Abstract
Background
Empty sella is the neuroradiological or pathological finding of an apparently empty sella turcica containing no pituitary tissue. The prevalence of primary empty sella, i.e., empty sella without any discernible cause, is not precisely known; estimates range from 2% to 20%. Technical advances in neuroradiology have made empty sella an increasingly common incidental finding. It remains unclear whether, and to what extent, asymptomatic adult patients with an incidentally discovered empty sella should undergo diagnostic testing for hormonal disturbances.
Methods
To answer this question, the authors carried out a systematic search in the PubMed and Web of Science databases for publications that appeared in the period 1995—2016 and that contained the search term “empty sella” (registration: PROSPERO 2015: CRD42015024550).
Results
The search yielded 1282 hits. After the exclusion of duplicates, pediatric reports, case reports, and veterinary studies, 120 publications on primary empty sella syndrome (PES) were identified. 4 of these dealt with the prevalence of pituitary insufficiency in patients with PES as an incidental finding. Among patients with PES, the relative frequency of pituitary insufficiency in the pooled analysis was 52% (95% confidence interval [38; 65]).
Conclusion
The data on PES as an incidental finding are too sparse to enable any evidence-based recommendation on the potential indications for hormone testing or its nature and extent. We advise basic neuroendocrinological testing (fasting cortisol, free thyroxine [fT4], estradiol or testosterone, insulin-like growth factor 1 [IGF-1], and prolactin). There is an unexplained discrepancy between the reported high prevalence of pituitary insufficiency among persons with PES and its low prevalence in epidemiologic studies. We suspect that the former may be high because of selection bias in the publications that we reviewed, or else the latter may be erroneously low.
We can assume that improvements in neuroradiological imaging techniques have resulted in an increase in the incidental finding of an “empty sella.” According to current data from India, an empty sella turcica without any detectable cause is an incidental finding in about 2% of all cerebral magnetic resonance imaging (MRI) scans (1). In asymptomatic adult patients, the question is whether and to what extent diagnostic hormone testing should be undertaken in an incidental finding of an empty sella, particularly as this does not necessarily have pathological significance. On the one hand, overdiagnosis should be avoided as this might upset patients unnecessarily, and it also incurs substantial expense to the healthcare system (2). On the other hand, treating hormonal dysregulation has a positive effect on morbidity and quality of life (3, 4).
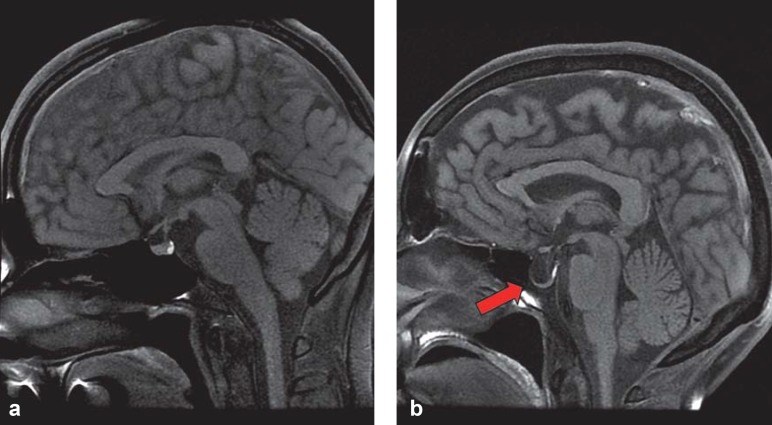
The term “empty sella” was first used in 1951 (5) for the neuroradiological or pathological-anatomical exposure of an apparently empty sella turcica. This impression arises from the fact that the sella turcica is filled with cerebrospinal fluid (CSF), and the hypophyseal tissue at its base is flattened (Figure 1). For this reason, an empty sella is often regarded as a herniation of the subarachnoid space into the sella turcica (6). In this setting, a distinction is made between:
Figure 1.
Cerebral MRI scan of normal sella turcica with regular pituitary gland (a) and empty sella (b)
Partial empty sella syndrome, with the sella turcica filled with CSF to a level of <50%
Complete empty sella syndrome, with the sella turcica filled with CSF to a level of >50% (7).
