Abstract
Low molecular weight reactive chemicals causing skin and respiratory allergies are known to activate dendritic cells (DC), an event considered to be a key step in both pathologies. Although generation of reactive oxygen species (ROS) is considered a major danger signal responsible for DC maturation, the mechanisms leading to cellular redox imbalance remain poorly understood. Therefore, the aim of this study was to unveil the origin and kinetics of redox imbalance elicited by 1-fluoro-2,4-dinitrobenzene (DNFB) and trimellitic anhydride chloride (TMAC), two golden standards of skin and chemical respiratory allergy, respectively. To track this goal, we addressed the time course modifications of ROS production and cellular antioxidant defenses as well as the modulation of MAPKs signaling pathways and transcription of pathophysiological relevant genes in THP-1 cells. Our data shows that the thiol-reactive sensitizer DNFB directly reacts with cytoplasmic glutathione (GSH) causing its rapid and marked depletion which results in a general increase in ROS accumulation. In turn, TMAC, which preferentially reacts with amine groups, induces a delayed GSH depletion as a consequence of increased mitochondrial ROS production. These divergences in ROS production seem to be correlated with the different extension of intracellular signaling pathways activation and, by consequence, with distinct transcription kinetics of genes such as HMOX1, IL8, IL1B and CD86. Ultimately, our observations may help explain the distinct DC phenotype and T-cell polarizing profile triggered by skin and respiratory sensitizers.
Keywords: ROS, Oxidative stress, Glutathione, Allergic contact dermatitis, Chemical respiratory allergy, Dendritic cells maturation
Graphical abstract
Highlights
-
•
Distinctive ROS origin and kinetics elicited by skin and respiratory sensitizers.
-
•
ROS production elicited by DNFB results primarily from direct GSH haptenation.
-
•
Distinct expression of genes involved in DC maturation and T-cell polarizing capacity.
1. Introduction
Contact and respiratory allergies to low molecular weight (LMW) chemicals are growing among general population in result of an increased exposure to environmental and industrial compounds present in toiletry and household products. Studies focusing in the physiopathology of allergy have pointed out common key molecular events triggered by contact and respiratory allergens that are crucial for the development of the so called adverse outcome pathway [1], [2]. The first assumption is that low molecular weight chemicals (LMW; < 1000 Da) are too small to be recognized by the immune system and must first react with a protein [3], [4]. Such chemicals behave as haptens and are either naturally protein-reactive or are rapidly metabolized into protein-reactive compounds. Covalent binding of an hapten to a protein is believed to be a relevant mechanism for immune recognition and further development of antigenic LMW-induced chemical allergies [4], [5]. The second assumption is that protein-hapten conjugates induce stress responses and xenoinflammation through release of damage-associated molecular patterns (DAMPs), such as reactive oxygen species (ROS), uric acid, hyaluronic acid fragments and extracellular ATP/ADP. These DAMPs are required for the activation of pattern recognition receptors (PRRs) and intracellular signaling pathways in antigen presenting cells such as dendritic cells (DCs), leading to their maturation. Then, and as third assumption, DCs process the conjugates and subsequently migrate to the draining lymph nodes where they prime naive T lymphocytes. T-cells become activated and expand into allergen-specific effector T-cells that disseminate systemically and elicit a strong inflammatory reaction upon later contact with the same chemical [6]. Respiratory tract sensitization has been associated with the development of a Th2 response (promoting immediate-type allergic hypersensitivity), which is consistent with the secretion of interleukin (IL)-4, IL-5, IL-6, IL-10, IL-13 and, for most chemicals, the production of IgE by B lymphocytes [6], [7], [8]. In contrast, skin sensitization is a cell-mediated, delayed type hypersensitivity reaction, involving a preferential polarization of Th1 and cytotoxic T cells with the secretion of interferon-gamma (IFN-γ), IL-2 and tumor necrosis factor β (TNF-β) [6]. An important clue to be further deciphered consists in the identification of the molecular mechanisms that first trigger these qualitatively distinct immunotoxic responses, although it has been shown that depending on DCs maturation state and cytokine/chemokine profiles, they are able to polarize naive T cells into distinct effector populations [9].
As stated previously, ROS (superoxide, O2•− and hydrogen peroxide, H2O2) as well as protein oxidation play an important role in allergen-induced sensitization [10], [11]. Indeed, there is growing evidence that redox equilibrium influences DCs ability to trigger T-cell activation and to regulate the polarity of the immune response [12]. Multiple signaling pathways involved in DC maturation are known to be redox-sensitive, including transcription factors such as NF-κB and AP-1, mitogen-activated protein kinases (MAPKs), and several phosphatases and proteins directly involved in oxidative stress detection such as Keap-1 [Kelch-like ECH-associated protein 1]/Nrf2, hypoxia inducible factor-1 and thioredoxin [13]. Despite the importance attributed to ROS in allergen-induced sensitization, little is known about their nature and formation kinetics. We hypothesize that different intracellular toxicity pathways evoked by respiratory and contact allergens may trigger divergent immune responses. This rational prompted us to investigate the potential sites of ROS generation triggered by respiratory and skin chemical sensitizers on the human monocytic cell line THP-1, as well as the activation of MAPKs signaling pathways (e.g., ERK, JNK and p38 MAPK) and the modulation of relevant genes such as HMOX1, NQO1, MDR1, IL1B, IL8, IL12B, IL18 and CD86. To accomplish this goal, we used 1-fluoro-2,4-dinitrobenzene (DNFB) and trimellitic anhydride chloride (TMAC), two golden standards of contact and respiratory allergies, respectively, that possess an equivalent immunogenic potential. Methyl salicylate (MeSA) was used as a respiratory and contact irritant (negative control) and bacterial lipopolysaccharide (LPS) as a non-allergen immunogenic compound.
2. Materials and methods
2.1. Materials
The chemicals l-Lysine, l-Cysteine hydrochloride, TMAC, DNFB, MeSA, LPS from Escherichia coli (serotype 026:B6), Dibromobimane (34025) and SOD determination Kit (19160) were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). The tetramethyl-rhodamine ethyl ester (TMRE) mitochondrial membrane potential assay kit (ab113852) was obtained from Abcam (Cambridge, UK). Amplex Red Xanthine/Xanthine Oxidase Assay Kit (a22182), hoechst 3342 (H3570), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; D399) for oxidative stress detection and MitoSOX (M36008) red mitochondrial superoxide indicator were obtained from Molecular Probes (Eugene, OR, USA). Phospho-p44/p42 MAPK (ERK1/ ERK2) (9101 S), phospho-p38 MAPK (9211S), phospho-SAPK/JNK (4668S), total p44/p42 MAPK (ERK1/ ERK2) (9102S), p38 MAPK (9212S) and SAPK/JNK (9252S) were from Cell Signaling Technologies (Danvers, MA, USA). The polyvinylidene difluoride (PVDF) membranes were obtained from Millipore Corp (Bedford, MA, USA). Alkaline phosphatase-conjugated secondary antibodies were purchased from GE Healthcare (Chalfont St. Giles, UK). Protease and phosphatase inhibitor cocktails were from Roche (Mannheim, Germany). TRIzol reagent was purchased from Invitrogen (Barcelona, Spain) and RNA Storage Solution was from Ambion (Foster City, CA, USA). The NZY First-Strand cDNA Synthesis Kit was obtained from NZYTech (Lisbon, Portugal) and custom oligonucleotide primers were from Eurofins MWG Operon (Ebersberg, Germany).
