Abstract
Introduction
Alzheimer's disease (AD) is characterized by the presence of amyloid β (Aβ) plaques, neurofibrillary tangles, and neurodegeneration, evidence of which may be detected in vivo via cerebrospinal fluid (CSF) sampling. Physical activity (PA) has emerged as a possible modifier of these AD-related pathological changes. Consequently, the aim of this study was to cross-sectionally examine the relationship between objectively measured PA and CSF levels of Aβ42 and tau in asymptomatic late-middle-aged adults at risk for AD.
Methods
Eighty-five cognitively healthy late-middle-aged adults (age = 64.31 years, 61.2% female) from the Wisconsin Registry for Alzheimer's Prevention participated in this study. They wore an accelerometer (ActiGraph GT3X+) for one week to record free-living PA, yielding measures of sedentariness and various intensities of PA (i.e., light, moderate, and vigorous). They also underwent lumbar puncture to collect CSF, from which Aβ42, total tau, and phosphorylated tau were immunoassayed. Regression analyses were used to examine the association between accelerometer measures and CSF biomarkers, adjusting for age, sex, and other relevant covariates.
Results
Engagement in moderate PA was associated with higher Aβ42 (P = .008), lower total tau/Aβ42 (P = .006), and lower phosphorylated tau/Aβ42 (P = .030). In contrast, neither light nor vigorous PA was associated with any of the biomarkers. Increased sedentariness was associated with reduced Aβ42 (P = .014).
Discussions
In this cohort, moderate PA, but not light or vigorous, was associated with a favorable AD biomarker profile, while sedentariness was associated with greater Aβ burden. These findings suggest that a physically active lifestyle may play a protective role against the development of AD.
Keywords: Alzheimer's disease, CSF biomarkers, Exercise, Physical activity, Sedentary behavior
1. Introduction
The preclinical stage of Alzheimer's disease (AD) is characterized by the emergence of pathognomonic brain changes in the absence of cognitive decline [1]. These brain alterations include amyloid β (Aβ) plaques, neurodegeneration, and neurofibrillary tangles [1]. Although these lesions are only definitively ascertained via histologic assessments at autopsy, it is now accepted that it is possible to probe their occurrence ante mortem by measuring specific cerebrospinal fluid (CSF) biomarkers intended to reflect these pathologies. These biomarkers include Aβ42, total tau (t-tau), and phosphorylated tau (p-tau), respectively [2]. Because persons in the preclinical stage of AD are yet asymptomatic, they collectively represent a unique target population for therapeutics aimed at slowing the progression of AD pathology and ultimately preventing the manifestation of clinical symptoms [3].
A vast body of work has shown that physical activity (PA) ameliorates cognitive dysfunction and the risk of dementia in the elderly [4], [5], [6], with a recent evidence review reporting that PA is the modifiable risk factor with the highest potential for arresting the increasing national prevalence of AD [7]. Spurred by encouraging data from animal models of AD, a growing number of human studies have sought to determine the extent to which PA might modulate core pathogenic markers of AD. The evolving evidence suggests that higher levels of PA associate with reduced Aβ and tau burden [8], [9], [10]. Furthermore, we found that, in a middle-aged group at increased risk for AD, those who were physically active exhibited fewer age-related alterations in Aβ deposition, glucose metabolism, hippocampal volume, and episodic memory relative to their less active peers [11]. In addition, PA may also have the potential to attenuate the adverse effects of genetic risk [12], [13] and poor diet [14] on Aβ deposition.
Although less studied, a growing body of evidence suggests that high levels of sedentary behavior (SB) may also serve as an independent risk factor for negative outcomes [15], [16]. Studies indicate that most older adults engage in high amounts of SB, up to 8.5 hours a day [17], and those at risk for AD due to family history exhibit higher levels of SB than those without a family history [18]. As such, it is especially important to examine these behaviors among those at risk for AD. Previous studies have examined SB among older adults in relation to cognitive performance [19], cerebral blood flow [20], and risk of AD [21]. However, to our knowledge, no study has yet examined the relationship between SB and CSF biomarkers in older adults at risk for AD.
Furthermore, although the foregoing studies are important for providing initial evidence for potentially disease-modifying effects of PA and SB in the AD cascade, many are limited by the use of self-report questionnaires for ascertaining such levels. These assessment approaches are vulnerable to measurement error stemming from faulty recall, social desirability, and other biases that might mask or inflate associations between PA, SB, and relevant outcomes [22], [23]. Recent evidence indicates accelerometer-based measures may be more sensitive to distinctions between PA intensities and SB than self-report and more valuable when examining AD outcomes [24]. Second, to our knowledge, no studies have assessed the association between objectively measured PA, SB, and CSF biomarkers in an asymptomatic, middle-aged cohort at risk for AD. Accordingly, the objective of this study was to investigate the relationship between accelerometer-measured PA, SB, and CSF biomarkers of AD among at-risk, late-middle-aged adults. We specifically seek to ascertain the intensity of PA most conducive to a favorable AD biomarker profile and examine whether SB confers an additional risk beyond that of low levels of PA.
