Summary
An impaired expression of α‐defensins (α‐Defs) in the ileal mucosa and, conversely, increased levels in plasma, have been reported in Crohn's disease (CD). However, the specificity and correlation of these findings with the degree of inflammation are unclear. We aimed to characterize the concentration and utility of ileal and plasma α‐Defs in CD and to analyse a potential epigenetic mechanism of α‐Def expression. Peripheral blood samples and ileal biopsies were obtained from patients at disease onset (aCD), from those who achieved remission (iCD) and from two control groups (healthy controls and non‐CD‐aetiology ileitis patients). Plasma α‐Defs 1–3 and 4 were detected by enzyme‐linked immunosorbent assay (ELISA); α‐Def 5 by immunolocalization. Methylation analysis of the α‐Def 5 gene was performed using the MassARRAY EpiTYPER system. Plasma α‐Defs 1–3 concentrations were significantly higher in aCD with ileal involvement (L1, L3) versus iCD or the control groups. The α‐Defs 1–3 concentrations were also similar to healthy controls in patients with non‐CD ileitis. There was a significant positive correlation between plasma α‐Defs 1–3 levels in aCD and the endoscopic index, as well as with C‐reactive protein (CRP) levels. The immunopositivity scoring showed significantly reduced α‐Def 5 expression in ileal inflamed (aCD) versus non‐inflamed mucosa (iCD and healthy controls). The α‐Def 5 gene showed a higher methylation status in CD patients than controls, regardless of the inflammation. Plasma α‐Defs 1–3 concentrations correlate with the degree of inflammation and appear to be specific biomarkers of ileal‐CD at diagnosis. Ileal α‐Def 5 expression is down‐regulated permanently by methylation.
Keywords: inflammation, innate lymphoid cells, mucosa
Introduction
Disruption of intestinal immune tolerance is associated increasingly with inflammatory bowel disease (IBD) 1, as confirmed by independent genome‐wide association studies 2. These studies have demonstrated the risk (up to a 17·1‐fold increased risk) for Crohn's disease (CD) development when certain polymorphisms are present in the nucleotide binding oligomerization domain containing 2/caspase recruitment domain‐containing protein 15 (NOD2/CARD15) gene 3. Mutations in this gene in intestinal epithelial cells have been related to a significant decrease in endogenous anti‐microbial peptides in these cells 4, 5, 6. Among these peptides, defensins play a role as endogenous anti‐microbials, an important part of the innate immune response against infectious pathogens 7. The defensins are expressed predominantly in neutrophils and epithelial cells and are classified into two primary categories: alpha (α)‐defensins and beta (β)‐defensins, depending on the pattern of linkage between the cysteine residues 2. In the gastrointestinal tract, α‐defensins (α‐Defs) are secreted specifically by Paneth cells in the small bowel, whereas β‐defensins are expressed predominantly in the epithelial cells of the colon 8.
The α‐Defs were first identified in 1989 by Ouellette et al. 9 as a large family of cysteine‐rich peptides expressed in ileal Paneth cells. These peptides, also termed cryptdins in mice, share strong structural homology and in‐vitro and in‐vivo broad‐spectrum anti‐bacterial activity 10, 11. To date, six human α‐Defs have been identified. The subtypes 1–4 are also known as human neutrophil peptides (HNP) due to their major expression in peripheral blood neutrophils, where they are stored in azurophil granules as mature and active forms. Peripheral α‐Defs 1–3 differ by only one amino acid residue and are major neutrophil components, comprising approximately 99% of the total defensin content, with traces of α‐Def 4, which is found in small amounts and has a different sequence. The other two subtypes, α‐Defs 5 and 6, are secreted specifically by goblet and Paneth cells (specialized secretory epithelial cells) in the luminal surface of the terminal ileum 2, 4, 12.
Defensins play a role in linking innate and adaptive immunity by attracting immature dendritic cells, helping in their maturation and promoting the subsequent activation of T cells 13. Defensins might therefore be key factors in modulating the immune response in several bowel diseases. To date, the most consistent associations between anti‐microbial peptides and IBD apply to α‐Defs in the small intestine of patients with CD 2. In particular, reduced α‐Defs 5 and 6 expression has been observed in the ileal mucosa of patients with CD. It has also been shown that variants in defensin genes are associated inversely with anti‐glycan antibodies, which are associated with the CD phenotype, supporting the role of these anti‐microbial peptides in the pathogenesis of CD 14. One of the mechanistic explanations for this finding is that defensin reduction could result in weakness of the mucosal anti‐microbial defence and/or in alterations in the composition of commensal microbiota 12, allowing bacteria to adhere to the intestinal mucosa and trigger the inflammatory response 15.