2.2. Cell culture and treatment
The THP-1 human monocytic cell line (ATCC TIB-202, American Type Culture Collection, Manassas, VA, USA) was cultured and maintained at a cell density between 0.2 × 106 and 1 × 106 cells/mL in RPMI 1640 supplemented with 10% inactivated fetal bovine serum, 25 mM glucose, 10 mM Hepes, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin and 0.05 mM 2-mercaptoethanol. Cells were subcultured every 3 or 4 days and kept in culture for a maximum of 2 months.
2.3. Chemical exposure
Since a certain level of cytotoxicity is essential for effective DC maturation [14], the concentrations of chemicals inducing up to 30% decrease in cell viability (EC30 value) were determined through the resazurin assay (Supplementary data, Fig. S1). In all subsequent experiments cells were exposed for the indicated times to the EC30 concentration of each chemical, corresponding to 7 μM for DNFB, 400 μM for TMAC and 600 μM for MeSA. In certain experiments, cysteine (Cys) or lysine (Lys) were pre-incubated in chemico with sensitizers. More specifically, we mixed Cys/Lys with sensitizers on microcentrifuge tubes (in chemico reaction) and allowed them to react for 1 h at 37 °C. After that, we stimulated THP-1 cells with the mixture (Cys/Lys + sensitizer) for the indicated times. The final concentration for Cys/Lys was 10 mM and for DNFB and TMAC, 7 μM and 400 μM respectively. Cells were also exposed to LPS (1 μg/mL) as a control for a non-allergen DC maturation inducer.
2.4. Oxidative stress evaluation
Chemical-induced ROS formation was assayed with ROS indicator 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). Briefly, 0.5 × 106 cells/mL were plated in a 12-well plate, exposed to chemicals during indicated times, washed with PBS and then loaded with 5 μM H2DCFDA and 0.5 μg/mL Hoechst in HBSS (in mM: 1.3 CaCl2, 0.5 MgCl2, 5.3 KCl, 0.44 KH2PO4, 4.2 NaHCO3, 138 NaCl, 0.34 Na2HPO4 and 5,5 Glucose, pH 7.4) for 30 min at 37 °C in the dark. Cells were then washed with PBS, transferred to µ-slides 8-well ibidiTreat (ibidi GmbH, München, Germany) for observation. Images were obtained using an Axio Observer.Z1 inverted microscope (Zeiss) and analyzed with Fiji software from ImageJ (http://fiji.sc/Fiji).
2.5. Mitochondrial membrane potential (MMP) integrity
The MMP integrity was evaluated by the TMRE mitochondrial membrane potential assay kit according to the manufacturer's instructions. Briefly, 1 × 106 cells/mL were plated in a 48-well plate and exposed to chemicals for 6 h. Cells were also incubated for 10 min, with 50 μM FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone), a protonophore that collapses the MMP, as a negative control. TMRE (1 mM) was then added for 30 min and cells were further collected, washed and TMRE fluorescence was read (λexc = 549 nm; λem = 575 nm).
2.6. Mitochondrial superoxide anion measurement
Mitochondrial O2− generation was determined using MitoSOX according to the manufacturer's instructions. Briefly, 0.5 × 106 cells/mL were plated in a 12-well plate, exposed to chemicals for the indicated times, washed with PBS and then loaded with 5 μM MitoSOX and 0.5 μg/mL Hoechst in HBSS for 10 min at 37 °C in the dark. Cells were then washed with PBS and transferred to µ-slides 8-well ibidiTreat (ibidi GmbH, München, Germany) for observation. Images were obtained using an Axio Observer.Z1 inverted microscope (Zeiss) and analyzed with Fiji software from ImageJ (http://fiji.sc/Fiji).
2.7. Determination of superoxide dismutase activity
THP-1 cells were plated at a density of 1 × 106 cells/mL, in a 6 well plate and treated as previously described (see Chemical exposure). Cells were further collected, centrifuged (300 g for 5 min, at 4 °C) and washed in ice-cold PBS. After a second centrifugation, the pellet was incubated in RIPA lysis buffer (50 mM Tris–HCl, pH 8.0, 1% Nonidet P-40, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA) for 30 min on ice. The nuclei and the insoluble cell debris were removed by centrifugation (10,000 g for 15 min, at 4 °C) and the supernatant collected and used as total cell lysates. Total SOD activity was then determined using the SOD Determination Kit, according to the manufacturer's instructions, with some modifications. Cell extract duplicates were also incubated with KCN (2 mM) to inhibit SOD 1, thus allowing the measurement of mitochondrial SOD 2. SOD 1 activity was then calculated by subtraction of SOD 2 from total SOD.
2.8. Quantification of xanthine oxidase or hypoxanthine
Xantine or hypoxantine levels were determined using the Amplex Red Xanthine/Xanthine Oxidase Assay Kit according to the manufacturer's instructions. Briefly, 1 × 106 cells/mL were plated in a 6-well plate and exposed to chemicals for 6 h. Briefly, 50 μL of cell extracts and controls were added to separate wells of a microplate and incubated with equal volume of Amplex Red reagent/HRP/xanthine oxidase (100 μM; 0.4 U/mL; 40 mU/mL) (100 or Amplex Red reagent/HRP/hypoxantine (100 μM; 0.4 U/mL; 200 μM) working solutions (100 for 48 h at 37 °C in the dark. Fluorescence was then measured in a microplate reader using (λexc = 530 nm; λem = 590 nm).
2.9. Glutathione (GSH) depletion assay
The effect of chemicals on cell GSH content was determined by fluorescence microscopy. Briefly, 0.5 × 106 cells/mL were plated in a 12-well plate, exposed to chemicals during indicated times, washed with PBS and then loaded with 30 μM dibromobimane for 30 min at 37 °C in the dark. Cells were then washed with PBS and transferred to µ-slides 8-well ibidiTreat (ibidi GmbH, München, Germany) for observation. Images were obtained using an Axio Observer. Z1 inverted microscope (Zeiss) and analyzed with Fiji software from ImageJ (http://fiji.sc/Fiji).
2.10. Analysis of gene transcription by quantitative real-time PCR
Total RNA was isolated from cells with TRIzol reagent according to the manufacturer's instructions. RNA concentration was determined by OD260 measurement using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and samples stored in RNA Storage Solution at − 80 °C until use. Briefly, 1 μg of total RNA was reverse-transcribed using the NZY First-Strand cDNA Synthesis Kit and quantitative real-time PCR (qPCR) reactions were performed, in duplicate for each sample, on a Bio-Rad MyCycler iQ5 as previously described [15]. After amplification, a threshold was set for each gene and Ct values were calculated for all samples. Gene expression changes were analyzed using the built-in iQ5 Optical system software. The results were normalized using HPRT1 as reference gene. Primer sequences were designed using Beacon Designer software version 7.7 (Premier Biosoft International, Palo Alto, CA, USA) (Supplementary data, Table S1) and thoroughly tested.
