Abstract
Objective
Although laparoscopic treatment of gallbladder cancer (GBC) has been explored in the last decade, long-term results are still rare. This study evaluates long-term results of intended laparoscopic treatment for suspected GBC confined to the gallbladder wall, based on our experience over 10 years.
Methods
Between August 2006 and December 2015, 164 patients with suspected GBC confined to the wall were enrolled in the protocol for laparoscopic surgery. The process for GBC treatment was analyzed to evaluate the feasibility of computed tomography (CT) and/or magnetic resonance imaging (MRI) combined with frozen-section examination in identifying GBC confined to the wall. Of 159 patients who underwent the intended laparoscopic radical treatment, 47 with pathologically proven GBC were investigated to determine the safety and oncologic outcomes of a laparoscopic approach to GBC.
Results
Among the 164 patients, 5 patients avoided further radical surgery because of unresectable disease and 12 were converted to open surgery; in the remaining 147 patients, totally laparoscopic treatment was successfully accomplished. Extended cholecystectomy was performed in 37 patients and simple cholecystectomy in 10. The T stages based on final pathology were Tis (n=6), T1a (n=2), T1b (n=9), T2 (n=26), and T3 (n=4). Recurrence was detected in 11 patients over a median follow-up of 51 months. The disease-specific 5-year survival rate of these 47 patients was 68.8%, and rose to 85% for patients with a normal cancer antigen 19-9 (CA19-9) level.
Conclusions
The favorable long-term outcomes demonstrate the feasibility of combined CT/MRI and frozen-section examination in the selection of patients with GBC confined to the gallbladder wall, confirm the oncologic safety of laparoscopic treatment in selected GBC patients, and favor measurement of preoperative CA19-9 in the selection of GBCs suitable for laparoscopic treatment.
Keywords: CA19-9, frozen-section diagnosis, gallbladder cancer, laparoscopic radical cholecystectomy, laparoscopic surgery, radical cholecystectomy
Introduction
Gallbladder cancer (GBC) is rare in our country (1), and GBC confined to the gallbladder wall was often histologically incidentally discovered after laparoscopic cholecystectomy (LC), with radical reoperation being recommended for such patients (2,3). Owing to the increasing utilization of multiple imaging studies in high-risk patients with gallbladder stones and polyps, preoperative detection of early GBC has become possible (3-6).
Preoperative suspicion of cancer has generally been regarded as a contraindication for laparoscopic treatment because of concerns about the risk of tumor cell dissemination and port-site metastasis as well as the feasibility of a radical laparoscopic approach (7,8). Most studies evaluating the risk of tumor cell dissemination and port-site metastasis stimulated by laparoscopy for GBC involve patients with unexpected GBCs, the incidence of which may be reduced by adherence to oncologic principles and the use of specimen bags in suspected GBC patients (9).
To date, only two prospective long-term studies examining the role of laparoscopic treatment for suspected GBCs have been published (10,11). The aim of this study is to share our 10-year experience of elective laparoscopic treatment for suspected GBC confined to the gallbladder wall.
Materials and methods
Since August 2006 in Peking University Third Hospital, the totally laparoscopic approach has been electively applied for patients with suspected GBC confined to the gallbladder wall. Suspicion of GBC confined to the wall was initially based on preoperative ultrasonography, after which further imaging studies, including enhanced computed tomography (ECT) and magnetic resonance imaging (MRI), were conducted to identify invasion confined to the gallbladder wall and the absence of extrahepatic bile duct involvement. Informed consent for the laparoscopic procedures was provided by each patient prior to surgery. We prospectively collected the clinicopathological data of 164 preoperatively suspect GBC patients who underwent surgery from August 2006 to December 2015. Pathological staging and the extent of lymph node dissection were defined according to guidelines of the American Joint Committee on Cancer, 7th edition (12). The study was approved by the Institutional Review Board of the Peking University Third Hospital.