There is some controversy as to whether this altered α‐Def expression is permanent and could therefore be a fundamental feature of the disease 6, 15 or could be due to loss of epithelium as a result of inflammatory changes 16. However, the systemic inflammatory response is also a crucial part of the host's immune reaction to various events occurring in CD and facilitated by a more permeable intestinal mucosa (such as bactDNA translocation) 17. Therefore, circulating levels of α‐Defs could be useful biomarkers for identifying disease activity 18. Factors such as their specificity and actual utility in this setting need to be explored.
The first aim of this study was therefore to characterize the expression of ileal and plasma‐circulating α‐Defs in patients with ileal CD at disease onset (and prior to any specific medication intake) and, later on, in inactivity. We examined the results from various control groups [healthy subjects, patients with non‐CD ileitis and patients with only colonic CD (L2)] to evaluate the utility and specificity of α‐Defs as a potential biomarker for identifying and assessing ileal CD activity. The second aim of the study was to analyse the potential role of epigenetic mechanisms in α‐Defs expression by analysing the methylation status of human defensin genes.
Materials and methods
Patients and sampling
In total, 58 patients were enrolled and distributed in four groups (two groups of CD patients – active and inactive – and two control groups), as follows:
Group 1: 25 new‐onset CD patients with active disease [of whom 15 had ileal involvement (L1 or L3, according to the Montreal Classification) and 10 had exclusively colonic disease (L2)]. The latter with colonic CD were included to analyse the specificity of α‐Defs with respect to ileal location in active disease.
Group 2: 10 patients with inactive CD (of the 15 ileal CD patients from the first group and enrolled in a secondary analysis once they achieved clinical, analytical and endoscopic remission under immunosuppressive treatment).
Group 3: 8 patients with ileitis of non‐CD aetiology.
Group 4: 15 healthy controls.
A flowchart of the study design, which includes the type of patients and the different groups analysed, is shown in the Fig. 1.
Figure 1.
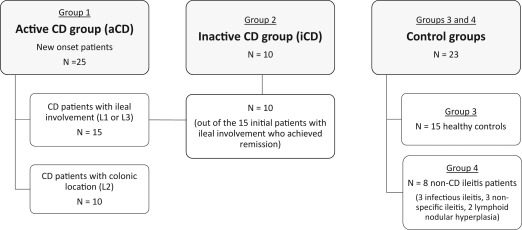
Flowchart of the study design. Peripheral blood samples (at inclusion) and ileal biopsies (at the time of ileocolonoscopy) were obtained from all the patients included (n = 58).
Consecutive peripheral blood samples and ileal biopsies were obtained from all the patients included. Blood samples of the active CD patients were collected at diagnosis and prior to any therapeutic procedure. Ileal biopsies were obtained from all patients at the time of the ileocolonoscopy. The diagnosis of CD was reached on the basis of the endoscopic, radiological, histological and clinical criteria provided by the European Crohn's and Colitis Organization 19. Clinical activity was evaluated by the Harvey–Bradshaw Index 20. Clinical remission was defined as a disease activity index less than or equal to 4, analytical remission by normalization of inflammation parameters [C‐reactive protein (CRP), fibrinogen and erythrocyte sedimentation rate] and endoscopic remission when there was mucosal healing, which was evaluated by ileocolonoscopy or, when needed, by magnetic resonance enterography (MRE) according to disease location and availability of ileal intubation. Endoscopic activity was scored with the Simplified Endoscopic Activity Score for Crohn's Disease 21 and, in cases assessed by MRE, with the quantitative Magnetic Resonance Index of Activity 22. The control group was composed of sex‐ and age‐matched healthy volunteers who underwent an ileocolonoscopy to investigate functional abdominal symptoms, which resulted in a normal endoscopy and biopsies. We also included another control group of patients (group 3) who had histologically confirmed terminal ileitis of non‐CD aetiology who had yet to take any specific medication.
Ethical considerations
All participants provided their written informed consent on the day of recruitment. The study was approved by the Clinical Research Ethics Committee of Hospital Universitari i Politècnic La Fe (ref: PI013/01936), in compliance with the Declaration of Helsinki.
Blood and tissue preparation
Anti‐coagulated blood [K3‐ethylenediamine tetraacetic acid (EDTA)] was obtained after 12 h of fasting, layered onto Histopaque 1077 solution (Sigma‐Aldrich, Poole, UK) at room temperature and centrifuged at 213 g for 30 min (without breaks). The upper‐layer phases containing the white cell‐rich plasma were removed, and the cells and platelets were subsequently separated from the plasma by spinning down at 2375 g for 10 min. Immediately after sampling, the mucosal biopsies were immersed in 4% buffered formalin and sent to the Department of Pathology to be processed and stained.