2. Methods
2.1. Participants
Eighty-five cognitively normal adults from the Wisconsin Registry for Alzheimer's Prevention (WRAP) participated in this study. WRAP is a longitudinal cohort consisting of approximately 1500 late-middle-aged adults who were cognitively healthy and between the ages of 40 and 65 years at study entry [25]. The cohort is enriched with persons who have a parental history of AD and/or carry ≥1 apolipoprotein ε4 (APOE ε4) alleles. Cognitive normalcy was determined based on intact performance on a comprehensive battery of neuropsychological tests, absence of functional impairment, and absence of neurologic/psychiatric conditions that might impair cognition [11], [25]. In addition, all participants were living and functioning independently. The 85 participants for the present study were selected based on their participation in two WRAP substudies that included a 7-day PA assessment using an accelerometer and collection of CSF via lumbar puncture. Similar to the larger WRAP cohort, these individuals overrepresented persons who had a parental family history of AD (81.2%) and were APOE ε4–positive (42.4%). Table 1 details participants' additional background characteristics. All study procedures were approved by the University of Wisconsin Institutional Review Board and each participant provided informed consent before participation.
Table 1.
Participant characteristics (N = 85)
| Characteristic | Value∗ |
|---|---|
| Age at PA assessment, years | 64.31 (5.44); range: 51.58–74.23 |
| Female, no. (%) | 52 (61.2) |
| APOE ε4–positive, No. (%) | 36 (42.4) |
| Family history of AD, No. (%) | 69 (81.2) |
| Education, years | 16.73 (2.82); range: 12–25 |
| Mini-Mental State Examination | 29.32 (0.98); range: 25–30 |
| Body mass index, kg/m2 | 28.33 (5.32); range: 17.65–44.11 |
| Total cholesterol, mg/dL | 206.29 (38.70); range: 142–353 |
| HDL cholesterol, mg/dL | 65.00 (20.34); range: 34–121 |
| Systolic blood pressure, mmHg | 128.18 (17.46); range: 98–166 |
| Diastolic blood pressure, mmHg | 75.44 (9.09); range: 55–97 |
| Hypertension, No. (%) | 20 (23.5) |
| Diabetes, No. (%) | 3 (3.5) |
| Smoker (ever), No. (%) | 39 (45.9) |
| Sedentary % | 72.35 (6.58); range: 49.00–86.36 |
| Light PA % | 18.32 (4.81); range: 7.76–30.21 |
| Moderate PA % | 7.98 (3.01); range: 2.83–18.63 |
| Vigorous PA % | 1.34 (1.18); range: .06–7.30 |
| Met weekly PA recommendations, no. (%) | 30 (35.3) |
| Total accelerometer wear time per day, hours | 15.29 (1.10); range: 12.00–17.80 |
Abbreviations: PA, physical activity; APOE ε4, apolipoprotein E ε4 allele; AD, Alzheimer's disease; HDL, high-density lipoprotein.
Values indicate mean and standard deviation, unless otherwise indicated.
2.2. PA assessment
A triaxial accelerometer (Actigraph GT3X+; Actigraph LLC, Pensacola, FL) measured the intensity of movement over seven consecutive days to objectively document participants' free-living PA and SB during that time period. A period of seven days is standard to ensure participants meet standard accelerometry inclusion criteria of at least 10 hours of valid wear time per day for a minimum of 3 weekdays and 1 weekend day [26]. Participants were instructed to place the accelerometer on their hip, affixed to an elastic belt, and to wear the device during all waking hours, except while swimming or bathing. Accelerometer data (in 1-second epochs) were processed using the sojourn-3 axis method [27] to calculate time spent engaging in SB and three well-established intensity categories of PA (i.e., light, moderate, and vigorous). This method uses information from all three axes (vertical, anterior-posterior, and medial-lateral) to identify independent bout intervals—the time between starting one activity (e.g., sitting, standing, walking, running, jumping, etc.) and transitioning to another—by instances of rapid acceleration or deceleration.
