996). In a model of corneal wound healing, galectin-3 deficient mice were found to exhibit reduced re-epithelialization rates relative to wild-type counterparts. Interestingly, galectin-3 did not alter proliferation rates of epithelial cells and elevated levels of galectin-3 were detected in the migrating epithelial front following injury, suggesting the protein promotes epithelial cell migration (Cao et al. 2002). This was supported by later studies showing that galectin-3 promotes cell scattering, lamellipodia formation, and motility in human corneal epithelial cells (Saravanan et al. 2009). Furthermore, studies in mouse corneas showed that galectin-3 knockout mice exhibit impaired re-epithelialization (Cao et al. 2002). The effect of the addition of exogenous galectin-3 has also been investigated in models of murine corneal healing, where the addition of exogenous galectin-3 increased re-epithelialization in wild type (WT) mice, but not galectin-3 deficient mice (Cao et al. 2002). The increase in re-epithelialization in WT mice was attributed to the modulation of galectin-7 by exogenous galectin-3, as galectin-7 was found to accelerate re-epithelialization in galectin-3 knockout mice and because mouse embryonic fibroblasts from galectin-3 knockout mice showed reduced levels of galectin-7 (Cao et al. 2002). Studies of epithelial wounds in monkey corneal explants also demonstrated enhanced re-epithelization when recombinant human galectin-3 was added exogenously to the media (Fujii et al. 2015).
Consistent with studies in the cornea, studies in skin have revealed that keratinocytes from galectin-3 knockout mice exhibit a migratory defect (Liu et al. 2012), and that re-epithelialization is delayed in galectin-3 deficient mice (Liu et al. 2012; Walker et al. 2016). However, in skin this defect was attributed to deficient EGFR endocytosis and recycling, which is controlled by cytosolic galectin-3 binding to ALG-2 interacting protein X (ALIX) (Liu et al. 2012). Additionally, there were no differences between levels of galectin-7 in wound tissue from WT and knockout mice at day 7 post injury, during which re-epithelialization was impaired (Walker et al. 2016). Therefore, as is evident in the regulation of inflammatory processes, the influence of galectin-3 deletion in skin appears to contrast with other tissues.
Galectin-3 in fibrosis
Galectin-3 has been implicated in different roles during fibrosis, a process which, much like chronic wound healing, involves excessive inflammation initially. However, this phase is overcome with inflammation yielding to myofibroblast differentiation, excessive ECM synthesis resulting in fibrotic tissue formation (Li et al. 2014). Thus, the mechanisms involved in fibrosis have been suggested to be a potential guide for the development of therapeutics for chronic wound healing, where a fibrotic response is absent (Elliott and Hamilton 2011).
Galectin-3 is associated with fibrosis in several organs, including heart (Gonzalez et al. 2014), lung (Mackinnon et al. 2012) and kidney (Chen and Kuo 2016), although the exact effect of the galectin-3 may differ in each tissue. Galectin-3 knockout mice showed increased fibrosis in a unilateral ureteral obstruction induced renal fibrosis model (Okamura et al. 2011), but decreased fibrosis in a bleomycin induced lung fibrosis model (Mackinnon et al. 2012). However, both models were associated with reduced myofibroblast number in the knockout mice, as determined by α-SMA expression (Okamura et al. 2011; Mackinnon et al. 2012). Interestingly in excisional skin healing, we have shown galectin-3 knockout mice show similar levels of myofibroblasts in vivo and the cells show the same differentiation ability in vitro as their wild type counterparts (Walker et al. 2016).
It has also been shown that galectin-3 can stimulate the proliferation, differentiation, and collagen synthesis of pulmonary adventitial fibroblasts in hypoxia-induced pulmonary arterial hypertension (Luo et al. 2017). Indeed, galectin-3 levels are predictive of mortality in pulmonary hypertension (Mazurek et al. 2017). Galectin-3 as a recombinant protein has also been shown to stimulate the proliferation, differentiation, collagen deposition, and Nox4 expression of cardiac fibroblasts (He et al. 2017). However, other research using the knockout suggests that galectin-3 is not a critical modulator of cardiac fibrosis but may delay the subsequent hypertrophic response (Frunza et al. 2016).
