Abstract
Aim
Increasing evidence has shown that long noncoding RNAs (lncRNAs) ANRIL may function as oncogenes in various types of malignancies. However, there is still a lack of knowledge concerning its role in osteosarcoma (OS). In this study, we aimed to investigate the influence of ANRIL on cell proliferation and invasion of OS and to determine its association with clinicopathological features of the patients.
Methods
The tumor specimens and the adjacent normal tissues were collected from 57 OS patients and the expression level of ANRIL was quantified by RT-qPCR. High expression of ANRIL was defined as a relative mRNA expression of > 1.5 fold (tumor/normal). Knockdown of ANRIL was performed in human OS cell lines to investigate its influence on cell proliferation, apoptosis and invasion. In addition, expression of downstream genes in the transfected cells were determined by Western blot.
Results
The expression level of ANRIL was significantly increased in OS tissues than in the adjacent normal tissues. 33 patients were included in the high expression group and the other 24 patients were included in the normal expression group. ANRIL expression was significantly associated with tumor size (5.7 cm ± 2.4 cm vs. 4.3 cm ± 1.7 cm, p = 0.02) and the 5-year survival rate (51.5% vs. 79.1%, p = 0.03). Knockdown of ANRIL could significantly induce cell apoptosis and inhibit cell proliferation and invasion. Moreover, knockdown of ANRIL could significantly decrease the expression level of phosphorylated PI3K and AKT in OS cells.
Conclusions
Upregulated expression of ANRIL is associated with the tumor development and prognosis of OS. ANRIL may regulate the function of OS cells through the AKT pathway.
Keywords: Long noncoding RNA, ANRIL, Osteosarcoma, AKT
1. Introduction
Osteosarcoma (OS) is one of the most common primary bone tumors that occur during the childhood [1], [2]. Currently therapeutic modalities of OS include surgical resection of the tumor combined with preoperative and postoperative chemotherapy, with the overall 5-year disease-free survival rate ranging from 60% to 70% [3], [4]. Although the survival rate has improved dramatically with the advance of chemotherapy, distant metastases may still develop in some patients whose survival rate has not improved much. Therefore, identification of biomarkers involved in the development of OS could facilitate personalized treatment strategy and contribute to better outcomes.
In recent years, increasing evidence has shown that long noncoding RNAs (lncRNAs) may function as oncogenes or tumor suppressors in various types of malignancies [5], [6], [7], [8]. Initially identified from familial melanoma patient, lncRNA ANRIL has been reported to be a risk factor in several human cancers such as breast, colorectal and bladder cancer [9], [10], [11]. Zou et al. [12] found that ANRIL could promote progression of nasopharyngeal via reprograming cell glucose metabolism. Sun et al. [13] reported that ANRIL is upregulated in colorectal cancer tissues, and is associated with cancer cell pathogenesis. Zhang et al. [14] found that ANRIL could promote the progression of cervical cancer via PI3K/AKT pathway and act as an indicator of poor prognosis. Chen et al. [15] reported that over-expression of ANRIL can promote pancreatic cancer by activating the ATM-E2F1 pathway.
Although ANRIL functions as a vital oncogene in many cancers, to the best of our knowledge, there is still a lack of knowledge concerning its role in OS. Wei et al. [16] reported that ANRIL is involved in hypoxia-induced aggressive phenotype in OS. In the present study, we analyzed the expression of ANRIL in OS tissues to determine its association with clinicopathological features and prognosis of the patients. In addition, loss-of-function experiments were performed to investigate the influence of ANRIL on cell proliferation and invasion of OS [16].
2. Methods
2.1. Patients and tissue samples
Under the approval of the Ethics Committee, 57 OS patients who underwent resection surgery from June 2008 to May 2015 were included in this study. All the patients had a minimum follow-up of 2 years. No chemotherapy was prescribed to the patient before the surgery. The tumor specimens and the corresponding adjacent normal tissues were collected for each patient. All the samples were immediately frozen and stored in liquid nitrogen for RNA extraction. Baseline characteristics of the patients were recorded, including age, gender, Enneking stage, tumor size, distant metastasis and survival rate.
2.2. Cell culture
Human OS cell lines MNNG/HOS and U2 OS were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin. Both cell lines were cytogenetically tested and authenticated before they were frozen. Cells were maintained in a humidified incubator at 37 °C with 5% CO2.
