Abstract
Background:
Metabolic control is a major concern in preventing diabetic complications. Saffron as a natural source of antioxidants could play a role in alleviating diabetes insults. The aim of this study was to investigate effect of saffron hydroalcoholic extract on metabolic control in type 2 diabetes (T2D) mellitus.
Materials and Methods:
This randomized triple blind study was included 54 T2D patients which randomly received either saffron (Group 1) or placebo (Group 2) twice daily other than routine antidiabetic treatments for 8 weeks. Serum concentration of fasting blood sugar (FBS), 2-h plasma glucose, hemoglobin A1c (HbA1c), total cholesterol, triglyceride (TG), low-density lipoprotein, and high-density lipoprotein were measured as the markers of metabolic control. Anthropometric measures and blood pressure were also measured at the baseline, every 2 weeks during the intervention and the end of the study. Data analyzed using repeated measure analysis of variance test.
Results:
The baseline metabolic parameters were the same in two group (P > 0.01). FBS serum level significantly decreased within 8 weeks in the saffron group (128.84 ± 31.86) as compared to the placebo (153.76 ± 41.23), (P < 0.001). There was no statistical difference in other metabolic parameters such as serum lipids, blood pressure, and HbA1c (P > 0.01).
Conclusion:
Saffron hydroalcoholic extract may improve blood glucose control by reducing FBS in T2D patients. However, saffron extract has no significant effect on other aspects of diabetic control in diabetic patients.
Keywords: Diabetes mellitus, fasting blood glucose, lipid, metabolic syndrome, saffron extract
INTRODUCTION
Type 2 Diabetes (T2D) is a chronic disease with considerable morbidity and mortality. T2D is considered as a public health problem globally.[1,2] Studies have shown a dramatic increase in the prevalence of diabetes in Iran during the last decade.[3] Hyperglycemia, hyperlipidemia, and hypertension are the most important complications of diabetes.[4] In addition, poorly controlled diabetes may cause some macro and microvascular complications, including neuropathy, retinopathy, atherosclerosis, and nephropathy which finally increase the risk of mortality.[5,6] Therefore, metabolic control is an effective preventive strategy in T2D to decrease the severe complications. Due to the high prevalence of diabetes and the wide range of catastrophic complications, the medical providers are continuously searching for additive and alternative remedies, especially in the field of herbal medicine, to use as an adjuvant for better diabetic control.[7,8]
The dried stigma of saffron (Crocus Sativus L.) is a native additive commonly used in Iran. Saffron has been used for many years in Iran as the most expensive traditional spice.[9,10,11] It contains three main secondary metabolites including soluble crocin, picrocrocin, and safranal as well as crocetin.[12,13] Antioxidant and anti-inflammatory properties of saffron extract have been attributed to the presence of these secondary metabolites; which in order result in major effects of saffron in different physiologic and psychological condition.[14,15]
To the best of our knowledge, no clinical trial has investigated the effect of saffron on serum lipid and glucose concentrations and on blood pressure in T2D patients, so far. In a recent study, daily consumption of 3 g saffron tea reduced blood lipids in T2D patients, however, it is not clear that these effects were due to saffron or tea. Furthermore, changes in blood glucose were not significant after consumption of saffron tea in that study.[16] In an animal model study, saffron injection reduced hyperglycemia, hyperlipidemia, and oxidative stress in diabetic rats;[17] however, it may be completely different in a humans.
Given these reasons and lack of study in this area, this randomized, placebo-controlled, and triple-blind clinical trial was done to investigate the effect of the saffron hydro-alcoholic extract on serum concentrations of glucose and lipids and blood pressure in patients with T2D mellitus.
MATERIALS AND METHODS
Participants
This study was an 8-week randomized triple-blind clinical trial which was done in accordance with the Declaration of Helsinki of 1975, as revised in 2008 and good clinical practice guidelines. The study was conducted on outpatients of Natanz Diabetes Society (NDS), Isfahan, Iran, between September 2015 and May 2016. NDS is a referral Center in Natanz where all diabetic patients are registered.
