Abstract
Across the years the CCNs have been increasingly implicated in the development of obesity, diabetes and its complications. Evidence for this is currently derived from their dysregulation in key metabolic pathological states in humans, animal and in vitro models, and also pre-clinical effects of their bioactivities. CCN2 is the best studied in this disease process and the other CCNs are yet to be better defined. Key steps where CCNs may play a pathogenic metabolic role include: (i) obesity and insulin resistance, where CCN2 inhibits fat cell differentiation in vitro and CCN3 may induce obesity and insulin resistance; (ii) elevated blood glucose levels to diabetes mellitus onset, where CCN2 may contribute to pancreatic beta cell and islet function; and (iii) in diabetes complications, such as nephropathy, retinopathy, liver disease (NAFLD/NASH), CVD and diabetes with heart failure. In contrast, CCN1, CCN2 and possibly CCN3, may have a reparative role in wound healing in diabetes, and CCN2 in islet cell development. In terms of CCN2 regulation by a diabetes metabolic environment and related mechanisms, the author’s laboratory and others have progressively shown that advanced glycation-end products, protein kinase C isoforms, saturated fatty acids, reactive oxygen species and haemodynamic factors upregulate CCN2 in relevant cell and animal systems. Recent data has suggested that CCN2, CCN3 and CCN6 may affect energy homeostasis including in regulating glycolysis and mitochondrial function. This paper will address the current data implicating CCNs in diabetes and its complications, focusing on recent aspects with translational clinical relevance and future directions.
Keywords: CCNs, Diabetes, Obesity, Insulin resistance, Complications
The metabolic conditions of overweight and obesity collectively affect the majority of adults in developed societies (Twigg and Wong 2015). In addition, those states characterised by hyperglycemia, namely pre-diabetes and diabetes mellitus are becoming increasingly common globally, being driven mainly by combinations of: an ageing population, sedentary lifestyle with obesity, especially in ethnically susceptible groups. The major concern with diabetes is that it commonly causes premature mortality and morbidity due to the microvascular and macrovascular complications of diabetes (Twigg and Wong 2015).
The CCN family of proteins have diverse roles in regulating cellular function and tissue pathology (Brigstock 2003). This article will address current knowledge in the regulation and bioactivity of this family of proteins and genes, sequentially in the disease states of obesity, diabetes and diabetes complications settings.
CCNs in overweight, obesity and insulin resistance
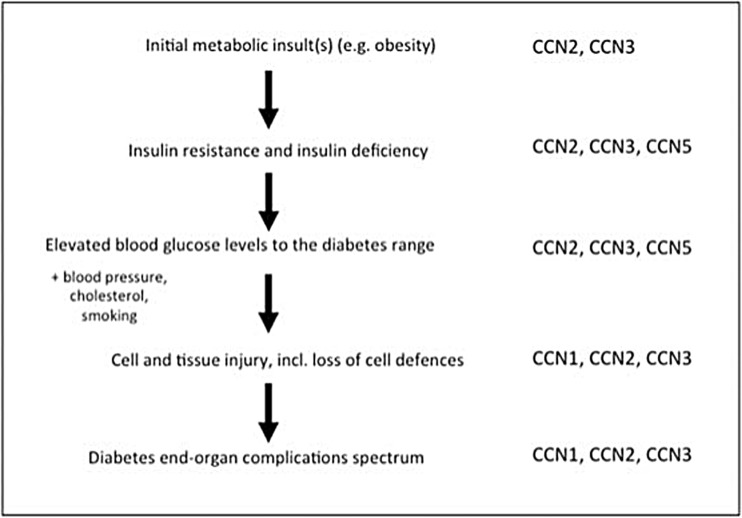
The current high prevalence of overweight and obesity in humans is thought to be related to an energy imbalance, where excessive calories are consumed relative to those expended, resulting in excess caloric storage mainly as lipid in adipose tissue. One evolving concept in that environment is that people vary in the degree to which their bodies are able to store the excess calories as fat and expend the calories through thermogenesis. Indeed, it may be that in people whose fat tissue is less prone to result in thermogenesis and less prone to store energy in subcutaneous fat, they are then at increased risk of low grade systemic inflammation and insulin resistance, in many cases leading to diabetes mellitus (Fig. 1).
Fig. 1.
Pathogenesis of diabetes and its complications, reflecting where CCN proteins may take part. An overview of a schematic linear pathway by which obesity may lead to diabetes mellitus, and subsequently, diabetes complications. The article refers sequentially to this figure and the potential role of CCNs at each step of the disease pathway. The CCNs currently implicated in affecting this pathogenesis and being dysregulated at various points, are indicated at the right of the figure; explanatory detail addressing promotion or protection by the respective CCN, is provided in the article text
Limited series of intriguing studies to date have been undertaken addressing the CCNs in fat tissue and insulin resistant states. CCN2 has been found to inhibit fat cell differentiation in murine NIH3T3L1 cells in vitro and in primary murine cultures of pre-adipocytes (Tan et al. 2008). This was shown to occur through TGF-beta pathway dependent mechanisms (Song et al. 2015), and to involve the key early transcription factors in adipocyte differentiation, the CCAAT/enhancer binding proteins, particularly CEBP-beta and CEBP-delta (Song et al. 2015). Studies in obesity induction in high fat diet environments have shown that adipose CCN2 mRNA is upregulated in adipose tissue in obese mice, and less so in mice less prone to gain weight (Tan et al. 2013). In humans CCN2 expression correlates positively with the amount of peri-adipocyte fibrosis in white adipose tissue from obese individuals, which itself may impair adipocyte function (Pellegrinelli et al. 2014). Studies by other groups have also implicated CCN2 in inhibition of commitment of mesenchymal stem cells to the adipocyte lineage and preferentially to the osteoblast lineage, through cellular mechanisms, involving Wnt signaling (Si et al. 2006), whereas more recent publication has indicated suppression of CCN2 expression potentiates adipocyte differentiation (Battula et al. 2013). Collectively, this data suggests that CCN2 in adipose tissue may variably affect mesenchymal stem cell differentiation into adipocytes, inhibit initial and terminal fat cell differentiation, and also contribute to obesity and adipoyte dysfunction. However, CCN2 knockout mice and overexpression mice, on a background of high fat and caloric feeding have not been reported, either for CCN2 globally dysregulated mice or for adipose tissue specific and time-regulated genetically modified mice, nor whether CCN2 may impact on browning of adipose tissue and thus affect energy expenditure through thermogenesis.