2.3. RNA extraction and qRT-PCR analysis
Total RNA was extracted from tissue samples with Trizol reagent (Invitrogen) following the instruction of the manufacture. The expression level of ANRIL was quantified using SYBR Master Mixture (TAKARA, Tokyo, Japan) on the LightCycler 480 (Roche Applied Science, Mannheim, Germany). The primers were as follows: forward 5′- TGCTCTATCCGCCAATCAGG -3′, reverse 5′- GGGCCTCAGTGGCACATACC -3′ for ANRIL, and forward 5′- GAGTCAACGGATTTGGTCGT -3′, reverse 5′ -TTGATTTTGGAGGGATCTCG- 3′ for Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) that was used as the internal control. Relative mRNA expression was analyzed using the ∆∆Ct method.
2.4. siRNA transfection
The siRNA targeting ANRIL (siANRIL) was purchased from Shanghai GenePharma Co., Ltd. The sequence of siANRIL was as follows: GGUCAUCUCAUUGCUCUAU. The negative control denoted as siCtrl was nonhomologous to any human genome sequence. The MNNG/HOS and U2 OS cells were plated in 6-well plates for 18 h and then transfected with 20 nM of the RNA duplex and 5 μL of Lipofectamine RNAiMAX (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions. The knockdown efficiency was evaluated with qRT-PCR as mentioned above.
2.5. Cell proliferation assays
Transfected cells were plated in 96-well plates and cultured for 5 days. For each day, the well was added with MTT (20 μL of 5 mg/mL; Genview) and incubated for 4 h at 37 °C. After the incubation, 200 μL DMSO was added to the cultured cells to dissolve the crystals. To test the cell proliferation, 2 × 103 cells were plated in 96-well plates at 72 h after transfection. The absorbance at 490 nm was read with the Spectramax M5 (Molecular Devices, Sunnyvale, USA) to count the cells for 5 consecutive days.
2.6. Cell apoptosis assay
Flow cytometry (FCM) analysis was used to determine the cell apoptosis. Briefly, after the incubation of transfected cell for 4 days, cell suspensions were generated and plated in 6-cm dishes for further culturing. Subsequently, cells were collected and fixed with pre-cold 70% alcohol for at least 1 h, then washed with PBS and stained with PI buffer. Cell apoptosis was assayed by staining with Annexin V-APC (eBioscience, San Diego, CA, USA) following manufacturer's instructions and the signal was detected by FACS Calibur (Becton-Dickinson, USA). Each experiment was performed in triplicates.
2.7. Cell invasion assay
Assay of cell invasion was performed using a Transwell chamber with an 8-μm pore size (Corning, Corning, NY, USA) in a 24-well plate. 200 μl of the cell suspension medium containing 1 × 105 cells were seeded into the upper chamber coated with Matrigel. DMEM supplemented with 10% FBS (800 μL) was used as the chemoattractant in lower chamber. After 48 h, the cells on the top surface of the membrane were mechanically removed. The cells that had moved to the basal side of the membrane were fixed in 95% ethanol and stained with 0.2% crystal violet solution, which was then imaged using an IX71 inverted microscope (Olympus, Tokyo, Japan).
2.8. Western blot analysis
Expression of 4 target proteins including p-PI3K, PI3K, p-AKT and AKT were determined by Western blot. Total cell proteins were prepared using cell lysis buffer. Equal amounts of cell lysates were separated on a 10–12% SDS polyacrylamide gel and electro-transferred to polyvinylidene fluoride membranes (Immobile P; Millipore). The membranes were then blocked with 5% nonfat dry milk in TBST for 1 h and incubated with the primary antibody (Abcam) overnight at 4 °C, followed by incubation with second antibody (Cell Signaling Technology) for 1 h at 37 4 °C. The signals were detected by enhanced chemiluminescence (Thermo). GAPDH was used as a loading control.
2.9. In vivo tumor growth
6-week-old male BALB/c nude mice were anesthetized with a 1:1 mixture of isoflurane gas and oxygen. MNNG/HOS cells transfected with siANRIL or siCtrl were injected into 20 nude mice subcutaneously. After 4 weeks of inoculation, the tumors were harvested and weighed for each mouse. Animals were purchased from the Shanghai Institute of Biological Sciences (Shanghai, China). Under the approval of the local Institutional Animal Care and Use Committee, all procedures were performed in compliance with the guidelines for the use of laboratory animals.
