Abstract
The inflammatory microenvironment plays a critical role in the development and progression of malignancies. In the present study, we aimed to evaluate the prognostic value of lymphocyte-related inflammation and immune-based prognostic scores in patients with chordoma after radical resection, including the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), and systemic immune-inflammation index (SII). A total of 172 consecutive patients with chordoma who underwent radical resection were reviewed. R software was used to randomly select 86 chordoma patients as a training set and 86 chordoma patients as a validation set. Potential prognostic factors were also identified, including age, sex, tumor localization, KPS, Enneking stage, tumor size, and tumor metastasis. Overall survival (OS) was calculated using the Kaplan–Meier method and multivariate Cox regression analyses. NLR, PLR, SII, Enneking stage, tumor differentiation and tumor metastasis were identified as significant factors from the univariate analysis in both the training and validation sets and were subjected to multivariate Cox proportional hazards analysis. The univariate analysis showed that NLR ≥1.65, PLR ≥121, and SII ≥370×109/L were significantly associated with poor OS. In the multivariate Cox proportional hazard analysis, SII, Enneking stage and tumor metastasis were significantly associated with OS. As noninvasive, low-cost, reproducible prognostic biomarkers, NLR, PLR and SII could help predict poor prognosis in patients with chordoma after radical resection. This finding may contribute to the development of more effective tailored therapy according to the characteristics of individual tumors.
Introduction
Chordoma, the fourth most common malignant neoplasm arising from notochordal remnant tissue, accounts for 1% to 4% of all bone malignancies [1]. Chordoma preferentially occurs in the axial skeleton and is most commonly found in the sacrum (50%-60%), the spheno-occipital region (25%-30%), cervical (10%) and thoracolumbar vertebrae (5%) [2]. Chordoma is insensitive to conventional radiotherapy [3] and chemotherapy [4]. The gold standard of treatment for chordoma is radical resection with the goal of negative margins [5]. However, chordoma tends to be locally aggressive and has a high rate of recurrence [6], and recurrent chordoma is nearly impossible to eradicate [7]. Metastases occur in 5% to 40% of patients [8]. Given the current therapeutic challenges, a better understanding of the factors that influence chordoma prognosis may help guide treatment planning to prolong survival [9].
In previous studies, several prognostic factors such as age, sex, location, resection margins and Enneking stages have been investigated [10]. The abovementioned prognostic markers are mainly based on the clinicopathological findings. It has increasingly been recognized that inflammation with the tumor microenvironment plays a critical role in the development and progression of malignancies [11]. Elevated C-reactive protein (CRP), as a marker of systemic inflammation, has been confirmed to be associated with decreased survival in patients with chordoma of the lumbar spine and sacrum [5]. Several inflammatory and immune-based prognostic scores, such as the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), and systemic immune-inflammation index (SII), have also been developed to predict survival and recurrence in hepatocellular carcinoma, gastric cancer and small cell lung cancer [12], [13], [14], [15]. However, the prognostic value of these inflammatory and hematological markers in patients with chordoma is still unclear.
In the present study, we aimed to evaluate the prognostic value of lymphocyte-related inflammation and the immune-based scores NLR, PLR, MLR and SII in patients with chordoma after radical resection.
Materials and Methods
Between January 1997 and October 2016, 319 consecutive patients with chordoma confirmed by postoperative histological pathology at Chinese PLA General Hospital were retrospectively reviewed. Treatment-naïve patients who did not receive other treatments before surgery and patients who underwent radical resection were included in this study. Patients with active infection or inflammatory disease within 1 month before blood test were excluded. In all, 172 patients were included in this study. This study has been approved by the Ethics Committee of Chinese PLA General Hospital and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants or their legal guardians.
The blood samples for the neutrophil, lymphocyte, monocyte, and platelet counts were obtained within 3 days before surgery. The various ratios were calculated as follows: NLR = N / L; PLR = P / L; MLR = M / L and SII = P ×N/L, where N, L, M and P were the preoperative absolute neutrophil, lymphocyte, monocyte, and platelet counts, respectively. Potential prognostic factors were identified, including age, sex, tumor localization, Karnofsky performance status (KPS), Enneking stage, tumor size, and tumor metastasis. Tumor stage was determined according to the Enneking stage [16], and tumor differentiation was classified as classical (conventional), chondroid or dedifferentiated [17].