2.11. Cell lysates and Western Blot analysis
Cells were plated at a density of 0.8 × 106 cell/mL, in a six-well plate with a final volume of 3 mL and treated as previously described (see Chemical exposure). After incubation with chemicals, for the indicated times, cells were collected, centrifuged (300 g, 5 min at 4 °C), and washed in ice-cold PBS. After a second centrifugation, the pellet was incubated in RIPA lysis buffer (50 mM Tris–HCl, pH 8.0, 1% Nonidet P-40, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA), freshly supplemented with 1 mM dithiothreitol (DTT) and protease and phosphatase inhibitor cocktails, for 30 min in ice. The nuclei and the insoluble cell debris were removed by centrifugation (12,000 g for 10 min, at 4 °C). The post-nuclear extracts were collected and used as total cell lysates. Protein concentration was determined using the bicinchoninic acid method, and the cell lysates were denatured at 95 °C, for 5 min, in sample buffer (0.125 mM Tris, pH 6.8; 2% w/v SDS; 100 mM DTT; 10% glycerol; and bromophenol blue) for subsequent use in Western blot analysis. Briefly, 25 μg of protein were electrophoretically separated on a 4–10% (v/v) sodium dodecyl sulfate – polyacrylamide gel and transferred to a PVDF membrane. The membranes were blocked with 5% (w/v) fat-free dry milk in Tris- buffered saline containing 0.1% (v/v) Tween 20 (TBS-T), for 1 h, at room temperature. Blots were then incubated overnight at 4 °C with the primary antibodies against phospho-p44/p42 MAPK (ERK1/ ERK2) (1:1000), phospho-p38 MAPK (1:1000) and phospho-SAPK/JNK(1:1000). The membranes were then washed for 30 min with TBS-T and incubated for 1 h at room temperature with alkaline phosphatase-conjugated anti-rabbit antibody (1:20,000). The immune complexes were detected by membrane exposure to the enhanced chemifluorescence reagent for 5 min, followed by scanning for blue excited fluorescence on the Typhoon imager (GE Healthcare). The generated signals were analyzed using TotalLab TL120. To test whether similar amounts of protein were loaded for each sample, the membranes were stripped and reprobed with antibodies to total ERK1/ ERK2, SAPK/JNK and p38 MAPK. The blots were then developed with alkaline phosphatase-conjugated secondary antibodies and visualized by enhanced chemifluorescence. Phosphorylated protein levels were calculated relative to total protein levels (p-ERK1/2/total ERK, p-p38/total p38 and p-JNK/total JNK).
2.12. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 for Mac OS X (GraphPad Software, San Diego, CA, USA; www.graphpad.com). For each experimental condition, the results are presented as the mean value ± SEM of at least 3 independent experiments. Comparisons between two groups were made by the two-tailed unpaired Student t-test and multiple group comparisons by one-way ANOVA analysis, with a Dunnett´s multiple comparison post-test. Significance levels are as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
3. Results
In this study, we aimed to further elucidate the mechanisms involved in skin and respiratory sensitization evoked by LMW chemicals, focusing on the oxidative stress toxicity pathways of THP-1 cells. The THP-1 cell line is frequently used as a DC surrogate in in vitro skin sensitization tests since, upon stimulation, cells display activation markers, such as increase in phosphotyrosine levels, up-regulation of cell surface co-stimulatory molecules and increase in cytokine and chemokine production [16], [17]. Because a certain level of cytotoxicity is essential for effective DC activation [14], THP-1 cells were exposed to allergens DNFB and TMAC and the irritant MeSA in concentrations that induced up to 30% cytotoxicity (EC30). Cells were also exposed to the non-sensitizing but immunogenic LPS, an immunostimulatory molecule from gram-negative bacteria cell wall that induces the maturation of DCs by binding to the transmembrane TLR4 [18], [19], [20], as a control for DC maturation induction by a non-allergen.
3.1. Both DNFB and TMAC induce ROS production, yet with different origins and kinetics
ROS production and protein oxidation are referred as early molecular events triggered during allergen-induced sensitization. Therefore, we attempted to decipher whether chemicals directly induce ROS production or interfere with the antioxidant defenses of the cell. Thus, we evaluated general ROS formation, mitochondrial membrane potential (MMP) integrity and mitochondrial superoxide levels at different time points. ROS production was addressed using the cell-permeant probe, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Fig. 1). H2DCFDA is non-fluorescent in its reduced state being converted to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) upon cleavage of the acetate groups by intracellular esterases and oxidation. The results demonstrate that only DNFB significantly increased ROS production at 1 h, which decreased at 6 h, although to values still above the control condition (Fig. 1).
Fig. 1.
Chemical-induced ROS production. Human THP-1 cells were exposed to LPS, DNFB, TMAC and MeSA for 1 h (a) and 6 h (b), and ROS production was evaluated by fluorescence microscopy using the cell-permeant dye H2DCFDA. Hoechst 3342 was used as a fluorescent marker for the nucleus. Images shown are representative of three independent experiments. Magnification: 63✕; Scale bar = 20 µm. Results are presented as the means ± SEM of cellular fluorescent intensity of at least 50 cells per experiment. Statistical analysis: one-way ANOVA with Dunnett's multiple comparison test, ***p < 0.001, ****p < 0.0001 compared to untreated cells; t-test, ##p < 0.01, ###p < 0.001.
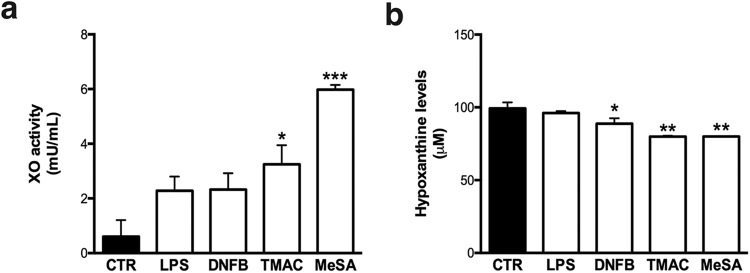
Next we evaluated the activity of the cytoplasmatic enzyme xanthine oxidase (XO), which generates superoxide through the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. All the stimuli tested increased the activity of xanthine oxidase, although only the irritant MeSA and the respiratory allergen TMAC reached statistical significance (p = 0.0219 and p = 0.002, respectively) (Fig. 2a). The increase in XO activity is accompanied by a concomitant consumption of its substrate hypoxanthine, as seen in Fig. 2b.
Fig. 2.
Xanthine oxidase activity (a) and hypoxantine levels (b) after exposure to different stimuli. Human THP-1 cells were exposed to LPS, DNFB, TMAC, and MeSA for 6 h. Xanthine oxidase activity and hypoxanthine levels were further evaluated with the Xanthine/Xanthine Oxidase Assay Kit. The bars in the graphs correspond to the means ± SEM of three independent experiments. Statistical analysis: one-way ANOVA with Dunnett's multiple comparison test; *p < 0.05, **p < 0.01, ***p < 0.001 compared to untreated cells.
Since mitochondrial respiratory chain is a major ROS source and ROS production is often associated with mitochondrial dysfunction, we proceeded to evaluate mitochondrial function using MitoSOX and TMRE (Fig. 3). MitoSOX red reagent is a fluorogenic dye specifically targeted to mitochondria in live cells. Once in the mitochondria, it is rapidly oxidized by superoxide but not by other ROS or reactive nitrogen species (RNS). The oxidized product is highly fluorescent upon binding to nucleic acid. TMRE is a cell permeant, positively charged, red-orange dye that readily accumulates in active mitochondria due to their relative negatively charged matrix. Depolarized or inactive mitochondria have decreased membrane potential and fail to sequester TMRE. FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone) was used as a positive control of depolarized mitochondria, since it is an ionophore uncoupler of oxidative phosphorylation able to mitigate mitochondrial membrane potential and TMRE staining. After 6 h of incubation with chemicals, mitochondrial superoxide levels were similar to those observed in untreated cells except for TMAC, which showed a 2.5-fold increase (Fig. 3b). MMP was not significantly altered by cell exposure to any of the chemicals tested (Fig. 3c).
Fig. 3.