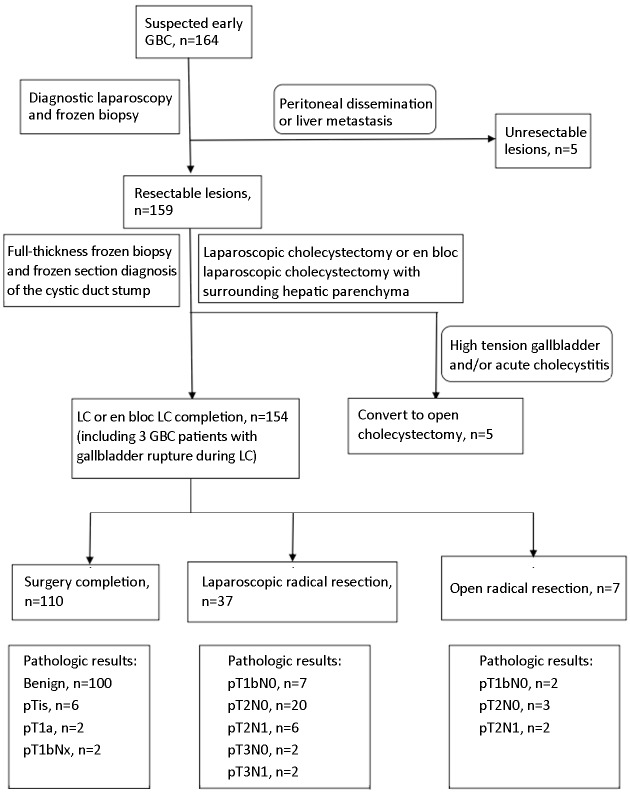
Algorithm of laparoscopic approach and extent of resection (Figure 1)
1.
Treatment algorithm of a laparoscopic approach for treating suspected early gallbladder cancer (GBC).
We initially performed laparoscopic exploration to exclude factors affecting the patients’ eligibility for curative resection. Simple LC was applied to all suspected GBCs confined to the wall up until 2012 in Peking University Third Hospital, at which time laparoscopic en bloc gallbladder resection with the gallbladder plate or surrounding liver was introduced for patients with suspected liver invasion or high-tension gallbladder (Figure 2). For all patients who undergo LC, a plastic bag is used to extract the gallbladder harboring the potential tumor. After intraoperative frozen-section examination of pathological diagnosis, depth of tumor invasion, and surgical margin of the cystic duct, laparoscopic radical resection is initiated. Laparoscopic radical cholecystectomy consists of 2-cm wedge hepatectomy of gallbladder fossa and hepatoduodenal lymphadenectomy; in some patients, particularly those with suspected stage T2 or T3 tumor, an additional posterosuperior pancreatic lymphadenectomy is performed (Figure 3).
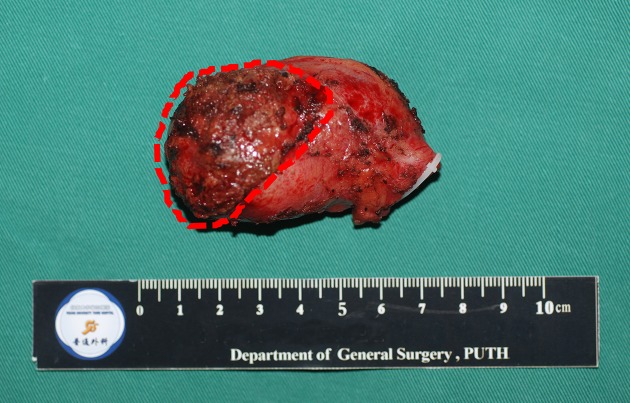
2.
Laparoscopic resected gallbladder with gallbladder plate and surrounding liver. Dotted line indicates gallbladder plate with limited hepatic surround.
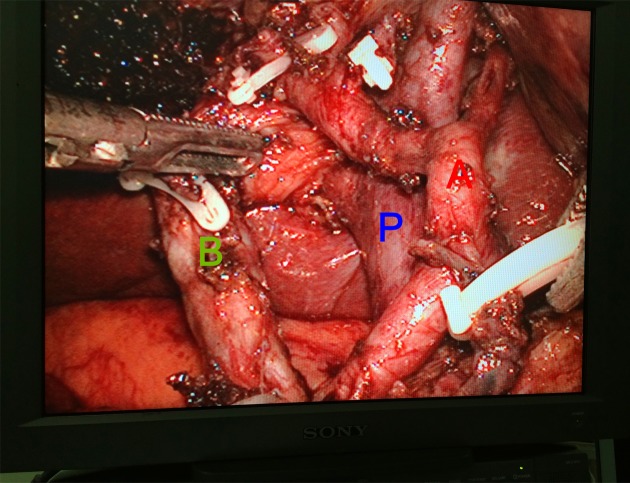
3.
View of laparoscopic limited liver resection and hepatoduodenal ligament dissection. A, proper hepatic artery; P, portal vein; B, bile duct.
