Abstract
Objective:
Cancer-associated inflammation and coagulation cascades play vital roles in cancer progression and survival. In this study, we investigated the significance of the combination of preoperative fibrinogen and the neutrophil-to-lymphocyte ratio (NLR) in predicting the survival of patients with non-small cell lung cancer (NSCLC).
Methods:
We retrospectively enrolled 589 patients with NSCLC who underwent surgery. The univariate and multivariate Cox survival analyses were used to evaluate the prognostic indicators, including the combination of fibrinogen and NLR (F-NLR). The cut-off values for fibrinogen, NLR, and clinical laboratory variables were defined by the receiver operating characteristic (ROC) curve analysis. According to the ROC curve, the recommended cut-off values for fibrinogen and the NLR were 3.48 g/L and 2.30, respectively. Patients with both a high NLR (≥ 2.30) and hyperfibrinogenemia (≥ 3.48 g/L) were given a score of 2, whereas those with one or neither were scored as 1 or 0, respectively.
Results:
Our results showed that F-NLR was an independent prognostic indicator for disease-free survival (DFS) [hazard ratio (HR), 1.466; 95% confidence interval (CI), 1.243–1.730; P < 0.001] and overall survival (OS) (HR, 1.512; 95% CI, 1.283–1.783; P < 0.001). The five-year OS rates were 66.1%, 53.5%, and 33.3% for the F-NLR = 0, F-NLR = 1, and F-NLR = 2, respectively ( P < 0.001). Correspondingly, their five-year DFS rates were 62.2%, 50.3%, and 30.4%, respectively ( P < 0.001). In the subgroup analyses of the pathological stages, the F-NLR level was significantly correlated with DFS and OS in stage I and IIIA cancers.
Conclusions:
Preoperative F-NLR score can be used as a valuable prognostic marker for patients with resectable early-stage NSCLC.
Keywords: Non-small cell lung cancer, neutrophil-to-lymphocyte ratio, fibrinogen; prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with non-small cell lung cancer (NSCLC) accounting for approximately 80% of all cases1. Despite the recent improvements in treatment and diagnosis of lung cancer, its prognosis remains unsatisfactory, with a low five-year survival rate of about 15% at diagnosis. Currently, the new strategy in tumor therapy focuses on using a suitable prognostic factor to make the appropriate risk classification of patients with tumors and to design subsequent treatment. Although multiple studies have found a large number of prognostic indicators for patients with NSCLC, the majority of these indicators are not available preoperatively.
Cancer-related inflammation plays an important role in tumor progression and survival2. Cancer-related inflammation, as the 7th hallmark of cancer, promotes the proliferation and invasion of tumor cells and accelerates metastasis3. Moreover, most systemic symptoms associated with cancer, including weight loss, cachexia, and anemia, are stimulated by inflammation4. The neutrophil-to-lymphocyte ratio (NLR), as a representative index, can be considered a useful marker to assess the inflammatory response5. An increased NLR promotes tumor progression and relates to poor prognoses in a variety of cancers, such as NSCLC, esophageal cancer, and gastric cancer6-8. In terms of the systemic inflammatory response, the coagulation cascade also plays a pivotal role in tumor progression and metastasis9. Liver-produced fibrinogen is an important acute phase protein. Fibrinogen, as a key factor in the coagulation cascade, is converted into fibrin under the action of activated thrombin. Hyperfibrinogenemia is involved in cancer aggressiveness in various types of malignancies10-12. Recently, several studies analyzed a novel prognostic index, that is, the combination of fibrinogen and NLR (F-NLR). F-NLR has been found to be a significant prognostic factor in different types of cancers, such as gastric cancer, esophageal carcinoma, and NSCLC13-16.
The present study aimed to evaluate the clinical significance of a novel prognostic system based on fibrinogen concentration and NLR in patients with NSCLC undergoing complete resection. This study also assessed the association between the three F-NLR groups and the clinicopathologic characteristics or the clinical laboratory variables.
Materials and methods
Patients
We performed a retrospective study of patients with NSCLC who underwent complete surgical resection at the Tianjin Medical University Cancer Institute and Hospital between January 2006 and December 2009. The major inclusion criteria were pathological confirmation of primary NSCLC and complete surgical resection. The exclusion criteria were as follows: preoperative treatment (including chemotherapy or radiotherapy), residual tumor cells in the surgical edge, continuous anticoagulant therapy, hematological disease, autoimmune disease, and infection. Patients with intravenous or arterial embolization within 3 months before the surgery were also excluded. Based on the inclusion and exclusion criteria, 589 patients were enrolled in our study. This study was approved by the Ethical Committees of Tianjin Medical University Cancer Institute and Hospital. Prior to the treatment, and written informed consent from all participants were acquired.
Based on the medical records, we collected the patients’ clinicopathologic parameters and laboratory inspections, such as age, sex, histopathology, TNM stage, and blood cell count. Tumor stages were determined according to the 7th edition of the TNM classification17.