An empty sella can have several causes (8). Increased intracranial pressure can lead to herniation of the subarachnoid space, which can in turn lead to compression of the pituitary gland. This pathomechanism has been observed in cerebral tumors or idiopathic intracranial hypertension (IIH), for example. That said, the pituitary gland may be atrophied subsequent to an earlier injury. Pituitary gland necrosis can occur, among others, in a setting of pituitary adenoma, postpartum (Sheehan’s syndrome), after craniocerebral trauma, or after radiotherapy. Whenever the empty sella turcica is a sequela of another disorder, the term “secondary empty sella syndrome” (SES) is used (Box). By contrast, in primary empty sella syndrome (PES), the etiology of the pituitary gland atrophy with a simultaneous increase in the CSF volume filling the sella turcica is not clear. Congenital anomalies are being discussed as a possible mechanism.
BOX. Possible causes of secondary empty sella syndrome:
Increase in intracranial pressure, for example, because of a cerebral tumor or hydrocephalus
Idiopathic intracranial hypertension
Surgery for pituitary tumor
Sheehan‘s syndrome (postpartum pituitary gland necrosis)
Sequela of craniocerebral trauma
Sequela of cerebral radiotherapy
The estimated prevalence of PES is 6–20%, based on unselective autopsy studies (9– 12). Most patients with PES are female and are affected by obesity, hypertension, headaches, and/or impaired vision (13, 14). This constellation has also been observed in IIH, a cause of SES (15). Additional symptoms, such as rhinorrhea or visual field defects, are rare (16). Our systematic literature search collects and evaluates the available evidence on the prevalence and extent of hormonal dysregulation in an incidental finding of PES as endpoints. The review aims to contribute to answering the question of whether in an incidental finding of PES, further hormone diagnostic testing is required—and if so, to what extent this should be done.
Methods
This systematic review was conducted according to the recommendations of the PRISMA statement (17) for systematic reviews and meta-analyses and was registered with PROSPERO (International Prospective Register of Systematic Reviews of the National Institute for Health Research, NIHR) under the title “Prevalence of pituitary insufficiency in primary empty sella syndrome (PES)” (PROSPERO 2015: CRD42015024550).
Search strategy
We used the general term “empty sella” to search the databases PubMed and Web of Science. Reviews and meta-analyses were excluded from our search. Two authors (MKA, AK) conducted the literature search independently of one another. In case of controversy/dispute or disagreement, these were discussed with a third author (MRS).
Inclusion criteria
Our research interest was in the prevalence of pituitary insufficiency at a particular point in time (cross-sectional study design). To this end, we also included case-control and cohort studies. We included only studies in which an empty sella was diagnosed by cerebral imaging using replicable radiological criteria. Because of advances in neuroradiological techniques and changed cut-off values in neuroendocrinological hormone diagnostics, we evaluated only studies published in the past 22 years (1 January 1995 through 31 December 2016). Diagnostic hormone testing had to have taken place after an incidental finding of PES. The diagnosis of pituitary failure had to be made by using an adequate stimulation test or guideline conform measuring of hormones. We included only studies whose full text was available and published in the English language.
Exclusion criteria
We excluded studies in children as hormone diagnostics in pediatric patients differs from that in adult patients. Furthermore, we excluded case reports with n = 3 patients from our evaluation, because of an assumption of a biased presentation of primarily positive findings in case reports. Consistent with our research question, we did not include studies of secondary empty sella syndrome in our data analysis.
Data extraction
Of those studies that met the inclusion criteria and did not meet any of the exclusion criteria at the level of the summary (abstract and title), we extracted further characteristics from the full text—such as study design, age and number of patients, sex ratios, radiological criteria, and the presence of hormonal dysregulation, including the method used for the diagnosis.
Assessing the methodological quality
We used the evaluation criteria of Guyatt et al. (GRADE working group) to assess the methodological quality of the studies (18, 19). The maximum score of 10 points reflects a high methodological quality.