Effect of stimuli over mitochondrial membrane potential and superoxide production. Human THP-1 cells were exposed to LPS, DNFB, TMAC and MeSA for 1 h (a) and 6 h (b and c). Mitochondrial O2- formation was evaluated by fluorescence microscopy using the MitoSOX superoxide indicator (a and b). Hoechst 3342 was used as a fluorescent marker for the nucleus. Magnification: 63✕; Scale bar = 20 µm. Results are presented as the means ± SEM of cellular fluorescent intensity of at least 50 cells per experiment and the images shown are representative of three independent experiments. MMP alterations due to chemical exposure were determined by TMRE fluorescence (c). FCCP (50 μM), a protonophore that collapses mitochondrial membrane potential, was used as a positive control. The bars in the graph correspond to means ± SEM of at least three independent experiments. Statistical analysis: one-way ANOVA with Dunnett's multiple comparison test; **p < 0.01 compared to untreated cells.
3.2. DNFB and TMAC deplete intracellular glutathione with different kinetics
We proceeded to investigate the effect of chemicals on major antioxidant defense systems, namely the soluble antioxidant glutathione (GSH) and the antioxidant enzymes superoxide dismutase (SOD) 1 and 2. Glutatione (GSH) is the most common source of thiol groups present in the cell, with concentrations reaching millimolar levels (1–10 mM). Traditional methods for measuring GSH usually rely on the reaction of compounds, such as dibromobimane, with GSH. Dibromobimage is a cross-linking reagent essentially nonfluorescent, that emits fluorescence when conjugated with several low molecular weight thiols, including glutathione. Indeed, this probe is broadly used to assess changes in GSH [21], [22], [23]. A significant depletion of GSH was observed in cells exposed to DNFB for 1 h (Fig. 4a and b). However, GSH levels recovered to basal values when DNFB treatment was prolonged to 6 h (Fig. 4a and c). These results have a similar trend to those observed for ROS production in cells exposed to DNFB (Fig. 1). In contrast, TMAC only significantly depleted GSH at 6 h (Fig. 4a and c), which is also in accordance with the observed increase in mitochondrial superoxide levels following 6 h of treatment (Fig. 3b). Next, we evaluated SOD activity and, as shown in Fig. 4d and e, only LPS significantly affected this enzymatic system.
Fig. 4.
Effect of stimuli on cellular antioxidant defenses. THP-1 cells were exposed to LPS, DNFB, TMAC and MeSA for 1 h (a and b) and 6 h (a, c, d and e). GSH depletion (a, b and c) was determined by fluorescence microscopy using the thiol reactive protein cross-linking reagent dibromobimane. Magnification: 63✕.; Scale bar = 20 µm. Results in the graphs are presented as the means ± SEM of cellular fluorescent intensity of at least 50 cells per experiment. SOD1 (d) and SOD2 (e) activities were evaluated with SOD determination Kit. The bars in the graphs correspond to the means ± SEM of at least three independent experiments. Statistical analysis: one-way ANOVA with Dunnett's multiple comparison test, *p < 0.05, ***p < 0.001, ****p < 0.0001 compared to untreated cells; t-test, ##p < 0.01. Pictures shown (a) are representative of at least three independent experiments.
3.3. Incubation with Cys or Lys blocks DNFB induced ROS production, GSH depletion and MAPK activation
It is well established that sensitizers are naturally highly reactive or are rapidly metabolized into compounds that react with thiol or primary amine groups present in proteins [4]. Given the rapid and extensive changes evoked by DNFB on cellular ROS and GSH levels we next evaluated whether pre-incubation of the sensitizer with cysteine or lysine blocked the observed effects. DNFB and its analogue DNCB were reported to have a mixed reactivity with both thiol and amine groups [24], [25]. Indeed, when DNFB was pre-incubated in chemico for 1 h with cysteine or lysine before addition to THP-1 cells, we did not observe an increase in ROS production (Fig. 5a) or GSH depletion (Fig. 5b). To assess MAPK activation, we first evaluated p38, JNK and ERK phosphorylation in THP-1 cells stimulated with chemicals for different periods of time by western blot analysis (Supplementary data, Figure S2). Results indicate that only p38 and JNK were phosphorylated at all times tested, with the peak occurring at 1 h. We then proceeded with 1 h of incubation for further experiments. Similarly to what was observed for ROS production and GSH depletion in cells treated with DNFB, MAPKs activation was also suppressed if DNFB and TMAC were previously incubated with lysine or cysteine (Fig. 5c and d). Regardless of previous studies reporting TMAC reactivity only towards lysine residues [24], our results suggest that TMAC reacts with both cysteine and lysine. Indeed, JNK and p38 phosphorylation was significantly lower when TMAC was pre-incubated with cysteine.
Fig. 5.
Effect of the pre-incubation of sensitizers with cysteine (CYS) or lysine (LYS) on ROS production, GSH depletion and MAPK activation. DNFB and TMAC were pre-incubated in chemico for 1 h with cysteine or lysine and later added to THP-1 cells for 1 h. ROS production (a) and GSH depletion (b) were evaluated by fluorescence microscopy, using the cell-permeant dye H2DCFDA and the thiol reactive protein cross-linking reagent dibromobimane, respectively. Hoechst 3342 was used as a fluorescent marker for the nucleus. Magnification: 63✕.; Scale bar = 20 µm. Evaluation of the JNK (c) and p38 (d) signaling pathways activation was performed by western blotting of total cell extracts. Data correspond to the means ± SEM of at least three independent experiments and is expressed as % relatively to untreated cells (CTR). Statistical analysis: one-way ANOVA with Dunnett's multiple comparison test: **p < 0.01, ***p < 0.001 ****p < 0.0001, compared to CTR; t-test: #p < 0.05, ##p < 0.01, ####p < 0.0001. Images shown (a and b) are representative of three independent experiments.
3.4. Gene modulation by contact and respiratory chemicals
We further investigated the transcription of genes related to DCs functions on the physiopathology of chemical allergy: genes containing antioxidant response elements (ARE), namely heme oxigenase 1 (HMOX1) and NADPH quinone oxidoredutase 1 (NQO1); multidrug resistance protein 1 (MDR1), an ATP-dependent drug efflux pump for xenobiotic compounds which is involved in DC migration; cytokines well-known to be modulated upon hapten stimulation (IL1B, IL8, IL12B, IL18, and IL8) [26], [27]; and CD86, a DC maturation marker. The effect of chemicals on the gene expression was evaluated by quantitative real-time PCR (qPCR) at 6 and 24 h post-treatment. We observed that the transcription of ARE-dependent genes was markedly induced by the contact allergen DNFB early after exposure, while TMAC elicited a delayed response (Fig. 6a and b). These results are in accordance with the observed rapid induction of oxidative stress by DNFB while TMAC seems to activate stress related toxicity pathways with a later profile. Previous studies demonstrated that skin DCs and T cells express MDR1, which has been described as being required for efficient DC maturation and T cell migration [28], [29]. Indeed, DNFB significantly increased MDR1 gene transcription compared to untreated cells and cells treated with TMAC at both time points tested (Fig. 6c). Concerning the transcription of cytokines, none of the chemicals tested significantly interfere with IL12B mRNA levels (Fig. 6f). On the other hand, there was an increase in IL1B and IL8 gene transcription in the presence of DNFB (Fig. 6d and e), which is consistent with the literature since IL8 expression is regulated by the transcription factor Nrf2 [30]. Among the compounds tested only DNFB increased the transcription of CD86 (p < 0.001 and p < 0.0001 for 6 h and 24 h respectively). According to our results, none of the genes studied were significantly modulated by the irritant MeSA at both time points tested.
Fig. 6.