Eligible participants were selected based on inclusion criteria: patients with T2D mellitus (fasting plasma glucose levels of ≥126 mg/dL), aged 40–65 years, body mass index (BMI) 18.5–30 kg/m2. Patients who smoked, were using insulin or medications rather than common used diabetes medications including a determined doses of metformin (up to 1.5 gr) or glibenclamide (10 mg), those with uncontrolled blood glucose (fasting blood sugar [FBS] >170 mg/dl), high physical activity, and patients with recent experience of hospitalization as well as subjects with frequent use of herbal medications, pregnant, or lactating women and those who had planned for pregnancy were excluded from the study. Finally, 54 patients were included in the current study. All participants signed an informed written consent after receiving the explanations for study purposes and design. The study protocol was approved by the Tehran University of Medical Sciences’ Ethics Committee (ir.tums.rec. 1394.9211468004-143703; research.tums.ac.ir). The trial was registered at Iranian Registry of Clinical Trials as IRCT2015082623776N1.
Study design
Totally, 54 patients (12 males and 42 females, age 54.59 ± 7.09 years) were randomly divided into two similar groups (n = 27) to receive either placebo or saffron extract capsules twice a day (at the morning and evening) for 8 weeks. The sample size was determined using suggested formulas for parallel clinical trials by considering type I (a) and type II errors (b) as 0.05 and 0.20 (study power = 80%). Randomization was performed with the use of computer-generated random numbers. The randomization was blinded from all project investigators, participants and data analyzers, except for the trained physician of the NDS who did the random allocation and assigned participants to the placebo or saffron capsules. Before random assignment, participants were stratified based on sex (male or female) and age (<50 or ≥ 50 years). Each capsule contained 15 mg placebo or saffron hydroalcoholic extracts. In this study, we used commercially available Safrotin. Safrotin contains 15 mg saffron hydroalcoholic extract. All safrotin and placebo capsules were provided by Green Plants of Life Co., (IMPIRAN; Tehran, Iran). Placebo capsules were similar to Safrotin capsules in the appearance, color, and size. The content of placebo capsules was starch, lactose, magnesium stearate, gelatin, and saffron essence.
Participants were asked not to change their diet, physical activity or medications during the intervention. Subjects were also asked to attend the clinic every 2 weeks (2nd, 4th, 6th, and 8th weeks) to take their capsules. To assess participants’ compliance, they were requested to bring their capsules’ boxes at each visit to determine a total number of capsules remaining. To increase the compliance, all patients were receiving short messages on their cell phones to remind taking supplements each day.
Biochemical tests
At the beginning and at the end of the intervention, all participants referred to central laboratory of Natanz, Isfahan, Iran. After 12 h fasting, participants’ blood samples were taken, then serum was isolated and kept at −70°C. Serum concentration of triacylglycerol (TG) and total cholesterol (TC) were measured using enzymatic methods (Pars Azmoun.co kit, Tehran, Iran). FBS was measured by autoanalyzer (Hitachi 911, Japan) using enzymatic and colorimetric method (Pars Azmoun.co kit, Tehran, Iran). Glucose tolerance of participants was estimated by measuring serum 2 h Plasma Glucose (2 hPG). For this purpose, all participants received oral 82.5 g glucose monohydrate solution (equivalent to 75 g dehydrated glucose) and their blood samples were taken after 2 h. In addition, serum concentration of hemoglobin A1C (HbA1C) was measured using Elisa kit (Bioassay Technology Laboratory, Elisa kit). Measurement of high-density lipoprotein (HDL)-cholesterol was done by auto-analyzer, using cholesterol oxidase method. Moreover, serum low-density lipoprotein (LDL)-cholesterol was estimated using Friedewalds’ formula.