The patients were followed up every 3 months during the first postoperative year and every 6 months thereafter. If recurrence was confirmed and the recurrent tumor was localized, a second resection was suggested. The last follow-up date was April 07, 2017. Overall survival (OS) was defined as the interval between the initial surgery and either death or the last follow-up.
Statistical Analysis
For normally distributed continuous data, Student’s t-test was used for assessing the significance of the difference between the means of two samples and the one-way ANOVA test was performed to compare mean values of three samples. For non-normally distributed continuous data, Mann-Whitney U-test was applied. Differences were considered statistically significant when p < 0.05. OS was calculated using the Kaplan-Meier method. Prognostic parameters associated with OS were assessed using both univariate and multivariate Cox analyses. Only variables proven to be significant in the univariate analysis were included in the multivariate model. R software was used to analyze the results of the regression analyses, receiver operating characteristic (ROC) curve analysis [18], [19], [20] to determine the cut-off values of the parameters associated with OS of patients with chordoma. ROC curve analysis was used to compare the sensitivity and specificity of the different models. The Clarke-Pearson test was used to compare the differences of the area under the curve (AUC). The cut-off value was determined by the AUC; the ROC curve with the highest AUC indicated that the corresponding model had the best sensitivity and specificity. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated as estimates of the correlations. Statistical analyses were carried out using IBM SPSS 20.0 statistical software (SPSS Inc., Chicago, IL).
Results
Patient Characteristics
A total of 172 patients with a median age of 50 years (ranging from 8-71 years) who were diagnosed with primary chordoma and underwent radical resection were included in this study. The baseline characteristics of patients and their predictive values on OS are shown in Tables 1 and 2. The mean follow-up period was 73.0 months with a minimum follow-up duration of 2 months. At the time of analysis, 68 (39.5%) patients died, and the median OS was 80 months (95%CI: 63.8-98.6).
Table 1.
The Baseline Characteristics of the Patients
| Variables | n | % |
|---|---|---|
| Total | 172 | 100 |
| Sex | ||
| Male | 114 | 66.3 |
| Female | 58 | 33.7 |
| Age | ||
| <60 years | 121 | 70.3 |
| ≥60 years | 51 | 29.7 |
| KPS | ||
| ≥80% | 102 | 59.3 |
| 60%–80% | 48 | 27.9 |
| <60% | 22 | 12.8 |
| Enneking stage | ||
| I | 96 | 55.8 |
| II–III | 76 | 44.2 |
| Tumor differentiation | ||
| Classical | 118 | 68.6 |
| Non-classical (chondroid, dedifferentiated) | 54 | 31.4 |
| Tumor size | ||
| ≤6 cm | 108 | 62.8 |
| >6 cm | 64 | 37.2 |
| Tumor metastasis | ||
| Without metastasis | 107 | 62.2 |
| With metastasis | 65 | 37.8 |
| Localization | ||
| Cranial | 79 | 45.9 |
| Spine | 43 | 25 |
| Sacrum | 50 | 29.1 |
KPS, Karnofsky performance status.
Table 2.