Chemical-induced gene expression. THP-1 cells were exposed to chemicals for 6 h (black bars) and 24 h (white bars) and mRNA levels of HMOX1 (a), NQO1 (b), MDR1 (c), IL1B (d), IL8 (e), IL12B (f) IL18 (g) and CD86 (h) were determined by qPCR. Data are represented as log2 of fold expression levels normalized to control cells (log2(1)= 0) of the respective timeline studied. Data depicted in the graphs correspond to the means ± SEM of at least three independent experiments. Statistical analysis: one-way ANOVA with Dunnett's multiple comparison *P < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to untreated cells; t-test (DNFB 6 h vs TMAC 6 h), #p < 0.05, ###p < 0.001, (DNFB 24 h vs TMAC 24 h), §p < 0.05, §§p < 0.01, §§§p < 0.001.
4. Discussion
ROS production and protein oxidation are early danger signals occurring in the sensitization phase of chemical-induced allergy [31], [32]. Indeed, several studies using monocyte-derived dendritic cells and other DC-cell models have shown that exposure to skin sensitizers rapidly induces oxidative stress [31], [33], [34] and that this event is important for DCs activation and maturation. Although their recognized role, the nature, origin and kinetics of ROS induced by chemical sensitizers remain elusive. To address this question, we analyzed in THP-1 cells the mechanisms of redox imbalance elicited by DNFB and TMAC, two golden standards of skin and chemical respiratory allergy, respectively. We found that both sensitizers increased ROS production, although with distinct origins and timings. This results in a different extent at which intracellular signaling pathways are activated and, if the results in THP-1 cells are confirmed in normal DCs, may be in part responsible for the distinct T-cell polarizing abilities attributed to DCs in skin or respiratory chemical allergies.
Under homeostatic conditions, cellular redox status is maintained by a dynamic equilibrium of processes that produce and eliminate ROS. Indeed, there are several known sources of cellular ROS, including NADPH oxidase, the mitochondrial respiratory cycle and xanthine oxidase, which generates superoxide through the oxidation of hypoxanthine to xanthine. To manage these deleterious oxidative molecules cells are equipped with a variety of antioxidants that can be enzymatic, such as SODs, catalase and glutathione peroxidase, and non-enzymatic, such as GSH [35]. We found that both the skin sensitizer DNFB and the respiratory sensitizer TMAC induce oxidative stress, though with temporal and intensity differences. DNFB, highly electrophilic and therefore reactive with thiol groups, rapidly reacts with GSH, inactivating it and subsequently leading to an increase in cytoplasmatic ROS. In contrast, for TMAC our data indicates that GSH depletion at later time points may be in part a consequence of the later increase in mitochondrial ROS and increased xanthine oxidase activity. Accordingly, Silva and colleagues showed that another respiratory sensitizer, hexamethylene diisocyanate, which also predominantly reacts with amine groups, increased mitochondrial ROS accumulation, which was relevant for further inducing the expression of cytoprotective genes and DC maturation markers [36]. Besides TMAC, the irritant MeSA also significantly increased xanthine oxidase activity, a result that is corroborated by the decreased hypoxanthine levels observed for the two compounds.
Regarding the modulation of cellular antioxidant defenses, none of the chemicals tested affected SOD1 or SOD2 activity. Although, in LPS-treated cells, we observed a decrease in SOD 1 activity and an increase in SOD 2 activity. Interestingly, several authors have shown that LPS potently increases the activity and the mRNA levels of MnSOD (SOD 2) but does not change or decreases those of Cu/ZnSOD (SOD 1). Studies by Frank and colleagues in rat renal mesangial cells and whole kidney homogenates from LPS-treated rats showed that induction of SOD 1 was clearly dependent on nitric oxide, as none of the many growth factors and inflammatory cytokines tested were able to induce SOD 1. By contrast, SOD 2 expression was clearly induced by LPS, TNF-α, and IL-1β in mesangial cells in vitro [37]. Also, several studies show that in contrast to mouse macrophages, human monocytes stimulated with cytokines or LPS fail to release NO [38], [39]. These evidences could account for the decrease SOD 1 activity and increased SOD 2 activity observed in cells treated with LPS.
We then proceeded to evaluate the contribution of the major cellular non-enzymatic soluble antioxidant GSH. GSH, present in millimolar concentrations in virtually all cells, donates electrons to H2O2 reducing it to H2O and O2 while being oxidized to GSSG. It is widely believed that strong contact sensitizers covalently bind to thiol or amino protein groups, with several studies reporting the maturation of DCs by 2,4-dinitrochlorobenzene (DNCB), a structural analogue of DNFB, as a consequence of glutathione depletion [31], [40], [41]. Recently, several works reported that DNCB rapidly and extensively reacts with GSH, cysteine and SH-containing peptides [24], [42], [43], [44]. In accordance to this, our results show that DNFB rapidly depletes GSH, which is coincident with the observed increased oxidative status 1 h after cells treatment. Given that major cellular sources of ROS are not affected by the chemical, we hypothesize that the observed oxidative stress is an event resulting from the direct haptenation of GSH leading to its incapacity to neutralize constitutive ROS production. Supporting our hypothesis, DNCB was shown to cause a decrease of almost 45% of intracellular GSH in human peripheral blood mononuclear cells, just 15 min after exposure[42]. Authors suggested that DNCB primarily depletes intracellular GSH, being the reminiscent chemical free to haptenate cellular proteins. In the case of TMAC we found that it causes a decrease of GSH levels only 6 h after cell exposure. Studies addressing TMAC reactivity are contradictory, with some works reporting reactivity towards lysine residues and GSH [24], [45] and others reporting a preferential reactivity with cysteine peptides [46]. Our results indicate that delayed GSH depletion caused by TMAC may be the consequence of GSH consumption in the detoxification of H2O2 produced from SOD activity due to high levels of O2−. Therefore, chemicals that preferentially react with thiol groups, as DNFB, will rapidly induce redox imbalance in consequence of direct GSH depletion, while chemicals that react more extensively with primary amines, such as TMAC, will cause a more delayed oxidative stress.
We then proceeded to evaluate the activation of MAPKs, intracellular signaling pathways known to be involved in sensitizers induced DC maturation [47], [48], [49]. Only sensitizers and LPS were able to modulate THP-1 MAPK signaling, whereas the non-sensitizer MeSA had no significant effect, as previous described in the literature [50]. ERK activation was not induced by the chemicals tested, while SAPK/JNK and p38 MAPKs were strongly modulated by the sensitizers and LPS, with an increased activation at 1 h post-treatment that fell over time but remained above basal values at 6 h. Accordingly, several studies reported the selective activation of p38 MAPK by contact sensitizers (DNFB, NiSO4) and not by irritants. The authors demonstrated that activation of p38 MAPK is involved in DNFB-induced DC up-regulation of CD86, IL-1β and IL-8 [48], [51], [52], [53]. Regarding the effects of chemicals on JNK pathway, we observed a selective and marked increase in phospho-JNK levels following exposure to sensitizers, even more robustly than the activation observed for p38 MAPK. These results are in line with previous studies reporting a sustained phosphorylation of p38 MAPK and JNK following the treatment of the mouse fetal skin-derived dendritic cell line FSDC with the sensitizers DNFB, oxazolone, 1,4-phenylenediamine and NiSO4 but not with irritants sodium dodecyl sulfate (SDS) and benzalkonium chloride (BC) [15]. Interestingly, a similar activation pattern was also observed in murine and human skin explants as well as in reconstituted skin models EST-100 and AST-200 [54]. Although the role of JNK in the immunobiology of DC remains less studied than that of p38 MAPK, several authors pointed out that its activation is implicated in the expression of CD83, CD86 and CCR7 [47].