Assessment of other variables
At the study beginning, general characteristics of participants were obtained using a questionnaire. In addition, a skilled nutritionist measured weight, height, and waist circumference (WC). Weight was measured by a digital scale (Sega 707, Hamburg, Germany) to the nearest 100 g using light clothes and without shoes; height by a stadiometer (Seca, Hamburg, Germany) without shoes to the nearest 0.1 cm, and WC at the narrowest level by a nonstretchable tape to the nearest 0.1 cm. BMI was calculated as weight (kg) divided by height squared (m2). Systolic and diastolic blood pressure were also measured twice by a standard barometer that was calibrated by Institute of Standard and Industrial Research of Iran in the right arm of the patients who were at sitting position for at least 10 min. All these measures were also examined at the periodical visits and at the end of the intervention. Three 24-hour dietary recalls were taken from all of the participants at the beginning, 4th week, and the end of the study, by a skilled nutritionist. Dietary intakes of energy and macronutrients were determined using nutritionist IV (N-IV) software (First Databank, San Bruno, CA, USA) modified for Iranian foods. In addition, physical activity of subjects was assessed by a valid and reliable (Alpha coefficient = 0.7) Persian version of the International physical activity questionnaire.[18] This assessment was repeated at the end of the study. Data on physical activity were expressed as metabolic Equivalents using available publications.[19,20]
Statistical analysis
Normal distribution of data was investigated using Kolmogorov–Smirnov test. Data were expressed as mean ± standard deviation (SD). Baseline characteristics of study participants, as well as their dietary intakes throughout the study were compared using student's t-test for continuous variables and Chi-square test for categorical variables. To examine time and time × group effects, we applied repeated measure of analysis of variance, and adjusted our findings for the potential confounders including participants’ physical activity, total energy intake, and BMI. P < 0.05 was considered statistically significant. All statistical analyses were done using the Statistical Package for Social Sciences for Windows version 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
Fifty-two patients completed the study. One person from the placebo and one from the saffron group voluntarily left the study during the intervention due to complications of the capsules, especially headache. Flowchart of the study has been indicated in Figure 1.
Figure 1.
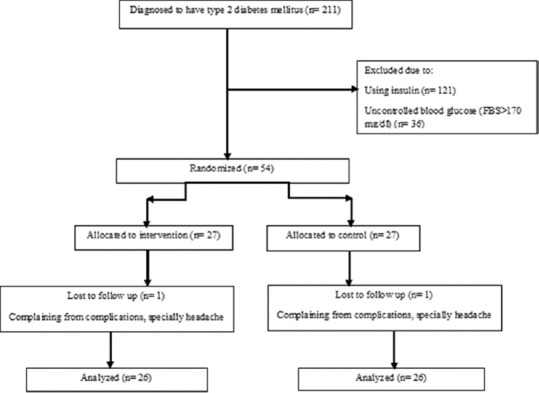
Flowchart of the study
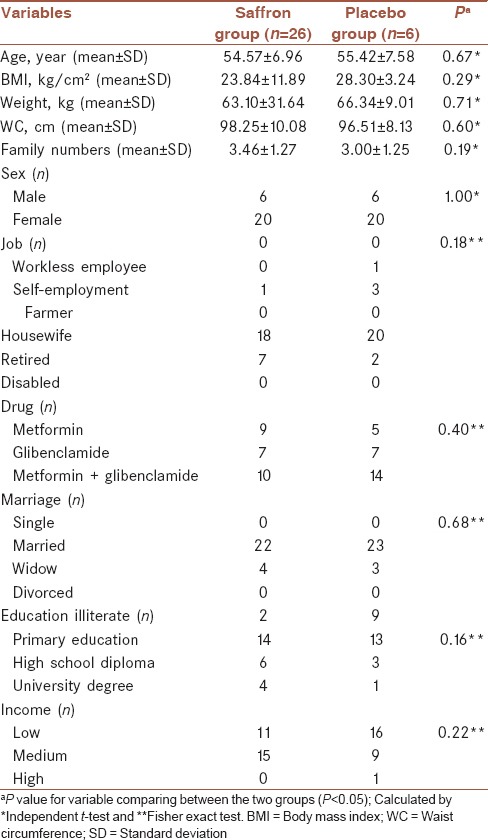
Baseline characteristics of participants are shown in Table 1. There were no significant differences between the two groups with regard to basic characteristics. Mean (±SD) age and BMI of participants were 55.00 ± 7.22 and 25.87 ± 7.29, respectively.
Table 1.