Within the sojourn-3 axis method [27], metabolic equivalents (METs) are determined for each bout interval using a validated neural network approach [28], as opposed to the traditional counts per minute approach. The MET values that correspond with each intensity level are as follows: sedentary = <1.5 METs, light = 1.5–2.99 METs, moderate = 3.0–6.0 METs, and vigorous = >6.0 METs [26]. The total minutes spent participating in SB and each PA intensity category during a single day were then divided by the total minutes of wear time for that day and averaged over all valid days to compute percent of day spent in SB and each PA category.
2.3. CSF collection and analysis
Lumbar puncture for collection of CSF samples was performed in the morning after a 12-hour fast with a Sprotte 24- or 25-gauge spinal needle at L3/4 or L4/5 using gentle extraction into polypropylene syringes. Each sample consisted of 22 mL of CSF, which was then combined, gently mixed, and centrifuged at 2000 g for 10 minutes. Supernatants were frozen in 0.5-mL aliquots in polypropylene tubes and stored at −80°C. The samples were immunoassayed for Aβ42, t-tau, and p-tau (at threonine 181) using INNOTEST enzyme-linked immunosorbent assays (Fujirebio, Gent, Belgium) by board-certified laboratory technicians who were blind to clinical data and used protocols accredited by the Swedish Board for Accreditation and Conformity Assessment as previously described [29].
Using these data, we additionally computed t-tau/Aβ42 and p-tau/Aβ42 ratios. These ratios reflect multiple aspects of AD pathology in a single measure and have been shown to possess improved predictive and diagnostic power compared to individual biomarkers [2], [30], [31]. Lower CSF Aβ42 levels indicate greater amyloid burden, while higher CSF t-tau and p-tau levels signal greater AD pathology. Therefore, increased t-tau/Aβ42 and p-tau/Aβ42 ratios indicate greater pathology. The mean time interval between CSF sampling and the collection of accelerometer data was 1.52 ± 1.26 years.
2.4. Data analysis
Tests for model assumptions [32] revealed that the PA and SB variables were nonnormally distributed. Therefore, we subjected them to a Blom transformation before model fitting [33]. To address the objective of this study, that is, an investigation into whether PA and SB are associated with levels of CSF biomarkers for AD, we fitted a series of covariate-adjusted linear regression models that included terms for age, sex, APOE ε4 status, the interval between CSF collection and accelerometer data, and assay batch. Covariates were selected based on their known associations with AD risk (age, sex, and APOE ε4 status) [34] or to control for interindividual variation among measures (collection interval and assay batch). The outcome variables were the five CSF biomarkers. A separate model was fit for each of the three PA intensity categories and SB. All analyses were conducted using IBM SPSS, version 24.0. Only findings with P ≤ .05 (two-tailed) were considered significant.
3. Results
3.1. Background characteristics
Participants had an average age of 64.31 ± 5.44 years at accelerometer data collection, with a majority of individuals being women (61.2%). Thirty-six (42.4%) participants were APOE ε4 positive and 69 (81.2%) had a family history of AD. Other relevant background characteristics are reported in Table 1.
3.2. PA intensity and CSF biomarkers of AD
Light PA did not associate with any of the CSF biomarkers, although the association between Aβ42 and light PA was in the hypothesized direction (P = .081). Moderate PA was significantly associated with higher Aβ42 (P = .008), lower t-tau/Aβ42 (P = .006), and lower p-tau/Aβ42 (P = .030). Vigorous PA did not associate with any of the CSF biomarkers. These findings are reported in Table 2.
Table 2.
Association between intensity of physical activity and CSF biomarkers
| Biomarker | Sedentary |
Light |
Moderate |
Vigorous |
||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |
| Aβ42 | −54.12 (21.52) | .014 | 39.47 (22.35) | .081 | 60.74 (22.27) | .008 | 35.42 (23.45) | .135 |
| t-tau | −2.89 (15.28) | .850 | 9.88 (15.53) | .526 | −24.31 (15.68) | .125 | −1.79 (16.25) | .913 |
| p-tau | −1.45 (1.56) | .356 | 1.57 (1.59) | .327 | −0.46 (1.63) | .779 | −0.11 (1.67) | .947 |
| t-tau/Aβ42 | −0.05 (0.03) | .109 | −0.03 (0.03) | .366 | −0.09 (0.03) | .006 | −0.05 (0.04) | .150 |
| p-tau/Aβ42 | 0.01 (0.01) | .161 | −0.01 (0.01) | .402 | −0.01 (0.01) | .030 | −0.01 (0.01) | .101 |
NOTE. Models were adjusted for age, sex, APOE ε4 status, interval between CSF collection and accelerometer data, and assay batch. The regression estimate (β) refers to the amount of change in each biomarker for every 1% change in time spent in each PA category. Bolded values refer to associations where P < .05. Italicized values refer to associations where .05 < P < .10.