Determination of α‐Defs 1–3 and α‐Def 4 concentrations in plasma samples
There were no commercially available individual enzyme‐linked immunosorbent assay (ELISA) kits for the determination of α‐Defs 1–3 concentrations in plasma at the time of these experiments. We therefore measured the total concentration of α‐Defs 1, 2 and 3 together in plasma samples by the only commercially available ELISA kit (HNP1–3, human, ELISA kit catalogue no. HK317‐01; Hycult Biotech, Uden, the Netherlands). The plasma concentration of α‐Def 4 was then assessed separately, using a different ELISA kit (human alpha defensin‐4, NP‐4 ELISA kit, ref: CSB‐E17958h; Cusabio, Wuhan, China). Previously, the optimal sample dilutions were tested (1/100 for α‐Defs 1–3 and 1/500 for α‐Def 4). Spectrophotometric analyses of the defensin quantity were performed at 450 nm. The concentration of each protein in the plasma samples was calculated according to a standard curve.
Immunohistochemistry of α‐Def 5 in tissue samples
Immunolocalization of α‐Def 5 in the granules of Paneth cells was assessed by an anti‐human defensin 5 monoclonal antibody (8C8) (Novus Biologicals, Littleton, CO, USA) in a subset of 10 ileal CD mucosa pairs (same patient, active and inactive) and in 10 normal control mucosa samples. In brief, 4‐μm sections were cut from paraffin‐embedded tissue samples, deparaffinized and rehydrated, and microwave‐heated to retrieve hidden antigens. Non‐specific binding was blocked by incubating the tissue sections with 5% serum in phosphate‐buffered saline‐Tween (PBS‐T) for 30 min. Sections were then incubated overnight at 4°C with the appropriate dilution (1 : 1000) of the mouse anti‐α‐Def 5 monoclonal antibody.
The expression of α‐Def 5 was scored by multiplying the intensity scores and the percentage area positively stained. The immunostaining was read in a semiquantitative manner. The percentage positivity was scored as: negative, fewer than 5% of Paneth cells stained; weakly positive (1+), between 5 and 10% of Paneth cells stained; moderately positive (2+), between 10 and 50% of Paneth cells stained; and strongly positive (3+), when more than 50% of the Paneth cells were stained. All paraffin sections from the patients with CD and the non‐IBD controls were scored blindly for inflammation by a gastrointestinal pathologist (D. R.).
DNA methylation analysis
We first searched in the Ensembl Genome Browser (http://www.ensembl.org) for the α‐Defs 1–3, 4 and 5 gene sequences. The gene sequences of α‐Defs 1–3 and 4 do not contain any cytosine–phosphate–guanosine (CpG) islands, so they were not included in the methylation analysis; thus, only the α‐Def 5 gene could be evaluated for methylation status.
Quantitative high‐throughput methylation analysis of CpG sites in the α‐Def 5 gene was performed using the MassARRAY® EpiTYPER system (Sequenom, San Diego, CA, USA). Briefly, genomic DNA was isolated from peripheral blood mononuclear cells of patients with CD (active and inactive) and controls using the PureLinkTM Genomic DNA midi kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). A 329 pb amplicon (region 6) was designed using EpiDesigner software (www.epidesigner.com), which covered all CpGs across the island. The primer sequences are shown in Supporting information, Table S1. The method employs a T7‐promoter‐tagged polymerase chain reaction (PCR) amplification of bisulphite‐converted DNA, followed by the generation of a single‐strand RNA molecule and subsequent base‐specific cleavage (3′ to either rUTP or rCTP) by RNase A. The mixture of cleavage products of differing length and mass were analysed by matrix‐assisted laser desorption ionization‐time of flight mass spectrometry (MALDI‐TOF‐MS). Methylation ratios were then quantified using EpiTYPER (Sequenom) and plotted from the mean value of two replicate amplicons using Heatmap.2 in r software.
Statistical analysis
Statistical analysis was performed using spss software version 17.0 (SPSS Inc., Chicago, IL, USA) and all graphics were generated using GraphPad Prism software version 5.03 for Windows (GraphPad Software Inc., San Diego, CA, USA). Grubb's test (GraphPad QuickCalcs, online calculator for scientists at http://www.graphpad.com/quickcalcs/) was employed as a preliminary test to detect significant outliers. All outliers were excluded from the statistical analyses. The data are shown as means ± standard deviation (s.d.). A P‐value of less than 0·05 was considered statistically significant.
First, the α‐Defs concentrations in plasma were compared among the various study groups using the Mann–Whitney U‐ and Kruskal–Wallis tests, as appropriate. A secondary analysis was then performed to correlate the plasma concentrations of α‐Defs 1–3 with the endoscopic score and the laboratory data. Kolmogorov–Smirnov and Shapiro–Wilk testing confirmed non‐Gaussian distribution for α‐Def levels; the Spearman's rank correlation was therefore applied.
Results
Demographic and clinical characteristics of the 58 enrolled patients are shown in Table 1.
Table 1.