Data collection
We examined the data collected from all patients admitted to the Peking University Third Hospital with suspected GBC between August 2006 and December 2015. A total of 164 patients with suspected GBC confined to the wall were identified over this 10-year period. The data were extracted from the database and from hospital and clinical records. Recorded data included patient demographics, operative procedures, perioperative outcomes, complications, pathology and staging of tumor, recurrence patterns, and survival time. Surgical mortality was defined as death within 30 days of surgery. Follow-up was based on regular visits to the clinic, or by personal contact with the patient and the patient’s family by telephone interviews whenever needed.
Statistical analysis
All statistical analyses were performed by IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA). Data were expressed as
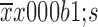 . The Kaplan-Meier method was used to estimate the overall and disease-free survival. The log-rank test was used to compare different survival curves. A two-tailed probability value of less than 0.05 was considered statistically significant. Patients who were lost to follow-up were censored at the time of the last follow-up. Deaths from other causes were censored.
. The Kaplan-Meier method was used to estimate the overall and disease-free survival. The log-rank test was used to compare different survival curves. A two-tailed probability value of less than 0.05 was considered statistically significant. Patients who were lost to follow-up were censored at the time of the last follow-up. Deaths from other causes were censored.
Results
Feasibility of combined preoperative CT/MRI and intraoperative frozen-section examination in diagnosis of GBC confined to the wall
Between August 2006 and December 2015, 164 patients with suspected GBC confined to the wall underwent an initial laparoscopic exploration, with 7 of these patients receiving additional intraoperative frozen-section biopsy for suspected metastasis on the peritoneal or liver surface. The outcomes of these biopsies were in accordance with the final pathology, namely 2 patients with benign lesions and 5 patients with malignant metastasis on the abdominal wall or liver surface. Excluding 5 patients who were converted to open cholecystectomy, LC and subsequent frozen-section examination of the gallbladder were then performed in the remaining 154 patients. Benign lesions were found in 102 patients, 2 of which were proved to be Tis and T1a malignant disease in the final pathology; the remaining 100 benign lesions consisted of 13 cholesterol polyps, 27 adenomas, 31 xanthogranulomatous cholecystitis, and 29 adenomyomatosis in final pathology. Of the 52 patients diagnosed with GBC, T stage was evaluated during intraoperative frozen-section examination in 40 patients, 17 of which were in accordance with the final pathology. The remaining 22 were lower than the final T stage, including 7 stage T1a, 14 stage T1b, and 1 stage T2; only one patient with stage T2 was diagnosed as stage T1b after final pathology (Table 1).
1.
Outcomes during selection of 159 patients with suspected early gallbladder cancer
| Variables | n (%) |
| Laparoscopic exploration and frozen biopsy outcomes (n=7) | |
| Benign (frozen biopsy:final pathology) (n=2) | 2:2 |
| Malignant (frozen biopsy:final pathology) (n=5) | 5:5 |
| Conversion reasons for laparoscopic cholecystectomy (n=5) | |
| Gallbladder with high tension and/or acute cholecystitis | 5 (100) |
| Frozen pathologic examination of gallbladder | |
| Pathologic diagnosis (n=154) | |
| Benign (frozen biopsy:final pathology) | 102:100 |
| Cholesterol polyp of the gallbladder | 13 (8.4) |
| Adenoma of the gallbladder | 27 (17.5) |
| Xanthogranulomatous cholecystitis | 31 (20.1) |
| Adenomyomatosis of gallbladder | 29 (18.8) |
| Malignant (frozen biopsy: final pathology) | 52:52 |
| T stage evaluation (n=52) | |
| T stage equivalent (final pathology: frozen biopsy) | 17 (32.7) |
| T stage elevated (final pathology: frozen biopsy) | 22 (42.3) |
| T stage degraded (final pathology: frozen biopsy) | 1 (1.9) |
| T stage not evaluated | 12 (23.1) |
| Conversion reasons for open radical surgery (n=7) | |
| Edema or errhysis of hepatoduodenal ligment | 4 (57.1) |
| Patient or surgeon tendency | 2 (28.6) |
| Positive cystic duct margin | 1 (14.3) |
Feasibility and safety of initial LC and laparoscopic lymphadenectomy plus limited liver resection
Among the 159 resectable lesions after laparoscopic exploration, 151 successfully underwent surgery. Thirteen GBC patients with tumor located at the hepatic side received simple LC (from July 2012 onward, 8 GBC patients with hepatic side tumor location received en bloc gallbladder resection with gallbladder plate or surrounding liver included), all with no local recurrence of the malignant lesions. In the remaining 8 patients, 5 were converted to open cholecystectomy due to the high-tension gallbladder and/or acute cholecystitis, which led to the difficulty exposing Calot’s triangle; the other 3 with GBC disease suffered gallbladder rupture in the process of simple LC, 2 of whom presented with peritoneal metastasis during follow-up. Detailed analysis revealed that the 2 patients with peritoneal metastasis had suspected floating tumors in the gallbladder, leading to subsequent high-tension gallbladder and acute cholecystitis (Table 1, 2; Figure 1).