2.10. Statistical analysis
The statistical analysis was carried out with the SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). High expression of ANRIL was defined as a relative mRNA expression of > 1.5 fold (tumor/normal). Patients were classified into high expression group and normal expression group. The inter-group comparison of clinical parameters of the patient was analyzed using the Student's t-test or the Chi-square test. Survival analysis was performed using the Kaplan-Meier method and the log-rank test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Overexpression of ANRIL was correlated with the prognosis of the patients
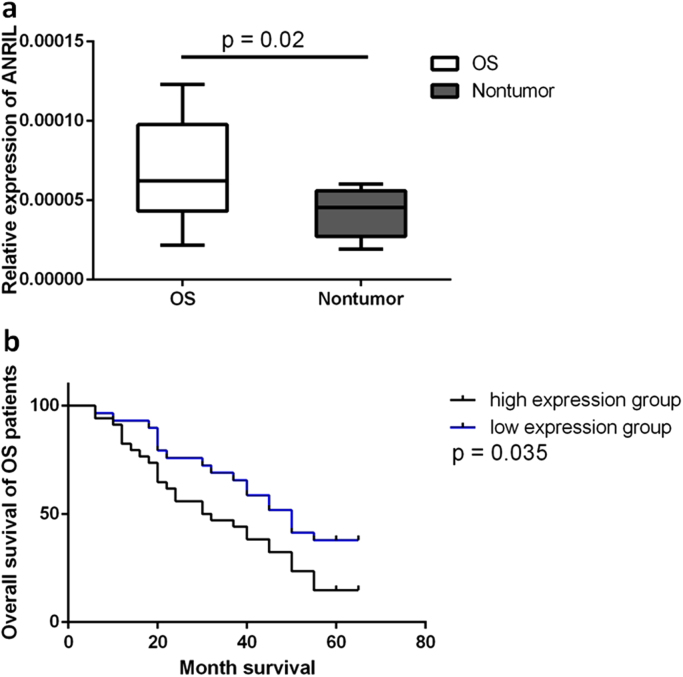
The expression level of ANRIL was significantly increased in OS tissues than in the adjacent normal tissues (Fig. 1a). 33 patients were included in the high expression group and the other 24 patients were included in the normal expression group. As shown in Table 1, we found that ANRIL expression was significantly associated with tumor size (5.7 cm ± 2.4 cm vs. 4.3 cm ± 1.7 cm, p = 0.02) and the 5-year survival rate (51.5% vs. 79.1%, p = 0.03). The survival curve showed that high ANRIL expression was a significant predictor of a reduced overall survival rate (Fig. 1b). No significant association was found between ANRIL expression and other clinical features such as Enneking stage and distant metastasis (Table 1).
Fig. 1.
Overexpression of ANRIL was associated with the prognosis of osteosarcoma (OS). (a) Quantification real-time PCR analysis showed significantly higher ANRIL mRNA level in OS tissues than normal tissues. (p < 0.001) (b) Log-rank tests showed that patients with high ANRIL expression had significantly lower survival rate than patients with normal ANRIL expression.
Table 1.
Relationship between ANRIL expression and clinical features of osteosarcoma.