Abbreviations: CSF, cerebrospinal fluid; β, regression estimate; SE, standard error; Aβ42, amyloid β 42; t-tau, total tau; p-tau, phosphorylated tau; APOE ε4, apolipoprotein E ε4 allele.
To further verify the robustness of the moderate PA findings, we refitted the moderate PA model while additionally including light PA and vigorous PA as covariates. Moderate PA remained significantly associated with lower t-tau/Aβ42 (P = .029), but not with Aβ42 (P = .068) or p-tau/Aβ42 (P = .156). Interestingly, a significant association between greater moderate PA and lower t-tau also emerged (β standard error [SE] = −38.57 [19.07], P = .047). However, these results should be interpreted with caution as the issue of multicollinearity may be a factor. Moderate PA was significantly correlated with both light PA (r = 0.239, P = .027) and vigorous PA (r = 0.555, P < .001). In addition, the moderate and vigorous PA variables suffered from low tolerance, another indicator of multicollinearity [32] (1 − R2 = 0.717; tolerance values were as follows: light PA = 0.815, moderate PA = 0.611, vigorous PA = .649).
3.3. SB and CSF biomarkers of AD
Greater SB was significantly associated with lower Aβ42 (P = .014) (Table 2), but not with any of the other biomarkers. To examine SB as an independent risk factor, we refitted the model to also include moderate PA as a covariate. Upon inclusion, SB was no longer associated with any CSF biomarkers. However, multicollinearity may again be an issue as SB and moderate PA are highly correlated (r = −0.677, P < .001) and both variables exhibited low tolerance (1 − R2 = 0.711; tolerance values were as follows: SB = 0.459, moderate PA = 0.425).
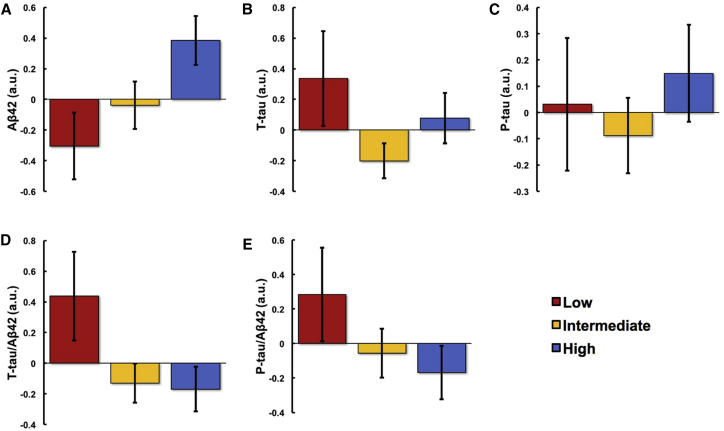
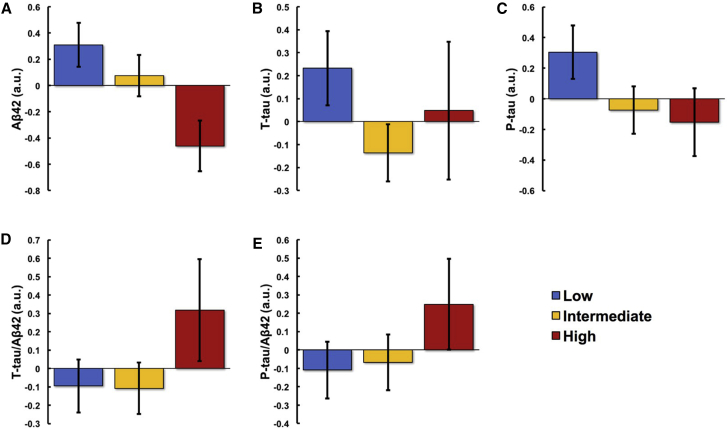
To display these moderate PA (Fig. 1) and SB (Fig. 2) findings, the participants were divided into three groups, implemented separately for moderate PA and SB: lowest 25th percentile (n = 21), middle 50th percentile (n = 43), and highest 25th percentile (n = 21). Mean ± SE levels of CSF biomarkers were plotted for each group.
Fig. 1.