Demographic and clinical features of the patients included
| Patients with CD | ||||
|---|---|---|---|---|
| Disease activity | Active (onset) | Inactive | Non‐CD ileitis | Controls |
| Number | 25 | 10 | 8 | 15 |
| Age (range), years | 31·1 ± 14·1 (18–74) | 32·4 ± 9·4 (21–46) | 27·4 ± 13·7 (18–57) | 37·6 ± 12·3 (27–67) |
| Sex (M/F) | 15/10 | 5/5 | 4/4 | 7/8 |
| Disease location, n | ||||
| L1 Ileal | 5 | 3 | Terminal ileum | – |
| L3 Ileocolonic | 10 | 7 | – | |
| L2 Colonic | 10 | – | – | |
| Disease behaviour, n | ||||
| B1 Inflammatory | 24 | 7 | – | – |
| B2 Stricturing | 1 | 2 | – | – |
| B3 Penetrating | 0 | 1 | – | – |
| P Peri‐anal disease | 9 | 3 | – | – |
| Harvey–Bradshaw Index | 8 | 3 | ||
| Concomitant treatment, n | ||||
| 5‐Aminosalicylates | – | 1 | – | – |
| Thiopurines | – | 4 | – | – |
| Anti‐TNF‐α | – | 2 | – | – |
| Double IS | – | 1 | – | – |
| None | – | 2 | – | – |
Mean ± standard deviation (s.d.). CD = Crohn's disease; IS = immunosuppression; TNF = tumour necrosis factor; M/F = male/female.
Plasma α‐Defs 1–3 and α‐Def 4 concentrations in patients with CD compared with non‐CD ileitis and controls
The α‐Defs 1–3 and 4 levels in plasma differed significantly among the study groups. The plasma α‐Defs 1–3 concentrations were significantly higher in the patients with active CD and ileal involvement (L1, L3) than in the patients with inactive CD or in the control group (P < 0·01 for each). However, when considering the patients with active CD and exclusively colonic involvement (L2), no significant differences were detected in the plasma concentrations compared with the inactive CD patients and the healthy controls (P = 0·1678) (Fig. 2a; numerical data in Table 2).
Figure 2.
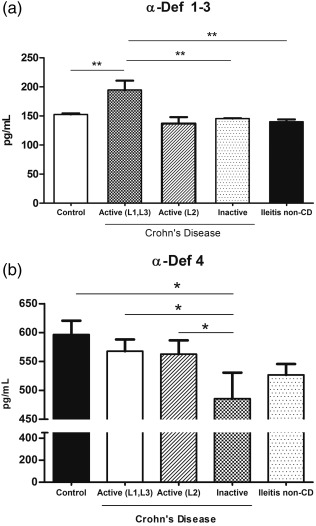
(a) Plasma α‐defensins 1–3 (α‐Defs) concentrations in patients with Crohn's disease (CD), non‐CD ileitis and healthy controls. (b) Plasma α‐Defs 4 concentrations in patients with CD, non‐CD ileitis and healthy controls.
Table 2.
Plasma α‐defensin (α‐Def) concentrations, laboratory data and α‐Def 5 gene methylation status in patients with ileal Crohn's disease (CD) compared with the control groups
| CD | ||||
|---|---|---|---|---|
| Disease activity | Active (onset) | Inactive | Non‐CD ileitis | Controls |
| Plasma concentrations* (pg/ml) | ||||
| α‐Defs 1–3 | 194·7 ± 60·1 | 145·5 ± 1·3 | 140·2 ± 10·4 | 152·5 ± 7·7 |
| α‐Def 4 | 567·8 ± 80·6 | 485·5 ± 119·8 | 526·8 ± 46·1 | 596·5 ± 93·3 |
| CRP (mg/dl) | 74·5 ± 76·5 | 2·9 ± 3·7 | 45·8 ± 16·9 | 1·3 ± 0·7 |
| WBC (×103/ul) | 9·313 ± 2·189 | 6·431 ± 2·213 | 9·362 ± 2·354 | 5·122 ± 1·262 |
| Neutrophils (×103/ul) | 6·213 ± 2·169 | 5·231 ± 1·738 | 8·982 ± 2·435 | 3·672 ± 1·934 |
| Fibrinogen (mg/dl) | 628 ± 95 | 346 ± 58 | 606 ± 61 | 378 ± 38 |
| α‐Def 5 gene methylation status (%) | ||||
| CpG_11 † | 36 ± 14 | 32 ± 09 | 22 ± 04 | 14 ± 10 |
| CpG_13 ‡ | 87 ± 09 | 83 ± 12 | 77 ± 04 | 58 ± 25 |
Mean ± standard deviation (s.d.). *aCD versus iCD and aCD versus controls group: P < 0·01 for each; †aCD, iCD and non‐CD ileitis versus controls: P = 0·003, P = 0·033 and P = 0·027, respectively; ‡aCD, iCD and non‐CD ileitis versus controls: P = 0·008, P = 0·05 and P = 0·04, respectively. aCD = active Crohn's disease; CRP = C‐reactive protein; iCD = inactive Crohn's disease; WBC = white blood cells.