2.
Clinicopathological characteristics of 47 GBC patients received totally laparoscopic treatment
| Variables | n (%) |
| GBC, gallbladder cancer; CA19-9, cancer antigen 19-9. | |
| Patient characteristics | |
Age (
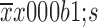 ) (year) ) (year)
|
63±13 |
| Sex (male:female) | 13:34 |
| Gallbladder with high tension | 6 (12.8) |
| Gallbladder with acute cholecystitis | 5 (10.6) |
| Tumor located in liver side | 21 (44.7) |
| CA19-9, elevated | 7 (14.9) |
| Perioperative outcomes | |
| Gallbladder rupture | 3 (6.4) |
Operative time (
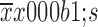 ) (min) ) (min)
|
233±51 |
Blood loss (
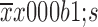 ) (mL) ) (mL)
|
177±340 |
Hospital stay (
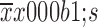 ) (d) ) (d)
|
12±7 |
| Complications | 4 (8.5) |
| Pathological outcomes | |
Number of lymph node retrieved (
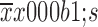 ) (range) ) (range)
|
4±3 (1–12) |
| R status, R0 | 47 (100) |
| T stage | |
| Tis and/or T1a | 8 (17.0) |
| T1b | 9 (19.1) |
| T2 | 26 (55.3) |
| T3 | 4 (8.5) |
| N stage | |
| Nx | 2 (4.3) |
| N0 | 37 (78.7) |
| N1 | 8 (17.0) |
| Histologic differentiation | |
| Well differentiation | 15 (31.9) |
| Moderate differentiation | 22 (46.8) |
| Poor differentiation | 10 (21.3) |
| Vessel invasion | 7 (14.9) |
| Perineural invasion | 7 (14.9) |
| Adjuvant therapy | 10 (21.3) |
After confirmation of diagnosis and cystic duct status by frozen-section examination, 7 patients were converted to open radical surgery, owing to edema and errhysis of the hepatoduodenal ligament in 4 patients and positive cystic duct margin in 1 patient; open curative surgery was decided upon for the other 2 patients after communication with a family member during the intraoperative period (Figure 1).
Mean operating time was 233±51 min with mean blood loss of 177±340 mL in patients with laparoscopic radical resection. Only one patient, who lost 2,100 mL of blood, required blood transfusion perioperatively because of resection of the gallbladder bed. The pringle maneuver was not required in any of the patients. The procedure was completed laparoscopically without conversion in all patients. The mean hospital stay was 12±7 days with no 30-day mortality (Table 2).
Postoperative complications occurred in 4 patients (Table 2). The first patient developed bile leak and complained of abdominal pain and fever 5 days after the operation. Ultrasonography showed a biloma in the right sub-hepatic space, which was drained by placing a central venous catheter percutaneously with antibiotic cover, and she recovered without requiring surgical intervention. The second patient returned to the emergency department 10 days after discharge, complaining of abdominal pain. CT showed a hematoma around the gallbladder bed space, and this patient recovered uneventfully after conservative treatment. Right infrapulmonary effusion and cerebral hemorrhage occurred in one patient respectively, who recovered after conservative treatment.
Oncologic outcomes of patients with pathologically proven GBC
Only patients with GBC who received totally laparoscopic treatment were enrolled for further pathological and survival analysis. The cohort comprised 13 men and 34 women with the median age of 66 (range, 23–80) years.
Resected liver and cyst margins were free in all patients. The mean number of lymph nodes retrieved was 4±3 (range, 1–12). In patients who underwent radical resection, pathological stage was pT1bN0 in 7, pT2N0 in 20, pT2N1 in 6, pT3N0 in 2, and pT3N1 in 2. Ten patients were given gemcitabine/5-fluorouracil-based adjuvant chemotherapy (Table 2).
Median follow-up was 51 (range, 14–110) months. Of the 11 patients who died during the follow-up period, 4 had lymph node-positive disease, 2 suffered gallbladder rupture during LC, and 1 had a T3 lesion; one patient who did not return to Peking University Third Hospital for regular re-examination had no radiological or pathological evidence of metastasis, while in the remainder we detected metastatic lymph node disease in 4 patients, multiple metastasis in 4, peritoneal disease in 2, and liver metastasis in 1 (Table 3).