Baseline characteristics of participants
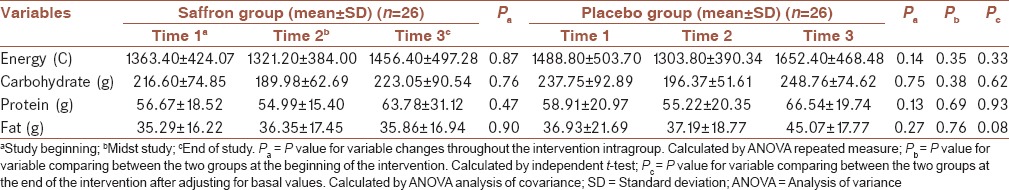
Dietary intake of participants is indicated in Table 2. There were no significant differences in dietary intakes between the two groups at the baseline. Moreover, differences in dietary intakes remained nonsignificant throughout the intervention.
Table 2.
Dietary intake of participants
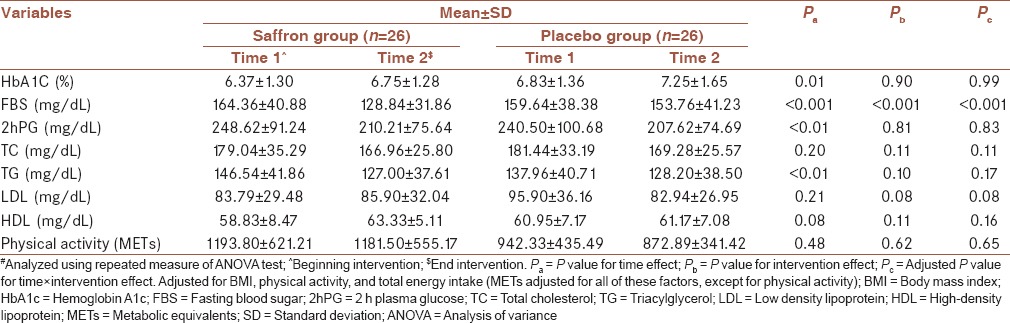
In addition, participants’ physical activity did not change significantly throughout the intervention. Significant reduction was found in serum concentration of FBS in the saffron group as compared to the controls (saffron group = 128.84 ± 31.86 mg/dL; placebo group = 153.76 ± 41.23 mg/dL, P < 0.001). This reduction remained significant after controlling for the potential confounders including physical activity, total energy intake, and BMI (P < 0.001). Changes in serum concentration of HbA1C (P = 0.90), 2 hPG (P = 0.81), TC (P = 0.11), TG (P = 0.10), LDL (P = 0.08), HDL (P = 0.11) were not significant after consumption of safrotin than placebo; and remained unchanged after controlling for the potential confounders [Table 3].
Table 3.
Serum concentration of glucose and lipids and physical activity of the study participants#
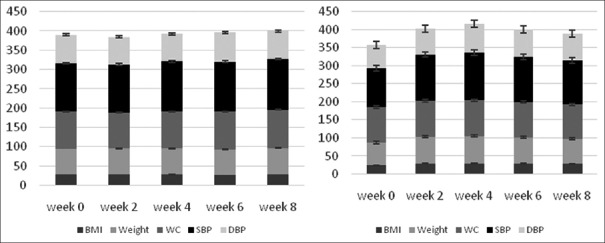
Changes in anthropometric measures and blood pressure throughout the study in both saffron and placebo groups are indicated in Figure 2. Although an increase was seen in systolic and diastolic blood pressures during the intervention, it was nonsignificant. In addition, differences in anthropometric measures between two groups were also not statistically significant (P = 0.46).
Figure 2.
Changes in anthropometric measures and blood pressure parameters throughout the study in the saffron (right) and placebo (left) groups. *Calculated by the analysis of variance repeated measure. Anthropometric measures and systolic and diastolic blood pressure did not change significantly during the study. BMI = Body mass index; WC = Waist circumference; SBP = Systolic blood pressure; DBP: Diastolic blood pressure
DISCUSSION
Our study showed that hydroalcoholic extract of saffron significantly decreases the serum levels of FBS in T2D patients comparing to the controls.