We then compared these α‐Defs 1–3 circulating levels with those in patients with terminal ileitis of non‐CD aetiology. The plasma α‐Defs 1–3 concentrations were significantly higher in the patients with active CD than in the patients with non‐CD ileitis (P = 0.0012), in whom the α‐Defs 1–3 levels did not differ from those found in the healthy controls.
In contrast, when we evaluated the plasma α‐Def 4 levels, we found no increase in the patients with active CD. A significant decrease was detected in the patients with inactive CD compared with the active CD patients and the control group (P < 0·05 for each) (Fig. 2b; numerical data in Table 2). Conversely, no significant differences were found in plasma α‐Def 4 concentrations between the patients with CD (whether active or inactive) and the non‐CD patients.
The increase in plasma α‐Defs 1–3 concentrations observed in patients with active ileal CD showed a strong positive correlation with the endoscopic score (SES‐CD), with a Spearman's correlation coefficient of r = 0·934 (P < 0·01) (Fig. 3a). We then investigated the correlation between the plasma α‐Defs 1–3 levels and the laboratory parameters associated with inflammatory activity in these patients. There was also a significant positive correlation between plasma α‐Defs 1–3 levels and the CRP levels, with a Spearman's correlation coefficient of r = 0·696 (P < 0·01) (Fig. 3b). By contrast, plasma α‐Defs 1–3 concentrations did not correlate with other laboratory data, such as fibrinogen levels or white blood cell counts.
Figure 3.
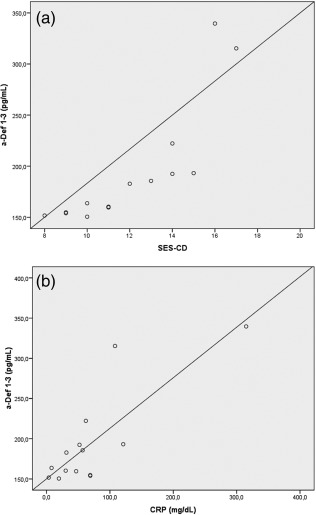
(a) Scatter‐plot of α‐defensins 1–3 (α‐Defs) concentrations in plasma of patients with active Crohn's disease (CD) against endoscopic score (Spearman's rank correlation = 0·934; P < 0·01). (b) Scatter‐plot of α‐Defs 1–3 concentrations in plasma of patients with active CD against serum C‐reactive protein (CRP) levels (Spearman's rank correlation = 0·696; P < 0·01).
α‐Def 5 expression in tissue samples
We also assessed specific Paneth cell α‐Def 5 expression in pairs of ileal biopsies from patients with active and inactive CD and from healthy controls. The immunoreactivity‐scoring results revealed significantly reduced α‐Def 5 expression in histologically confirmed inflamed mucosa (active CD patients, < 5%) compared with non‐inflamed ileal mucosa (inactive CD patients, 30–50%; healthy controls, > 50% of Paneth cells stained). We also observed that this overall decrease in ileal Paneth cell–α‐Def 5 expression in patients with active CD was more pronounced with an increasing histological degree of inflammation. This lower defensin expression is partially recovered in the non‐inflamed mucosa from patients with inactive disease, although clearly without returning to control levels (Fig. 4a–c).
Figure 4.
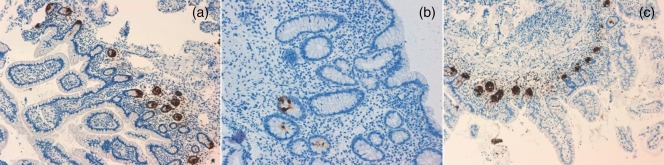
Immunohistochemistry of α‐Def 5 in normal control mucosa (a), and in the non‐inflamed (c) and inflamed (b) mucosa of a patient with Crohn's disease (CD).
DNA methylation profile of α‐Def 5 gene
We next analysed the methylation status of CpG sites in the α‐Def 5 gene. CpG island 11 showed a different methylation status in patients with active CD, inactive CD and non‐CD ileitis group versus healthy controls (P = 0·003, P = 0·033 and P = 0·027, respectively), although no differences were observed between the two patient groups (Fig. 5a). CpG island 13 showed similar results (active CD versus controls: P = 0·008; inactive CD versus controls: P = 0·05; non‐CD ileitis versus controls: P = 0·04) (Fig. 5b). In both CpG islands, a higher methylation status was observed in patients with CD, regardless of the activity of the disease. The methylation percentages are shown in Table 2.
Figure 5.