Curiously, although TMAC and DNFB elicit different kinetics of ROS production, they share similar activation profiles for p38 and JNK MAPKs. This indicates that rather than ROS themselves, direct interaction of chemical sensitizers with cellular proteins would evoke the intracellular signaling events involved in DC maturation. To clarify this, we evaluated if pre-incubation of sensitizers with cysteine or lysine would hamper their capacity to activate THP-1 intracellular signaling events. Indeed, when sensitizers were pre-incubated with cysteine or lysine, we did not observe GSH depletion, ROS production or MAPKs activation. Accordingly, several studies emphasize the relationship between sensitizers reactivity with specific amino acid residues from critical proteins and the modulation of signaling pathways involved in DC maturation. Bruchhausen et. al, demonstrated that the thiol antioxidant N-acetyl-cysteine (NAC) revokes trinitrochlorobenzene-induced tyrosine phosphorylation and p38 MAPK activation in human monocyte-derived dendritic cells (MoDC) by preventing the binding of the sensitizer to proteins [40]. These results, together with the inability of radical scavengers to prevent tyrosine phosphorylation, led them to hypothesize that ROS may not be essential for DCs activation by sensitizers [40]. Reinforcing this hypothesis, our group recently identified, though a proteomics–based approach, several intracellular proteins that are directly targeted by fluorescein isothiocyanate (FITC), a sensitizer that preferentially reacts towards primary amines. Among these proteins, we found that FITC directly haptenizes mixed-lineage protein kinase kinase kinase in THP-1 cells, directly modulating the activation state of p38 and JNK pathways [55].
Finally, we analyzed the effects of chemicals on the transcription of several genes related to cytoprotection, DC maturation and T-cell polarizing capacities. We found that both allergens induce the transcription of HMOX1 and NQO1 detoxifying genes but with different kinetics. DNFB caused an early and marked transcription while TMAC a delayed one. These genes are under regulation of the Nrf2-Keap1-ARE pathway, a signaling cascade that functions primarily as a sensor for electrophilic stress and that has been explored for the identification of cysteine-reactive skin sensitizers in vitro [56], [57], [58]. Briefly, in a steady state, Keap1 which contains highly reactive Cys residues, targets Nrf2 for Cul3-mediated ubiquitinylation and proteolytic degradation in the proteasome. Covalent modification of the reactive Cys residues of Keap1 leads to its dissociation from the transcriptional regulator Nrf2, which then accumulates in the nucleus and activates genes having an ARE domain in their promoter sequence [59], [60]. Besides electrophilic stress, oxidative stress was also shown to activate Nrf2-Keap1-ARE pathway [61]. This may explain the different activation kinetics observed for DNFB and TMAC. While early induction by DNFB results from its strong and direct reactivity toward the Cys residues on Keap1, later induction by TMAC is probably caused by cellular oxidative stress.
Regarding the transcription of pro-inflammatory cytokines, we observed that IL1B, IL8 and IL18 are rapidly and robustly induced by the skin sensitizer DNFB and this effect is maintained over time. In turn, the respiratory sensitizer TMAC caused a modest induction in IL1B at 6 h, which is decreased to basal levels after 24 h. These cytokines, namely IL-12 and IL-18, play an important role in the DC-induced polarization of T-cells into Th1 type subset [62], [63]. In fact, IL-18 was shown to play an important role in allergic contact sensitization, favoring a Th1 type immune response by enhancing the secretion of pro-inflammatory mediators such as TNF-α, IL-8 and IFN-γ [64]. Moreover, contact sensitizers, including pro-haptens, but not irritants or respiratory sensitizers, were shown to induce IL18 expression in the human keratinocyte cell line NCTC2455 [65]. Major differences were also found in the transcription of the co-stimulatory molecule CD86 and MDR1, a membrane transporter with important roles in DC maturation and migration. Therefore, we may hypothesize that skin sensitizers such as DNFB (preferentially thiol-reactive) evoke a sustained transcription of pro-inflammatory cytokines and co-stimulatory molecules in DCs promoting a Th1 polarization, while modest and transitory transcription caused by respiratory sensitizers such as TMAC (preferentially amine-reactive) lead to Th2 responses.
5. Conclusions
Overall, the present study brought new insights about the origin, nature, kinetics and role of redox imbalance triggered by respiratory and skin sensitizers in the human monocytic cell line THP-1.
According to our data, DNFB, a preferentially thiol-reactive skin sensitizer, induces an early depletion of GSH with a concomitant increase in general ROS levels, while TMAC, a preferentially amine-reactive respiratory sensitizer, induces a delayed GSH depletion in consequence of increased mitochondrial ROS production. Our results indicate that the preferential reactivity of sensitizers over thiol or primary amine groups determines the quickness and extent at which danger signals are generated, conditioning the transcription kinetics of genes such as HMOX1, IL1B, IL8, IL18 and CD86 (Fig. 7). Ultimately, these events may account for the distinct DC phenotypes and T-cell polarizing profiles triggered by skin and respiratory sensitizers.
Fig. 7.
Origin, nature, kinetics and role of redox imbalance triggered by respiratory and skin sensitizers in the human monocytic cell line THP-1. Our data shows that the thiol-reactive sensitizer DNFB directly reacts with cytoplasmic glutathione (GSH) causing its rapid and marked depletion which results in a general increase in reactive oxygen species (ROS) accumulation. In turn, TMAC, which preferentially reacts with amine groups, induces a delayed GSH depletion as a consequence of increased mitochondrial ROS production. These divergences in ROS production seem to be correlated with the different extension of intracellular signaling pathways activation observed and, by consequence, with distinct transcription kinetics of genes such as HMOX1, IL8, IL1B and CD86.
Acknowledgements
This work was financed by The Johns Hopkins Center for Alternatives to Animal Testing, Project no: 2014-07, by the Portuguese Foundation for Science and Technology (FCT) and FEDERCOMPETE, through POCI-01–0145-FEDER-007440 and by Regional Operational Programmes (CENTRO-01-0145-FEDER-000012: HealthyAging2020). Isabel Ferreira and João D. Martins were funded through FCT PhD fellowships refs: SFRH/BD/110717/2015 and SFRH/BD/73065/2010, respectively.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.002.
Contributor Information
Isabel Ferreira, Email: isabelcvf@student.uc.pt.
Maria Teresa Cruz, Email: trosete@ff.uc.pt.
Appendix A. Supplementary material
Supplementary material
References
- 1.OECD, The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 1: Scientific Evidence, 2012.
- 2.OECD, The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 2: Use of the AOP to Develop Chemical Categories and Integrated Assessment and Testing Approaches, 2012.