| Number | ANRIL expression |
P | ||
|---|---|---|---|---|
| Normal expression (n = 24) | High expression (n = 33) | |||
| Age (years) | ||||
| > 20 | 26 | 11 (45.8%) | 15 (45.5%) | 0.92 |
| ≤ 20 | 31 | 13 (54.2%) | 18 (54.5%) | |
| Mean ± S.D. | 41.5 ± 21.3 | 42.6 ± 22.5 | 0.85 | |
| Gender | ||||
| Male | 35 | 13 (54.2%) | 22 (66.7%) | 0.33 |
| female | 22 | 11 (45.8%) | 11 (33.3%) | |
| Enneking stages | ||||
| I | 10 | 3 (12.5%) | 7 (21.2%) | 0.45 |
| IIA | 15 | 6 (25.0%) | 9 (27.3%) | |
| IIB | 24 | 10 (41.7%) | 14 (42.4%) | |
| III | 8 | 5 (20.8%) | 3 (9.1%) | |
| Tumor size (cm) | ||||
| > 5 | 27 | 9 (37.5%) | 18 (54.5%) | 0.21 |
| ≤ 5 | 30 | 15 (62.5%) | 15 (45.5%) | |
| Mean ± S.D. | 4.3 ± 1.7 | 5.7 ± 2.4 | 0.02 | |
| Tumor metastasis | ||||
| Presence | 25 | 7 (29.2%) | 18 (54.5%) | 0.06 |
| Absence | 32 | 17 (70.8%) | 15 (45.5%) | |
| Survival rate (%) | ||||
| Death | 21 | 5 (20.8%) | 16 (48.5%) | 0.03 |
| Survival | 36 | 19 (79.2%) | 17 (51.5%) | |
3.2. Knockdown of ANRIL significantly induced cell apoptosis and inhibited cell proliferation in vivo and in vitro
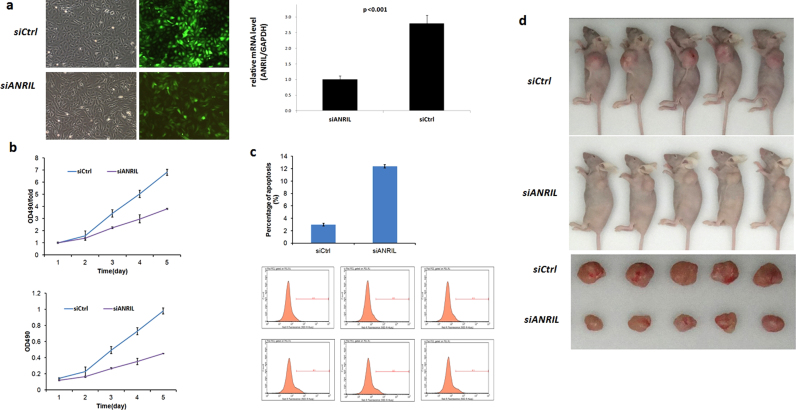
Stable clones of MNNG/HOS and U2OS cells transfected with siANRIL or siCtrl were developed to further investigate the biological role of ANRIL in OS. A knockdown efficiency of more than 70% was confirmed by qRT-PCR (Fig. 2a). Knockdown of ANRIL was found to result in a significantly decreased cell proliferation rate (p < 0.001) (Fig. 2b). Moreover, FCM analysis showed remarkably increased cell apoptosis following knockdown of ANRIL in both cell lines (p < 0.001) (Fig. 2c). Nude mice injected with siANRIL-transfected MNNG/HOS cells were observed to generate obviously smaller xenografts as compared with those in the control group (Fig. 2d).
Fig. 2.
Knockdown of ANRIL induced cell apoptosis and inhibited cell proliferation of OS. (a) MNNG/HOS cells were transfected with lentivirus expressing siANRIL or shCtrl. qRT-PCR showed significantly decreased ANRIL expression in the cell lines transfected with siANRIL. (b) ANRIL-knockdown cells show significantly lower proliferation. (c) Flow cytometry showed an increased apoptosis rate of shANRIL-transfected cells as indicated by the representative diagrams of the annexin-V/PI assay. (d) MNNG/HOS cells transfected with the siANRIL or siCtrl were injected subcutaneously into the nude mice. At the 28th day after inoculation, mice of the siANRIL group were observed to have remarkably smaller tumor size than those in the control group (p < 0.05).
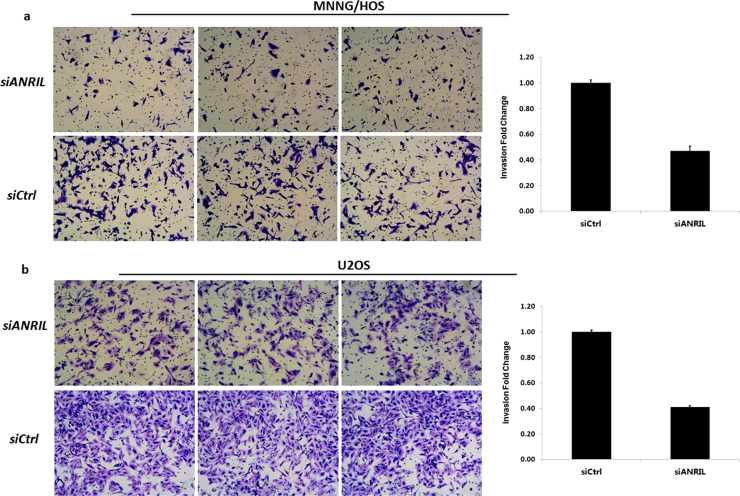
3.3. Knockdown of ANRIL remarkably inhibited cell invasion
For MNNG/HOS cell lines, the invasion rate of the control cells was 2.2 fold of that of the transfected cells (p < 0.001) (Fig. 3a). For U2OS cell lines, the invasion rate of the control cells was 2.5 fold of that of the transfected cells (p < 0.001) (Fig. 3b).