3.
Recurrence patterns after laparoscopic treatment of gallbladder cancer
| No. | Age
(year), sex |
Gallbladder
with high tension/ rapture |
Tumor
location |
Elevated
CA19-9 |
T
stage |
Lymph
node metastasis |
Vessel/Neural
invasion |
Adjuvant therapy | Site of recurrence | Time to
recurrence (month) |
Survival duration (month) | Status |
| CA19-9, cancer antigen 19-9; F, female; M, male; Y, yes; N, no; P, peritoneal side; L, liver side; M, mixed type with both liver and peritoneal location. | ||||||||||||
| 1 | 66, F | N/N | L | Y | 2 | Y | Y/N | N | Liver and celiac lymph node | 10 | 26 | Dead |
| 2 | 63, F | Y/N | P | Y | 2 | N | Y/N | N | Liver | 10 | 67 | Dead |
| 3 | 50, F | Y/N | L | N | 2 | Y | N/N | Y | Retroperitoneal lymph node | 18 | 30 | Dead |
| 4 | 37, M | N/N | M | Y | 3 | Y | Y/Y | N | – | – | 5 | Dead |
| 5 | 73, F | Y/Y | M | N | 1b | N | N/N | Y | Peritoneal metastasis | 9 | 25 | Dead |
| 6 | 72, M | N/N | P | N | 2 | Y | N/N | N | Retroperitoneal lymph node | 48 | 71 | Dead |
| 7 | 66, F | Y/Y | M | N | 2 | N | N/N | Y | Peritoneal metastasis | 12 | 18 | Dead |
| 8 | 66, F | N/N | P | N | 2 | N | Y/Y | N | Retropancreatic lymph node | 8 | 10 | Dead |
| 9 | 51, M | N/N | L | N | 2 | N | N/N | N | Liver and retroperitoneal lymph node | 30 | 42 | Dead |
| 10 | 67, F | N/N | L | Y | 2 | N | N/N | Y | Retroperitoneal lymph node | 19 | 29 | Dead |
| 11 | 69, F | N/N | M | Y | 2 | N | N/N | N | Liver and retroperitoneal lymph node | 5 | 8 | Dead |
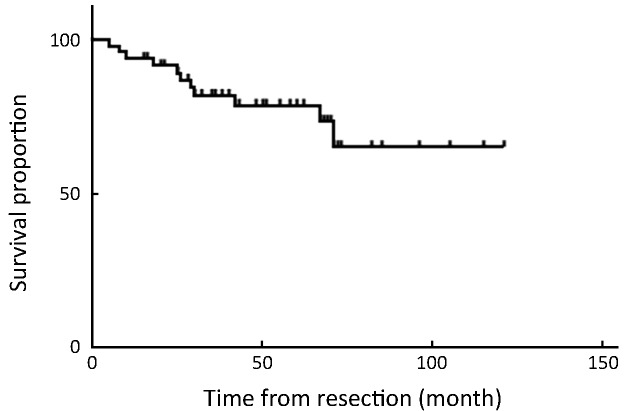
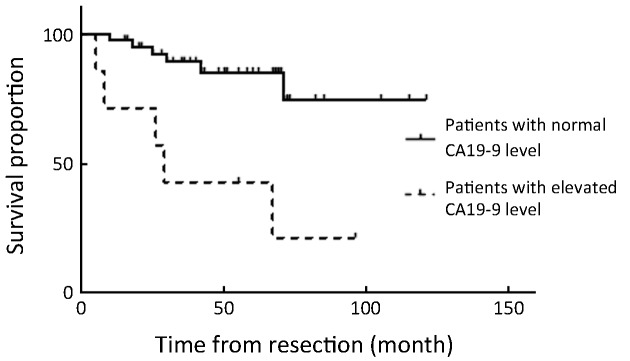
Overall 5-year survival rate was 68.8%. All patients with stage ≤T1b have survived without recurrence until now, except for 1 patient with T1b who died of peritoneal recurrence (Table 3). The disease-specific actual survival rate in 22 patients whose postoperative follow-up period exceeded 5 years was 72.7% at 5 years, 52.5% of this cohort having T2 or T3 stage lesions. In our series there were 7 patients with elevated cancer antigen 19-9 (CA19-9); patients with normal CA19-9 achieved 85% disease-specific survival, whereas those with elevated CA19-9 attained only 29% survival, with relapse occurring in 5 of 7 patients, a statistically significant difference (P=0.001) (Figure 4, 5).