- 3.Lalko J.F., Kimber I., Dearman R.J., Gerberick G.F., Sarlo K., Api A.M. Chemical reactivity measurements: potential for characterization of respiratory chemical allergens. Toxicol. Vitr. 2011;25:433–445. doi: 10.1016/j.tiv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Chipinda I., Hettick J.M., Siegel P.D. Haptenation: chemical reactivity and protein binding. J. Allergy. 2011;2011:839682. doi: 10.1155/2011/839682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landsteiner K., Jacobs J. Studies on the sensitization of animals with simple chemical compounds II. J. Exp. Med. 1936;64:643–657. doi: 10.1084/jem.64.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dearman R.J., Betts C.J., Humphreys N., Flanagan B.F., Gilmour N.J., Basketter D.A., Kimber I. Chemical allergy: considerations for the practical application of cytokine profiling. Toxicol. Sci. 2003;71:137–145. doi: 10.1093/toxsci/71.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Kimber I., Basketter D.A., Gerberick G.F., Ryan C.A., Dearman R.J. Chemical allergy: translating biology into hazard characterization. Toxicol. Sci. 2011;120(suppl):S238–S268. doi: 10.1093/toxsci/kfq346. [DOI] [PubMed] [Google Scholar]
- 8.Kimber I., Dearman R.J. What makes a chemical a respiratory sensitizer? Curr. Opin. Allergy Clin. Immunol. 2005;5:119–124. doi: 10.1097/01.all.0000162302.82233.93. [DOI] [PubMed] [Google Scholar]
- 9.Steinman R.M., Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 10.Byamba D., Kim T.G., Kim D.H., Je J.H., Lee M.-G., Byamba D., Gyun Kim T., Hyun Kim D., Hwan Je J. The roles of reactive oxygen species produced by contact allergens and irritants in monocyte-derived dendritic cells. Ann. Dermatol. 2010;22:269–278. doi: 10.5021/ad.2010.22.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets - Inflamm. Allergy. 2005;4:517–519. doi: 10.2174/1568010054526386. [DOI] [PubMed] [Google Scholar]
- 12.Matsue H., Edelbaum D., Shalhevet D., Mizumoto N., Yang C., Mummert M.E., Oeda J., Masayasu H., Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J. Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J.R. Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulette B.C., Ryan C.A., Gildea L.A., Gerberick G.F. Relationship of CD86 surface marker expression and cytotoxicity on dendritic cells exposed to chemical allergen. Toxicol. Appl. Pharmacol. 2005;209:159–166. doi: 10.1016/j.taap.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Neves B.M., Rosa S.C., Martins J.D., Silva A., Gonçalo M., Lopes M.C., Cruz M.T. Development of an in vitro dendritic cell-based test for skin sensitizer identification. Chem. Res. Toxicol. 2013;26:368–378. doi: 10.1021/tx300472d. [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa M., Ito Y., Yoshida Y., Sakaguchi H., Suzuki H. Phenotypic alterations and cytokine production in THP-1 cells in response to allergens. Toxicol. Vitr. 2007;21:428–437. doi: 10.1016/j.tiv.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.dos Santos G.G., Reinders J., Ouwehand K., Rustemeyer T., Scheper R.J., Gibbs S. Progress on the development of human in vitro dendritic cell based assays for assessment of the sensitizing potential of a compound. Toxicol. Appl. Pharmacol. 2009;236:372–382. doi: 10.1016/j.taap.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Granucci F., Ferrero E., Foti M., Aggujaro D., Vettoretto K., Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 19.Perrin-Cocon L., Aublin-Gex A., Sestito S.E., Shirey K.A., Patel M.C., André P., Blanco J.C., Vogel S.N., Peri F., Lotteau V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017;7:40791. doi: 10.1038/srep40791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaisho T., Akira S. Critical roles of Toll-like receptors in host defense. Crit. Rev. Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 21.Yakubu S.I., Yakasai I.A., Musa A. Spectrofluorimetric assay method for glutathione and glutathione transferase using monobromobimane. J. Basic Clin. Pharm. 2011;2:151–158. 〈http://www.ncbi.nlm.nih.gov/pubmed/24826016〉 accessed 17, 2018. [PMC free article] [PubMed] [Google Scholar]
- 22.Hedley D.W., Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 23.Cox D.P., Cardozo-Pelaez F. High throughput method for assessment of cellular reduced glutathione in mammalian cells. J. Environ. Prot. Sci. 2007;1:23–28. 〈http://www.ncbi.nlm.nih.gov/pubmed/20463849〉 [PMC free article] [PubMed] [Google Scholar]
- 24.Gerberick G.F., Vassallo J.D., Foertsch L.M., Price B.B., Chaney J.G., Lepoittevin J.-P. Quantification of chemical peptide reactivity for screening contact allergens: a classification tree model approach. Toxicol. Sci. 2007;97:417–427. doi: 10.1093/toxsci/kfm064. [DOI] [PubMed] [Google Scholar]
- 25.Lalko J.F., Kimber I., Dearman R.J., Api A.M., Gerberick G.F. The selective peptide reactivity of chemical respiratory allergens under competitive and non-competitive conditions. J. Immunotoxicol. 2013;10:292–301. doi: 10.3109/1547691X.2012.725784. [DOI] [PubMed] [Google Scholar]
- 26.De Smedt A.C., Heuvel R.L. Van Den, Zwi Berneman N., Schoeters G.E. Modulation of phenotype, cytokine production and stimulatory function of CD34+-derived DC by NiCl(2) and SDS. Toxicol. Vitr. 2001;15:319–325. doi: 10.1016/s0887-2333(01)00029-7. [DOI] [PubMed] [Google Scholar]
- 27.Aiba S., Manome H., Nakagawa S., Mollah Z.U.A., Mizuashi M., Ohtani T., Yoshino Y., Tagami H. p38 Mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J. Invesig. Dermatol. 2003;120:390–399. doi: 10.1046/j.1523-1747.2003.12065.x. [DOI] [PubMed] [Google Scholar]
- 28.Randolph G.J., Beaulieu S., Pope M., Sugawara I., Hoffman L., Steinman R.M., Muller W.A. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. USA. 1998;95:6924–6929. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pendse S.S., Behjati S., Schatton T., Izawa A., Sayegh M.H., Frank M.H. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am. J. Transplant. 2006;6:2884–2893. doi: 10.1111/j.1600-6143.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X., Chen X., Song H., Chen H.-Z., Rovin B.H. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur. J. Immunol. 2005;35:3258–3267. doi: 10.1002/eji.200526116. [DOI] [PubMed] [Google Scholar]
- 31.Mizuashi M., Ohtani T., Nakagawa S., Aiba S. Redox imbalance induced by contact sensitizers triggers the maturation of dendritic cells. J. Investig. Dermatol. 2005;124:579–586. doi: 10.1111/j.0022-202X.2005.23624.x. [DOI] [PubMed] [Google Scholar]
- 32.Mokra D., Drgova A., Pullmann R., Mikolka P., Antosova M., Mokry J. Changes in several inflammatory and oxidation markers after ovalbumin-sensitization in a guinea pig model of allergic asthma - a pilot study. Acta Med. Martiniana. 2012;12:5–11. [Google Scholar]
- 33.Esser P.R., Wölfle U., Dürr C., von Loewenich F.D., Schempp C.M., Freudenberg M.A., Jakob T., Martin S.F. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One. 2012;7:e41340. doi: 10.1371/journal.pone.0041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matos T.J., Duarte C.B., Gonçalo M., Lopes M.C. Role of oxidative stress in ERK and p38 MAPK activation induced by the chemical sensitizer DNFB in a fetal skin dendritic cell line. Immunol. Cell Biol. 2005;83:607–614. doi: 10.1111/j.1440-1711.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 35.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva A., Nunes C., Martins J., Dinis T.C.P., Lopes C., Neves B., Cruz T. Respiratory sensitizer hexamethylene diisocyanate inhibits SOD 1 and induces ERK-dependent detoxifying and maturation pathways in dendritic-like cells. Free Radic. Biol. Med. 2014;72:238–246. doi: 10.1016/j.freeradbiomed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Frank S., Zacharowski K., Wray G.M., Thiemermann C., Pfeilschifter J. Identification of copper/zinc superoxide dismutase as a novel nitric oxide-regulated gene in rat glomerular mesangial cells and kidneys of endotoxemic rats. FASEB J. 1999;13:869–882. doi: 10.1096/fasebj.13.8.869. [DOI] [PubMed] [Google Scholar]