More recent publication addressing CCN3 dysregulation and its bioactivity, has identified that this protein may be involved in the induction of obesity, and insulin resistance (Martinerie et al. 2016). Studies in humans showed that high blood levels of CCN3 correlate strongly with adiposity and insulin resistance, and circulating levels fall with weight loss (Pakradouni et al. 2013). Subsequently, CCN3 was found to be produced by adipocytes and CCN3−/− knockout mice in the presence of a high fat diet ad libitum, were less prone to gain weight, less obese and less insulin resistant than wild type mice, with reduced adipose tissue inflammatory changes and adipose gene expression profile suggesting greater thermogenesis (Martinerie et al. 2016). These data, especially if independently confirmed by other research groups, provide a rationale to target CCN3 and its bioactivity, especially in an adipokine setting for CCN3, to prevent obesity-induced insulin resistance.
To date, studies of members of the CCN family other than CCN2 and CCN3, are lacking and are required to complete the profile of regulation and potential action of CCNs in obesity and insulin resistant states.
CCNs in pancreatic endocrine function linking to insulin secretion
In order for diabetes and the less hyperglycemic condition of pre-diabetes, to develop, the insulin secretion from pancreatic beta-cells needs to become impaired. This is the case in the pathogenesis of any type of diabetes mellitus (Fig. 1), including type 1 diabetes where autoimmune destruction of pancreatic beta-cells occurs, or in type 2 diabetes, where resistance to the action of insulin is thought to contribute to pancreatic beta cell exhaustion in genetically susceptible individuals.
Current published data addressing CCNs in pancreatic islets, and specifically in pancreatic beta-cells, suggests multiple roles of the CCNs in differing stages of pancreatic beta-cell development and pathology. While the original studies of CCN2 homozygous global gene knockout did not find an overt pancreatic abnormality (Ivkovic et al. 2003), more recent publications have interrogated and found an important role for CCN2 in endocrine pancreatic development prenatally, indicating it is required for beta-cell proliferation, differentiation, and islet morphogenesis during development (Charrier and Brigstock 2013), (Crawford et al. 2009), as well as beta- cell function gestationally (Pasek et al. 2016), (Pasek et al. 2017). CCN2 also mediated adult beta-cell proliferation in the setting of a murine model of partial beta-cell ablation in a macrophage dependent manner, suggesting CCN2 may have a role in preventing persistent adult islet dysfunction linked to inflammation (Riley et al. 2015).
Reports have overall indicated that CCN3 dysregulates pancreatic endocrine function. One group demonstrated by in vitro studies in rat pancreatic beta-cells, that CCN3 is a transcriptional target for the key transcription factor, FoxO1, and that CCN3 inhibits pancreatic beta-cell function, in that CCN3 decreases glucose oxidation, which translates into inhibition of glucose-stimulated Ca(2+) entry and impaired insulin secretion (Paradis et al. 2013). CCN3 also impaired β-cell proliferation concomitantly with a reduction in cAMP levels (Paradis et al. 2013). A more recent publication studying CCN3−/− knockout mice reportedly did not show dysregulated blood insulin levels in normal chow fed mice, and in isolated islets from global CCN3−/− mice, pancreatic insulin content and insulin release was described as being similar in knockout and wild-type mice (Martinerie et al. 2016). Thus whether CCN3 may directly impair islet cell function and insulin secretion in humans, as well as through secondary effects on insulin resistance, remains to be better determined.
Studies of members of the CCN family other than CCN2 and CCN3 in pancreatic endocrine development and function, are limited. CCN5 which is expressed in pancreatic beta-cells, has been reported to promote pancreatic beta-cell proliferation and cell survival against streptozotocin-induced cell death, thus indicating some similarity in function in this cell type to that of CCN2 (Liu et al. 2017).
A summary of CCNs in obesity, insulin resistance and pancreatic beta-cell deficiency, is given in Table 1, including CCN action, proposed mechanisms, and CCN regulation.
Table 1.
Summarised CCN Reports in Obesity, Insulin resistance and Pancreatic Beta-Cell Dysfunction
| CCN species | CCN function | CCN mechanism(s) of action | Relevant CCN regulation |
|---|---|---|---|
| CCN1 | NR | NR | NR |
| CCN2 | Inhibits mesenchymal stem cell commitment into adipogenesis; and inhibits fat cell differentiation | Wnt signaling dependent; TGF-beta pathway and CCEBP-beta and -delta dependent mechanisms | Levels fall in parallel with adipocyte differentiation in vitro; adipose tissue levels are increased in obese, insulin resistant wild type mice |
| CCN2 | Is required for pancreatic beta-cell proliferation, differentiation, and islet morphogenesis during development; CCN2 mediated adult beta-cell proliferation in the setting of a murine model of partial beta-cell ablation | Direct effects of CCN2 on pancreatic beta-cell proliferation - CCN2 is both required and sufficient to induce proliferation of embryonic pancreatic beta-cells; also indirect effects of CCN2 via reparative macrophages to cause beta-cell immaturity and shortening cell replicative refractory period | CCN2 is induced during normal embryonic pancreatic islet morphogenesis. Expression and protein levels are much lower in postnatal life. |
| CCN3 | Induction of obesity and insulin resistance, and impairment of adipocyte differentiation | Induction of pro-inflammatory cytokines in adipose tissue inflammation and possibly lesser thermogenesis during high fat feeding in mice | Blood levels rise with obesity, possibly from CCN3 produced by adipocytes in fat tissue. |
| CCN3 | Inhibits both: pancreatic beta-cell proliferation, and glucose-stimulated Ca(2+) entry thus impairing insulin secretion | CCN3 decreases glucose oxidation; CCN3 also impairs β-cell proliferation concomitantly with a reduction in cAMP levels | Present in pancreatic beta-cells and increased CCN3 protein levels in db/db and Irs2−/− mouse islets; the key transcription factor FoxO1 binds to a conserved response element in the CCN3 promoter to regulate its expression in beta-cells |
| CCN4 | NR | NR | NR |
| CCN5 | Promotes pancreatic beta- cell (insulinoma) proliferation and cell survival against streptozotocin-induced cell death | Direct effect of CCN5 on pancreatic beta-cells to promote cell proliferation induces AKT phosphorylation and cyclin D1 levels | Present in pancreatic beta-cells, stimulated by IGF-1 together with Wnt signaling. No reports of pancreatic expression in obesity or diabetes |
| CCN6 | NR | NR | NR |
NR no reports. For relevant references refer to the text
CCNs in microvascular complications of diabetes
The traditional microvascular organ complications of diabetes are diabetic retinopathy, diabetic kidney disease, and diabetic neuropathy often linking in to foot disease, (Fig. 1). Studies in this type of diabetes complication series have reported dysregulation of CCN family members and have implicated them in prevention of, or potentiating, certain complications.