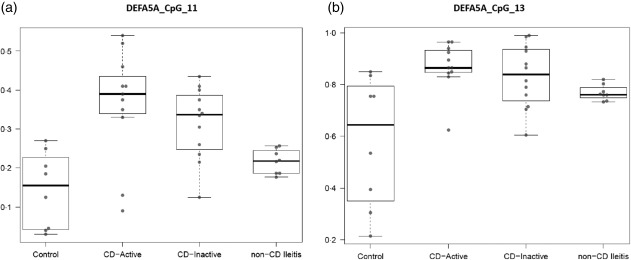
DNA methylation status of cytosine–phosphate–guanosine (CpG) island 11 (a) and CpG island 13 (b) in the α‐defensins (α‐Def) 5 gene (DEFA5) in patients with Crohn's disease (CD) (active and inactive) and non‐CD ileitis versus controls.
Discussion
The α‐Defs are not only potent anti‐microbial peptides with broad‐spectrum activity, but also immune regulators between innate and adaptive immune responses; therefore, interest in their potential role in IBD pathogenesis has increased in recent years 13, 23. In this study, we assessed the possibility that α‐Defs could be used for the specific diagnosis of ileal CD and as a possible pathogenic element contributing to the mechanism of CD.
We found that α‐Defs 1–3 plasma concentrations are increased in patients with active CD (at disease onset) compared with the inactive CD patients and healthy controls. Therefore, there seems to be a differential expression in α‐Defs 1–3 circulating levels between active and remission phases of the disease. Our study shows that this increase appears to depend upon the disease location, given the fact that it was observed specifically in patients with active CD with ileal involvement (L1 or L3) and not in those with exclusively colonic involvement (L2).
Few studies to date have evaluated peripheral α‐Defs expression in patients with CD, and the limited data available are from the Japanese population. To our knowledge, this is the first study that has reported α‐Defs plasma concentrations in Caucasian patients with CD. Two previous Japanese studies 18, 24 have reported similar findings to ours, consisting of an increase in plasma α‐Defs 1–3 concentrations in patients with IBD. The correlation of this increase with inflammatory parameters suggests its potential utility as an indicator of disease activity in patients with CD.
Whether the rise in serum α‐Defs 1–3 levels is specific for CD or whether it occurs under other inflammatory conditions has not been addressed. We compared plasma α‐Defs 1–3 levels with other ileal non‐IBD related inflammatory conditions (non‐CD ileitis group) to evaluate their specificity as indicators of CD activity. We showed that plasma α‐Defs 1–3 concentrations were high only in ileal CD, suggesting the potential role of plasma α‐Defs 1–3 levels as specific indicators of disease activity in patients with CD and ileal involvement. No specific markers have been ever proposed previously for identifying CD diagnosis. This would help in clinical situations when ileitis is presented as a first episode, and doubts arise whether it is due to an initiating chronic CD condition or to another different cause.
In terms of plasma α‐Def 4 levels, however, no increase was detected in the patients with active CD; instead, there was a significant decrease in plasma α‐Def 4 levels in the patients with inactive CD. We cannot clarify the origin of this decrease with our data; it could be secondary to the immunosuppressive treatment that we apply to the patients, although this hypothesis needs further research. Equally, whether this decrease in α‐Def 4 levels could potentially become a biomarker of remission remains to be explored, together with the pathophysiological role that peripheral α‐Defs might play in CD.
Focusing upon small intestinal CD pathogenesis, it has been postulated that the lack of α‐Defs could be a primary pathogenic factor 6, 15. We compared this peripheral leucocyte–α‐Defs expression with that of the specific Paneth cell–α‐Def 5 in the intestinal tissue of the same patients by assessing α‐Def 5 expression in pairs of mucosa samples from patients with CD (active versus inactive) compared with controls. We found that α‐Def 5 expression is reduced in the inflamed ileal mucosa of CD, in agreement with previous studies 6, 15, 16.
A number of authors have studied local defensin expression extensively in intestinal IBD mucosa, although with controversial results regarding the cause of this deficiency. Simms et al. 16 postulated that these low α‐Defs levels in ileal mucosa from patients with CD are due most probably to the loss of epithelium as a consequence of inflammatory changes. Conversely, Wehkamp et al. 6, 15 proposed that this decreased Paneth cell–defensin expression was an intrinsic feature of the disease, associated with the NOD2 genotype, and was not only a result of tissue inflammation. In our patients we observed a partial recovery, which was due probably to the healing of ulcerated tissue and crypt regeneration with development of new Paneth cells. However, recovery was incomplete and there were clearly fewer Paneth cells over time than in the healthy control subjects.
Paneth cells originate from intestinal stem cells under the control of TCF4, a Wnt signalling transcription factor 25. TCF4 expression has been found to be reduced in ileal CD but not in colonic CD or ulcerative colitis 26, and is therefore linked to a specific absence of α‐Defs, especially in patients with NOD2 mutations 27. More recently, another Wnt effector, TCF‐1, has been identified as a transcriptional regulator of α‐Defs 5 and 6 gene expression, which has been found to be decreased significantly in the small intestine of CD patients. However, TCF‐1 binding alone is insufficient for inducing transcriptional activation, suggesting that both TCF‐4 and TCF‐1 can contribute to transcription activation, as this can only be achieved upon complex formation with β‐catenin 28. Furthermore, monocytes arriving at the intestine from the peripheral blood appear to be inductors of Wnt ligands, an ability that is impaired in monocytes from patients with CD 29. All these data show that regulation of α‐Defs expression is complex, and that different pathogenic mechanisms could be interacting.