Even though there are varied results on the role of galectin-3 in fibrosis, its role in activating fibroblasts into myofibroblasts could be important as this differentiation is reduced in a chronic wound (Elliott and Hamilton 2011; Elliott et al. 2015) and its potential to increase ECM expression needs to be investigated in the context of a chronic wound.
Future directions: Galectin-3 as a therapeutic in impaired skin healing
Chronic skin wounds are subdivided into three categories: pressure sores, venous ulcers and diabetic foot ulcers (Elliott and Hamilton 2011). In the first stage of treatment, debridement, achieved enzymatically, mechanically, or using insect larvae (Gethin et al. 2015), is performed to remove necrotic tissue and excessive inflammatory-cell infiltrate, which if allowed to persist, leads to an extremely toxic wound environment (Falanga 1992; Elliott et al. 2015). While debridement by itself can stimulate repair mechanisms (Steed et al. 1996), it is not sufficient to cause wound resolution and a plethora of advanced wound care products have been developed, ranging from living-tissue equivalents (e.g., Dermagraft® (Bowering 1998), Apligraf® (Streit and Braathen 2000), Graftskin (Veves et al. 2001)) to growth-factor delivery (Robson 1997; Goldman 2004). Despite weak evidence of clinical efficacy (Chaby et al. 2007), synthetic dressings (i.e. films, foams, hydrocolloids, and hydrogels) still dominate the marketplace – accounting for 46% of the wound-care market. The similarly low clinical efficacy of growth factor technology was unforeseen and surprising based on their described functions (Elliott and Hamilton 2011). Alternative strategies to induce wound closure are clearly needed. As described above, galectin-3 has been implicated in several processes associated with wound healing, including modulating inflammation and contributing to re-epithelialization, making it a possible adjunct therapy for wound healing (Cao et al. 2002; MacKinnon et al. 2008; Danella Polli et al. 2013). In this review, we will focus on these processes, both of which are significantly impaired in human chronic skin wounds.
Of major significance, we were the first group to investigate galectin-3 expression surrounding and within human chronic wound tissue (Pepe et al. 2014). In healthy skin, galectin-3 is strongly expressed in the epidermis and the vasculature, but at the edge of chronic wounds, this expression is diminished. Based on the defined role of galectin-3 on regulation of inflammatory processes, this data was surprising, as it suggests that galectin-3 is not required for inflammatory cell recruitment in this particular pathology. The inflammatory cell profile in chronic wounds is associated with variable macrophage phenotypes and neutrophil infiltration, which is dependent on the individual wound examined (Walker et al. 2016).
Overall, our data shows that mRNA levels for galectin-3 are low in the wound bed compared to non-involved tissue in the same limb. Moreover, localization of M1 and M2 macrophage markers was independent of galectin-3 expression, and negatively correlated with neutrophil infiltration. Given the identified roles of galectin-3 in neutrophil recruitment (Bhaumik et al. 2013), monocyte migration and chemoattraction (Sano et al. 2000; Danella Polli et al. 2013), and macrophage polarization (MacKinnon et al. 2008), it is possible that galectin-3 is not sufficient to stimulate a pro-regenerative phenotype in chronic wounds or the expression levels of the protein are too low. In dermal fibroblasts in vitro, galectin-3 is downregulated by both TGFβ1 and TNFα (Walker et al. 2016), both of which are abundant in chronic wounds. If these growth factors act in a similar manner on inflammatory cells, it provides a potential explanation for the lack of galectin-3 mRNA in the wound bed tissue, combined with a reduced expression by mesenchymal cells. As endogenous expression of galectin-3 surrounding the chronic wound does not appear to influence the inflammatory processes, it provides a strong rationale to assess whether addition of exogenous galectin-3 could reset the inflammatory process and push the wound phenotype towards a pro-regenerative M2 polarization.