In diabetic retinopathy, both CCN1 and CCN2 have been implicated as pathogenic factors. CCN1 acts as an angiogenic mediator of ocular neovascularization in vitro and in vivo, and it is implicated as a causative factor upregulating vascular endothelial growth factor (VEGF) leading to proliferative retinopathy in a mouse model of hyperoxia induced retinopathy (You et al. 2009). Interestingly in a similar mouse model, targeting of pericyte, rather than endothelial, CCN1, significantly decreased pathological retinal neovascularization, indicating that cell type of origin may affect CCN1 bioactivity (Lee et al. 2017).
CCN2 was found to be induced in diabetic rodent retinae compared with controls (Hughes et al. 2007), especially in the deep retinal neuronal or Muller cell layer (Tikellis et al. 2004), where it is thought to be a pathogenic factor in non-proliferative diabetic retinopathy preclinical models and in vitro, contributing to thickening of the retinal capillary basal lamina, and it is involved in loss of retinal vascular pericytes (Van Geest et al. 2014), (Klaassen et al. 2015). CCN2 is increased in the human retina especially in retinal pericytes, moreso than microglial cells in people with diabetes (Kuiper et al. 2004), and in the severe vision threatening complication of proliferative diabetic retinopathy (Kuiper et al. 2008), (Ciprian 2015). Furthermore, intraocular administration of a dominant negative fragment of CCN2 prevented retinal neovascularization in the hyperoxia induced retinopathy model (Pi et al. 2012). Concern has been raised that blocking of the action of the growth factor, VEGF, using intraocular anti-VEGF monoclonal antibodies may result in excess CCN2 action and retinal and vitreous fibrosis (Kuiper et al. 2008). However, to date intraocular anti-VEGF therapy has been shown to result in generally sustained improvement in visual acuity, when administered in a repeated manner, with transient pro-fibrotic changes that may be CCN2 linked; indeed CCN2 is thought to be pro-fibrotic factor in diabetic proliferative retinopathy, and a shift in the balance between CCN2 and VEGF parallels the switch from angiogenesis to fibrosis in proliferative retinopathy (Van Geest et al. 2012), (Klaassen et al. 2015). As intraocular anti-VEGF therapy may induce CCN2 and other profibrotic proteins in the diabetic retina (Zhang et al. 2016), there is increasing interest in simultaneously inhibiting both intraocular VEGF-A and CCN2 action to prevent abnormalities in early (Hu et al. 2014) and fibro-proliferative pathology in advanced diabetic retinopathy (Bagheri et al. 2015).
Across the totality of diabetes complications, it is diabetic kidney disease where the CCNs have been most extensively studied. The reader is referred to a more expansive review of this topic, relating CCN2 regulation and action in various stages of diabetic kidney disease, across various renal cell types and parts of the nephron (Twigg 2010). To highlight seminal publications, CCN2 was originally shown to be induced in vitro in human renal mesangial cells in response to elevated extracellular D-glucose through a classical PKC dependent isoform and in a TGF-β1 dependent manner (Murphy et al. 1999). Subsequent work by others showed that circulating CCN2 is elevated in people with diabetic kidney disease, and in post-translationally modified forms in urine (Riser et al. 2003), (Gilbert et al. 2003), and also that higher circulating CCN2 (Nguyen et al. 2008) and CCN2 mRNA in human kidneys (Adler et al. 2001) each predict progressive diabetic nephropathy. We and many others studied the cellular mechanisms by which CCN2 may be up-regulated in diabetes, including in renal mesangial cells, podocytes, and tubular epithelial cells: factors downstream of glucose - advanced glycation-end products, protein kinase C isoforms, as well as saturated fatty acids, and reactive oxygen species, may each induce CCN2 (Twigg 2010), (van Nieuwenhoven et al. 2005). In addition, haemodynamic factors such as endothelin-1, angiotensin-II, and aldosterone, each up-regulate CCN2 (Twigg 2010). CCN2 may work through inhibition of (matrix metalloproteinase) MMP action and tissue inhibitor of MMP-1 (TIMP-1) upregulation, to cause renal extracellular matrix induction (McLennan et al. 2004).
Studies of inhibition of CCN2 bioactivity have implicated CCN2 in diabetic nephropathy. In CCN2+/− whole body heterozygous mice rendered diabetic compared with wild type controls, and in anti-CCN2 neutralising antibody studies in diabetic rodents, the diabetes induced GBM thickening was reported to be prevented by the CCN2 inhibition strategies (van Nieuwenhoven et al. 2005). A recent study using anti-CCN2 neutralising antibody prevented structural and functional renal pathology in a diabetic mouse model of nephropathy (Dai et al. 2017). The most definitive direct preclinical evidence for a role of renal CCN2 in mediating diabetic kidney disease is shown by studies that target CCN2 specifically in the kidney: over-expression of CCN2 in podocytes worsens diabetic nephropathy in mice (Yokoi et al. 2008), and inhibition of CCN2 expression in diabetes by antisense oligonucleotide administered to the kidney attenuates structural and functional changes of nephropathy in mouse models of diabetes (Guha et al. 2007). Moreover, a phase 1b clinical trial showed that an anti-CCN2 neutralising antibody had promise in being well tolerated and in possibly preventing albuminuria progression (Adler et al. 2010). Clearly, later phase studies in people with diabetic kidney disease, especially pathological albuminuria, are required as a next step to address potential clinical utility of targeting CCN2 in incipient, and more overt, diabetic nephropathy.