- 38.Zembala M., Siedlar M., Pryjma J., Marcinkiewicz J. Human monocytes are stimulated for nitric oxide releasein vitro by some tumor cells but not by cytokines and lipopolysaccharide. Eur. J. Immunol. 1994;24:435–439. doi: 10.1002/eji.1830240225. [DOI] [PubMed] [Google Scholar]
- 39.Arias M., Zabaleta J., Rodríguez J.I., Rojas M., París S.C., García L.F. Failure to induce nitric oxide production by human monocyte-derived macrophages. manipulation of biochemical pathways. Allergol. Immunopathol. 1997;25:280–288. 〈http://www.ncbi.nlm.nih.gov/pubmed/9469204〉 accessed 19, 2018. [PubMed] [Google Scholar]
- 40.Bruchhausen S., Zahn S., Valk E., Knop J., Becker D. Thiol antioxidants block the activation of antigen-presenting cells by contact sensitizers. J. Investig. Dermatol. 2003;121:1039–1044. doi: 10.1046/j.1523-1747.2003.12510.x. [DOI] [PubMed] [Google Scholar]
- 41.Becker D., Valk E., Zahn S., Brand P., Knop J. Coupling of contact sensitizers to thiol groups is a key event for the activation of monocytes and monocyte-derived dendritic cells. J. Investig Dermatol. 2003;120:233–238. doi: 10.1046/j.1523-1747.2003.12026.x. [DOI] [PubMed] [Google Scholar]
- 42.Pickard C., Smith A.M., Cooper H., Strickland I., Jackson J., Healy E., Friedmann P.S. Investigation of mechanisms underlying the T-cell response to the hapten 2,4-dinitrochlorobenzene. J. Investig. Dermatol. 2007;127:630–637. doi: 10.1038/sj.jid.5700581. [DOI] [PubMed] [Google Scholar]
- 43.Jacquoilleot S., Sheffield D., Olayanju A., Sison-Young R., Kitteringham N.R., Naisbitt D.J., Aleksic M. Glutathione metabolism in the HaCaT cell line as a model for the detoxification of the model sensitisers 2,4-dinitrohalobenzenes in human skin. Toxicol. Lett. 2015;237:11–20. doi: 10.1016/j.toxlet.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Megherbi R., Kiorpelidou E., Foster B., Rowe C., Naisbitt D.J., Goldring C.E., Park B.K. Role of protein haptenation in triggering maturation events in the dendritic cell surrogate cell line THP-1. Toxicol. Appl. Pharmacol. 2009;238:120–132. doi: 10.1016/j.taap.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Lalko J.F., Kimber I., Gerberick G.F., Foertsch L.M., Api A.M., Dearman R.J. The direct peptide reactivity assay: selectivity of chemical respiratory allergens. Toxicol. Sci. 2012;129:421–431. doi: 10.1093/toxsci/kfs205. [DOI] [PubMed] [Google Scholar]
- 46.Jaworska J., Harol A., Kern P.S., Gerberick G.F. Integrating non-animal test information into an adaptive testing strategy - skin sensitization proof of concept case. ALTEX. 2011;28:211–225. doi: 10.14573/altex.2011.3.211. [DOI] [PubMed] [Google Scholar]
- 47.Boislève F., Kerdine-Römer S., Pallardy M. Implication of the MAPK pathways in the maturation of human dendritic cells induced by nickel and TNF-alpha. Toxicology. 2005;206:233–244. doi: 10.1016/j.tox.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Arrighi J.F., Rebsamen M., Rousset F., Kindler V., Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 49.Nakahara T., Moroi Y., Uchi H., Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Trompezinski S., Migdal C., Tailhardat M., Le B. Varlet, P. Courtellemont, M. Haftek, M. Serres, Characterization of early events involved in human dendritic cell maturation induced by sensitizers: cross talk between MAPK signalling pathways. Toxicol. Appl. Pharmacol. 2008;230:397–406. doi: 10.1016/j.taap.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Brand P., Plochmann S., Valk E., Zahn S., Saloga J., Knop J., Becker D. Activation and translocation of p38 mitogen-activated protein kinase after stimulation of monocytes with contact sensitizers. J. Investig. Dermatol. 2002;119:99–106. doi: 10.1046/j.1523-1747.2002.01791.x. [DOI] [PubMed] [Google Scholar]
- 52.Mitjans M., Galbiati V., Lucchi L., Viviani B., Marinovich M., Galli C.L., Corsini E. Use of IL-8 release and p38 MAPK activation in THP-1 cells to identify allergens and to assess their potency in vitro. Toxicol. Vitr. 2010;24:1803–1809. doi: 10.1016/j.tiv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Nukada Y., Miyazawa M., Kosaka N., Ito Y., Sakaguchi H., Nishiyama N. Production of IL-8 in THP-1 cells following contact allergen stimulation via mitogen-activated protein kinase activation or tumor necrosis factor-alpha production. J. Toxicol. Sci. 2008;33:175–185. doi: 10.2131/jts.33.175. [DOI] [PubMed] [Google Scholar]
- 54.Koeper L.-M., Schulz A., Ahr H.J., Vohr H.-W. In vitro differentiation of skin sensitizers by cell signaling pathways. Toxicology. 2007;242:144–152. doi: 10.1016/j.tox.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 55.Guedes S., Neves B., Vitorino R., Domingues R., Cruz M.T., Domingues P. Contact dermatitis: in pursuit of sensitizer's molecular targets through proteomics. Arch. Toxicol. 2017;91:811–825. doi: 10.1007/s00204-016-1714-y. [DOI] [PubMed] [Google Scholar]
- 56.Natsch A., Emter R. Skin sensitizers induce antioxidant response element dependent genes: application to the in vitro testing of the sensitization potential of chemicals. Toxicol. Sci. 2008;102:110–119. doi: 10.1093/toxsci/kfm259. [DOI] [PubMed] [Google Scholar]
- 57.Emter R., Ellis G., Natsch A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol. Appl. Pharmacol. 2010;245:281–290. doi: 10.1016/j.taap.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Natsch A. The Nrf2-Keap1-ARE Toxicity Pathway as a Cellular Sensor for Skin Sensitizers—Functional Relevance and a Hypothesis on Innate Reactions to Skin Sensitizers. Toxicol. Sci. 2010;113:284–292. doi: 10.1093/toxsci/kfp228. [DOI] [PubMed] [Google Scholar]
- 59.Dinkova-Kostova A.T., Holtzclaw W.D., Kensler T.W. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 60.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.-I., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi A., Kang M.-I., Watai Y., Tong K.I., Shibata T., Uchida K., Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 63.Tominaga K., Yoshimoto T., Torigoe K., Kurimoto M., Matsui K., Hada T., Okamura H., Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int. Immunol. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 64.Cumberbatch M., Dearman R.J., Antonopoulos C., Groves R.W., Kimber I. Interleukin (IL)-18 induces Langerhans cell migration by a tumour necrosis factor-alpha- and IL-1beta-dependent mechanism. Immunology. 2001;102:323–330. doi: 10.1046/j.1365-2567.2001.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corsini E., Mitjans M., Galbiati V., Lucchi L., Galli C.L., Marinovich M. Use of IL-18 production in a human keratinocyte cell line to discriminate contact sensitizers from irritants and low molecular weight respiratory allergens. Toxicol. Vitr. 2009;23:789–796. doi: 10.1016/j.tiv.2009.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material