Data analysis and synthesis
We used models with fixed and random effects to calculate pooled prevalence rates with accompanying 95% confidence intervals, with individual estimators weighted on the basis of the inverse values of their variances, as well as, in addition, on the basis of DerSimonian-Laird estimators. We used the I2 statistic and the appropriate significance tests to check for heterogeneity of the individual study results. We conducted our meta-analysis by using the software package R-Package meta (20), version 4.3–2, for R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Search results
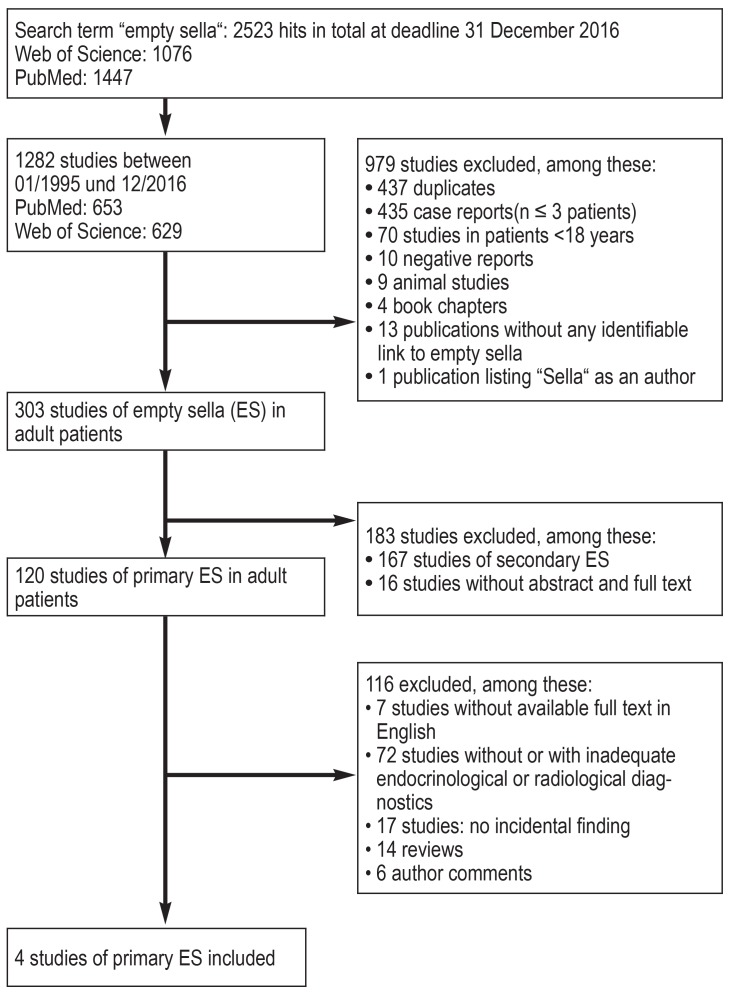
We identified 2523 publications by using the search term “empty sella”. 1282 of these were published between 1995 and 2016. Applying further exclusion criteria yielded 120 remaining studies of PES. Only four of these studies dealt with PES as an incidental finding and had used appropriate endocrinological and radiological diagnostic evaluation methods (Figure 2).
Figure 2.
PRISMA flow diagram showing the literature search; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Study characteristics
eTable 1 provides an overview of the evaluated studies. In addition to a prospective cohort study (21), we included three case-control studies (22– 24). A total of 303 patients (mean age 49 ± 1.2 years) with PES were examined at four centers. eTable 2 provides a detailed overview of the diagnostic methods.
eTable 1. Included studies and their characteristics (in alphabetical order).
| Reference | Country | Study design | Study period |
Number of patients with empty sella |
Age (mean ± SD) |
Symptoms |
Initially imaging, followed by endocrinologic diagnostics |
Radiological method |
Radiological diagnostic criteria empty sella present |
Study quality*1 |
| Cannavo 2002 (23) |
Italy | Case-control study*2 | Not available | 43 | 48 ± 12 | Headache, impaired vision | Yes | MRI | Yes | 8 |
| Colao 2013 (24) |
Italy | Case-control study*3 | Not available | 94 | 50.1 ± 9.3 | Not available | Yes (78%)*4 | CT, MRI | Yes | 10 |
| Lupi 2011 (22) |
Italy | Case-control study*5 | 2006–2009 | 85 (PES), 16 (SES) |
48 ± 1 | Headache, impaired vision, sexual dysfunction, oligomenorrhea, rhinoliquorrhea*6 |
Yes | MRI | Yes | 10 |
| Zuhur 2014 (21) |
Turkey | Prospective cohort study | 2011–2012 | 81 | 49.9 ± 14.5 | Headache, fatigue, arthralgia, myalgia, nausea, weight loss, sexual dysfunction, amenorrhea*7 |
Yes | MRI | Yes | 9 |
*1 Based on the quality assessment criteria of Guyatt et al. (GRADE working group) (18, 19) (0 = low to 10 = high quality)
*2 43 patients (10 men, 33 women) with PES and 40 controls (9 men, 31 women) without goiter or prior intake of thyroid hormone
*3 94 patients (39 men, 55 women) with PES, of which 78% had an incidental finding, and 94 controls from a cohort of 1484 individuals with normal pituitary function, matched for age (± 1 year), body mass index, and sex
*4 In 78% incidental, in 22% imaging for pituitary disorder
*5 85 patients (18 men, 67 women) with PES and 214 healthy controls (48 men, 166 women) with normal findings on examination and normal pituitary function, as well as 16 patients with autoimmune hypophysitis, of these at least 14 histologically confirmed
*6 No differentiation between PES, SES, and hypophysitis
*7 A non-incidental finding has to be assumed in <10% CT, computed tomography; MRI, magnetic resonance imaging; PES, primary empty sella syndrome; SD, standard deviation; SES, secondary empty sella syndrome
eTable 2. Prevalence of pituitary insufficiency [?] in an incidental finding of empty sella.