CCN3 is often found to have actions in cells and tissues which are apposite to those of CCN2. Indeed, CCN3 is said to be the balancing ‘yang’ to the ‘ying’ effects of CCN2 including in in vitro models of diabetic kidney disease where CCN3 inhibited the expression of CCN2 mRNA and protein and profibrotic changes (Riser et al. 2009). In rodent diabetic kidney disease, CCN3 is down-regulated (Mason 2013), and it is yet to be demonstrated if CCN3 as therapy, alone or combined with anti-CCN2 strategies, may prevent preclinical diabetic kidney disease.
In contrast to diabetic kidney and eye disease, there is a notable lack of data reported about CCNs in diabetic neuropathy, including the classical peripheral neuropathy that can lead to loss of sensation in the feet. There is certainly ample rationale to explore CCN regulation in nerve biopsies in humans who have varying degrees of attendant diabetic neuropathy, and in studying roles of CCNs in this metabolic neuropathy - recent publication that CCN3 may have important neuroprotective roles, albeit for the central nervous system via immune based T-Regulatory cells (Dombrowski et al. 2017), supports this consideration.
In foot ulceration in diabetes at a wound microenvironment level, a lack of trophic factors such as platelet derived growth factor (PDGF), in the presence of the observed persistent wound inflammation, may contribute to delayed wound healing (Twigg and Wong 2015). Our studies of CCN2 in wounds showed that: in diabetic compared with non-diabetic baboons levels of that tissue protein were lacking in subcutaneously placed drums (Thomson et al. 2010); that protein CCN2 is induced in post-debridement wound fluid as human diabetic foot ulcers heal (Henshaw et al. 2015); and that rhCCN2 as topical therapy accelerates wound healing in diabetic full thickness rat wounds (Henshaw et al. 2015). Known bioactivities of CCN2, including macrophage chemotaxis, pro-angiogenesis, induction of ECM including relevant collagens and induction of keratinocyte proliferation, may all be relevant to the evidence that CCN2 can aid wound healing in diabetes, as it also did in a pre-clinical burns model (Penn et al. 2012). Further studies will be required to examine other CCNs in a diabetes wound setting, especially CCN1 which is dysregulated in diabetic skin wounds (Ge et al. 2015) with recent promise of it as a local treatment in wounds including in diabetic rodents including to aid neutrophil wound clearance or efferocytosis (Jun et al. 2015). CCN3 which is induced in physiological cutaneous wound healing, promotes proangiogenic activities in vascular endothelial cells through integrin receptors, induces neovascularization in vivo, and induces responses in primary fibroblasts consistent with promotion of wound healing (Lin et al. 2005).
CCNs in macrovascular complications of diabetes
Some of the CCN family members have been found to be present in atheromatous plaques, the later which form the basis for cardiovascular and cerebrovascular disease events and peripheral arterial disease. CCN2 protein is increased in the fibrotic areas of human atheromatous plaques (Cicha et al. 2005), in the fibrous cap in particular (Leeuwis et al. 2010). Subsequent studies using advanced glycation end-products (AGEs) cross-link breakers have shown that CCN2 mRNA and protein is inhibited as plaque amount is reduced in a chronic diabetic rodent ApoE−/− deficient model (Candido et al. 2002). As CCN2 may induce pro-inflammatory macrophage chemotaxis and CXCL2 (Cicha et al. 2005) and high shear stress induces greater platelet CCN2 release (Cicha et al. 2006), it remains unclear whether CCN2 may prevent or promote plaque instability, although data overall certainly suggests it may well promote plaque lesion complexity (Cicha et al. 2005). Human carotid atheroma series relate higher plaque CCN2 presence to more stable clinical disease following a cerebrovascular event (Leeuwis et al. 2010). Studies targeting CCN2 regulation are required to address this issue; notably a recent small study in ApoE−/− (albeit, non-diabetic) mice using a carotid artery partial-ligation model, showed that anti-CCN2 antibody administered for 3 weeks, reduced macrophage deposition and lesion plaque volume (Yao et al. 2017). Studies of other CCNs in human atheromatous plaques and in diabetes preclinical models are required to address the presence, regulation and role of these CCNs. A recent report also in the ApoE−/− atheromatous model using adoptive transfer methods crossed with CCN3−/− mice, implicated bone marrow derived CCN3 in causing enhanced atheromatous lesions through regulation of macrophage foam cells (Shi et al. 2017), suggesting CCN3 may indirectly promote atheromatous disease in its earliest stages, of lipid insudation into the sub-endothelial space and lipid-laden plaque.
CCNs in less traditional diabetes organ complications
In recent years, diabetes complications which are common and cause significant morbidity and mortality, yet which are not routinely screened for at present, have been investigated. In diabetic cardiomyopathy, where diabetes directly injures the myocardium causing dysfunction and in some cases, heart failure, myocardial CCN2 is induced in fibroblasts and myocytes in preclinical rodent models (Way et al. 2002), (Wang et al. 2009), (Ivanovic-Matic et al. 2010), and it is also increased in other cardiomyopathies, likely contributing to myocardial fibrosis and remodeling (Chatzifrangkeskou et al. 2016), (Gabrielsen et al. 2007), (Tsoutsman et al. 2013). CCN2 has been reported in vitro in murine H9C2 cardiomyocytes, to mediate pathological myocyte hypertrophy and also apoptosis, when each are induced by the saturated fatty acid, palmitate (Wang et al. 2009). It has been suggested that inhibition of cardiac CCN2 may be a mechanism by which the thiazolidenedione diabetes medicines (Ihm et al. 2010) and angiotensin pathways modulators (Sukumaran et al. 2017) prevent diabetic and other cardiomyopathy, and CCN2 was more recently reported to prevent dilated cardiomyopathy, albeit in a non-diabetic mouse model (Koshman et al. 2015). In contrast, our group could not find evidence of increased myocardial CCN2 protein in diabetes cases of dead in bed syndrome which is thought to be arrhythmia mediated (Tu et al. 2010). At a circulating level, CCN2 increases with overt heart failure presence in humans suggesting that it may have some utility as a blood marker of this condition (Yagi et al. 2012), although some publications have suggested that it may have less utility than other circulating profibrosis markers in some forms of cardiomyopathy (Rubis et al. 2017) and in subclinical diabetic cardiomyopathy (Brooks et al. 2008). Thus the field remains in some conflict, as to the importance of CCN2 in mediating diabetic cardiomyopathy and it is role as a circulating marker of fibrosis in the same condition. In terms of the other CCNs, CCN1 as well as CCN2 steady state mRNA levels, have recently been found to be up-regulated by the conserved hippo pathway in failing human hearts with ischemic heart disease (some with diabetes) and idiopathic dilated cardiomyopathy, and in mouse desmin-related cardiomyopathy, implicating Yap1/Taz signaling (Hou et al. 2017). In the case of CCN3, CCN3−/− global deletions in mice cause a specific form of cardiomyopathy (Heath et al. 2008), and whether CCN3 presence in the heart may help to protect from clinical diabetic cardiomyopathy in mice and man with diabetes, is yet to be reported.