Among the different factors that have been implicated in CD, the environment appears to have an impact on the pathogenesis, although no key environmental element has been found. The environment can influence the expression of many genes through various epigenetic mechanisms, including DNA methylation. DNA methylation within the CpG islands in the promoter region is associated typically with inhibition of gene expression. We learned that the gene sequences of α‐Defs 1–3 and 4 do not contain any CpG islands, precluding the methylation mechanism in those genes. The gene encoding for α‐Def 5 contains CpG islands susceptible to methylation. A good correlation between methylation levels in peripheral blood samples and the intestinal biopsies of patients with IBD has been reported 30. We found a higher methylation status of α‐Def 5 in patients with CD than in healthy controls, regardless of the disease activity, supporting the idea of a permanent reduction in α‐Def production in patients with ileal CD. This grants the epigenetic regulation a possible role in the altered expression of ileal α‐Defs. The regulation of α‐Defs expression is complex, as stated above, and the fact that the methylation is permanent could be interpreted as being a facilitator for down‐regulation of the gene but not the trigger. Thus, it would be a condition that facilitates the α–Defs deficiency when other factors come into play.
The analysis of α‐Def 5 methylation shows that the grade of methylation is significantly higher in active ileal CD patients than in the non‐CD ileitis group; however, α‐Def 5 methylation was also risen significantly in this latter group compared to the healthy control group. Thus, inflammation itself or the predisposition of patients to become inflamed in the ileum could be associated with Def‐5 methylation by means of a mechanistic pathway that we cannot clarify with the data of this study.
Permanent α‐Defs deficiency in the mucosa from patients with ileal CD contrasts with the selective increase of plasma α‐Defs when CD is active. We hypothesize that this apparent contradiction could be due to a compensatory increase mechanism in circulating defensins at disease onset, in an attempt to maintain host defence and intestinal immune homeostasis. In fact, active secretion of α‐Defs 1–3 by neutrophils in the mucosa from patients with active IBD has also been reported 31. These data support the hypothesis that, in states of mucosal inflammation, α‐Defs 1–3 could be taken up by enterocytes from adjacent neutrophils. The plasma α‐Defs increase could be a compensatory response, given that mucosal defensin deficiency leads to an impaired innate immunity, which facilitates the entry of commensal bacteria and bacterial material translocation 17, which could trigger a response in the blood compartment.
In conclusion, our findings show that α‐Defs 1–3 plasma concentrations can reflect the degree of inflammation in CD, suggesting their implication in pathogenic pathways and their potential utility as specific biomarkers in patients with active ileal CD at diagnosis. Among other mechanisms, α‐Def 5 ileal mucosa expression is down‐regulated, at least in part, by methylation, supporting the role of epigenetic mechanisms in the pathogenesis of CD. Methylation appears to remain permanently, supporting the fact that α‐Def 5 expression cannot totally be recovered in ileal tissue when remission is achieved. Further studies in larger samples are necessary to confirm these results, in order to clarify the pathogenic mechanisms involved and to evaluate the clinical application of these peptides as new biomarkers in ileal CD.
Disclosure
The authors declare that they have no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Primer sequences used in the DNA methylation analysis
Acknowledgements
This study was supported by grants from the Healthcare Research Institute (Instituto de Investigación Sanitaria) of La Fe University Hospital (2012_0121_CPR), the Healthcare Institute Carlos III (PI14/01702, CA10/01027) and the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) (X Geteccu‐Otsuka Grant).