Based on the best of our knowledge, there was no study investigated the effect of saffron on metabolic control including serum lipid profile and glucose concentrations in T2D mellitus. However, Azimi et al. have shown a significant decrease in TC and LDL and increase in HDL among T2D patients after drinking daily three glasses of saffron tea (containing 1 g saffron) for 8 weeks. However, no significant effect was seen on blood glucose profiles.[16] However, this effect may be due to tea instead of saffron. In another study by Fadai et al., 12 weeks consumption of saffron (30 mg/day) in patients with schizophrenia who suffered from metabolic syndrome induced significant reduction only in FBS.[21] In an animal study by Samarghandian et al. on diabetic rats suffered from encephalopathy, saffron at doses of 40 and 80 mg/kg significantly reduced TC, TG, LDL, and blood glucose and increased HDL after 28 days.[17]
We could not found significant changes in TG and HDL serum concentrations throughout the intervention. In addition, we also failed to find a significant effect of saffron on the blood pressure. This finding is in a line with Azimi et al. study in which daily consumption of three glasses saffron tea (containing 1 g saffron) for 8 weeks did not effect on blood pressure in T2D patients.[22] In an animal model study, 5 weeks administration of three doses saffron aqueous extract (10, 20, and 40 mg/Kg/day) did not change blood pressure in normotensive rats.[23]
The mechanisms through which dietary saffron extract might influence the metabolic control in T2D mellitus are lacking. Saffron is considered as a natural source of dietary antioxidants. Antioxidants are hypothesized to modulate diabetes by reducing its complications. Such that, antioxidants may improve endothelial function, reduce platelet aggregation, lower blood glucose, and might induce anti-inflammatory effects.[24,25] It becomes so important when we know systemic inflammation as a risk factor in multiple aspects of diabetes etiology and pathology.[26]
This study is the first randomized clinical trial investigating effect of saffron hydroalcoholic extract on metabolic control in T2D mellitus. Several other strengths of the current study need to be highlighted; including having detailed data on diet and physical activity. In addition, matching subjects for age and sex and controlling medications using by the participants improved our conclusions. Given these strengths, some limitations must also be taken into account. We measured some serum glucose and lipid indicators in this study. Assessment of insulin resistance, serum concentration of insulin, and important serum indicators of inflammation might improve our conclusions. In addition, as there was no human study available in this area, we took a saffron daily dose and our study sample size and duration similar to the clinical trials investigated the effect of saffron on depression.[25,26,27,28,29] Therefore, further studies by using different doses of saffron, with larger sample sizes and longer durations are recommended for the future.
CONCLUSIONS
Given together, it should be said that saffron hydroalcoholic extract may considerably improve blood glucose control by reducing FBS serum concentrations in T2D patients. However, saffron extract have no significant effect on lipids serum concentrations and blood pressure among patients with T2D. Further studies with different dosages of saffron extract and duration and larger sample size are required to confirm these findings.
Financial support and sponsorship
The study was financially supported by the Tehran University of Medical Sciences (TUMS). Safrotin and placebo capsules used in this study was dedicated by IMPIRAN Co.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to gratefully acknowledge Green Plants of Life Co., and Natanz Health Center and Khatamolanbia hospital staff, especially Dr. Alireza Chavoshzadeh and Hossein Yeganeh, in Natanz, Iran. The study has supported by Tehran University of Medical Sciences.