In the common metabolic condition of non-alcoholic fatty liver disease (NAFLD), diabetes presence in humans and in mouse models is known to induce the more severe fibrotic form of NAFLD known as non-alcoholic steatohepatitis (NASH), especially with fibrosis (Williams et al. 2013). In fibrotic liver, CCN2 mRNA and protein are produced by fibroblasts, myofibroblasts, hepatic stellate cells (HSCs), endothelial cells, and bile duct epithelial cells (Rachfal and Brigstock 2003). In a mouse model of high fat feeding with diabetes, CCN2 mRNA and protein are induced particularly in hepatocytes (Lo et al. 2011), and in the same model CCN3 is reduced (Twigg 2017a, b); blockade of CCN2 protein using a neutralising polyclonal anti-CCN2 antibody prevents, albeit partially, the fibrosis in the diabetes NASH model (Twigg 2017a, b). Other studies indicate that CCN2 levels are elevated in human NASH fibrosis livers and in blood (V. Paradis et al. 2001). In mice with hepatocyte hCCN2 overexpression, the transgene exacerbates pathogenic stimuli such as carbon tetrachloride and bile duct ligation to cause increased liver fibrosis (Tong et al. 2009). Recent data has suggested that key micro RNA species through exosomes may regulate CCN2 expression in the pro-fibrotic activated hepatic stellate cells (Chen et al. 2014), implicating that methodology as a future approach in NASH fibrosis in diabetes. For other CCNs, CCN1 therapy systemically in NAFLD helps to prevent progressive disease and fibrosis in a high fat feeding non-diabetic murine model (Kim et al. 2013), and no studies have been undertaken to date combining CCNs as targets in NAFLD.
Thus, overall, CCN2 is implicated in potentially contributing to pathology in NASH fibrosis in diabetes, and in diabetic cardiomyopathy, and strategies to inhibit CCN2 action especially in a tissue specific manner, hold promise in these conditions, which currently have no specific treatment clinically. Regulation of CCN1 and CCN3 may also be of utility in NAFLD including that linked to diabetes. A summary of CCNs in diabetes end-organ complications, is provided in Table 2, currently reflecting that only CCN1, CCN2, and CCN3 have been reported in this context.
Table 2.
Summarised CCN Bioactivities and Mechanisms of Action in Diabetes End-Organ Complications
| Diabetes complication type | CCN1 action | CCN1 main proposed mechanism | CCN2 action | CCN2 main proposed mechanism | CCN3 action | CCN3 main proposed mechanism |
|---|---|---|---|---|---|---|
| Diabetic retinopathy | Pos | Proangiogenic via CCN1 esp. in vascular pericytes | Pos | Retinal capillary basal lamina thickening, and loss of retinal vascular pericytes | NR | NR |
| Diabetic kidney disease | NR | NR | Pos | Pro-fibrotic - glomerular and renal interstitial including via mesangial cell TIMP-1 | Prev | Inhibition of CCN2 mRNA induction |
| Diabetic foot ulceration /wounds | Prev (+ulcer treatment) | Promotes wound neutrophil effero-cytosis | Prev +ulcer treatment | Granulation tissue induction | Prev | Proangio-genic via integrin receptors; induces cutaneous primary fibroblast action |
| Cardiovasc-ular disease | NR | NR | Pos (for the complex lesions) | Induction of matrix in plaques | Pos (for lipid laden lesions) | Marrow derived CCN3 with macrophage early plaque infiltration |
| Cerebrovasc-ular disease | NR | NR | Pos (for complex lesions) | Induction of matrix in plaques | NR | NR |
| Diabetic cardiomyop-athy | NR | NR | Pos | Profibrotic; proapoptotic, proinflammat-ory and hypertrophic in cardiomyo-cytes | NR | NR |
| Non-alcoholic fatty liver disease | Prev | Induction of activated stellate cell senescence | Pos | Induction of activated stellate cell action | NR | NR |
For relevant references refer to the text. Please note that no bioactivities in diabetes organ complications have been reported for CCN4, CCN5 or CCN6. In addition, no effects for any CCNs have been reported to date in diabetic peripheral neuropathy or peripheral arterial disease.
Key: Pos promotes the complication, Prev inhibits the complication, NR no reports
CCNs, metabolic disease and future directions
Potential roles of CCNs acutely in regulation of macronutrients of protein, lipids and carbohydrate, and cellular downstream pathways, for example of glucose metabolism, have been minimally examined. Of much intrigue, a recent abstract identified CCN6 as protein that localizes to mitochondria and may act as a regulator mitochondrial electron transport in eukaryotic cells (Patra et al. 2017). At the same recent international CCN Workshop, it was reported that in chondrocytes CCN2 and CCN3 may differentially regulate glycolysis in a sub-acute time course (Kubota et al. 2017). Collectively, this work suggests that the CCNs may have as yet only partially defined roles in cellular macronutrient and energy homeostasis.
The reports of CCNs in obesity and diabetes and its complications described in this article reflect that for most of the CCN family of proteins and genes, their regulation and potential role as disease mediators and modifiers including in end-organ complications, is yet to be defined. However, the data which is better developed suggests that CCN1 may be important in vascular complications and in NAFLD, CCN2 is implicated as a mediator of end-organ complications such as nephropathy, cardiomyopathy and NASH fibrosis, and alternatively in diabetic wounds as a treatment (as is CCN1), whereas CCN3 may induce obesity and insulin resistance, and may be protective to diabetes end-organ complications. The research field in exploring roles of this fascinating family of genes and proteins in obesity and diabetes remains in its relative infancy, and the data reported to date continue to suggest clinically relevant pathophysiological roles in these common metabolic disease states for CCNs as markers, as well as mediators or protective factors.