Association between moderate PA and CSF biomarkers. Participants were divided into lowest 25th (n = 21), middle 50th (n = 43), and highest 25th (n = 21) percentiles according to moderate PA levels; and mean Aβ42 (A), t-tau (B), p-tau (C), t-tau/Aβ42 (D), and p-tau/Aβ42 (E) levels of each group were plotted. Figures reflect values adjusted for age, sex, APOE ε4 status, the interval between CSF collection and accelerometer data, and assay batch. Abbreviations: PA, physical activity; CSF, cerebrospinal fluid; Aβ42, amyloid β 42; t-tau, total tau; p-tau, phosphorylated tau; a.u., arbitrary units; APOE ε4, apolipoprotein E ε4 allele.
Fig. 2.
Association between sedentariness and CSF biomarkers. Participants were divided into lowest 25th (n = 21), middle 50th (n = 43), and highest 25th (n = 21) percentiles according to sedentariness levels; and mean Aβ42 (A), t-tau (B), p-tau (C), t-tau/Aβ42 (D), and p-tau/Aβ42 (E) levels of each group were plotted. Figures reflect values adjusted for age, sex, APOE ε4 status, the interval between CSF collection and accelerometer data, and assay batch. Abbreviations: CSF, cerebrospinal fluid; Aβ42, amyloid β 42; t-tau, total tau; p-tau, phosphorylated tau; a.u., arbitrary units; APOE ε4, apolipoprotein E ε4 allele.
3.4. Supplemental analyses
Because the literature often looks at moderate and vigorous PA in combination as a measure of health-enhancing behavior [35], [36], [37], [38], we reanalyzed the data after pooling together moderate and vigorous PA into a composite measure of moderate-to-vigorous PA (MVPA) as the PA intensity of interest. The results remained essentially the same, with MVPA associated with higher Aβ42 (β [SE] = 62.16 [22.43], P = .007), lower t-tau/Aβ42 (β [SE] = −0.09 [0.03], P = .007), and lower p-tau/Aβ42 (β [SE] = −0.01 [0.01], P = .020). Given these results and the moderate PA findings reported previously, it would appear moderate PA is the more potent of the two PA intensities and is driving the associations between MVPA and these CSF biomarkers.
4. Discussion
To our knowledge, this is the first study to investigate the association between objectively measured PA, SB, and CSF biomarkers in a cognitively unimpaired, middle-aged cohort at risk for AD. Our findings show that increased moderate PA is associated with a favorable biomarker profile (i.e., higher levels of CSF Aβ42 and lower t-tau/Aβ42 and p-tau/Aβ42 ratios), while SB is associated with greater AD pathophysiology (i.e., lower levels of CSF Aβ42). We did not observe any significant associations between light or vigorous PA and CSF biomarkers, indicating a moderate level of PA may be the most beneficial intensity of PA for forestalling alterations in AD pathophysiology.
These findings are broadly consistent with previous studies. Specifically, past reports have found PA to be beneficial in promoting a favorable AD biomarker profile, including decreased Aβ42 and tau burden [8], [9], [10] and fewer age-related changes in Aβ deposition, glucose metabolism, hippocampal volume, and episodic memory [11]. Furthermore, our findings of the benefits of moderate-intensity PA in particular are supported by previous research. Geda et al. [39] found moderate-intensity PA in both midlife and late life to be associated with a reduced risk of developing cognitive impairment later in life, while light- and vigorous-intensity PA did not confer the same benefits. In addition to cognitive advantages, moderate PA has been favorably correlated with other AD-related markers. A recent study by our group [40] found moderate PA to be associated with increased cerebral glucose metabolism in 6 brain regions of interest, while vigorous-intensity PA was only associated with one region and light-intensity with none. In addition, in a study by Doi et al. [41], objectively measured MVPA was associated with less brain atrophy among older adults with mild cognitive impairment, while light PA was not. A study by the same group also found moderate, but not light PA, to be associated with increased hippocampal volume in the same mild cognitive impairment cohort [42]. These studies complement our moderate PA finding, suggesting that moderate-intensity activity may be the most advantageous level of PA for older adults to see benefits.
Although our study did not directly examine potential mechanisms, moderate PA may protect against adverse biomarker changes by increasing cerebral blood flow; reducing insulin resistance; reducing the risk of cardiovascular disease (CVD) and cerebrovascular disease; or increasing production of growth factors, such as brain-derived neurotrophic factor [39], [43]. It is possible that light PA on its own is not sufficient to produce these benefits. This is consistent with the PA recommendations set by the American College of Sports Medicine and the American Heart Association, which suggest a minimum of 150 minutes of moderate PA or 60 minutes of vigorous PA per week, in addition to the light-intensity activities of daily living [44]. It should also be noted that our lack of findings for vigorous PA should be interpreted with caution; our analyses are limited by our sample's restricted range on time spent engaging in vigorous PA (Table 1). Older adults are overall less likely to engage in vigorous PA [26], and this limitation has been observed by others as well [45].