Fig. 3.
Knockdown of ANRIL inhibited the invasion of OS cells. Transwell assay showed a significantly reduced number of invasion cells for both MNNG/HOS and U2 OS cell lines. (a) For MNNG/HOS cell lines, the invasion rate of the control cells were 2.2 times higher than that of the transfected cells (p < 0.001). (b) For U2 OS cell lines, the invasion rate of the control cell were 2.5 times higher than that of the transfected cells (p < 0.001).
3.4. The relationship between ANRIL and PI3K/AKT pathway
To determine the mechanism by which ANRIL regulated the progression of OS cells, western blot analysis was used to explore the influence of ANRIL knockdown on the PI3K/AKT pathway. Our data showed that knockdown of ANRIL could significantly decrease the expression levels of phosphorylated PI3K (p-PI3K) and AKT (p-AKT) in OS cells (Fig. 4).
Fig. 4.
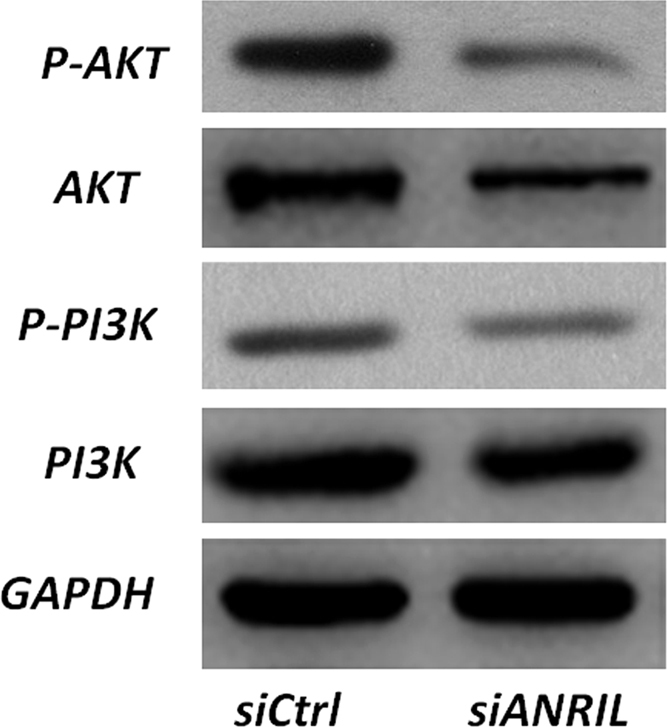
ANRIL may influence the function of OS cells through the AKT pathway. Western blot analysis was used to detect the expression of PI3K, AKT, p-AKT, and P-PI3k in both ANRIL-silenced and siCtrl-transfected MNNG/HOS cells. P-PI3k and p-AKT were confirmed to have remarkably lower protein expression in the ANRIL-silenced OS cells.
4. Discussion
ANRIL is transcribed in anti-sense direction of the primary INK4 and ARF transcripts [17]. Recently, emerging evidence has shown that upregulated expression of ANRIL is associated with the tumorigenesis of several types of cancers [9], [10], [11]. For the first time, we observed that ANRIL expression was upregulated in OS tissues. Patients with higher expression of ANRIL were found to have significantly larger tumor size. In addition, as indicated by a decreased survival time, high expression of ANRIL was also found to be associated with a poor prognosis of OS patients. These findings preliminarily supported that upregulated ANRIL expression could be involved in the development and progression of OS.
It has been well documented that high-proliferative activity can add to the risk of tumor progression [18], [19]. The influence of ANRIL on the viability and proliferation of cancer cells has been widely reported in previous studies [20], [21]. Zhang et al. [20] reported that overexpression of ANRIL in gastric cancer could promote cancer cell proliferation by epigenetic suppression of miR-449a/miR-99a. Nie et al. [21] reported that in non–small cell lung cancer cell, ANRIL could promote cell proliferation and inhibit apoptosis by silencing KLF2 and P21 expression. In this study, we performed knockdown of ANRIL in OS cell lines and observed a significant decrease of cell proliferation. The in vivo experiment showed that ANRIL-knockdown cells could generate smaller tumor xenografts in nude mice. Resistance to cell death could act as a factor of tumor proliferation. It has been reported that silencing of ANRIL could promote cell apoptosis in multiple cancer cell lines [14], [21]. Similarly, we confirmed in this study that knockdown of ANRIL could result in a significant increase of OS cell apoptosis.