F-NLR definition
Hematological indexes, including lymphocyte count, neutrophil count, and fibrinogen concentration, were obtained from the routine blood test administered a week prior to the surgery. The neutrophil count divided by the lymphocyte count was defined as the NLR. Receiver operating characteristic (ROC) curve analysis was used to determine the cut-off values for the preoperative fibrinogen concentration and NLR. According to the ROC curve analysis, the most appropriate cut-off point for NLR was 2.30, with an area under the curve of 0.635. Therefore, we recommended 2.30 as the cut-off value for NLR. Similarly, the optimal point based on the ROC curve showed a cut-off value of 3.48 g/L for fibrinogen, with an area under the curve of 0.595. Consequently, we defined 3.48 g/L as the optimal cut-off value for fibrinogen.
Based on these cut-off values, we calculated the F-NLR score. Patients with both a high NLR (≥ 2.30) and hyperfibrinogenemia (≥ 3.48 g/L) were given a score of 2. Patients with either high NLR (≥ 2.30) or hyperfibrinogenemia (≥ 3.48 g/L) were given a score of 1. Patients without either abnormality were scored 0.
Statistical analysis
Chi-square and Kruskal-Wallis tests were applied to evaluate the differences between the three F-NLR groups and the clinicopathologic characteristics or clinical laboratory variables. Continuous variables are presented as mean ± SD. We carried out the ROC curve analysis to select the appropriate cut-off values for NLR, fibrinogen, and the clinical laboratory variables. These clinical laboratory variables included fibrinogen, lactate dehydrogenase (LDH), D-dimer, neutrophil ratio, monocyte ratio, lymphocyte ratio, white blood cell (WBC) count, platelet count, hemoglobin (Hb), and alkaline phosphatase (ALP). The outcomes of this study were disease-free survival (DFS) and overall survival (OS). DFS was defined as the time in months from the date of surgery to the date of first progression or last follow-up. OS was defined as the time in months from the date of surgery to the date of death or last follow-up. Survival analysis was performed using the Kaplan-Meier survival curve. Univariate and multivariate analyses, which were carried out by Cox regression models, were used to determine the prognostic factors. SPSS version 18.0 (SPSS Inc., Chicago, IL) was utilized for statistical analyses. A P value of < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 589 patients who were pathologically diagnosed with NSCLC were included in this study. All patients underwent surgery for early-stage NSCLC. Tables 1 and 2 illustrate the relationship of the clinicopathologic variables and clinical laboratory parameters with patients grouped by their F-NLR score. The present study included 390 (66.2%) men and 199 (33.8%) women, ranging 24–82 years (median age: 60 years). The allocation of the F-NLR score was as follows: F-NLR = 0, 207 (35.1%) patients; F-NLR = 1, 154 (26.2%) patients; and F-NLR = 2, 228 (38.7%) patients. A total of 278, 120, and 191 patients presented with pathological stages I, II, and IIIA, respectively. The median and mean follow-up periods were 44 and 44.3 months, respectively. The five-year OS rate in the entire study population was 50.3%.
1.
Correlation between preoperative F-NLR and clinicopathologic characteristics of patients with NSCLC
| F-LMR score, n(%) | P | |||
| 0 | 1 | 2 | ||
| NSCLC: non-small cell lung cancer; SqCC: squamous cell carcinoma; F-NLR: combination of fibrinogen concentration and neutrophil to lymphocyte ratio. | ||||
| Age, years | 0.005 | |||
| ≤ 60 | 115 (55.6) | 60 (39.0) | 120 (52.6) | |
| > 60 | 92 (44.4) | 94 (61.0) | 108 (47.4) | |
| Gender | 0.006 | |||
| Female | 84 (40.6) | 55 (35.7) | 60 (26.3) | |
| Male | 123 (59.4) | 99 (64.3) | 168 (73.7) | |
| Smoking | 0.014 | |||
| Yes | 121 (58.5) | 108 (70.1) | 161 (70.6) | |
| No | 86 (41.5) | 46 (29.9) | 67 (29.4) | |
| Tumor location | 0.496 | |||
| Right | 121 (58.5) | 87 (56.5) | 142 (62.3) | |
| Left | 86 (41.5) | 67 (43.5) | 86 (37.7) | |
| Lesion | <0.001 | |||
| Peripheral | 178 (86.0) | 108 (70.1) | 138 (60.5) | |
| Central | 29 (14.0) | 46 (29.9) | 90 (39.5) | |
| Resection type | <0.001 | |||
| Pneumonectomy | 10 (4.8) | 18 (11.7) | 41 (18.0) | |
| Lobectomy | 197 (95.2) | 136 (88.3) | 187 (82.0) | |
| Pathological stage | <0.001 | |||
| I | 131 (63.3) | 71 (46.1) | 76 (33.3) | |
| II | 18 (8.7) | 33 (21.4) | 69 (30.3) | |
| IIIA | 58(28.0) | 50 (32.5) | 83 (36.4) | |
| Histology | <0.001 | |||
| SqCC | 62 (30.0) | 74 (48.1) | 137 (60.1) | |
| Adenocarcinoma | 122 (58.9) | 53 (34.4) | 66 (28.9) | |
| Others | 23 (11.1) | 27 (17.5) | 25 (11.0) | |
| Lymph node metastasis | 0.028 | |||
| Yes | 71 (34.3) | 64 (41.6) | 107 (46.9) | |
| No | 136 (65.7) | 90 (58.4) | 121 (53.1) | |
| Tumor size, cm | <0.001 | |||
| <4 | 128 (61.8) | 64 (41.6) | 67 (29.4) | |
| ≥4 | 79 (38.2) | 90 (58.4) | 161 (70.6) | |
2.