Galectin-3 has also been shown to play a significant role in re-epithelialization; studies have shown that although galectin-3 knock-out mice do not exhibit altered wound closure kinetics (Walker et al. 2016), models of both corneal and skin wound healing reveal that galectin-3 deficient mice exhibit delayed re-epithelialization (Cao et al. 2002; Liu et al. 2012; Walker et al. 2016). Despite unaltered wound closure kinetics in galectin-3 knockout mice, studies in mouse and monkey corneas reveal that the addition of exogenous galectin-3 enhanced wound re-epithelialization in WT mice (Cao et al. 2002; Fujii et al. 2015). Whether exogenous galectin-3 has a similar effect in WT mice on re-epithelialization during skin is unknown. However, it must be considered that effects of genetic deletion of galectin-3 does not eliminate the possibility that protein could influence cell behavior when added exogenously, and whether this would extend to a model of impaired wound healing, although intriguing, has not been investigated.
Summary
Galectin-3 is involved in a great variety of additional interactions that also seem to be highly context and localization dependent. Research utilizing the knockout mouse has shown that the effects of galectin-3 on cell behavior are very much dependent on the organ or tissue being studied (Table 1). While genetic deletion of galectin-3 has given important insights into the role of the protein in several cellular processes, less information is available on the response of cells to exogenous galectin-3. Future studies should focus on this area, particularly for potential development of therapeutics for impaired skin healing.
Table 1.
Influence of Galectin-3 deletion on tissue remodeling and pathology in different organ systems
| Tissue | Knockout Phenotype | Model | References |
|---|---|---|---|
| Skin | Migratory defect in keratinocytes | Single-cell migration assay | (Liu et al. 2012) |
| Impaired re-epithelialization | 3 mm full-thickness skin wounds; 6 mm full-thickness skin wounds |
(Liu et al. 2012; Walker et al. 2015) | |
| Cornea | Impaired re-epithelialization | Excimer laser ablations, alkali-burn wounds | (Cao et al. 2002) |
| Heart | Increased sized of myocardial infarction Reduced fibrosis, collagen deposition and macrophage infiltration at site of myocardial infarction |
Permanent coronary artery ligature | (Gonzalez et al. 2014) |
| Accelerated cardiac hypertrophy | Transverse aortic constriction | (Frunza et al. 2016) | |
| Lung | Reduced fibrosis, collagen levels and levels of myofibroblasts | Bleomycin induced lung fibrosis | (Mackinnon et al. 2012) |
| Kidney | Increased fibrosis and interstitial collagen levels Decreased renal tubular integrity Reduced levels of interstitial myofibroblasts Disorganized interstitial matrix pattern |
Unilateral ureteral obstruction | (Okamura et al. 2011) |
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (Operating grant RN247506) to D. W. Hamilton.
References
- Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279(12):10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- Barondes SH. Galectins: a family of β-Galactoside-binding Lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Bhaumik P, St-Pierre G, Milot V, St-Pierre C, Sato S. Galectin-3 facilitates neutrophil recruitment as an innate immune response to a parasitic protozoa cutaneous infection. J Immunol. 2013;190(2):630–640. doi: 10.4049/jimmunol.1103197. [DOI] [PubMed] [Google Scholar]
- Bowering CK. Dermagraft in the treatment of diabetic foot ulcers. J Cutan Med Surg. 1998;3(Suppl 1):29–32. [PubMed] [Google Scholar]
- Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178(1):19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277(44):42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Chaby G, Senet P, Vaneau M, Martel P, Guillaume JC, Meaume S, Teot L, Debure C, Dompmartin A, Bachelet H, et al. Dressings for acute and chronic wounds: a systematic review. Arch Dermatol. 2007;143(10):1297–1304. doi: 10.1001/archderm.143.10.1297. [DOI] [PubMed] [Google Scholar]