NR = no reports. For relevant references refer to the text.
Key: Pos = promotes the complication; Prev = inhibits the complication; NR = no reports.
For relevant references refer to the text. Please note that no bioactivities in diabetes organ complications have been reported for CCN4, CCN5 or CCN6. In addition, no effects for any CCNs have been reported to date in diabetic peripheral neuropathy or peripheral arterial disease.
References
- Adler SG, Kang SW, Feld S, Cha DR, Barba L, Striker L, et al. Glomerular mRNAs in human type 1 diabetes: biochemical evidence for microalbuminuria as a manifestation of diabetic nephropathy. Kidney Int. 2001;60(6):2330–2336. doi: 10.1046/j.1523-1755.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin J Am Soc Nephrol. 2010;5(8):1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri A, Soheili ZS, Ahmadieh H, Samiei S, Sheibani N, Astaneh SD, et al. Simultaneous application of bevacizumab and anti-CTGF antibody effectively suppresses proangiogenic and profibrotic factors in human RPE cells. Mol Vis. 2015;21:378–390. [PMC free article] [PubMed] [Google Scholar]
- Battula VL, Chen Y, Cabreira Mda G, Ruvolo V, Wang Z, Ma W, et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood. 2013;122(3):357–366. doi: 10.1182/blood-2012-06-437988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178(2):169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, Twigg SM. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10(9):739–746. doi: 10.1111/j.1463-1326.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- Candido R, Jandeleit-Dahm KA, Cao Z, Nesteroff SP, Burns WC, Twigg SM, et al. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation. 2002;106(2):246–253. doi: 10.1161/01.CIR.0000021122.63813.32. [DOI] [PubMed] [Google Scholar]
- Charrier A, Brigstock DR. Regulation of pancreatic function by connective tissue growth factor (CTGF, CCN2) Cytokine Growth Factor Rev. 2013;24(1):59–68. doi: 10.1016/j.cytogfr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzifrangkeskou M, Le Dour C, Wu W, Morrow JP, Joseph LC, Beuvin M, et al. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum Mol Genet. 2016;25(11):2220–2233. doi: 10.1093/hmg/ddw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25(5):1008–1013. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- Cicha I, Yilmaz A, Suzuki Y, Maeda N, Daniel WG, Goppelt-Struebe M, Garlichs CD. Connective tissue growth factor is released from platelets under high shear stress and is differentially expressed in endothelium along atherosclerotic plaques. Clin Hemorheol Microcirc. 2006;35(1–2):203–206. [PubMed] [Google Scholar]
- Ciprian D. The pathogeny of proliferative vitreoretinopathy. Rom J Ophthalmol. 2015;59(2):88–92. [PMC free article] [PubMed] [Google Scholar]
- Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, et al. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23(3):324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HY, Ma LN, Cao Y, Chen XL, Shi H, Fan YP, Yang B. Protection of CTGF antibody against diabetic nephropathy in mice via reducing glomerular beta-catenin expression and podocyte epithelial-mesenchymal transition. J Cell Biochem. 2017;118(11):3706–3712. doi: 10.1002/jcb.26017. [DOI] [PubMed] [Google Scholar]
- Dombrowski Y, O'Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674–680. doi: 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A, Lawler PR, Yongzhong W, Steinbruchel D, Blagoja D, Paulsson-Berne G, et al. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J Mol Cell Cardiol. 2007;42(4):870–883. doi: 10.1016/j.yjmcc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Ge K, Wu JJ, Qian L, Wu MJ, Wang FL, Xu B, Xie T. Bioinformatic analysis of the effect of type II diabetes on skin wound healing. Genet Mol Res. 2015;14(2):4802–4811. doi: 10.4238/2015.May.11.12. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Akdeniz A, Weitz S, Usinger WR, Molineaux C, Jones SE, et al. Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care. 2003;26(9):2632–2636. doi: 10.2337/diacare.26.9.2632. [DOI] [PubMed] [Google Scholar]
- Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21(12):3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8:18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw FR, Boughton P, Lo L, McLennan SV, Twigg SM. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res. 2015;2015:236238. doi: 10.1155/2015/236238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou N, Wen Y, Yuan X, Xu H, Wang X, Li F, Ye B. Activation of Yap1/Taz signaling in ischemic heart disease and dilated cardiomyopathy. Exp Mol Pathol. 2017;103(3):267–275. doi: 10.1016/j.yexmp.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Zhang Y, Zeng Q, Han Q, Zhang L, Liu M, Li X. Intravitreal injection of ranibizumab and CTGF shRNA improves retinal gene expression and microvessel ultrastructure in a rodent model of diabetes. Int J Mol Sci. 2014;15(1):1606–1624. doi: 10.3390/ijms15011606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Kuiper EJ, Klaassen I, Canning P, Stitt AW, Van Bezu J, et al. Advanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retina. Diabetologia. 2007;50(5):1089–1098. doi: 10.1007/s00125-007-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihm SH, Chang K, Kim HY, Baek SH, Youn HJ, Seung KB, Kim JH. Peroxisome proliferator-activated receptor-gamma activation attenuates cardiac fibrosis in type 2 diabetic rats: the effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res Cardiol. 2010;105(3):399–407. doi: 10.1007/s00395-009-0071-x. [DOI] [PubMed] [Google Scholar]