Correlation between preoperative F-NLR and clinical laboratory characteristics of patients with NSCLC
| Variables | F-NLR=0 (n=207) | F-NLR=1 (n=154) | F-NLR=2 (n=228) | P |
| F-NLR: combination of fibrinogen concentration and neutrophil to lymphocyte ratio; NLR: neutrophil to lymphocyte ratio; WBC: white blood cell; PLT: platelet count; ALP: alkaline phosphatase; Hb: hemoglobin; LDH: lactate dehydrogenase. | ||||
| Age, years | 59.3±9.4 | 62.6±9.5 | 60.0±9.3 | 0.001 |
| Maximum tumor diameter (cm) | 3.3±1.4 | 4.4±1.8 | 5.1±2.4 | <0.001 |
| NLR | 1.6±0.4 | 1.7±0.4 | 3.2±1.0 | <0.001 |
| Fibrinogen (g/L) | 2.8±0.4 | 4.3±0.6 | 4.1±1.0 | <0.001 |
| Neutrophil ratio (%) | 53.8±6.5 | 55.8±6.2 | 67.3±4.8 | <0.001 |
| Monocyte ratio (%) | 7.6±2.3 | 7.9±2.1 | 7.9±2.2 | 0.333 |
| Lymphocyte ratio (%) | 35.2±6.1 | 33.5±6.7 | 22.3±4.3 | <0.001 |
| D-dimer (mg/L) | 0.16±0.09 | 0.21±0.27 | 0.20±0.17 | 0.119 |
| WBC count (×103/μL) | 6.1±1.6 | 7.0±1.9 | 7.4±1.5 | <0.001 |
| PLT (×109/L) | 225.6±63.1 | 265.5±80.9 | 252.7±72.4 | <0.001 |
| ALP (U/L) | 70.4±23.4 | 77.5±22.7 | 78.0±31.6 | <0.001 |
| Hb (g/L) | 140.2±18.2 | 138.0±13.4 | 137.8±14.6 | 0.269 |
| LDH (U/L) | 178.3±53.6 | 180.3±47.8 | 185.4±55.4 | 0.284 |
| Survival period (months) | 51.3±22.7 | 44.7±24.4 | 37.6±25.6 | <0.001 |
Correlation between the clinicopathologic variables or clinical laboratory parameters and F-NLR
The association between the F-NLR and clinicopathologic indexes of patients with NSCLC is shown in Table 1. We found significant correlation of F-NLR with age (P = 0.005), gender (P = 0.006), smoking (P = 0.014), lesion (P < 0.001), resection type ( P < 0.001), pathological stage ( P < 0.001), histology ( P < 0.001), lymph node metastasis ( P = 0.028), and tumor size (P < 0.001).
The clinical laboratory variable distribution in the three F-NLR groups is presented in Table 2. Significant differences among these three groups were demonstrated in the following indexes: age (P = 0.001), maximum tumor diameter (P < 0.001), NLR ( P < 0.001), fibrinogen ( P < 0.001), neutrophil ratio ( P < 0.001), lymphocyte ratio ( P < 0.001), WBC count ( P < 0.001), platelet count ( P < 0.001), ALP ( P < 0.001), and survival period ( P < 0.001).