| Reference |
Partial or complete ES (number) |
Women (n, %) |
BMI (kg/m2) |
Somatotropic insufficiency n (%) |
Somatotropic insufficiency confirmed by |
Corticotropic insufficiency n (%) |
Corticotropic insufficiency confirmed by |
Thyrotropic insufficiency n (%) |
Thyrotropic insufficiency confirmed by |
Gonadotropic insufficiency n (%) |
Gonadotropic insufficiency confirmed by |
Hyperprolactinemia n (%) |
Diabetes insipidus (n, %) |
Prevalence of pituitary insufficiency |
| Cannavo 2002 (23) |
Partial (21) | 16 (76%) |
29.3 ± 6.9 | 5 (24%) |
Pyridostigmine + GHRH test |
2 (9%) |
CRH test | 1 (<5%) |
TRH test | 4 (19%) |
LHRH test | 4 (19%) |
– | 23 (53%) |
| Complete (22) | 17 (77%) |
10 (45%) |
3 (14%) |
1 (<5%) |
7 (32%) |
1 (4%) |
||||||||
| Colao 2013 (24) |
Complete (94) | 55 (58.5%) |
28.8 ± 5.2 | 56 (87.5%) |
GHRH- arginine |
24 (37.5%) |
ACTH test | 35 (54.6%) |
TSH+fT4 + fT3 |
32 (50%) |
LH, FSH, testosterone/ amenorrhea |
– | 0 | 64 (68%) |
| Lupi 2011 (22) |
Partial (59), Complete (26) | 67 (79%) |
29 ± 1 | – | GHRH- arginine |
– | ACTH test | – | TRH test | – | LH, FSH, testosterone/ estradiol |
8 (9%) |
4 (5%) |
42 (49%) |
| Zuhur 2014 (21) |
Partial (47) | 38 (81%) |
29.5 ± 5.6 | 4 (8.5%) |
ITT | 2 (4.3%) |
ITT | 2 (4.3%) |
TSH, fT3,fT4, TRH test |
5 (10.,6%) |
LH, FSH, testosterone/estradiol, GnRH test |
7 (15%) |
0 | 7 (14.9%) |
| Complete (34) | 32 (94%) |
28.9 ± 6.7 | 20 (58.8%) |
5 (14.7%) |
16 (47.1%) |
19 (55.9%) |
6 (17.6%) |
23 (67.4%) |
ACTH, adrenocorticotropic hormone; BMI, body mass index; CRH, corticotropin releasing hormone; ES, empty sella; FSH, follicle stimulating hormone; fT3, triiodothyronine; fT4, Tetraiodothyronine; GHRH, growth hormone releasing hormone;
GnRH, gonadotropin releasing hormone; ITT, insulin tolerance test; LH, luteinizing hormone; LHRH, luteinizing hormone releasing hormone; TRH, thyrotropin releasing hormone; TSH, thyroid stimulating hormone
Study quality
The quality of the four studies was categorized as good to very good (8–10 points), according to the evaluation criteria of Guyatt et al. (eTable 1). All studies were included in the meta-analysis.
Radiological definition of an empty sella
The studies in our meta-analysis did not yield a uniform definition for the radiological diagnosis of an empty sella. Lupi et al. found in 26 of 85 patients a “complete” primary empty sella, defined as pituitary tissue of <3mm exposed on an MRI scan (22). Colao et al. (24) and Zuhur et al. (21) diagnosed a complete empty sella if more than 50% of the sella was filled by CSF and the pituitary tissue on CT/MRI scanning was = 2 mm. In the study by Zuhur et al., a partial empty sella was defined as <50% of the sella filled with CSF and exposed pituitary tissue = 3 mm (21). Cannavo et al. in their study differentiated complete or partial empty sella by whether more or less than 60% of the hypophyseal fossa was filled by CSF (23).
Prevalence of pituitary insufficiency according to meta-analysis
eTable 2 provides a detailed listing of the prevalence of hypopituitarism. In the case-control study including 85 patients reported by Lupi et al. (22), pituitary insufficiency was diagnosed in 49%. Two or more hormone axes were affected in 31%; 16% had an isolated growth hormone deficiency, 1% each isolated thyrotropin or gonadotropic deficiency (22). Neither the clinical symptoms nor the prevalence of pituitary failure differed significantly between patients with a partial and complete empty sella.