4.
Overall 5-year survival rate of 47 patients who underwent totally laparoscopic treatment.
5.
Overall 5-year survival rate of 47 patients who underwent totally laparoscopic treatment based on preoperative cancer antigen 19-9 (CA19-9) level (P=0.001).
Discussion
Although entirely laparoscopic treatment for suspected GBC has been explored in different centers in several countries over the past decade (10,11,13-15) and has achieved acceptable results with regard to feasibility of technique and oncologic outcomes, the present study is the largest long-term prospective series from a single center, which in comparison with multicenter or retrospective studies decreases the bias related to patient selection and treatment and allows for a more thorough assessment of outcomes.
First, preoperative CT and/or MRI combined with intraoperative frozen pathological examination were feasible in directing totally laparoscopic treatment for suspected GBC confined to the gallbladder wall. Currently there are insufficient definitive data available for the purpose of specifying an imaging and workup protocol for patients to undergo totally laparoscopic treatment for suspected GBC confined to the gallbladder wall. Only 2 prospective long-term studies examining the role of laparoscopic treatment for suspected GBCs introduced laparoscopic ultrasonography (LUS) and/or endoscopic ultrasonography (EUS) to their protocols (10,11). However, in this regard our study differs from these 2 studies. There are no patients with T stage greater than T3 in our cohort and only 4 patients with stage T3, indicating the feasibility of preoperative CT and/or MRI in order to exclude patients with extensive liver invasion or involvement of the extrahepatic bile duct. Furthermore, as GBC confined to the wall has a tendency to resemble benign gallbladder lesions such as adenoma, adenomyosis, or xanthogranulomatous cholecystitis, intraoperative frozen-section examination of the pathology and depth of tumor invasion before a radical operation should help to avert surgical morbidity in patients with a benign lesion or early GBC of less than stage T1b. In our cohort there were 100 cases with benign diseases and 8 patients with Tis/T1a malignant lesions that precluded extended resection for suspected GBC confined to the wall. While for patients diagnosed with malignant lesions, intraoperative frozen-section examination of the gallbladder usually underestimated the stage of GBC, only one case was overdiagnosed. Thus the frozen-section diagnosis is reliable in distinguishing cancer from benign lesions, but unsatisfactory in evaluating the depth of invasion. These results are in accordance with those of Yamaguchi et al. (16). However, the patients scheduled to receive radical resection were not obviously influenced by such bias. In fact most of the underestimated patients were stage T1b. Fortunately, in our cohort only 2 patients with final T1b stage who did not undergo radical resection were assessed as T1a and not given a T stage, respectively, upon frozen gallbladder examination. Therefore in a scenario of diagnostic malignancy with stage T1b or greater, radical en bloc resection is advisable if the expertise is available. Given that LUS and EUS are not widely available, our protocol represents an alternative for selection of suspected GBCs confined to the gallbladder wall, especially in the context of a promising novel method described by Park et al. (17) that combined intraoperative specimen ultrasonography with frozen-section examination acquired satisfactory results in determining the depth of gallbladder invasion.
Second, initial simple LC is only appropriate for selected patients with clear margins and an unbroken gallbladder, whereas en bloc gallbladder resection along with the gallbladder plate or surrounding liver is recommended for patients with high-tension gallbladder or tumor located on the hepatic side. In our study cohort, simple LC was applied to all suspected GBCs confined to the wall during the early period; unfortunately gallbladder rupture occurred in 3 patients, and 2 had peritoneal recurrence at follow-up. Detailed analysis of these 2 patients revealed floating tumors in the gallbladder. As previous studies have also shown that perforation of the gallbladder during LC can be responsible for peritoneal metastasis and a worse prognosis (18), an intact gallbladder is warranted, especially one with high tension. Although laparoscopic en bloc gallbladder resection is also employed by other authors (10,11), whereby especially the technique described by Itano et al. avoids grasping the gallbladder and thus protecting it from perforation (10), in some patients with high-tension gallbladder the exposure of Calot’s triangle is difficult. For this reason, initial application of simple LC should be prudent in patients with high-tension gallbladder or with tumor located on the hepatic side, and even conversion to open surgery should be considered for some high-tension gallbladders.