Kaplan-Meier Analyses (log-rank test) of the Predictive Value of Baseline Characteristics on OS
| Variables | OS (months) | 95%CI | P |
|---|---|---|---|
| Total | 80 | (63.8-98.6) | |
| Sex | |||
| Male | 77.4 | (52.6-88.3) | .913 |
| Female | 78.7 | (57.3-96.8) | |
| Age | |||
| <60 years | 79.6 | (59.5-89.7) | .757 |
| ≥60 years | 63 | (46.4-78.5) | |
| KPS | |||
| ≥80% | 78.4 | (65.7-94.8) | .033 |
| 60%-80% | 66.5 | (55.8-78.4) | |
| <60% | 43.2 | (37.3-54.9) | |
| Enneking stage | |||
| I | 81.3 | (73.2-99.1) | <.001 |
| II–III | 34.5 | (26.7-48.5) | |
| Tumor differentiation | |||
| Classical | 75.7 | (65.3-88.4) | .042 |
| Non-classical (chondroid, dedifferentiated) | 43.5 | (22.4-53.1) | |
| Tumor size | |||
| <6 cm | 70.4 | (53.7-80.7) | .633 |
| ≥6 cm | 48.1 | (37.6-53.4) | |
| Tumor metastasis | |||
| Without metastasis | 75.9 | (59.7-91.3) | .008 |
| With metastasis | 23.5 | (17.1-38.9) | |
| Localization | |||
| Cranial | 66.8 | (47.5-79.9) | .641 |
| Spine | 71.2 | (49.9-89.6) | |
| Sacrum | 55.8 | (39.9-75.7) | |
| Lymphocyte cells count | |||
| <1.8*10^10 | 77.8 | (53.8-85.9) | .893 |
| ≥1.8*10^10 | 65 | (51.3-77.9) | |
| Neutrophil cells count | |||
| <3.4*10^9 | 77.3 | (53.5-86.2) | .317 |
| ≥3.4*10^9 | 64 | (43.4-78.6) | |
| NLR | |||
| <1.65 | 82.3 | (73.5-91.9) | .023 |
| ≥1.65 | 56.4 | (37.4-78.8) | |
| PLR | |||
| <121 | 81.7 | (77.3-93.7) | .024 |
| ≥121 | 58.6 | (47.9-85.4) | |
| MLR | |||
| <0.36 | 81.3 | (55.7-91.4) | .635 |
| ≥0.36 | 67.4 | (46.7-83.5) | |
| SII | |||
| <370*10^9 | 81.6 | (73.1-89.4) | .008 |
| ≥370*10^9 | 55.9 | (39.8-76.7) |
OS, Overall survival; CI, Confidence interval; KPS, Karnofsky performance status; NLR, Neutrophil-lymphocyte ratio; PLR, Platelet-lymphocyte ratio; MLR, Monocyte-lymphocyte ratio; SII, Systemic immune-inflammation index.
Association of NLR, PLR and SII with OS
R software was used to randomly select 86 chordoma patients as a training set and 86 chordoma patients as a validation set. All the clinicopathological features, blood routine parameters, and lymphocyte-related indexes (including the NLR, PLR, MLR and SII) were included in the Cox univariate analyses. In both sets, the following parameters were significantly associated with chordoma OS: NLR, PLR, SII, Enneking stage, tumor differentiation and tumor metastasis (all P < .05). The data are shown in Table 3.
Table 3.
Association Between Routine Blood Test Parameters and Overall Survival in Chordoma Patients Based on Univariate Cox Regression Analyses
| Training Set, n=86 |
Validation Set, n=86 |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR(95% CI) | P | |
| Lymphocyte cell counts, per increase of 1000 cells/mm3 |
0.52 (0.22-1.5) | .74 | 0.74 (0.25-1.6) | .86 |
| NLR, per increase of 1 unit | 1.4 (0.77-2.2) | .025 | 1.6 (0.97-2.7) | .037 |
| PLR, per increase of 1 unit | 1.4 (0.94-2.5) | .031 | 1.8 (1.0-2.8) | .044 |
| SII, per increase of 1 unit | 2.9 (1.2-3.7) | .009 | 3.0(1.1-4.9) | .015 |
| MLR, per increase of 1 unit | 1.3 (0.97-3.1) | .654 | 1.6 (1.5-2.9) | .712 |
| Enneking stage, stage I vs. stage II-III | 6.9 (3.5-8.9) | <.001 | 7.1 (5.0-9.8) | <.001 |
| Tumor differentiation, classical vs. non-classical | 3.2 (2.8-6.0) | .049 | 4.2 (3.0-7.2) | .051 |
| Tumor metastasis, with vs. without | 4.0 (2.6-6.8) | .013 | 3.6 (2.3-6.3) | .006 |
NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; SII, systemic immune-inflammation index; HR, hazard ratios; CI, confidence intervals.