REFERENCES
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus – Present and future perspectives. Nat Rev Endocrinol. 2011;8:228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine. 2010;38:602–6. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rashedi V, Asadi-Lari M, Delbari A, Fadayevatan R, Borhaninejad V, Foroughan M, et al. Prevalence of diabetes type 2 in older adults: Findings from a large population-based survey in Tehran, Iran (Urban HEART-2) Diabetes Metab Syndr. 2017;11(Suppl 1):S347–50. doi: 10.1016/j.dsx.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Luk AOY, Hui EMT, Sin MC, Yeung CY, Chow WS, Ho AYY, et al. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: The Hong Kong diabetes database. Diabetes Care. 2017;40:928–35. doi: 10.2337/dc16-2354. [DOI] [PubMed] [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 6.Forbes JM, Fotheringham AK. Vascular complications in diabetes: Old messages, new thoughts. Diabetologia. 2017;60:2129–38. doi: 10.1007/s00125-017-4360-x. [DOI] [PubMed] [Google Scholar]
- 7.Kesavadev J. Efficacy and safety concerns regarding complementary and alternative medicine use among diabetes patients. J Pak Med Assoc. 2017;67:316–9. [PubMed] [Google Scholar]
- 8.Nahas R, Moher M. Complementary and alternative medicine for the treatment of type 2 diabetes. Can Fam Physician. 2009;55:591–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Milajerdi A, Jazayeri S, Bitarafan V, Hashemzadeh N, Shirzadi E, Derakhshan Z, et al. The effect of saffron (Crocus sativus L.) hydro-alcoholic extract on liver and renal functions in type 2 diabetic patients: A double-blinded randomized and placebo control trial. J Nutr Intermed Metab. 2017;9:6–11. [Google Scholar]
- 10.Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: A 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35:581–8. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 11.Modabbernia A, Sohrabi H, Nasehi AA, Raisi F, Saroukhani S, Jamshidi A, et al. Effect of saffron on fluoxetine-induced sexual impairment in men: Randomized double-blind placebo-controlled trial. Psychopharmacology (Berl) 2012;223:381–8. doi: 10.1007/s00213-012-2729-6. [DOI] [PubMed] [Google Scholar]
- 12.Hausenblas HA, Saha D, Dubyak PJ, Anton SD. Saffron (Crocus sativus L.) and major depressive disorder: A meta-analysis of randomized clinical trials. J Integr Med. 2013;11:377–83. doi: 10.3736/jintegrmed2013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milajerdi A, Bitarafan V, Mahmoudi M. A review on the effects of saffron extract and its constituents on factors related to neurologic, cardiovascular and gastrointestinal diseases. J Med Plants. 2015;3:9–28. [Google Scholar]
- 14.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world's most expensive spice: Saffron. Food Res Int. 2010;43:1981–9. [Google Scholar]
- 16.Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud. 2014;11:258–66. doi: 10.1900/RDS.2014.11.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samarghandian S, Azimi-Nezhad M, Samini F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed Res Int. 2014;2014:920857. doi: 10.1155/2014/920857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian version of International Physical Activity Questionnaire (IPAQ) in Iran: Content and construct validity, factor structure, internal consistency and stability. World Appl Sci. 2012;18:1073–80. [Google Scholar]
- 19.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. 2011 compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 21.Fadai F, Mousavi B, Ashtari Z, Ali beigi N, Farhang S, Hashempour S, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacopsychiatry. 2014;47:156–61. doi: 10.1055/s-0034-1382001. [DOI] [PubMed] [Google Scholar]
- 22.Azimi P, Ghiasvand R, Feizi A, Hosseinzadeh J, Bahreynian M, Hariri M, et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press. 2016;25:133–40. doi: 10.3109/08037051.2015.1111020. [DOI] [PubMed] [Google Scholar]
- 23.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H, et al. The effect of chronic administration of saffron (Crocus sativus) stigma aqueous extract on systolic blood pressure in rats. Jundishapur J Nat Pharm Prod. 2013;8:175–9. doi: 10.17795/jjnpp-12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talebnejad MR, Soltanzadeh K, Masoomeh E, Yasemi M, Khalili MR, Maryam S, et al. Effect of intraperitoneal injection of saffron on the treatment of experimental endotoxin induced uveitis in the rabbit. J Clin Diagn Res. 2017;11:NC01–NC04. doi: 10.7860/JCDR/2017/23266.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milajerdi A, Djafarian K, Hosseini B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J Nutr Intermed Metab. 2016;3:23–32. [Google Scholar]
- 26.Van Dyke AL, Lang Kuhs KA, Shiels MS, Koshiol J, Trabert B, Loftfield E, et al. Associations between self-reported diabetes and 78 circulating markers of inflammation, immunity, and metabolism among adults in the United States. PLoS One. 2017;12:e0182359. doi: 10.1371/journal.pone.0182359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L. In the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–51. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 28.Moshiri E, Basti AA, Noorbala AA, Jamshidi AH, Hesameddin Abbasi S, Akhondzadeh S, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13:607–11. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Akhondzadeh Basti A, Moshiri E, Noorbala AA, Jamshidi AH, Abbasi SH, Akhondzadeh S, et al. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:439–42. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]