Third, totally laparoscopic hepatoduodenal ligament dissection combined with limited liver wedge resection is feasible and produced acceptable oncologic results. Postoperative complications occurred in 4 patients who recovered without necessitating any surgical intervention, indicating the feasibility and safety of radical laparoscopic treatment of GBC confined to the gallbladder wall. The extent of liver resection for GBC confined to the wall is controversial, even in patients with stage T2 (19). In our cohort limited liver wedge resection was applied to all patients, none of whom experienced local liver recurrence. A more limited method including a 2-mm gallbladder bed was employed in the cohort of Yoon et al. (11) and produced similar results. Laparoscopic lymph node dissection was performed for most patients diagnosed with malignancy after intraoperative frozen-section examination unless there were microscopic indications highly suggestive of Tis/T1a or serious comorbidity. In our series, a mean harvested lymph node count of 4±3 was achieved (range, 1–12), meeting the Western criteria of adequate lymphadenectomy (20,21) while failing to meet the more strict criteria reported from Korea (22). The overall 5-year survival rate was 68.8%. The disease-specific actual survival rate of 22 patients whose postoperative follow-up period exceeded 5 years was 72.7% at 5 years, 52.5% of this cohort having stage T2 or T3 lesions. It is widely appreciated that extensive lymphadenectomy for GBC with lymph node metastasis exhibiting aggressive features and poor prognosis (21,23) cannot be justified for most patients (21). However, there is no reliable technique that can preoperatively distinguish patients with or without lymph node metastasis. Fortunately, our data and the other 5 studies relating to laparoscopic treatment for GBC demonstrated that the lymph node metastasis rate is low in preoperative suspected early GBC, even in stage T2 lesions (10,11,14,15,24). Of the 68 T2 patients from these previous 5 studies, only 11 had lymph node metastasis. Moreover, our data show that preoperative elevated CA19-9 may serve as an indicator for patients at high risk of relapse. In our series, of the 7 patients with elevated CA19-9 only 2 (29%) achieved disease-specific survival, with relapse occurring in the other 5. In brief, relatively conservative laparoscopic radical surgery is feasible and beneficial for most of the selected early GBC patients.
Two previous prospective studies have shown acceptable oncologic results after radical laparoscopic treatment (10,11). The first evaluated 19 patients with suspected T2 GBC treated at two Japanese centers; the second included 83 patients (45 of whom proved to be GBCs) who underwent totally laparoscopic treatment. Recurrence was found in none and 4 of these patients, respectively, and mainly involved patients with T2N1 disease. However, the present study differs from these 2 previous ones in several aspects. To our knowledge,this is the largest series from a single center, which, in comparison with the first multicenter study, decreases bias related to patient selection and treatment and allows for a more thorough assessment of outcomes. In addition, this is the first study to simply combine preoperative CT/MRI and intraoperative frozen-section examination as a protocol for selection and evaluation of patients with suspected GBC confined to the gallbladder wall. More importantly, in our study we discovered that preoperative elevated CA19-9 may serve as an indicator for high risk of relapse in patients with suspected GBC confined to the wall. Furthermore, evidence published by the Memorial Sloan-Kettering Cancer Center shows potential benefit from the adoption of preoperative neoadjuvant chemotherapy (23), which may indicated for those with preoperative elevated CA19-9.
Several limitations of this study must be recognized, especially in stage T2 GBCs. First, the question of whether hepatoduodenal ligament dissection is appropriate for patients with stage T2 GBC, although suggested by the data presented herein, cannot be answered definitively by this study, given its limited data and the absence of a control group. Second, this study does not change the recommendation for hepatic segment 4b/5 resection for stage T2 GBC, but rather argues for a re-evaluation of current treatment recommendations. In addition, the number of patients in stage T2 was low, and the results must be confirmed in larger cohorts.
Conclusions
The favorable long-term outcomes demonstrated in this study show the feasibility of combined CT/MRI and frozen examination in identifying GBC confined to the gallbladder wall, confirm the oncologic safety of totally laparoscopic treatment in selected patients with GBC, and support the preoperative CA19-9 level as a potentially distinguishing factor for long-term survival after laparoscopic treatment for GBC confined to the wall.