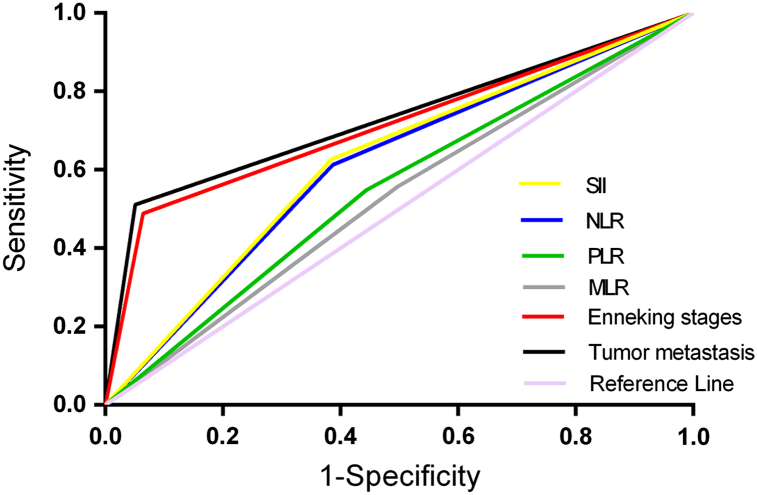
The ROC analyses was used to determine the cut-off values of the NLR, PLR, SII and MLR for predicting OS based on the data in the training set (Figure 1). According to these analyses, the AUCs of the NLR, PLR, SII and MLR in predicting OS in the training set were 0.675, 0.619, 0.683 and 0.517, respectively. Based on the ROC curves, the best cut-off value for the NLR was 1.65 with a sensitivity of 62.7% and a specificity of 61.5% for OS; for PLR, the cut-off was 121 with a sensitivity of 59.4% and a specificity of 55.6% for OS; for SII, the cut-off was 370 ×109/L with a sensitivity of 61.3% and a specificity of 65.9% for OS. These data are shown in Table 4. Based on the cut-off values, the continuous data were transformed to dichotomous data. Significant factors identified in the univariate analysis in both the training and validation sets, including the NLR, PLR, SII, Enneking stage, tumor differentiation and tumor metastasis, were subjected to multivariate Cox proportional hazards analysis. In this analysis, SII, Enneking stage and tumor metastasis were significantly associated with OS. These data are shown in Table 5.
Figure 1.
The ROC analyses of the NLR, PLR, SII and MLR.
ROC curves of the SII, NLR, PLR, MLR, tumor metastasis and Enneking stage for OS, with a median survival time of 80 months.
Table 4.
Predictive Value of the NLR, PLR, SII, MLR, New Predictive Variable and 5 Point Scale Variable for Overall Survival of Chordoma Patients
| Variables | Cut-off | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV | Accuracy | P |
|---|---|---|---|---|---|---|---|---|
| NLR | 1.65 | 0.675 (0.553-0.724) | 0.627 | 0.615 | 0.652 | 0.589 | 62.6 | .004 |
| PLR | 121 | 0.619 (0.491-0.675) | 0.594 | 0.556 | 0.606 | 0.543 | 58.1 | .035 |
| SII | 370 | 0.683 (0.569-0.738) | 0.613 | 0.659 | 0.674 | 0.597 | 63.7 | .003 |
| MLR | 0.36 | 0.517 (0.454-0.619) | 0.503 | 0.557 | 0.566 | 0.493 | 52.5 | .147 |
AUC, area under the curve; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; SII, systemic immune-inflammation index; MLR, monocyte-lymphocyte ratio; CI, Confidence intervals; PPV, positive predictive value; NPV, negative predictive value.
Table 5.
Association Between Routine Blood Test Parameters and Overall Survival of Chordoma Patients Based on Multivariate Cox Regression Analyses
| Variables | Category | Overall Survival |
|
|---|---|---|---|
| HR (95% CI) | P | ||
| SII | <370 vs. ≥370 | 1.9 (1.2-3.0) | .015 |
| Enneking stage Tumor metastasis |
I vs. II-III with vs. without |
6.2 (3.8-9.8) 3.2 (2.2-4.5) |
<.001 .002 |
SII, systemic immune-inflammation index; HR, hazard ratios; CI, confidence intervals.