Previous studies have shown that ANRIL can regulate tumor cell invasion and metastasis in various cancer entities [22], [23]. Qiu et al. [22] reported that silencing of ANRIL in ovarian cancer suppressed migration and invasion of the cells. Huang et al. [23] observed that knockdown of ANRIL in vitro and in vivo could reduce cell invasion and induce cell apoptosis. In this study, we found that the invasion of OS cell was obviously inhibited after the knockdown of ANRIL. To investigate the potential regulatory pathway through which ANRIL exerted its influence on OS cells, we investigated the gene expression alteration of MNNG/HOS cells transfected with siANRIL. We found that the knockdown of ANRIL may result in significantly decreased expression of phosphorylated PI3K (p-PI3K) and AKT (p-AKT) in OS cells. Previous studies have shown that AKT pathway is involved in the cell proliferation and apoptosis of OS [24], [25]. It has recently been identified as a key regulator in the process of Epithelial-Mesenchymal Transition (EMT), which may play a critical role in tumor metastasis [26]. Therefore, we speculated that ANRIL may promote the invasion of OS cell via activation of EMT, and further investigations are warranted to clarify the underlying mechanism connecting ANRIL and the AKT pathway.
The primary limitation of this study was that the sample size of the OS patients was relatively small due to a rare incidence of this type of cancer. A multi-center study is therefore warranted to investigate the influence of ANRIL on the overall survival of OS patients with stronger statistical power.
To conclude, our study provided the first evidence demonstrating that the upregulated expression of ANRIL is associated with the tumor progression and clinical outcome of OS. Moreover, ANRIL was identified as a key regulator of cell proliferation, apoptosis and invasion in OS possibly through the AKT pathway. Our data indicated that ANRIL could be used as a prognostic marker for OS patients.
Acknowledgment
We wish to thank all the patients included in this study.
Acknowledgments
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Marko T.A., Diessner B.J., Spector L.G. Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison. Pediatr. Blood Cancer. 2016;63:1006–1011. doi: 10.1002/pbc.25963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aljubran A.H., Griffin A., Pintilie M., Blackstein M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. / ESMO. 2009;20:1136–1141. doi: 10.1093/annonc/mdn731. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G., Longhi A., Versari M., Mercuri M., Briccoli A., Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 4.Ogura K., Fujiwara T., Yasunaga H., Matsui H., Jeon D.G., Cho W.H. Development and external validation of nomograms predicting distant metastases and overall survival after neoadjuvant chemotherapy and surgery for patients with nonmetastatic osteosarcoma: a multi-institutional study. Cancer. 2015;121:3844–3852. doi: 10.1002/cncr.29575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J., Cheng L. Long non-coding RNA CCAT1/miR-148a axis promotes osteosarcoma proliferation and migration through regulating PIK3IP1. Acta Biochim. Et. Biophys. Sin. 2017;49:503–512. doi: 10.1093/abbs/gmx041. [DOI] [PubMed] [Google Scholar]
- 6.Botti G., Collina F., Scognamiglio G., Aquino G., Cerrone M., Liguori G. LncRNA HOTAIR polymorphisms association with cancer susceptibility in different tumor types. Curr. Drug Targets. 2017 doi: 10.2174/1389450118666170622091940. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y., Song D., Xiao K., Yang C., Ding Y., Deng W. LncRNA GAS5 contributes to lymphatic metastasis in colorectal cancer. Oncotarget. 2016;7:83727–83734. doi: 10.18632/oncotarget.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin Y., Li Z., Shen J., Chan M.T., Wu W.K. CCAT1: a pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif. 2016;49:255–260. doi: 10.1111/cpr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khorshidi H.R., Taheri M., Noroozi R., Sarrafzadeh S., Sayad A., Ghafouri-Fard S. ANRIL genetic variants in Iranian breast cancer patients. Cell J. 2017;19:72–78. doi: 10.22074/cellj.2017.