Furthermore, our findings show that increased SB is associated with a less favorable AD biomarker profile, specifically lower levels of CSF Aβ42. SB refers to any activity that is done seated or in a reclined posture, including watching television, using a computer, or sitting in a car [16]. Emerging studies suggest that SB may serve as an independent risk factor for adverse outcomes [15]. Many individuals meet recommended PA guidelines, while also engaging in significant amounts of SB, which may have a deleterious effect on various health outcomes independent from the beneficial effect of PA [16]. Previous studies have found increased sedentary time to be associated with a higher risk of all-cause mortality [46], [47], [48] and death due to CVD [47], [49], even after accounting for PA. Not only does SB contribute to risk of death, it is also associated with a greater risk of CVD [50] and CVD risk factors [51], including high triacylglycerol level, low high-density lipoprotein (HDL)-cholesterol, and high fasting blood glucose. Among older adults, one study found SB to be independently associated with worsened cognitive function [52]. To date, no studies have examined SB and CSF biomarkers. Our results support previous literature indicating the negative effects of SB, while also offering new information about its adverse relationship with AD pathophysiology specifically.
Even so, this study is not without limitations. Its cross-sectional design restricts our ability to establish a causal relationship between PA and biomarker levels. Future studies within the context of a prospective design would be valuable in further elucidating the association between PA, SB, and CSF biomarkers of AD. Another limitation is the lack of contemporaneous lifestyle data, such as dietary habits and sleep hygiene, which may benefit future studies in identifying other lifestyle factors that may influence biomarker levels or play a role in these disease processes. In addition, the time interval between PA assessment and CSF collection (1.52 ± 1.26 years) poses another limitation to our study. By controlling for this time interval, we hoped to limit its effect on our results. Future studies might benefit from collecting all data more contemporaneously. Finally, the generalizability of our results to the larger population is limited. First, the level of PA in our sample is much higher than the general population of late-middle-aged adults: 35.3% of our sample met the weekly PA recommendations [53], while the reported national average is less than 5% [26]. In addition, our sample is relatively homogenous with regard to race and education, as most participants were non-Hispanic whites and well educated.
In conclusion, this study presents novel findings regarding the relationship between objectively measured PA and CSF biomarkers of AD. Our results suggest that limiting SB and engaging in moderate PA may produce beneficial effects on CSF biomarkers of AD, particularly among late-middle-aged adults at risk for the disease. However, given the novelty of our study, future investigations examining objectively measured PA and biomarkers of AD pathophysiology are needed to further validate our findings. In particular, additional exploration of the effects of moderate PA, versus light and vigorous, and the unique risk conferred by SB would be extremely valuable. Overall, this study reinforces previous literature on the benefits of PA for overall health and suggests PA may be an important tool in staving off adverse midlife pathophysiological changes related to AD.
Research in context.
-
1.
Systematic review: We searched PubMed using the following search terms: “physical activity,” “actigraphy,” “accelerometry,” “cerebrospinal fluid,” “CSF biomarkers,” “sedentary behavior,” “sedentariness,” and “Alzheimer's disease.” We also reviewed the references for each article identified.
-
2.
Interpretation: Midlife interventions to slow or prevent pathological changes related to Alzheimer's disease are of great scientific interest. Our findings show that, among cognitively healthy, late-middle-aged adults, levels of moderate physical activity and sedentariness are associated with cerebrospinal fluid levels of amyloid and tau. These results are consistent with previous studies suggesting a physically active lifestyle may play a protective role against the development of Alzheimer's disease.
-
3.
Future directions: Future studies should further investigate the unique influence of moderate-intensity physical activity, versus light or vigorous, and sedentary behavior on cerebrospinal fluid biomarkers. Studies in the context of a prospective design would be of particular value.
Acknowledgments
This work was supported by National Institute on Aging grants K23 AG045957 (O.C.O.), R21 AG051858 (O.C.O.), R01 AG027161 (S.C.J.), R01 AG031790 (C.M.C.), R01 AG021155 (S.C.J.), and P50 AG033514 (S.A.); and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Extendicare Foundation, Alzheimer's Association, Wisconsin Alumni Research Foundation, Helen Bader Foundation, Northwestern Mutual Foundation, the Veterans Administration, including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI, and the Swedish Research Council, the European Research Council, the Torsten Söderberg Foundation, the Swedish Brain Foundation, and the Wallenberg Academy. The authors thank the staff and study participants of the Wisconsin Registry for Alzheimer's Prevention and the laboratory technicians at the Clinical Neurochemistry Laboratory, Mölndal, Sweden, without whom this work would not be possible.