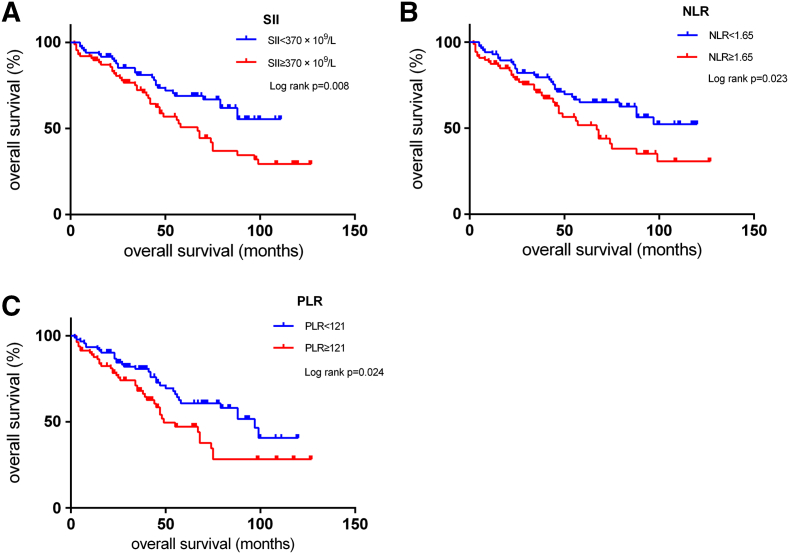
Additionally, we performed a Kaplan-Meier analysis of the parameters identified as significantly associated and OS. Using the cut-off values of 1.65, 121 and 370 × 109/L, we grouped the NLR, PLR and SII indexes, respectively, into normal groups and elevated groups. Our conclusion was that elevated NLR, PLR and SII indexes were significantly associated with poor OS (log-rank P < 0.05, Figure 2).
Figure 2.
Kaplan-Meier survival curves for OS according to inflammation-based scores in 172 chordoma patients.
(A). A total of 84 patients with an SII ≥ 370×109/L had a shorter median OS than 88 patients with an SII <370×109 (55.9 vs. 81.6 months, P = 0.008).
(B). A total of 85 patients with an NLR ≥ 1.65 had a shorter median OS than 87 patients with an NLR <1.65 (56.4 vs. 82.3 months, P = 0.023).
(C). A total of 89 patients with a PLR ≥ 121 had a shorter median OS than 83 patients with a PLR <121 (58.6 vs. 81.7 months, respectively; P = 0.024).
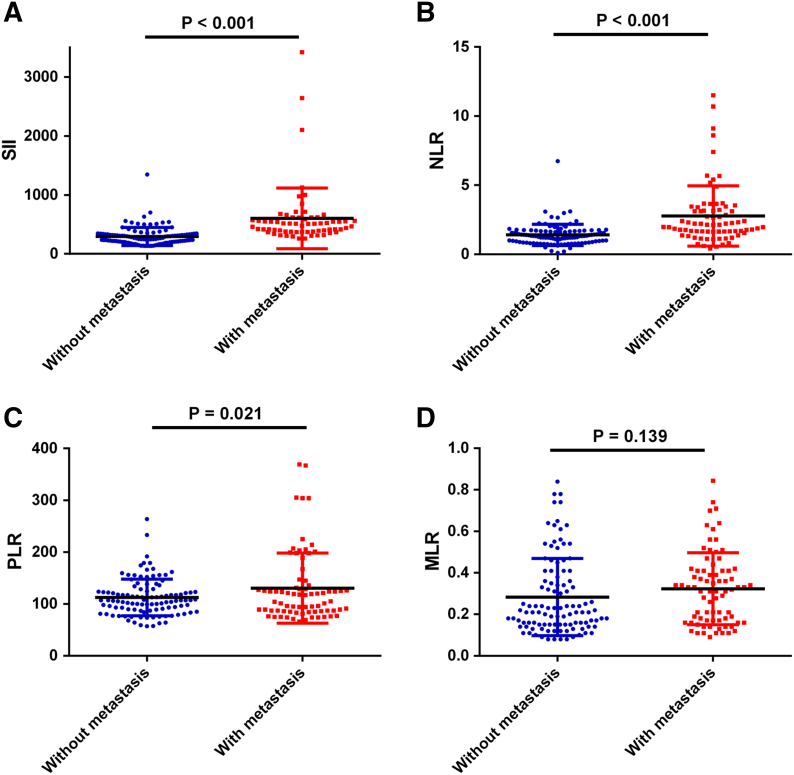
Association of the NLR, PLR, SII and MLR with Tumor Metastasis
As is commonly known, tumor metastasis was significantly associated with patient outcome in chordoma; thus, we evaluated the association between the NLR, PLR, SII, MLR and tumor metastasis. We found that the NLR, PLR and SII scores in chordoma patients with metastasis were higher than those in chordoma patients without metastasis (P<0.001, P = 0.021 and P<0.001, respectively, Figure 3). Therefore, we suggest that NLR, PLR, and SII may predict the invasiveness and progression of chordoma.