- Ivanovic-Matic S, Mihailovic M, Dinic S, Martinovic V, Bogojevic D, Grigorov I, Poznanovic G. The absence of cardiomyopathy is accompanied by increased activities of CAT, MnSOD and GST in long-term diabetes in rats. J Physiol Sci. 2010;60(4):259–266. doi: 10.1007/s12576-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130(12):2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Kim KH, Lau LF. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun. 2015;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33(10):2078–2090. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO. The role of CTGF in diabetic retinopathy. Exp Eye Res. 2015;133:37–48. doi: 10.1016/j.exer.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Koshman YE, Sternlicht MD, Kim T, O'Hara CP, Koczor CA, Lewis W, et al. Connective tissue growth factor regulates cardiac function and tissue remodeling in a mouse model of dilated cardiomyopathy. J Mol Cell Cardiol. 2015;89(Pt B):214–222. doi: 10.1016/j.yjmcc.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Hara ES, Akashi S, Ono M, Nishida T, Hattori T et al (2017) Small compounds that turn on CCN family genes. Ninth international workshop on the CCN family of genes, Saint-Malo, France, November 2 to 7
- Kuiper EJ, Witmer AN, Klaassen I, Oliver N, Goldschmeding R, Schlingemann RO. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol. 2004;88(8):1082–1087. doi: 10.1136/bjo.2003.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper, E. J., Van Nieuwenhoven, F. A., de Smet, M. D., van Meurs, J. C., Tanck, M. W., Oliver, N., … Schlingemann, R. O. (2008). The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One, 3(7), e2675 [DOI] [PMC free article] [PubMed]
- Lee S, Elaskandrany M, Lau LF, Lazzaro D, Grant MB, Chaqour B. Interplay between CCN1 and Wnt5a in endothelial cells and pericytes determines the angiogenic outcome in a model of ischemic retinopathy. Sci Rep. 2017;7(1):1405. doi: 10.1038/s41598-017-01585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwis JW, Nguyen TQ, Theunissen MG, Peeters W, Goldschmeding R, Pasterkamp G, Vink A. Connective tissue growth factor is associated with a stable atherosclerotic plaque phenotype and is involved in plaque stabilization after stroke. Stroke. 2010;41(12):2979–2981. doi: 10.1161/STROKEAHA.110.589036. [DOI] [PubMed] [Google Scholar]
- Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280(9):8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- Liu JL, Kaddour N, Chowdhury S, Li Q, Gao ZH. Role of CCN5 (WNT1 inducible signaling pathway protein 2) in pancreatic islets. J Diabetes. 2017;9(5):462–474. doi: 10.1111/1753-0407.12507. [DOI] [PubMed] [Google Scholar]
- Lo L, McLennan SV, Williams PF, Bonner J, Chowdhury S, McCaughan GW, et al. Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. J Hepatol. 2011;55(2):435–444. doi: 10.1016/j.jhep.2010.10.039. [DOI] [PubMed] [Google Scholar]
- Martinerie C, Garcia M, Do TT, Antoine B, Moldes M, Dorothee G, et al. NOV/CCN3: a new Adipocytokine involved in obesity-associated insulin resistance. Diabetes. 2016;65(9):2502–2515. doi: 10.2337/db15-0617. [DOI] [PubMed] [Google Scholar]
- Mason RM. Fell-Muir lecture: connective tissue growth factor (CCN2) -- a pernicious and pleiotropic player in the development of kidney fibrosis. Int J Exp Pathol. 2013;94(1):1–16. doi: 10.1111/j.1365-2613.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan SV, Wang XY, Moreno V, Yue DK, Twigg SM. Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathy. Endocrinology. 2004;145(12):5646–5655. doi: 10.1210/en.2004-0436. [DOI] [PubMed] [Google Scholar]
- Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem. 1999;274(9):5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Tarnow L, Jorsal A, Oliver N, Roestenberg P, Ito Y, et al. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care. 2008;31(6):1177–1182. doi: 10.2337/dc07-2469. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhoven FA, Jensen LJ, Flyvbjerg A, Goldschmeding R. Imbalance of growth factor signalling in diabetic kidney disease: is connective tissue growth factor (CTGF, CCN2) the perfect intervention point? Nephrol Dial Transplant. 2005;20(1):6–10. doi: 10.1093/ndt/gfh570. [DOI] [PubMed] [Google Scholar]
- Pakradouni J, Le Goff W, Calmel C, Antoine B, Villard E, Frisdal E, et al. Plasma NOV/CCN3 levels are closely associated with obesity in patients with metabolic disorders. PLoS One. 2013;8(6):e66788. doi: 10.1371/journal.pone.0066788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34(4 Pt 1):738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- Paradis R, Lazar N, Antinozzi P, Perbal B, Buteau J. Nov/Ccn3, a novel transcriptional target of FoxO1, impairs pancreatic beta-cell function. PLoS One. 2013;8(5):e64957. doi: 10.1371/journal.pone.0064957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek RC, Dunn JC, Elsakr JM, Aramandla M, Matta AR, Gannon M. Connective tissue growth factor is critical for proper beta-cell function and pregnancy-induced beta-cell hyperplasia in adult mice. Am J Physiol Endocrinol Metab. 2016;311(3):E564–E574. doi: 10.1152/ajpendo.00194.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pasek RC, Dunn JC, Elsakr JM, Aramandla M, Matta AR, Gannon M. Vascular-derived connective tissue growth factor (Ctgf) is critical for pregnancy-induced beta cell hyperplasia in adult mice. Islets. 2017;9(6):150–158. doi: 10.1080/19382014.2017.1356963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra M, Mahata SK, Padham DK, Sengupta A, Semn M (2017) CCN6 is a regulator of mitochondrial function. Ninth international workshop on the CCN family of genes, Saint-Malo, France, November 2 to 7
- Pellegrinelli V, Heuvingh J, du Roure O, Rouault C, Devulder A, Klein C, et al. Human adipocyte function is impacted by mechanical cues. J Pathol. 2014;233(2):183–195. doi: 10.1002/path.4347. [DOI] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2(1):18–28. [PMC free article] [PubMed] [Google Scholar]