Cannavo et al. documented hypopituitarism in 23 out of 44 patients (53%); of these, they documented isolated hormonal disturbances in 40% and multiple insufficiencies in 14%, but no complete insufficiency (23). In contrast to the results reported by Lupi et al. (22), differences were reported between patients with partial and complete empty sella; only for the prevalence of secondary hypothyroidism did the difference not reach significance. The somatotropic axis was most often affected, followed by the gonadotropic axis (eTable 2).
The conclusion that pituitary insufficiency is more common in a scenario of complete empty sella than in partial empty sella is also drawn by Zuhur et al. (21). Their cohort study of 81 PES patients found a significant difference in pituitary function in patients with partial empty sella compared with those with complete empty sella (14.9% vs 67.4%); here, too, the somatotropic and gonadotropic axes were affected most often (eTable 2).
In the case-control study reported by Colao et al., 19 of 94 patients with a complete empty sella (30%) had isolated pituitary insufficiency; as in the preceding studies, isolated growth hormone deficiency was documented most often (24). Altogether the prevalence of hypopituitarism was high in the population analyzed in this study, at 68% (eTable 2).
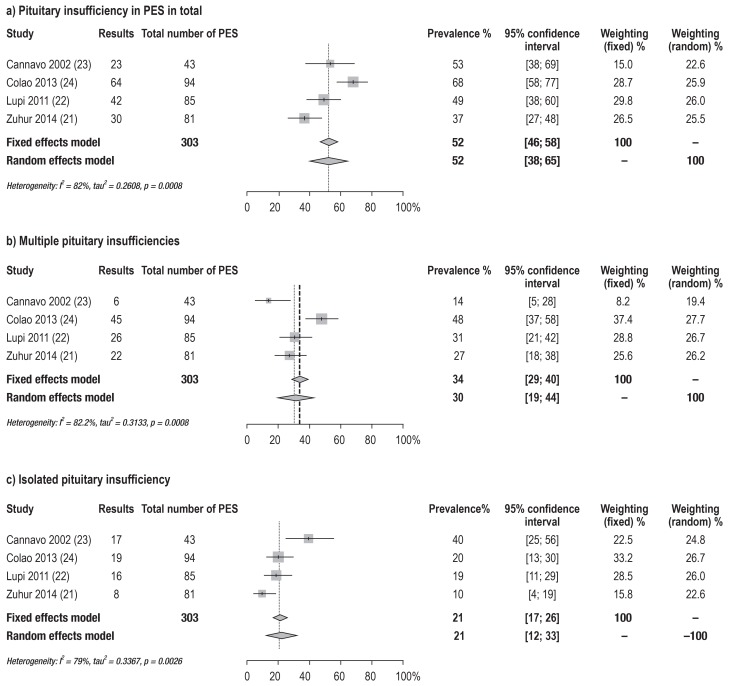
The prevalence of hypopituitarism in the individual studies is between 15% and 68%. The analysis of the pooled data yielded a prevalence of 52% (95% confidence interval [38; 65]). Multiple instances of axis dysfunction, with an estimated prevalence of 30% [19; 44], were more common than isolated pituitary insufficiency, with a prevalence of 21% [12; 33] (Figure 3).
Figure 3.
Forest plots of meta-analyses of the prevalence rates of a) pituitary insufficiency total, b) multiple pituitary insufficiencies, and c) isolated pituitary insufficiency
PES, primary empty sella syndrome
Risk factors for endocrine dysregulation in empty sella
Across all studies, a higher prevalence of hormonal disturbances was seen in women. In addition to female sex, a higher body mass index (BMI) and hypertension were named as risk factors. Lupi et al. (22) investigated the association between overweight (BMI 25 to <30kg/m2) and obesity (BMI = 30 kg/m2) and pituitary insufficiency in PES. Hypopituitarism had been diagnosed in 24 of 39 (62%) overweight patients and in 15 of 33 (45%) obese patients, but in only 2 of 13 (15%) patients with a normal weight. In the study reported by Colao et al., patients with PES had a higher cardiovascular risk profile, independently of their BMI, especially a higher Framingham score and poorer glucose and lipid profiles than the control cohort (24).