Survival analysis of F-NLR
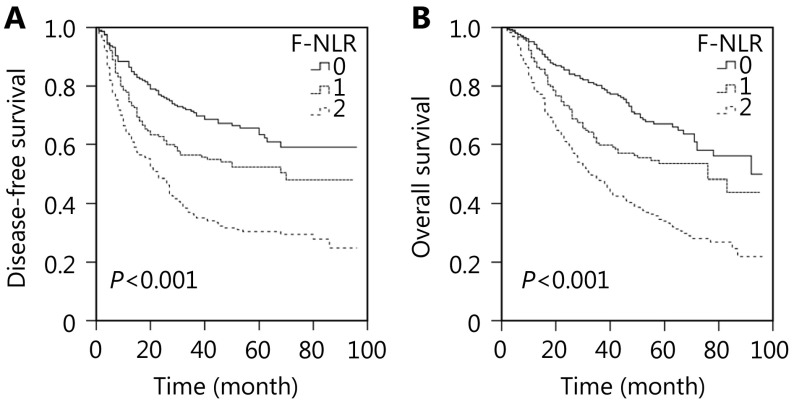
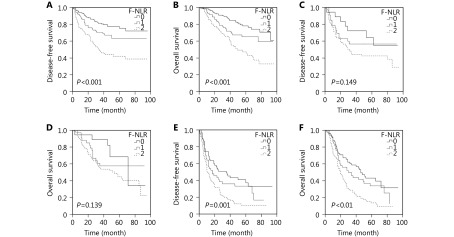
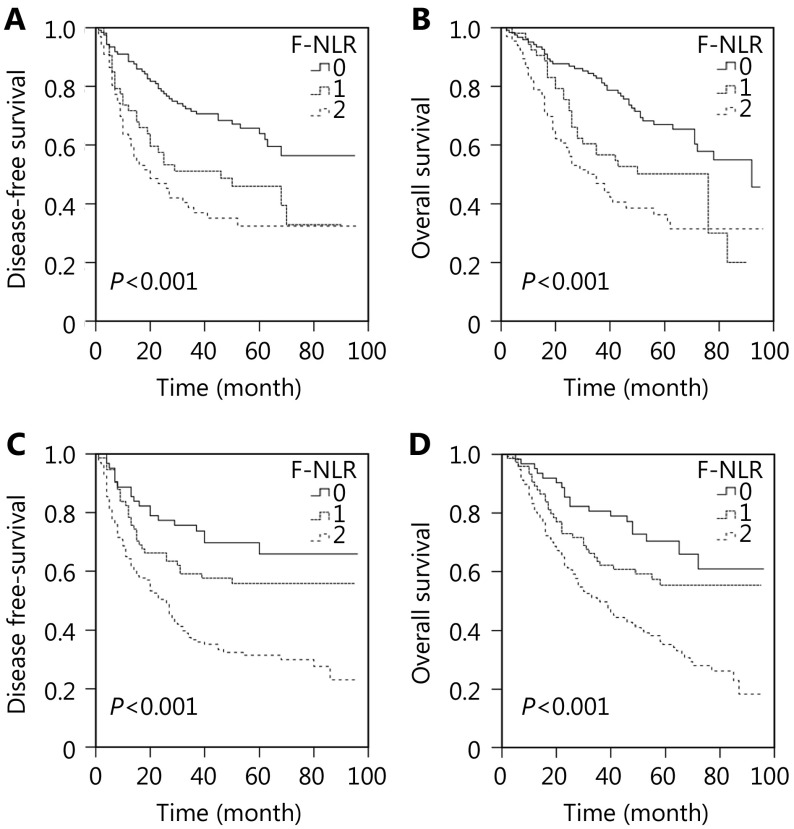
We performed the Kaplan-Meier analysis and log-rank test to determine the survival differences among the three groups classified by F-NLR score. The five-year DFS rate and the median survival in patients with F-NLR = 2 were significantly lower than those in patients with F-NLR = 1 or F-NLR = 0 [30.4% vs. 50.3% or 62.2% (22.5 vs. 36.0 or 42.0 months), P < 0.001; Figure 1A]. The five-year OS rates were 66.1%, 53.5%, and 33.3%, and the median survival times were 51.0, 46.0, and 33.0 months for F-NLR = 0, F-NLR = 1, and F-NLR = 2, respectively (P < 0.001, Figure 1B). When the pathological stages (I, II, and IIIA) were analyzed separately, the DFS and OS of patients with F-NLR = 0 were higher than those with F-NLR = 1 or F-NLR = 2 in stages I and IIIA (stage I: P < 0.001 for DFS, P < 0.001 for OS, Figures 2A and 2B; stage IIIA: P = 0.001 for DFS, P < 0.001 for OS, Figures 2E and 2F). However, no significant relationship was observed between F-NLR and prognosis in patients with stage II NSCLC (P = 0.149 for DFS and P = 0.139 for OS, Figures 2C and 2D). Further analyses were conducted in subgroups (adenocarcinoma and squamous carcinoma). We demonstrated that patients with F-NLR = 0 displayed a higher DFS and OS than those with F-NLR = 1 or F-NLR = 2 in the adenocarcinoma and squamous carcinoma subgroups (adenocarcinoma: P < 0.001 for DFS, P < 0.001 for OS, Figures 3A and 3B; squamous carcinoma: P < 0.001 for DFS, P < 0.001 for OS, Figures 3C and 3D).
1.
Survival curves of patients with non-small cell lung cancer (NSCLC) (stages I-IIIA) in the combination of fibrinogen and neutrophil-to-lymphocyte ratio (F-NLR). (A) Disease-free survival (DFS) curve of patients with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001). (B) Overall survival (OS) curve of patients with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001).
2.