Figure 3.
Association of the SII (A), NLR (B), PLR (C), and MLR (D) with tumor metastasis in 172 chordoma patients.
Discussion
Given the low-grade nature of malignant lesions, the overall survival of chordoma patients is still relatively poor. Previous studies have reported five-year and ten-year survival rates ranging from 45% to 77% and 28% to 50%, respectively [1], [21], [22]. The cumulative probability of local recurrence at five years and ten years after diagnosis was 46% and 54%, respectively [23]. Several studies have analyzed the prognostic factors of chordoma patients. However, the results are inconsistent [10], [24], [25]. In our research, our data suggested that sex, age, tumor size and localization were not independent prognostic factors for postoperative OS of chordoma. However, Enneking stage, tumor differentiation and metastasis were significantly associated with OS. Consistent with previous study by Meng et al. [10], we also found that a KPS ≥80% had a profound influence on OS but did not affect the recurrence of chordoma. Adequacy of the surgical resection margins is also an important prognostic indicator, and the gold standard of care for chordoma is en bloc resection [26], however, anatomic constraints make achieving this goal technically demanding and difficult. More importantly, total en bloc spondylectomy may lead to significant functional compromise, as a large chordoma in the upper cervical spine and sacrum cannot be excised in an ideal en bloc manner because of its proximity to vital neurovascular structures [27]. Radical resection was thought to be an ideal surgical treatment that offers the best oncological outcomes for chordoma [28]. Although all the patients in this study underwent radical resection, achieving long-term survival remains a challenge. Therefore, aside from preventing recurrence, increasing OS after surgery is an important issue that should be addressed.
In the past decade, new perspectives regarding tumor biology, microenvironment, and tumor surveillance have been widely investigated, and breakthroughs in immunotherapy in various cancers have led to renewed interest in altering the host immune system response to develop new methods of treating tumors. It is well known that early in the neoplastic process, inflammatory cells are powerful tumor promoters because they produce an attractive environment for tumor growth, facilitate genomic instability and promote angiogenesis [29], [30]. Cytotoxic lymphocytes and other lymphocytes play a fundamental role in cell-mediated immunologic destruction of cancer cells [31]. Neutrophils can promote adhesion and tumoral seeding by secreting circulating growth factors [32]. Platelets can directly interact with tumor cells, synergistically activate the TGF beta/Smad and NF-kappa B pathways in cancer cells, induce an epithelial-to-mesenchymal-like transition and promote metastasis [33]. An emerging link between circulatory cytokines and an increased NLR in cancer patients may reflect increased tumor burden and aggressiveness as well as consequent systemic pro-inflammatory effects [34]. Elevated concentrations of circulating interleukin (IL)-1 receptor antagonist, IL-6, IL-7, IL-8, IL-12, monocyte chemoattractant protein-1 and platelet-derived growth factor-BB were found to be associated with a high NLR, while a highly significant association was also found between serum IL-8 and TNM stage in colorectal cancer [35]. In another study conducted by Richard A. Smith et al. [36], a clear trend towards poorly differentiated tumors presenting a greater PLR was observed. They concluded that an elevated preoperative PLR reflected an enhanced host inflammatory response to more aggressive tumor biology. SII represents a score based on the combination of the platelet count and neutrophil-lymphocyte ratio, which reflects the systemic inflammatory response [12]. An elevated SII due to high levels of neutrophils and platelets with concomitant low levels of lymphocytes, usually suggests a stronger inflammatory response and a weaker immune response in patients, and it may be associated with the invasion and metastasis of cancer cells and thus lead to poor survival [15].