Discussion
Summary of the evidence
The literature on hypopituitarism in incidental PES is sparse. The assumed estimated prevalence is 52% [38%; 65%]. Insufficiencies in multiple pituitary axes are more common, with an estimated prevalence of 30% [19%; 44%] than isolated such events, with a prevalence of 21% [12%; 44%]. The somatotropic axis seems most commonly affected, followed by the gonadotropic axis.
According to our results, 1–10% of all humans are affected by hypopitutarism. This is in direct contradiction to general epidemiological data, which assume a prevalence of 50/100 000. The discrepancy may be the result of a selection bias in the studies included in our meta-analysis or of an underestimate of the general prevalence of the disorder.
Strengths and limitations
The general search term “empty sella” identified most studies that focused on the subject. To evaluate the prevalence of hypopitutarism in patients with PES, we pooled data from high-quality studies with clear diagnostic criteria.
The assumed publication bias requires critical attention, as the published studies mostly reported positive findings. As a result, the calculated total prevalence may be too high. In order to restrict bias, we excluded case reports from the data analysis. Another limitation is the fact that we included only studies published in the English language. Furthermore, the data are extremely heterogeneous, which limits their comparability.
The question of whether and to what extent hormone diagnostics should be undertaken in individual cases of PES can be answered with a very limited degree of certainty because of the sparse data. In the published studies, it was often not possible to clearly differentiate between incidental and non-incidental findings, as mixed patient cohorts with a diagnosis of PES were described (partly suspected hormonal aberration—for example, amenorrhea—before initial imaging). Risk factors for hypopitutarism in incidental PES seem to include female sex, overweight, and the presence of cardiovascular risk factors. Interestingly, all these are also risk factors for IIH, and it should be questioned whether this disorder was excluded to a satisfactory degree and whether patients with SES were possibly included in the analysis. Another limitation to the comparability lies in the inconsistent criteria for the radiological diagnosis of an empty sella.
Implications for future research
In order to be able to recommend a clear approach, further studies are required that systematically investigate patients in a cross-sectional study design by using stimulation tests. Only then will it be possible to issue a clear evidence-based recommendation as to if, and to what extent, patients with an incidental finding of PES should be referred for hormone diagnostics. In this context, publication bias is a problem. For this reason, it should be possible also to publish normal hormone test results in cross sectional studies.
Implications for clinical practice
In the setting of an incidental finding of an empty sella, secondary causes (Box) should be excluded first. On the basis of the data evaluation of this systematic review, which yielded a pooled prevalence of 52% for hypopituitarism, additional hormone diagnostic tests are recommended even in asymptomatic patients.
Because of the sparse data, no evidence based recommendations are possible for the type and extent of potential hormone tests. In our expert opinion, for pragmatic reasons, a basic diagnostic evaluation should be undertaken even in asymptomatic patients. This screening should comprise several measurements: morning cortisol concentrations, free thyroxine (fT4), estradiol in women (who do not have a regular cycle) and testosterone in men, insulin-like growth factor (IFG)-1, and prolactin. This approach is consistent with the general recommendations for conducting pituitary tests (25) and seems to have the best cost-benefit effect. If abnormalities are found, the following measurements should be taken additionally: thyrotropin releasing hormone (TSH) for the thyrotropic axis, and follicle stimulating hormone (FSH) and luteinizing hormone (LH) for the gonadotropic axis. For a detailed assessment of the somatotropic axis, a stimulation test is required (for example, the insulin-induced hypoglycemia test or growth hormone releasing hormone [GHRH]-L-arginine test). A stimulation test is also recommended for the corticotropic axis—for example, the insulin-induced hypoglycemia test or the adrenocorticotropic hormone [ACTH] test, although, strictly speaking, the latter tests only adrenal function. These recommendations are supported by a recently published review article (14), which refers to incidental and symptomatic PES; they are also confirmed by further review article (13, 26). In the basic diagnostic evaluation, potential factors that influence hormone concentrations have to be considered. For example, the use of oral contraceptives can lower the IGF-1 value below the sex and age specific normal value. With regard to rational weighing-up of costs and benefits, it should be mentioned that isolated growth hormone deficiency has to be confirmed by using at least two stimulation tests before health insurers will cover hormone substitution treatment in Germany at this point in time. Furthermore, in a setting of isolated growth hormone deficiency, substitution treatment with growth hormone does not have license approval. The reason is that the phase III (registration) trials to date did not study isolated deficiency states. In principle, growth hormone therapy can be assumed to be beneficial even in isolated growth hormone deficiency (27, 28), even though at the moment this constitutes off-label treatment.