References
- 1.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs213. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sofi F., Valecchi D., Bacci D., Abbate R., Gensini G.F., Casini A. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahlskog J.E., Geda Y.E., Graff-Radford N.R., Petersen R.C. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamer M., Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 7.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang K.Y., Mintun M.A., Fagan A.M., Goate A.M., Bugg J.M., Holtzman D.M. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown B.M., Peiffer J.J., Taddei K., Lui J.K., Laws S.M., Gupta V.B. Physical activity and amyloid-beta plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry. 2013;18:875–881. doi: 10.1038/mp.2012.107. [DOI] [PubMed] [Google Scholar]
- 10.Stillman C.M., Lopez O.L., Becker J.T., Kuller L.H., Mehta P.D., Tracy R.P. Physical activity predicts reduced plasma beta amyloid in the Cardiovascular Health Study. Ann Clin Transl Neurol. 2017;4:284–291. doi: 10.1002/acn3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okonkwo O.C., Schultz S.A., Oh J.M., Larson J., Edwards D., Cook D. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83:1753–1760. doi: 10.1212/WNL.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz S.A., Boots E.A., Darst B.F., Zetterberg H., Blennow K., Edwards D.F. Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology. 2017;88:1650–1658. doi: 10.1212/WNL.0000000000003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Head D., Bugg J.M., Goate A.M., Fagan A.M., Mintun M.A., Benzinger T. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker L.D., Bayer-Carter J.L., Skinner J., Montine T.J., Cholerton B.A., Callaghan M. High-intensity physical activity modulates diet effects on cerebrospinal amyloid-beta levels in normal aging and mild cognitive impairment. J Alzheimer's Dis. 2012;28:137–146. doi: 10.3233/JAD-2011-111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen N., Sparling P.B., Healy G.N., Dunstan D.W., Matthews C.E. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Same R.V., Feldman D.I., Shah N., Martin S.S., Al Rifai M., Blaha M.J. Relationship between sedentary behavior and cardiovascular risk. Curr Cardiol Rep. 2016;18:6. doi: 10.1007/s11886-015-0678-5. [DOI] [PubMed] [Google Scholar]
- 17.Harvey J.A., Chastin S.F., Skelton D.A. Prevalence of sedentary behavior in older adults: a systematic review. Int J Environ Res Public Health. 2013;10:6645–6661. doi: 10.3390/ijerph10126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loprinzi P.D. Need for increased promotion of physical activity among adults at risk for Alzheimer's disease: a brief report. J Phys Act Health. 2015;12:1601–1604. doi: 10.1123/jpah.2014-0554. [DOI] [PubMed] [Google Scholar]
- 19.Falck R.S., Davis J.C., Liu-Ambrose T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br J Sports Med. 2017;51:800–811. doi: 10.1136/bjsports-2015-095551. [DOI] [PubMed] [Google Scholar]
- 20.Zlatar Z.Z., Wierenga C.E., Bangen K.J., Liu T.T., Jak A.J. Increased hippocampal blood flow in sedentary older adults at genetic risk for Alzheimer's disease. J Alzheimers Dis. 2014;41:809–817. doi: 10.3233/JAD-132252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindstrom H.A., Fritsch T., Petot G., Smyth K.A., Chen C.H., Debanne S.M. The relationships between television viewing in midlife and the development of Alzheimer's disease in a case-control study. Brain Cogn. 2005;58:157–165. doi: 10.1016/j.bandc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Dyrstad S.M., Hansen B.H., Holme I.M., Anderssen S.A. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46:99–106. doi: 10.1249/MSS.0b013e3182a0595f. [DOI] [PubMed] [Google Scholar]
- 23.Celis-Morales C.A., Perez-Bravo F., Ibanez L., Salas C., Bailey M.E., Gill J.M. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7:e36345. doi: 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchman A.S., Wilson R.S., Bennett D.A. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry. 2008;16:697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- 25.Sager M.A., Hermann B., La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 26.Troiano R.P., Berrigan D., Dodd K.W., Masse L.C., Tilert T., Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 27.Lyden K., Keadle S.K., Staudenmayer J., Freedson P.S. A method to estimate free-living active and sedentary behavior from an accelerometer. Med Sci Sports Exerc. 2014;46:386–397. doi: 10.1249/MSS.0b013e3182a42a2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manini T., Knaggs J., Larkin K., Lorow M., Staudenmayer J. An artificial Neural network approach to estimate physical activity energy expenditure and physical activity type from an accelerometer in older adults. Gerontologist. 2011;51:560–561. [Google Scholar]