In the present study, we first verified the role of these lymphocyte-related inflammatory and immune-based prognostic scores, such as NLR, PLR, and SII, prior to treatment initiation in predicting OS in patients with chordoma. We found that higher NLR, PLR, and SII indexes were associated with poor prognosis of chordoma in the Cox univariate analyses and that SII was shown to be significantly associated with the prognosis of chordoma in the multivariate Cox proportional hazard analysis. Recently, two studies conducted by Feng et al. [37] and Mathios et al. [38] reported that the programmed death 1 (PD-1) protein and its ligands PD-L1 and PD-L2 are expressed in chordoma. It is suggested that PD-1/PD-L1/PD-L2 and the cytotoxic T lymphocyte antigen 4 (CTLA4)/B7 interactions play a role in chordoma pathogenesis, which make these signaling axis as possible targets for immune checkpoint blockade therapies in chordoma patients. Another study showed that chordomas overexpress chondroitin sulfate proteoglycan 4 (CSPG4) [39], and Schoenfeld et al. [40] reported that CSPG4 expression in chordoma was correlated with an increased risk of metastasis and mortality. It is believed the CSPG4 could be a vital prognostic biomarker in the treatment of chordoma and that treatments targeting CSPG4 may be of significant value in treating chordoma. In addition, Heery et al. [41] suggested that chordoma patients have an immune response to brachyury, which may offer another option for treating chordoma patients with tumors with intact HLA class I expression. Therefore, identifying lymphocyte-related inflammatory and immune-based prognostic scores as biomarkers in patients with chordoma may be an important aspect for selecting patients for immunotherapy.
The limitations of this study include its retrospective nature and the small sample size owing to the low incidence of chordoma. However, to the best of our knowledge, our study is the largest series of chordoma to date. Another limitation is that this study could not analyze the prognostic value of CRP because it is not measured in the routine examination. Further research is needed to evaluate the predictive performance of CRP combined with these inflammation-based scores.
In conclusion, as noninvasive, low-cost, reproducible prognostic biomarkers, the NLR, PLR and SII could help predict poor prognosis in patients with chordoma after radical resection. This finding may contribute to tailoring more effective therapies in accordance with the characteristics of individual tumors.
Acknowledgments
Acknowledgement
We would like to acknowledge the individuals and families that participated in this research. We thank Liqiang Zheng and Wenzhe Cao for their helpful comments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Samson IR, Springfield DS, Suit HD, Mankin HJ. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476–1484. doi: 10.2106/00004623-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–1350. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 3.Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, Wunder J. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67–72. doi: 10.1016/s0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 4.Azzarelli A, Quagliuolo V, Cerasoli S, Zucali R, Bignami P, Mazzaferro V, Dossena G, Gennari L. Chordoma: natural history and treatment results in 33 cases. J Surg Oncol. 1988;37:185–191. doi: 10.1002/jso.2930370311. [DOI] [PubMed] [Google Scholar]
- 5.Hobusch GM, Bodner F, Walzer S, Marculescu R, Funovics PT, Sulzbacher I, Windhager R, Panotopoulos J. C-reactive protein as a prognostic factor in patients with chordoma of lumbar spine and sacrum--a single center pilot study. World J Surg Oncol. 2016;14:111. doi: 10.1186/s12957-016-0875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88:1532–1539. doi: 10.2106/JBJS.D.02533. [DOI] [PubMed] [Google Scholar]
- 7.Patel SS, Schwab JH. Immunotherapy as a potential treatment for chordoma: a review. Curr Oncol Rep. 2016;18:55. doi: 10.1007/s11912-016-0543-8. [DOI] [PubMed] [Google Scholar]
- 8.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85:878–883. [PubMed] [Google Scholar]
- 9.Zou MX, Huang W, Wang XB, Li J, Lv GH, Deng YW. Prognostic factors in spinal chordoma: A systematic review. Clin Neurol Neurosurg. 2015;139:110–118. doi: 10.1016/j.clineuro.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Meng T, Yin H, Li B, Li Z, Xu W, Zhou W, Cheng M, Wang J, Zhou L, Yang X. Clinical features and prognostic factors of patients with chordoma in the spine: a retrospective analysis of 153 patients in a single center. Neuro-Oncology. 2015;17:725–732. doi: 10.1093/neuonc/nou331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 12.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297–304. doi: 10.1620/tjem.236.297. [DOI] [PubMed] [Google Scholar]