Conclusion
In asymptomatic patients with an incidental finding of an empty sella, secondary causes should be excluded first. According to current evidence, additional hormone diagnostic testing is recommended for asymptomatic patients too, on the basis of a pooled prevalence of 52% for hypopituitarism. The basis diagnostic evaluation comprises measuring morning cortisol concentrations and concentrations of fT4, estradiol or testosterone, IGF-1, and prolactin. If abnormalities are found or symptoms that after further exploration may indicate a hormonal disturbance, additional hormone diagnostic tests using stimulation are recommended.
Key Messages.
Because of a pooled prevalence of 52% for hypopituitarism (38, 65) in PES, we recommend in spite of sparse data to undertake basic hormone diagnostic evaluation in asymptomatic patients.
Reported prevalence rates of primary empty sella—that is, an empty sella without identifiable cause—are diffuse and range from 2% to 20%. According to our results, therefore, some 1-10% of all people are probably affected by hypopituitarism. This contradicts general epidemiological data; these assume a prevalence of 50/100 000.
The discrepancy in reported prevalence rates may be associated with selection bias in the studies included in our review or with an underestimate of the general prevalence of the disorder.
Risk factors for pituitary insufficiency in PES seem to include female sex, overweight, and the presence of cardiovascular risk factors.
If further abnormalities are observed, additional diagnostic evaluation is recommended, by using stimulation tests if required.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Acknowledgment
We thank Dr. med. Philipp Sämann and Dr. rer. nat. Michael Czisch for the image of the cerebral MRI scan. The present study was supported by a scholarship awarded by the Bavarian Gender Equality Grant scheme to Dr med Kopczak.
Footnotes
Conflict of interest
Dr Auer has received consultancy fees from Shire and has been reimbursed conference delegate fees, participant fees for educational events, and travel expenses by Pfizer, Ipsen, and Lilly.
Dr Stieg has received consultancy fees from Shire and has been reimbursed conference delegate fees, participant fees for educational events, and travel expenses by Pfizer, Novartis, and Shire. She has received funding from Pfizer for a research project that she initiated.
Dr Sievers has received speaker honoraria and has been reimbursed conference delegate fees, fees for educational events, and travel expenses by Pfizer.
Prof Stalla has received consultancy fees and/or reimbursements of delegate fees for conferences/educational events and/or travel expenses and/or funding for research projects from Pfizer, Ipsen, Lilly, Shire, Novartis, Sandoz, NovoNordisk, and HRA.
The remaining authors declare that no conflict of interests exists.
References
- 1.Debnath J, Ravikumar R, Sharma V, et al. „Empty sella“ on routine MRI studies: an incidental finding or otherwise? Med J Armed Forces India. 2016;72:33–37. doi: 10.1016/j.mjafi.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee S, Chalkidou K, Doust J, et al. Evidence for overuse of medical services around the world. Lancet. 2017;390:156–168. doi: 10.1016/S0140-6736(16)32585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb SM, Crespo I, Santos A, Resmini E, Aulinas A, Valassi E. Management of endocrine disease: quality of life tools for the management of pituitary disease. Eur J Endocrinol. 2017;177:R13–R26. doi: 10.1530/EJE-17-0041. [DOI] [PubMed] [Google Scholar]
- 4.Giavoli C, Profka E, Verrua E, et al. GH replacement improves quality of life and metabolic parameters in cured acromegalic patients with growth hormone deficiency. J Clin Endocrinol Metab. 2012;97:3983–3988. doi: 10.1210/jc.2012-2477. [DOI] [PubMed] [Google Scholar]
- 5.Busch W. Morphology of sella turcica and its relation to the pituitary gland. Virchows Arch. 1951;320:437–458. doi: 10.1007/BF00957474. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan MSF, Williams ED, Doyle FH. Applied anatomy of the pituitary gland and fossa: aradiological and histopathological study based on 50 necropsies. Br J Radiol. 1968;41:782–788. [Google Scholar]
- 7.De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A. Primary empty sella. J Clin Endocrinol Metab. 2005;90:5471–5477. doi: 10.1210/jc.2005-0288. [DOI] [PubMed] [Google Scholar]