- Pi L, Shenoy AK, Liu J, Kim S, Nelson N, Xia H, et al. CCN2/CTGF regulates neovessel formation via targeting structurally conserved cystine knot motifs in multiple angiogenic regulators. FASEB J. 2012;26(8):3365–3379. doi: 10.1096/fj.11-200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26(1):1–9. doi: 10.1016/S1386-6346(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Riley KG, Pasek RC, Maulis MF, Dunn JC, Bolus WR, Kendall PL, et al. Macrophages are essential for CTGF-mediated adult beta-cell proliferation after injury. Mol Metab. 2015;4(8):584–591. doi: 10.1016/j.molmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser BL, Cortes P, DeNichilo M, Deshmukh PV, Chahal PS, Mohammed, et al. Urinary CCN2 (CTGF) as a possible predictor of diabetic nephropathy: preliminary report. Kidney Int. 2003;64(2):451–458. doi: 10.1046/j.1523-1755.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, et al. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol. 2009;174(5):1725–1734. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubis P, Wisniowska-Smialek S, Dziewiecka E, Rudnicka-Sosin L, Kozanecki A, Podolec P. Prognostic value of fibrosis-related markers in dilated cardiomyopathy: a link between osteopontin and cardiovascular events. Adv Med Sci. 2017;63(1):160–166. doi: 10.1016/j.advms.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Shi H, Zhang C, Pasupuleti V, Hu X, Prosdocimo DA, Wu W, et al. CCN3 regulates macrophage foam cell formation and atherosclerosis. Am J Pathol. 2017;187(6):1230–1237. doi: 10.1016/j.ajpath.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si, W., Kang, Q., Luu, H. H., Park, J. K., Luo, Q., Song, W. X., … He, T. C. (2006). CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol, 26(8), 2955–2964 [DOI] [PMC free article] [PubMed]
- Song WW, McLennan SV, Tam C, Williams PF, Baxter RC, Twigg SM. CCN2 requires TGF-beta signalling to regulate CCAAT/enhancer binding proteins and inhibit fat cell differentiation. J Cell Commun Signal. 2015;9(1):27–36. doi: 10.1007/s12079-014-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran V, Tsuchimochi H, Tatsumi E, Shirai M, Pearson JT. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE-2/ANG 1-7/mas receptor cascade. Biochem Pharmacol. 2017;144:90–99. doi: 10.1016/j.bcp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Tan JT, McLennan SV, Song WW, Lo LW, Bonner JG, Williams PF, Twigg SM. Connective tissue growth factor inhibits adipocyte differentiation. Am J Physiol Cell Physiol. 2008;295(3):C740–C751. doi: 10.1152/ajpcell.00333.2007. [DOI] [PubMed] [Google Scholar]
- Tan JT, McLennan SV, Williams PF, Rezaeizadeh A, Lo LW, Bonner JG, Twigg SM. Connective tissue growth factor/CCN-2 is upregulated in epididymal and subcutaneous fat depots in a dietary-induced obesity model. Am J Physiol Endocrinol Metab. 2013;304(12):E1291–E1302. doi: 10.1152/ajpendo.00654.2012. [DOI] [PubMed] [Google Scholar]
- Thomson SE, McLennan SV, Hennessy A, Boughton P, Bonner J, Zoellner H, et al. A novel primate model of delayed wound healing in diabetes: dysregulation of connective tissue growth factor. Diabetologia. 2010;53(3):572–583. doi: 10.1007/s00125-009-1610-6. [DOI] [PubMed] [Google Scholar]
- Tikellis C, Cooper ME, Twigg SM, Burns WC, Tolcos M. Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology. 2004;145(2):860–866. doi: 10.1210/en.2003-0967. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50(3):939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoutsman T, Wang X, Garchow K, Riser B, Twigg S, Semsarian C. CCN2 plays a key role in extracellular matrix gene expression in severe hypertrophic cardiomyopathy and heart failure. J Mol Cell Cardiol. 2013;62:164–178. doi: 10.1016/j.yjmcc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Tu E, Bagnall RD, Duflou J, Lynch M, Twigg SM, Semsarian C. Post-mortem pathologic and genetic studies in "dead in bed syndrome" cases in type 1 diabetes mellitus. Hum Pathol. 2010;41(3):392–400. doi: 10.1016/j.humpath.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Twigg SM. Mastering a mediator: blockade of CCN-2 shows early promise in human diabetic kidney disease. J Cell Commun Signal. 2010;4(4):189–196. doi: 10.1007/s12079-010-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg SM (2017a) Regulation and bioactivity of the CCN family of proteins and genes in Diabees and its complications. Ninth international workshop on the CCN family of genes, Saint-Malo, France
- Twigg SM (2017b) Regulation and bioactivity of the CCN family of proteins and genes in diabetes and its complications. Ninth international workshop on the CCN family of genes. Conference Proceedings. Saint-Malo, France
- Twigg SM, Wong J. The imperative to prevent diabetes complications: a broadening spectrum and an increasing burden despite improved outcomes. Med J Aust. 2015;202(6):300–304. doi: 10.5694/mja14.01234. [DOI] [PubMed] [Google Scholar]
- Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, et al. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96(4):587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geest RJ, Leeuwis JW, Dendooven A, Pfister F, Bosch K, Hoeben KA, et al. Connective tissue growth factor is involved in structural retinal vascular changes in long-term experimental diabetes. J Histochem Cytochem. 2014;62(2):109–118. doi: 10.1369/0022155413512656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol. 2009;297(6):C1490–C1500. doi: 10.1152/ajpcell.00049.2009. [DOI] [PubMed] [Google Scholar]
- Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, et al. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51(9):2709–2718. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34(1):84–129. doi: 10.1210/er.2012-1009. [DOI] [PubMed] [Google Scholar]
- Yagi H, Toyama T, Kasama S, Koitabashi N, Arai M, Yokoyama T, et al. Relation between connective tissue growth factor and cardiac sympathetic nerve activity in heart failure in DCM patients. Int Heart J. 2012;53(5):282–286. doi: 10.1536/ihj.53.282. [DOI] [PubMed] [Google Scholar]
- Yao Y, Li B, Fu C, Teng G, Ma G, Liu N. Anti-connective tissue growth factor detects and reduces plaque inflammation in early-stage carotid atherosclerotic lesions. Nanomedicine. 2017;13(8):2385–2394. doi: 10.1016/j.nano.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73(4):446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- You JJ, Yang CH, Chen MS, Yang CM. Cysteine-rich 61, a member of the CCN family, as a factor involved in the pathogenesis of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(7):3447–3455. doi: 10.1167/iovs.08-2603. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Qi Y, Chen L, Shi X, Bai Y, Huang L, et al. The relationship between anti-vascular endothelial growth factor and fibrosis in proliferative retinopathy: clinical and laboratory evidence. Br J Ophthalmol. 2016;100(10):1443–1450. doi: 10.1136/bjophthalmol-2015-308199. [DOI] [PubMed] [Google Scholar]