- 29.Palmqvist S., Zetterberg H., Blennow K., Vestberg S., Andreasson U., Brooks D.J. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 30.Racine A.M., Koscik R.L., Nicholas C.R., Clark L.R., Okonkwo O.C., Oh J.M. Cerebrospinal fluid ratios with Aβ42 predict preclinical brain beta-amyloid accumulation. Alzheimers Dement (Amst) 2016;2:27–38. doi: 10.1016/j.dadm.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e712. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Tabachnick B.G., Fidell L.S. 5th ed. Pearson/Allyn & Bacon; Boston: 2007. Using Multivariate Statistics. [Google Scholar]
- 33.Blom G. Wiley; New York,: 1958. Statistical Estimates and Transformed Beta-Variables. [Google Scholar]
- 34.Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W., Wadley V.G., Howard V.J., Hutto B., Blair S.N., Hooker S.P. Objectively measured physical activity and cognitive function in older adults. Med Sci Sports Exerc. 2017;49:47–53. doi: 10.1249/MSS.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr J., Marshall S.J., Patterson R.E., Marinac C.R., Natarajan L., Rosenberg D. Objectively measured physical activity is related to cognitive function in older adults. J Am Geriatr Soc. 2013;61:1927–1931. doi: 10.1111/jgs.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehlers D.K., Aguinaga S., Cosman J., Severson J., Kramer A.F., McAuley E. The effects of physical activity and fatigue on cognitive performance in breast cancer survivors. Breast Cancer Res Treat. 2017;165:699–707. doi: 10.1007/s10549-017-4363-9. [DOI] [PubMed] [Google Scholar]
- 38.Loprinzi P.D., Danzl M.M., Ulanowski E., Paydo C. A pilot study evaluating the association between physical activity and cognition among individuals with Parkinson's disease. Disabil Health J. 2017;11:165–168. doi: 10.1016/j.dhjo.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Geda Y.E., Roberts R.O., Knopman D.S., Christianson T.J., Pankratz V.S., Ivnik R.J. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dougherty R.J., Schultz S.A., Kirby T.K., Boots E.A., Oh J.M., Edwards D. Moderate Physical Activity is Associated with Cerebral Glucose Metabolism in Adults at Risk for Alzheimer's Disease. J Alzheimers Dis. 2017;58:1089–1097. doi: 10.3233/JAD-161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doi T., Makizako H., Shimada H., Tsutsumimoto K., Hotta R., Nakakubo S. Objectively measured physical activity, brain atrophy, and white matter lesions in older adults with mild cognitive impairment. Exp Gerontol. 2015;62:1–6. doi: 10.1016/j.exger.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Makizako H., Liu-Ambrose T., Shimada H., Doi T., Park H., Tsutsumimoto K. Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2015;70:480–486. doi: 10.1093/gerona/glu136. [DOI] [PubMed] [Google Scholar]
- 43.Brown B.M., Peiffer J.J., Martins R.N. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol Psychiatry. 2013;18:864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 44.Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 45.Brown B.M., Peiffer J.J., Sohrabi H.R., Mondal A., Gupta V.B., Rainey-Smith S.R. Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry. 2012;2:e191. doi: 10.1038/tp.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koster A., Caserotti P., Patel K.V., Matthews C.E., Berrigan D., Van Domelen D.R. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7:e37696. doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katzmarzyk P.T., Church T.S., Craig C.L., Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 48.Stamatakis E., Hamer M., Dunstan D.W. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57:292–299. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 49.Warren T.Y., Barry V., Hooker S.P., Sui X., Church T.S., Blair S.N. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42:879–885. doi: 10.1249/MSS.0b013e3181c3aa7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chomistek A.K., Manson J.E., Stefanick M.L., Lu B., Sands-Lincoln M., Going S.B. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women's Health Initiative. J Am Coll Cardiol. 2013;61:2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gennuso K.P., Gangnon R.E., Thraen-Borowski K.M., Colbert L.H. Dose-response relationships between sedentary behaviour and the metabolic syndrome and its components. Diabetologia. 2015;58:485–492. doi: 10.1007/s00125-014-3453-z. [DOI] [PubMed] [Google Scholar]
- 52.Edwards M.K., Loprinzi P.D. Combined associations of sedentary behavior and cardiorespiratory fitness on cognitive function among older adults. Int J Cardiol. 2017;229:71–74. doi: 10.1016/j.ijcard.2016.11.264. [DOI] [PubMed] [Google Scholar]
- 53.Nelson M.E., Rejeski W.J., Blair S.N., Duncan P.W., Judge J.O., King A.C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]