- Chen SC, Kuo PL. The role of Galectin-3 in the kidneys. Int J Mol Sci. 2016;17(4):565. doi: 10.3390/ijms17040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil BJ, Chaitovitz S, Wong C, Pillai S. Molecular cloning of a human macrophage lectin specific for galactose. Proc Natl Acad Sci U S A. 1990;87(18):7324–7328. doi: 10.1073/pnas.87.18.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danella Polli C, Alves Toledo K, Franco LH, Sammartino Mariano V, de Oliveira LL, Soares Bernardes E, Roque-Barreira MC, Pereira-da-Silva G. Monocyte migration driven by Galectin-3 occurs through distinct mechanisms involving selective interactions with the extracellular matrix. ISRN Inflamm. 2013;2013:259256. doi: 10.1155/2013/259256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760(4):616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Dvorankova B, Szabo P, Lacina L, Gal P, Uhrova J, Zima T, Kaltner H, Andre S, Gabius HJ, Sykova E, et al. Human galectins induce conversion of dermal fibroblasts into myofibroblasts and production of extracellular matrix: potential application in tissue engineering and wound repair. Cells Tissues Organs. 2011;194(6):469–480. doi: 10.1159/000324864. [DOI] [PubMed] [Google Scholar]
- Elliott CG, Forbes TL, Leask A, Hamilton DW. Inflammatory microenvironment and tumor necrosis factor alpha as modulators of periostin and CCN2 expression in human non-healing skin wounds and dermal fibroblasts. Matrix Biol. 2015;43:71–84. doi: 10.1016/j.matbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Elliott CG, Hamilton DW. Deconstructing fibrosis research: do pro-fibrotic signals point the way for chronic dermal wound regeneration? J Cell Commun Signal. 2011;5(4):301–315. doi: 10.1007/s12079-011-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V. Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol. 1992;19(11):667–672. doi: 10.1111/j.1346-8138.1992.tb03756.x. [DOI] [PubMed] [Google Scholar]
- Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148(3):861–867. [PubMed] [Google Scholar]
- Fritsch K, Mernberger M, Nist A, Stiewe T, Brehm A, Jacob R. Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer. 2016;16:502. doi: 10.1186/s12885-016-2546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frunza O, Russo I, Saxena A, Shinde AV, Humeres C, Hanif W, Rai V, Su Y, Frangogiannis NG. Myocardial Galectin-3 expression is associated with remodeling of the pressure-overloaded heart and may delay the hypertrophic response without affecting survival, dysfunction, and cardiac fibrosis. Am J Pathol. 2016;186(5):1114–1127. doi: 10.1016/j.ajpath.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii A, Shearer TR, Azuma M. Galectin-3 enhances extracellular matrix associations and wound healing in monkey corneal epithelium. Exp Eye Res. 2015;137:71–78. doi: 10.1016/j.exer.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funasaka T, Raz A, Nangia-Makker P. Nuclear transport of galectin-3 and its therapeutic implications. Semin Cancer Biol. 2014;27:30–38. doi: 10.1016/j.semcancer.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gethin G, Cowman S, Kolbach DN (2015) Debridement for venous leg ulcers. Cochrane Database Syst Rev(9): CD008599 [DOI] [PMC free article] [PubMed]
- Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care. 2004;17(1):24–35. doi: 10.1097/00129334-200401000-00012. [DOI] [PubMed] [Google Scholar]
- Gonzalez GE, Cassaglia P, Noli Truant S, Fernandez MM, Wilensky L, Volberg V, Malchiodi EL, Morales C, Gelpi RJ. Galectin-3 is essential for early wound healing and ventricular remodeling after myocardial infarction in mice. Int J Cardiol. 2014;176(3):1423–1425. doi: 10.1016/j.ijcard.2014.08.011. [DOI] [PubMed] [Google Scholar]
- He J, Li X, Luo H, Li T, Zhao L, Qi Q, Liu Y, Yu Z. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens. 2017;11(5):275–289. doi: 10.1016/j.jash.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Honig E, Schneider K, Jacob R. Recycling of galectin-3 in epithelial cells. Eur J Cell Biol. 2015;94(7–9):309–315. doi: 10.1016/j.ejcb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Christenson K, Matlak M, Bjorstad A, Brown KL, Telemo E, Salomonsson E, Leffler H, Bylund J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology. 2009;19(1):16–20. doi: 10.1093/glycob/cwn104. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91(9):3430–3438. [PubMed] [Google Scholar]
- Kasper M, Hughes RC. Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol. 1996;179(3):309–316. doi: 10.1002/(SICI)1096-9896(199607)179:3<309::AID-PATH572>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156(10):3939–3944. [PubMed] [Google Scholar]
- Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther. 2014;351(2):336–343. doi: 10.1124/jpet.114.218370. [DOI] [PubMed] [Google Scholar]