Survival curves of patients with NSCLC (stage I-IIIA) in F-NLR. (A) DFS curve of patients with stage I NSCLC with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001). (B) OS curve of patients with stage I NSCLC with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001). (C) DFS curve of patients with stage II NSCLC with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P = 0.149). (D) OS curve of patients with stage II NSCLC with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P = 0.139). (E) DFS curve of patients with stage IIIA NSCLC with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P = 0.001). (F) OS curve of patients with stage IIIA NSCLC with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001).
3.
Survival curves of patients with adenocarcinoma or squamous carcinoma in F-NLR. (A) DFS curve of patients with adenocarcinoma with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001). (B) OS curve of patients with adenocarcinoma with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001). (C) DFS curve of patients with squamous carcinoma with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001). (D) OS curve of patients with squamous carcinoma with F-NLR=0, F-NLR=1, and F-NLR=2 (log-rank test, P > 0.001).
Univariate and multivariate analyses of variables are shown in Tables 3 and 4, respectively. Based on the cut-off values, we separated the patients into different groups. Univariate analysis demonstrated that lesion (P = 0.023 for DFS and P = 0.014 for OS), resection type (P = 0.041 for DFS and P = 0.038 for OS), pathological stage (P < 0.001 for DFS and P < 0.001 for OS), tumor size ( P < 0.001 for DFS and P < 0.001 for OS), adjuvant radiotherapy ( P = 0.001 for DFS and P = 0.004 for OS), F-NLR score (P < 0.001 for DFS and P < 0.001 for OS), NLR ( P < 0.001 for DFS and P < 0.001 for OS), fibrinogen ( P < 0.001 for DFS and P < 0.001 for OS), LDH ( P = 0.003 for DFS and P = 0.005 for OS), D-dimer (P < 0.001 for DFS and P = 0.002 for OS), neutrophil ratio (P < 0.001 for DFS and P < 0.001 for OS), monocyte ratio ( P = 0.008 for DFS and P = 0.006 for OS), lymphocyte ratio (P < 0.001 for DFS and P < 0.001 for OS), WBC count ( P = 0.007 for DFS and P = 0.008 for OS), ALP (P = 0.002 for DFS and P = 0.003 for OS), and Hb (P = 0.022 for DFS and P = 0.040 for OS) were correlated with DFS and OS.
3.
Univariate analysis for DFS and OS
| Item | DFS | OS | |||||
| P | HR | 95% CI | P | HR | 95% CI | ||
| DFS: disease-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; SqCC: squamous cell carcinoma; F-NLR: combination of fibrinogen concentration and neutrophil to lymphocyte ratio; NLR: neutrophil to lymphocyte ratio; LDH: lactate dehydrogenase; WBC: white blood cell; ALP: alkaline phosphatase; Hb: hemoglobin. | |||||||
| Age, years (≤ 60, >60) | 0.478 | 1.085 | 0.866–1.360 | 0.391 | 1.104 | 0.881–1.384 | |
| Gender (male, female) | 0.714 | 0.956 | 0.751–1.217 | 0.355 | 0.892 | 0.701–1.136 | |
| Smoking (yes, no) | 0.783 | 0.967 | 0.761–1.229 | 0.375 | 0.897 | 0.705–1.141 | |
| Histology (adenocarcinoma, SqCC, others) | 0.109 | 1.146 | 0.970–1.353 | 0.111 | 1.144 | 0.970–1.350 | |
| Tumor location (left, right) | 0.604 | 0.941 | 0.749–1.183 | 0.562 | 0.935 | 0.744–1.175 | |
| Lesion (peripheral, central) | 0.023 | 1.324 | 1.040–1.686 | 0.014 | 1.355 | 1.064–1.726 | |
| Resection type (pneumonectomy, lobectomy) | 0.041 | 1.400 | 1.013–1.933 | 0.038 | 1.407 | 1.019–1.943 | |
| Pathological stage (I, II, IIIA) | <0.001 | 1.788 | 1.569–2.038 | <0.001 | 1.783 | 1.564–2.031 | |