- 13.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 14.Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Liu S, Lei Y, Wang K, Xu M, Chen Y, Liu B, Chen Y, Fu Q, Zhang P. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016;7:44185–44193. doi: 10.18632/oncotarget.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986:9–24. [PubMed] [Google Scholar]
- 17.Stacchiotti S, Casali PG, Lo Vullo S, Mariani L, Palassini E, Mercuri M, Alberghini M, Pilotti S, Zanella L, Gronchi A. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17:211–219. doi: 10.1245/s10434-009-0740-x. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Zhang J, Lu Y, Xu Q, Tang B, Wang Q, Zhang W, Chen S, Lu L, Chen X. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015;6:43090–43098. doi: 10.18632/oncotarget.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer? Oncotarget. 2017 doi: 10.18632/oncotarget.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Wang X, Zhang W, Ying H, Xu Y, Zhang J, Min Q, Chen J. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are 2 new inflammatory markers associated with pulmonary involvement and disease activity in patients with dermatomyositis. Clin Chim Acta. 2017;465:11–16. doi: 10.1016/j.cca.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Smith J, Ludwig RL, Marcove RC. Sacrococcygeal chordoma. A clinicoradiological study of 60 patients. Skelet Radiol. 1987;16:37–44. doi: 10.1007/BF00349926. [DOI] [PubMed] [Google Scholar]
- 22.Sundaresan N. Chordomas. Clin Orthop Relat Res. 1986:135–142. [PubMed] [Google Scholar]
- 23.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–2216. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 24.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 25.Varga PP, Szoverfi Z, Fisher CG, Boriani S, Gokaslan ZL, Dekutoski MB, Chou D, Quraishi NA, Reynolds JJ, Luzzati A. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J. 2015;24:1092–1101. doi: 10.1007/s00586-014-3728-6. [DOI] [PubMed] [Google Scholar]
- 26.Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M, Chevalley F, Gasbarrini A, Picci P, Weinstein JN. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 27.Osaka S, Osaka E, Kojima T, Yoshida Y, Tokuhashi Y. Long-term outcome following surgical treatment of sacral chordoma. J Surg Oncol. 2014;109:184–188. doi: 10.1002/jso.23490. [DOI] [PubMed] [Google Scholar]
- 28.Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW. A review of the surgical management of sacral chordoma. Eur J Surg Oncol. 2014;40:1412–1420. doi: 10.1016/j.ejso.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffelt SB, de Visser KE. Cancer: Inflammation lights the way to metastasis. Nature. 2014;507:48–49. doi: 10.1038/nature13062. [DOI] [PubMed] [Google Scholar]
- 31.Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045–4051. doi: 10.1200/JCO.2010.27.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS. Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene. 2013;32:1752–1760. doi: 10.1038/onc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K, Begum N, Thacoor A, Green B, Sarveswaran J. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113:311–320. doi: 10.1038/bjc.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantola T, Klintrup K, Vayrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Napankangas J, Makela J, Karttunen TJ. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729–1736. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 37.Feng Y, Shen J, Gao Y, Liao Y, Cote G, Choy E, Chebib I, Mankin H, Hornicek F, Duan Z. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget. 2015;6:11139–11149. doi: 10.18632/oncotarget.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathios D, Ruzevick J, Jackson CM, Xu H, Shah S, Taube JM, Burger PC, McCarthy EF, Quinones-Hinojosa A, Pardoll DM. PD-1, PD-L1, PD-L2 expression in the chordoma microenvironment. J Neuro-Oncol. 2015;121:251–259. doi: 10.1007/s11060-014-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwab JH, Boland PJ, Agaram NP, Socci ND, Guo T, O'Toole GC, Wang X, Ostroumov E, Hunter CJ, Block JA. Chordoma and chondrosarcoma gene profile: implications for immunotherapy. Cancer Immunol Immunother. 2009;58:339–349. doi: 10.1007/s00262-008-0557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenfeld AJ, Wang X, Wang Y, Hornicek FJ, Nielsen GP, Duan Z, Ferrone S, Schwab JH. CSPG4 as a prognostic biomarker in chordoma. Spine J. 2016;16:722–727. doi: 10.1016/j.spinee.2015.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heery CR, Singh BH, Rauckhorst M, Marte JL, Donahue RN, Grenga I, Rodell TC, Dahut W, Arlen PM, Madan RA. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor brachyury. Cancer Immunol Res. 2015;3:1248–1256. doi: 10.1158/2326-6066.CIR-15-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]