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naemura M., Tsunoda T., Inoue Y., Okamoto H., Shirasawa S., Kotake Y. ANRIL regulates the proliferation of human colorectal cancer cells in both two- and three-dimensional culture. Mol. Cell. Biochem. 2016;412:141–146. doi: 10.1007/s11010-015-2618-5. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Fernandez M., Feber A., Duenas M., Segovia C., Rubio C., Fernandez M. Analysis of the Polycomb-related lncRNAs HOTAIR and ANRIL in bladder cancer. Clin. Epigenet. 2015;7:109. doi: 10.1186/s13148-015-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Z.W., Ma C., Medoro L., Chen L., Wang B., Gupta R. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7:61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y., Zheng Z.P., Li H., Zhang H.Q., Ma F.Q. ANRIL is associated with the survival rate of patients with colorectal cancer, and affects cell migration and invasion in vitro. Mol. Med. Rep. 2016;14:1714–1720. doi: 10.3892/mmr.2016.5409. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D., Sun G., Zhang H., Tian J., Li Y. Long non-coding RNA ANRIL indicates a poor prognosis of cervical cancer and promotes carcinogenesis via PI3K/Akt pathways. Biomed. Pharmacother. 2017;85:511–516. doi: 10.1016/j.biopha.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 15.Chen S., Zhang J.Q., Chen J.Z., Chen H.X., Qiu F.N., Yan M.L. The over expression of long non-coding RNA ANRIL promotes epithelial-mesenchymal transition by activating the ATM-E2F1 signaling pathway in pancreatic cancer: an in vivo and in vitro study. Int. J. Biol. Macromol. 2017;102:718–728. doi: 10.1016/j.ijbiomac.2017.03.123. [DOI] [PubMed] [Google Scholar]
- 16.Wei X., Wang C., Ma C., Sun W., Li H., Cai Z. Long noncoding RNA ANRIL is activated by hypoxia-inducible factor-1alpha and promotes osteosarcoma cell invasion and suppresses cell apoptosis upon hypoxia. Cancer Cell Int. 2016;16:73. doi: 10.1186/s12935-016-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Pasmant E., Laurendeau I., Heron D., Vidaud M., Vidaud D., Bieche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67:3963–3969. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- 18.Wangsa D., Ryott M., Avall-Lundqvist E., Petersson F., Elmberger G., Luo J. Ki-67 expression predicts locoregional recurrence in stage I oral tongue carcinoma. Br. J. Cancer. 2008;99:1121–1128. doi: 10.1038/sj.bjc.6604633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teufel A., Marquardt J.U., Galle P.R. Novel insights in the genetics of HCC recurrence and advances in transcriptomic data integration. J. Hepatol. 2012;56:279–281. doi: 10.1016/j.jhep.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Zhang E.B., Kong R., Yin D.D., You L.H., Sun M., Han L. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5:2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie F.Q., Sun M., Yang J.S., Xie M., Xu T.P., Xia R. Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol. Cancer Ther. 2015;14:268–277. doi: 10.1158/1535-7163.MCT-14-0492. [DOI] [PubMed] [Google Scholar]
- 22.Qiu J.J., Lin Y.Y., Ding J.X., Feng W.W., Jin H.Y., Hua K.Q. Long non-coding RNA ANRIL predicts poor prognosis and promotes invasion/metastasis in serous ovarian cancer. Int. J. Oncol. 2015;46:2497–2505. doi: 10.3892/ijo.2015.2943. [DOI] [PubMed] [Google Scholar]
- 23.Huang M.D., Chen W.M., Qi F.Z., Xia R., Sun M., Xu T.P. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell proliferation by epigenetic silencing of KLF2. J. Hematol. Oncol. 2015;8:57. doi: 10.1186/s13045-015-0153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong D., Wang Y. Knockdown of lncRNA HULC inhibits proliferation, migration, invasion, and promotes apoptosis by sponging miR-122 in osteosarcoma. J. Cell. Biochem. 2017 doi: 10.1002/jcb.26273. [DOI] [PubMed] [Google Scholar]
- 25.Jia D., Niu Y., Li D., Liu Z. LncRNA C2dat1 Promotes Cell Proliferation, Migration, and Invasion by Targeting MiR-34a-5p in Osteosarcoma Cells. Oncol. Res. 2017 doi: 10.3727/096504017X15024946480113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Grille S.J., Bellacosa A., Upson J., Klein-Szanto A.J., van Roy F., Lee-Kwon W. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]