| Tumor size, cm (< 4, ≥4) | <0.001 | 1.796 | 1.415–2.281 | <0.001 | 1.755 | 1.382–2.228 | |
| Adjuvant chemotherapy (yes, no) | 0.138 | 1.187 | 0.946–1.488 | 0.217 | 1.153 | 0.920–1.445 | |
| Adjuvant radiotherapy (yes, no) | 0.001 | 1.685 | 1.235–2.300 | 0.004 | 1.571 | 1.151–2.143 | |
| F-NLR (0, 1, 2) | <0.001 | 1.644 | 1.431–1.888 | <0.001 | 1.647 | 1.434–1.891 | |
| NLR (≥2.30, <2.30) | <0.001 | 2.196 | 1.751–2.754 | <0.001 | 2.199 | 1.753–2.757 | |
| Fibrinogen (<3.48 g/L,≥ 3.48 g/L) | <0.001 | 1.707 | 1.352–2.155 | <0.001 | 1.733 | 1.373–2.188 | |
| LDH (≥195.5, <195.5 U/L) | 0.003 | 1.443 | 1.134–1.835 | 0.005 | 1.415 | 1.112–1.799 | |
| D-dimer (≥0.15, <0.15 mg/L) | <0.001 | 1.546 | 1.232–1.939 | 0.002 | 1.437 | 1.144–1.803 | |
| Neutrophil ratio (≥62.35, <62.35%) | <0.001 | 2.015 | 1.607–2.526 | <0.001 | 2.007 | 1.601–2.517 | |
| Monocyte ratio (≥8.97, <8.97%) | 0.008 | 1.399 | 1.093–1.791 | 0.006 | 1.412 | 1.103–1.807 | |
| Lymphocyte ratio (≤ 26.55, >26.55%) | <0.001 | 0.450 | 0.359–0.565 | <0.001 | 0.451 | 0.359–0.565 | |
| WBC count (≥7.805, <7.805×10 3/μL) | 0.007 | 1.386 | 1.092–1.760 | 0.008 | 1.379 | 1.087–1.751 | |
| Platelet count (≥202, <202×10 9/L) | 0.140 | 1.216 | 0.938–1.577 | 0.160 | 1.205 | 0.929–1.563 | |
| ALP (≥66.5, <66.5 U/L) | 0.002 | 1.465 | 1.152–1.862 | 0.003 | 1.430 | 1.126–1.818 | |
| Hb (≤137.5, >137.5 g/L) | 0.022 | 0.769 | 0.613–0.964 | 0.040 | 0.789 | 0.630–0.989 | |
4.
Multivariate analysis for DFS and OS
| Item | DFS | OS | |||||
| P | HR | 95% CI | P | HR | 95% CI | ||
| DFS: disease-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; F-NLR: combination of fibrinogen concentration and neutrophil to lymphocyte ratio; LDH: lactate dehydrogenase; WBC: white blood cell; ALP: alkaline phosphatase; Hb: hemoglobin. | |||||||
| Lesion (peripheral, central) | 0.642 | 0.931 | 0.687–1.260 | 0.603 | 0.923 | 0.682–1.249 | |
| Resection type (pneumonectomy, lobectomy) | 0.265 | 1.246 | 0.846–1.836 | 0.246 | 1.257 | 0.854–1.851 | |
| Pathological stage (I, II, IIIA) | <0.001 | 1.602 | 1.379–1.862 | <0.001 | 1.648 | 1.419–1.914 | |
| Tumor size (< 4 cm, ≥4 cm) | 0.951 | 0.991 | 0.751–1.309 | 0.878 | 0.978 | 0.741–1.292 | |
| Adjuvant radiotherapy (yes, no) | 0.195 | 1.239 | 0.896–1.714 | 0.377 | 1.157 | 0.837–1.601 | |
| F-NLR (0, 1, 2) | <0.001 | 1.466 | 1.243–1.730 | <0.001 | 1.512 | 1.283–1.783 | |
| LDH (≥195.5, <195.5 U/L) | 0.186 | 1.196 | 0.917–1.560 | 0.294 | 1.152 | 0.885–1.500 | |
| D-dimer (≥0.15, <0.15 mg/L) | 0.007 | 1.403 | 1.096–1.796 | 0.012 | 1.371 | 1.071–1.756 | |
| Monocyte ratio (≥8.97, <8.97%) | 0.079 | 1.279 | 0.972–1.684 | 0.057 | 1.304 | 0.992–1.714 | |
| WBC count (≥7.805, <7.805×10 3/μL) | 0.564 | 1.080 | 0.832–1.402 | 0.687 | 1.055 | 0.812–1.370 | |
| ALP (≥66.5, <66.5 U/L) | 0.071 | 1.283 | 0.979–1.683 | 0.093 | 1.260 | 0.962–1.650 | |
| Hb (≤137.5, >137.5 g/L) | 0.337 | 0.887 | 0.695–1.133 | 0.302 | 0.879 | 0.688–1.123 | |
Multivariate analysis of independent prognostic indicators
To determine the independent predictive indexes, further Cox multivariate analysis, which included the variables mentioned above, was performed. As shown in Table 4, multivariate analysis revealed that F-NLR was significantly related to DFS and OS [hazard ratio (HR), 1.466; 95% confidence interval (CI), 1.243–1.730; P < 0.001 for DFS and HR, 1.512; 95% CI, 1.283–1.783; P < 0.001 for OS, respectively] along with pathological stage and D-dimer. Therefore, multivariate analysis demonstrated that F-NLR was considered an independent prognostic indicator for DFS and OS.