- Liu W, Hsu DK, Chen HY, Yang RY, Carraway KL, 3rd, Isseroff RR, Liu FT. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol. 2012;132(12):2828–2837. doi: 10.1038/jid.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Liu B, Zhao L, He J, Li T, Zha L, Li X, Qi Q, Liu Y, Yu Z. Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension. J Am Soc Hypertens. 2017;11(10):673–683. doi: 10.1016/j.jash.2017.07.009. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180(4):2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185(5):537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286(34):29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010;207(9):1981–1993. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. J Biol Chem. 2000;275(46):36311–36315. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- Mazurek JA, Horne BD, Saeed W, Sardar MR, Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ. 2017;26(11):1208–1215. doi: 10.1016/j.hlc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Melo FH, Butera D, Junqueira Mde S, Hsu DK, da Silva AM, Liu FT, Santos MF, Chammas R. The promigratory activity of the matricellular protein galectin-3 depends on the activation of PI-3 kinase. PLoS One. 2011;6(12):e29313. doi: 10.1371/journal.pone.0029313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Res. 2006;66(20):9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2002;19(7–9):527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Okamura DM, Pasichnyk K, Lopez-Guisa JM, Collins S, Hsu DK, Liu FT, Eddy AA. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol. 2011;300(1):F245–F253. doi: 10.1152/ajprenal.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani N. Role of galectins in re-epithelialization of wounds. Ann Transl Med. 2014;2(9):89. doi: 10.3978/j.issn.2305-5839.2014.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe D, Elliott CG, Forbes TL, Hamilton DW. Detection of galectin-3 and localization of advanced glycation end products (AGE) in human chronic skin wounds. Histol Histopathol. 2014;29(2):251–258. doi: 10.14670/HH-29.251. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Rubinstein N, Toscano MA. Role of galectins in inflammatory and immunomodulatory processes. Biochim Biophys Acta. 2002;1572(2–3):274–284. doi: 10.1016/S0304-4165(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Robertson MW, Albrandt K, Keller D, Liu FT. Human Ige-binding protein - a soluble Lectin exhibiting a highly conserved interspecies sequence and differential recognition of Ige Glycoforms. Biochemistry. 1990;29(35):8093–8100. doi: 10.1021/bi00487a015. [DOI] [PubMed] [Google Scholar]
- Robson MC. The role of growth factors in the healing of chronic wounds. Wound Repair Regen. 1997;5(1):12–17. doi: 10.1046/j.1524-475X.1997.50106.x. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165(4):2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Liu FT, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J Cell Sci. 2009;122(Pt 20):3684–3693. doi: 10.1242/jcs.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-a resolution. J Biol Chem. 1998;273(21):13047–13052. doi: 10.1074/jbc.273.21.13047. [DOI] [PubMed] [Google Scholar]
- Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic ulcer study group. J Am Coll Surg. 1996;183(1):61–64. [PubMed] [Google Scholar]
- Streit M, Braathen LR. Apligraf--a living human skin equivalent for the treatment of chronic wounds. Int J Artif Organs. 2000;23(12):831–833. [PubMed] [Google Scholar]
- Veves A, Falanga V, Armstrong DG, Sabolinski ML, S. Apligraf Diabetic Foot Ulcer Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- Walker JT, Elliott CG, Forbes TL, Hamilton DW. Genetic deletion of Galectin-3 does not impair full-thickness Excisional skin healing. J Invest Dermatol. 2016;136(5):1042–1050. doi: 10.1016/j.jid.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Walker JT, Kim S, Michelsons S, Creber K, Elliott CG, Leask A, Hamilton DW. Cell-matrix interactions governing skin repair: matricellular proteins as diverse modulators of cell function. Res Rep Biochem. 2015;5:73–88. [Google Scholar]
- Wesley UV, Vemuganti R, Ayvaci ER, Dempsey RJ. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 2013;1496:1–9. doi: 10.1016/j.brainres.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995;154(7):3479–3487. [PubMed] [Google Scholar]