Acknowledgements
The authors would like to give special thanks to Mr. Yimu Jia for his valuable contribution in preparing the intraoperative illustrations for the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isambert M, Leux C, Métairie S, et al. Incidentally-discovered gallbladder cancer: When, why and which reoperation? J Visc Surg. 2011;148:e77–84. doi: 10.1016/j.jviscsurg.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Lee JY, Baek JH, et al. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am J Roentgenol. 2015;204:W150–9. doi: 10.2214/AJR.13.11992. [DOI] [PubMed] [Google Scholar]
- 5.Jang JY, Kim SW, Lee SE, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009;250:943–9. doi: 10.1097/SLA.0b013e3181b5d5fc. [DOI] [PubMed] [Google Scholar]
- 6.Xu Q, Tao LY, Wu Q, et al. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB (Oxford) 2012;14:373–81. doi: 10.1111/j.1477-2574.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wullstein C, Woeste G, Barkhausen S, et al. Do complications related to laparoscopic cholecystectomy influence the prognosis of gallbladder cancer? Surg Endosc. 2002;16:828–32. doi: 10.1007/s00464-001-9085-7. [DOI] [PubMed] [Google Scholar]
- 8.Matthews JB. Planned laparoscopic approach for early-stage gallbladder cancer: the glass is one-third full. Arch Surg. 2010;145:133. doi: 10.1001/archsurg.2009.262. [DOI] [PubMed] [Google Scholar]
- 9.Hou C, Xu Z, Lu S, et al. A clinical study on unsuspected gallbladder carcinoma identified during or following laparoscopic cholecystectomy and immediate or two stage radical resection of the gallbladder carcinoma. Zhonghua Pu Tong Wai Ke Za Zhi (in Chinese) 2008;23:16–9. [Google Scholar]
- 10.Itano O, Oshima G, Minagawa T, et al. Novel strategy for laparoscopic treatment of pT2 gallbladder carcinoma. Surg Endosc. 2015;29:3600–7. doi: 10.1007/s00464-015-4116-y. [DOI] [PubMed] [Google Scholar]
- 11.Yoon YS, Han HS, Cho JY, et al. Is laparoscopy contraindicated for gallbladder cancer? A 10-year prospective cohort study. J Am Coll Surg. 2015;221:847–53. doi: 10.1016/j.jamcollsurg.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th editon. New York: Springer, 2010.
- 13.Agarwal AK, Javed A, Kalayarasan R, et al. Minimally invasive versus the conventional open surgical approach of a radical cholecystectomy for gallbladder cancer: a retrospective comparative study. HPB (Oxford) 2015;17:536–41. doi: 10.1111/hpb.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palanisamy S, Patel N, Sabnis S, et al. Laparoscopic radical cholecystectomy for suspected early gall bladder carcinoma: thinking beyond convention. Surg Endosc. 2016;30:2442–8. doi: 10.1007/s00464-015-4495-0. [DOI] [PubMed] [Google Scholar]
- 15.Shirobe T, Maruyama S. Laparoscopic radical cholecystectomy with lymph node dissection for gallbladder carcinoma. Surg Endosc. 2015;29:2244–50. doi: 10.1007/s00464-014-3932-9. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Chijiiwa K, Saiki S, et al. Reliability of frozen section diagnosis of gallbladder tumor for detecting carcinoma and depth of its invasion. J Surg Oncol. 1997;65:132–6. doi: 10.1002/(sici)1096-9098(199706)65:2<132::aid-jso11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Kim YH, Kim H, et al. Determining the extent of cholecystectomy using intraoperative specimen ultrasonography in patients with suspected early gallbladder cancer. Surg Endosc. 2016;30:4229–38. doi: 10.1007/s00464-015-4733-. [DOI] [PubMed] [Google Scholar]
- 18.Z’Graggen K, Birrer S, Maurer CA, et al. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery. 1998;124:831–8. [PubMed] [Google Scholar]
- 19.Horiguchi A, Miyakawa S, Ishihara S, et al. Gallbladder bed resection or hepatectomy of segments 4a and 5 for pT2 gallbladder carcinoma: analysis of Japanese registration cases by the study group for biliary surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:518–24. doi: 10.1007/s00534-012-0584-9. [DOI] [PubMed] [Google Scholar]
- 20.Coburn NG, Cleary SP, Tan JC, et al. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008;207:371–82. doi: 10.1016/j.jamcollsurg.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Ito K, D’Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320–5. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Chong JU, Lim JH, et al. Optimal assessment of lymph node status in gallbladder cancer. Eur J Surg Oncol. 2016;42:205–10. doi: 10.1016/j.ejso.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Creasy JM, Goldman DA, Dudeja V, et al. Systemic chemotherapy combined with resection for locally advanced gallbladder carcinoma: surgical and survival outcomes. J Am Coll Surg. 2017;224:906–16. doi: 10.1016/j.jamcollsurg.2016.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho SY, Park SJ, Kim SH, et al. Comparative analysis between clinical outcomes of primary radical resection and second completion radical resection for T2 gallbladder cancer: single-center experience. World J Surg. 2010;34:1572–8. doi: 10.1007/s00268-010-0522-4. [DOI] [PubMed] [Google Scholar]