Discussion
Although substantial developments have been made in the treatment and diagnosis of lung cancer, the median survival of NSCLC remains unsatisfactory. An appropriate prognostic factor may enable the suitable risk classification of patients with tumors and allow the assignment of appropriate prospective treatment. Cancer progression and survival are not determined solely by the tumor characteristics. Patient-related factors also play a crucial role in survival. Based on the preoperative blood specimens collected from 589 patients, we investigated the prognostic significance of F-NLR. We also analyzed the association between F-NLR and clinicopathologic or clinical laboratory characteristics.
In the last few decades, inflammation has been increasingly accepted as a hallmark of cancer3. Inflammation can increase the risk of cancer by producing bioactive molecules from the cells infiltrating the tumor microenvironment. Inflammation-related cells introduce crucial cytokines to the tumor microenvironment, thereby promoting the growth, angiogenesis, invasion, metastasis, and survival of cancer cells18-20. Increasing evidence has revealed that systemic inflammation responses are crucial prognostic indicators21. NLR is a systemic inflammation index, which is calculated by dividing the neutrophil count by the lymphocyte count. Lymphocytes, as key components of the host’s anticancer immunity, perform important functions in immunosurveillance and immunoediting and contribute to the inhibition of tumor cell proliferation and migration21. T lymphocytes exert a killing effect on target cells and help induce tumor cell apoptosis in cancer patients22. Increased amounts of circulating blood lymphocytes are an advantageous prognostic index in resected NSCLC23,24. Similar to lymphocytes, neutrophils are recognized as important components of tumor inflammation and immunology. Circulating neutrophils can produce a variety of cytokines, including tumor necrosis factor-α, vascular endothelial growth factor (VEGF), and interleukin, which can promote tumor progression25,26. Neutrophil extracellular traps, which are secreted by neutrophils, can contribute to tumor metastasis by sequestering the tumor cell27. Donskov28 reported that increased neutrophil levels infiltrating the tumor tissue and circulating in the blood are insufficient prognostic indexes in several cancers, including colorectal cancer, lung cancer, and head and neck cancer. With the combination of neutrophil and lymphocyte counts, NLR can be used as a representative index to indicate a systemic inflammatory response in patients with various cancers6-8.
Hyperfibrinogenemia is involved in tumor aggressiveness in various malignancies10-12. Although many studies have investigated the causes of hyperfibrinogenemia in malignant tumors, the underlying mechanism remains unclear. Liver-produced fibrinogen is a major acute-phase protein. When a malignant neoplasm or systemic inflammation is present, the fibrinogen level in the plasma is increased; this fibrinogen can be transformed into fibrin by activated thrombin. Yamaguchi et al.29 indicated that cancer cells can produce interleukin-6, which accelerates the secretion of fibrinogen in patients with lung cancer. Similarly, Sahni et al.30 found that tumor cells can synthesize fibrinogen. Fibrinogen eventually stimulates tumor proliferation and angiogenesis by its interaction with VEGF and fibroblast growth factor-230,31. When fibrinogen is converted, fibrin is involved in metastasis and new vessel formation32,33. Palumbo et al.34 demonstrated that the fibrin formed around circulating tumor cells can prevent natural killer cells from killing tumor cells.
Hence, F-NLR presents a good prognostic indicator for patients with cancer. Fibrinogen or NLR alone may exert a limited effect on tumor progression. F-NLR increases the unfavorable effect of fibrinogen and NLR, which eventually increases the predicted significance for patients with cancer. Recently, Wang et al.16 reported that patients with a low F-NLR score may exhibit a better prognosis than those with a high F-NLR score and that the preoperative F-NLR score can be considered a useful independent prognostic marker, consistent with the results of our study. In the present study, multivariate analysis using the characteristics selected in univariate analysis revealed that preoperative F-NLR was significantly correlated with DFS and OS, as well as pathological stage and D-dimer. According to the results of the Kaplan-Meier analysis and log-rank test, our study revealed that the preoperative F-NLR level can stratify the patients into different risk categories. Moreover, when the patients with different pathological stages were analyzed separately, the DFS and OS in the patients with F-NLR = 0 were higher than those with F-NLR = 1 or F-NLR = 2 in stage I and IIIA. However, in patients with stage II NSCLC, the correlation between F-NLR and prognosis was insignificant, which indicates that F-NLR may be more predictive in stage I or IIIA cancers than in stage II. Our study also found that the preoperative F-NLR level was significantly correlated with both DFS and OS in patients with adenocarcinoma or squamous carcinoma. Furthermore, a close relationship was observed between F-NLR and pathological stage, lesion, lymph node metastasis, and tumor size. To reveal the pathological status of tumor progression, preoperative F-NLR levels calculated from blood specimens should be evaluated. The advantage of the F-NLR score was based on the fibrinogen concentration and NLR, which were obtained from the routine blood sample analysis. Therefore, F-NLR may serve as a more inexpensive and widespread hematologic marker than other tumor markers.
This study had some limitations. First, this study was a retrospective analysis and all data were obtained from a single institute. Second, although we restricted the influence of other factors, blood cell counts can be influenced by a variety of factors.