References
- 1. Artis D. Epithelial‐cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008; 8:411–20. [DOI] [PubMed] [Google Scholar]
- 2. Jäger S, Stange EF, Wehkamp J. Inflammatory bowel disease: an impaired barrier disease. Langenbecks Arch Surg 2013; 398:1–12. [DOI] [PubMed] [Google Scholar]
- 3. Strober W, Asano N, Fuss I, Kitani A, Watanabe T. Cellular and molecular mechanisms underlying NOD2 risk‐associated polymorphisms in Crohn's disease. Immunol Rev 2014; 260:240–60. [DOI] [PubMed] [Google Scholar]
- 4. Meisch JP, Nishimura M, Vogel RM et al Human beta‐defensin 3 peptide is increased and redistributed in Crohn's ileitis. Inflamm Bowel Dis 2013; 19:942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 2003; 3:521–33. [DOI] [PubMed] [Google Scholar]
- 6. Wehkamp J, Salzman NH, Porter E et al Reduced Paneth cell α‐defensins in ileal Crohn's disease. Proc Natl Acad Sci USA 2005; 102:18129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao L, Lu W. Defensins in innate immunity. Curr Opin Hematol 2014; 21:37–42. [DOI] [PubMed] [Google Scholar]
- 8. Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol 2008; 1:S67–74. [DOI] [PubMed] [Google Scholar]
- 9. Ouellette AJ, Greco RM, James M, Frederick D, Naftilan J, Fallon JT. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol 1989; 108:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganz T, Selsted ME, Szklarek D et al Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 1985; 76:1427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keshav S. Paneth cells: leukocyte‐like mediators of innate immunity in the intestine. J Leukoc Biol 2006; 80:500–8. [DOI] [PubMed] [Google Scholar]
- 12. Bevins CL. Innate immune functions of α‐defensins in the small intestine. Dig Dis 2013; 31:299–304. [DOI] [PubMed] [Google Scholar]
- 13. Peyrin‐Biroulet L, Chamaillard M. NOD2 and defensins: translating innate to adaptive immunity in Crohn's disease. J Endotoxin Res 2007; 13:135–9. [DOI] [PubMed] [Google Scholar]
- 14. Lakatos PL, Altorjay I, Mándi Y et al Interaction between seroreactivity to microbial antigens and genetics in Crohn's disease: is there a role for defensins? Tissue Antigens 2008; 71:552–9. [DOI] [PubMed] [Google Scholar]
- 15. Wehkamp J, Stange EF. Paneth's disease. J Crohns Colitis 2010; 4:523–31. [DOI] [PubMed] [Google Scholar]
- 16. Simms LA, Doecke JD, Walsh MD, Huang N, Fowler EV, Radford‐Smith GL. Reduced α‐defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut 2008; 57:903–10. [DOI] [PubMed] [Google Scholar]
- 17. Gutiérrez A, Holler E, Zapater P et al Antimicrobial peptide response to blood translocation of bacterial DNA in Crohn's disease is affected by NOD2/CARD15 genotype. Inflamm Bowel Dis 2011; 17:1641–50. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi N, Isomoto H, Mukae H et al Concentrations of alpha‐ and beta‐defensins in plasma of patients with inflammatory bowel disease. Inflamm Res 2009; 58:192–7. [DOI] [PubMed] [Google Scholar]
- 19. Van Assche G, Dignass A, Panes J et al The second European evidence‐based Consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010; 4:7–27. [DOI] [PubMed] [Google Scholar]
- 20. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet 1980; 1:514. [DOI] [PubMed] [Google Scholar]
- 21. Daperno M, D'Haens G, Van Assche G et al Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES‐CD. Gastrointest Endosc 2004; 60:505–12. [DOI] [PubMed] [Google Scholar]
- 22. Rimola J, Ordás I, Rodriguez S et al Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 2011; 17:1759–68. [DOI] [PubMed] [Google Scholar]
- 23. Hazlett L, Wu M. Defensins in innate immunity. Cell Tissue Res 2011; 343:175–88. [DOI] [PubMed] [Google Scholar]
- 24. Kanmura S, Uto H, Numata M et al Human neutrophil peptides 1–3 are useful biomarkers in patients with active ulcerative colitis. Inflamm Bowel Dis 2009; 15:909–17. [DOI] [PubMed] [Google Scholar]
- 25. van Es JH, Jay P, Gregorieff A et al Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol 2005; 7:381–6. [DOI] [PubMed] [Google Scholar]
- 26. Wehkamp J, Wang G, Kübler I et al The Paneth cell alpha‐defensin deficiency of ileal Crohn's disease is linked to Wnt / Tcf‐4. J Immunol 2007; 179:3109–18.] [DOI] [PubMed] [Google Scholar]
- 27. Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med 2012; 271:421–8. [DOI] [PubMed] [Google Scholar]
- 28. Beisner J, Teltschik Z, Ostaff MJ et al TCF‐1‐mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD‐5 and −6: implications for Crohn's disease. Am J Physiol Gastrointest Liver Physiol 2014; 307:G487–98. [DOI] [PubMed] [Google Scholar]
- 29. Courth LF, Ostaff MJ, Mailänder‐Sánchez D, Malek NP, Stange EF, Wehkamp J. Crohn's disease‐derived monocytes fail to induce Paneth cell defensins. Proc Natl Acad Sci USA 2015; 112:14000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karatzas PS, Mantzaris GJ, Safioleas M, Gazouli M. DNA methylation profile of genes involved in inflammation and autoimmunity in inflammatory bowel disease. Medicine 2014; 93:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cunliffe RN, Kamal M, Rose FRAJ, James PD, Mahida YR. Expression of antimicrobial neutrophil defensins in epithelial cells of active inflammatory bowel disease mucosa. J Clin Pathol 2002; 55:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Primer sequences used in the DNA methylation analysis