- 8.Lenz AM, Root AW. Empty sella syndrome. Pediatr Endocrinol Rev. 2012;9:710–715. [PubMed] [Google Scholar]
- 9.Bergland RM, Ray BS, Torack RM. Anatomical variations in the pituitary gland and adjacent structures in 225 human autopsy cases. J Neurosurg. 1968;28-130:93–99. 1–25. doi: 10.3171/jns.1968.28.2.0093. [DOI] [PubMed] [Google Scholar]
- 10.Bjerre P. The empty sella A reappraisal of etiology and pathogenesis. Acta Neurol Scand Suppl. 1990;130:1–25. [PubMed] [Google Scholar]
- 11.Manavela MP, Goodall CM, Katz SB, Moncet D, Bruno OD. The association of Cushing`s disease and primary empty sella turcica. Pituitary. 2001;4:145–151. doi: 10.1023/a:1015310806063. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal NK, Jain P, Garg S. Primary empty sella with isolated ACTH deficiency and microprolactinoma. Gynecol Endocrinol. 2012;28:568–569. doi: 10.3109/09513590.2011.650663. [DOI] [PubMed] [Google Scholar]
- 13.Guitelman M, Garcia Basavilbaso N, Vitale M, et al. Primary empty sella (PES): a review of 175 cases. Pituitary. 2013;16:270–274. doi: 10.1007/s11102-012-0416-6. [DOI] [PubMed] [Google Scholar]
- 14.Chiloiro S, Giampietro A, Bianchi A, et al. Diagnosis of endocrine disease: primary empty sella: a comprehensive review. Eur J Endocrinol. 2017;177:R275–R285. doi: 10.1530/EJE-17-0505. [DOI] [PubMed] [Google Scholar]
- 15.Julayanont P, Karukote A, Ruthirago D, Panikkath D, Panikkath R. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87–99. doi: 10.2147/JPR.S60633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallardo E, Schächter D, Cáceres E, et al. The empty sella: results of treatment in 76 successive cases and high frequency of endocrine and neurological disturbances. Clin Endocrinol (Oxf) 1992;37:529–533. doi: 10.1111/j.1365-2265.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 e1000097. [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Sackett DL, Cook DJ. Users‘ guides to the medical literature II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1993;270:2598–2601. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Sackett DL, Cook DJ. Users‘ guides to the medical literature II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA. 1994;271:59–63. doi: 10.1001/jama.271.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 21.Zuhur SS, Kuzu I, Ozturk FY, Uysal E, Altuntas Y. Anterior pituitary hormone deficiency in subjects with total and partial primary empty sella: do all cases need endocrinological evaluation? Turk Neurosurg. 2014;24:374–379. doi: 10.5137/1019-5149.JTN.8671-13.0. [DOI] [PubMed] [Google Scholar]
- 22.Lupi I, Manetti L, Raffaelli V, et al. Pituitary autoimmunity is associated with hypopituitarism in patients with primary empty sella. J Endocrinol Invest. 2011;34:e240–e244. doi: 10.3275/7758. [DOI] [PubMed] [Google Scholar]
- 23.Cannavo S, Curto L, Venturino M, et al. Abnormalities of hypothalamic-pituitary-thyroid axis in patients with primary empty sella. J Endocrinol Invest. 2002;25:236–239. doi: 10.1007/BF03343996. [DOI] [PubMed] [Google Scholar]
- 24.Colao A, Cotta OR, Ferone D, et al. Role of pituitary dysfunction on cardiovascular risk in primary empty sella patients. Clin Endocrinol. 2013;79:211–216. doi: 10.1111/cen.12122. [DOI] [PubMed] [Google Scholar]
- 25.Petersenn S, Quabbe HJ, Schöfl C, Stalla GK, von Werder K, Buchfelder M. The rational use of pituitary stimulation tests. Dtsch Arztebl Int. 2010;107:437–443. doi: 10.3238/arztebl.2010.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giustina A, Aimaretti G, Bondanelli M, et al. Primary empty sella: why and when to investigate hypothalamic-pituitary function. J Endocrinol Invest. 2010;33:343–346. doi: 10.1007/BF03346597. [DOI] [PubMed] [Google Scholar]
- 27.Gardner CJ, Mattsson AF, Daousi C, Korbonits M, Koltowska-Haggstrom M, Cuthbertson DJ. GH deficiency after traumatic brain injury: improvement in quality of life with GH therapy: analysis of the KIMS database. Eur J Endocrinol. 2015;172:371–381. doi: 10.1530/EJE-14-0654. [DOI] [PubMed] [Google Scholar]
- 28.van Bunderen CC, van den Dries CJ, Heymans MW, et al. Effect of long-term GH replacement therapy on cardiovascular outcomes in isolated GH deficiency compared with multiple pituitary hormone deficiencies: a sub-analysis from the Dutch National Registry of Growth Hormone Treatment in Adults. Eur J Endocrinol. 2014;171:151–160. doi: 10.1530/EJE-14-0069. [DOI] [PubMed] [Google Scholar]