Conclusions
The preoperative F-NLR score can be considered a valuable prognostic indicator in patients with NSCLC after surgery. A close relationship between F-NLR and cancer progression was also observed in patients with NSCLC who underwent surgery. Thus, F-NLR may be considered for routine clinical use as a reliable and low-cost biomarker.
Acknowledgments
This work was supported by grants from National Key R&D Program of China (Grant No. 2016YFC0905501) and the Tianjin Science and Technology Major Project, China (Grant No. 12ZCDZSY15400).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Siegel R, Ma JM, Zou ZH, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 4.Scott HR, McMillan DC, Forrest LM, Brown DJF, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–7. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Horio H, Hato T, Harada M, Matsutani N, Morita S, et al. Prognostic significance of preoperative neutrophil-lymphocyte ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol. 2015;22:1324–31. doi: 10.1245/s10434-015-4735-5. [DOI] [PubMed] [Google Scholar]
- 7.Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, et al. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–9. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17:703–10. doi: 10.1007/s10120-013-0330-2. [DOI] [PubMed] [Google Scholar]
- 9.Zacharski LR, Meehan KR, Algarra SM, Calvo FA. Clinical trials with anticoagulant and antiplatelet therapies. Cancer Metastasis Rev. 1992;11:421–31. doi: 10.1007/BF01307191. [DOI] [PubMed] [Google Scholar]
- 10.Son HJ, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20:2908–13. doi: 10.1245/s10434-013-2968-8. [DOI] [PubMed] [Google Scholar]
- 11.Lu K, Zhu Y, Sheng LM, Liu LY, Shen L, Wei QC. Serum fibrinogen level predicts the therapeutic response and prognosis in patients with locally advanced rectal cancer. Hepatogastroenterology. 2011;58:1507–10. doi: 10.5754/hge11133. [DOI] [PubMed] [Google Scholar]
- 12.Sheng LM, Luo M, Sun XJ, Lin NM, Mao WM, Su D. Serum fibrinogen is an independent prognostic factor in operable non-small cell lung cancer. Int J Cancer. 2013;133:2720–5. doi: 10.1002/ijc.28284. [DOI] [PubMed] [Google Scholar]
- 13.Arigami T, Uenosono Y, Matsushita D, Yanagita S, Uchikado Y, Kita Y, et al. Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncol Lett. 2016;11:1537–44. doi: 10.3892/ol.2015.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arigami T, Uenosono Y, Ishigami S, Okubo K, Kijima T, Yanagita S, et al. A novel scoring system based on fibrinogen and the neutrophil-lymphocyte ratio as a predictor of chemotherapy response and prognosis in patients with advanced gastric cancer. Oncology. 2016;90:186–92. doi: 10.1159/000444494. [DOI] [PubMed] [Google Scholar]
- 15.Arigami T, Okumura H, Matsumoto M, Uchikado Y, Uenosono Y, Kita Y, et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma: a promising blood marker of tumor progression and prognosis. Medicine (Baltimore) 2015;94:e1702. doi: 10.1097/MD.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HY, Zhao J, Zhang MY, Han LJ, Wang M, Li XD. The combination of plasma fibrinogen and neutrophil lymphocyte ratio (F-NLR) is a predictive factor in patients with resectable non small cell lung cancer. J Cell Physiol. 2017; doi: 10.1002/jcp.26239. (in Press)
- 17.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 18.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–16. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 22.Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding W, Lotze M, et al. Decreased ζ chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma . Clin Cancer Res. 2001;7:947S–57S. [PubMed] [Google Scholar]
- 23.Zhang J, Huang SH, Li H, Li Y, Chen XL, Zhang WQ, et al. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol. 2013;30:352. doi: 10.1007/s12032-012-0352-3. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi N, Usui S, Kikuchi S, Goto Y, Sakai M, Onizuka M, et al. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer. 2012;75:223–7. doi: 10.1016/j.lungcan.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Ulich TR, del Castillo J, Keys M, Granger GA, Ni RX. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-α-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987;139:3406–15. [PubMed] [Google Scholar]
- 26.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 27.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–7. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Yamamoto Y, Yokota S, Nakagawa M, Ito M, Ogura T. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28:740–4. doi: 10.1093/jjco/28.12.740. [DOI] [PubMed] [Google Scholar]
- 30.Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2) J Thromb Haemost. 2008;6:176–83. doi: 10.1111/j.1538-7836.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- 31.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–8. [PubMed] [Google Scholar]
- 32.Wojtukiewicz MZ, Sierko E, Klemont P, Rak J. The hemostatic system and angiogenesis in malignancy. Neoplasia. 2001;3:371–84. doi: 10.1038/sj.neo.7900184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiarug V, Ruggiero M, Magnelli L. Angiogenesis and the unique nature of tumor matrix. Mol Biothechnol. 2002;21:85–90. doi: 10.1385/mb:21:1:085. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo JS, Talmage KE, Massari JV, La jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]