Abstract
Background:
There is a paucity of literature regarding outcome of critically ill patients with tuberculosis (TB) from India. Herein, we describe our experience of patients with active TB admitted to a Respiratory Intensive Care Unit (RICU) of a tertiary care hospital.
Methods:
This was a retrospective analysis of all the patients admitted with active TB. The baseline clinical, demographic, ICU parameters and mortality were recorded. A multivariate logistic regression analysis was performed to identify factors predicting mortality.
Results:
A total 3630 patients were admitted to the ICU during the study period; of these, 63 (1.7%) patients (mean [standard deviation (SD)] age 37.3 [19] years, 55.6% females) were admitted with active TB. Fifty-seven patients were mechanically ventilated (56, invasive and 1, noninvasive) for a mean (SD) duration of 7.5 (9.1) days. Respiratory failure was the most common indication for mechanical ventilation. TB-related acute respiratory distress syndrome was seen in 18 (28.6%) patients. There were 28 deaths (44.4%) during the study period. On a multivariate logistic regression analysis, a high baseline Acute Physiology and Chronic Health Evaluation II (APACHE II) score (odds ratio [OR] [95 confidence interval (CI)], 1.12 [1.02–1.23]) and delta Sequential Organ Failure Assessment (SOFA) (OR [95 CI], 1.39 [1.00–1.94]) were the independent predictors of mortality.
Conclusion:
TB was an uncommon cause of ICU admission even in a high TB burden country. Critically ill patients with TB had high mortality. A higher APACHE II score and delta SOFA were independent predictors of ICU mortality.
Keywords: Acute respiratory distress syndrome, human immunodeficiency virus, invasive mechanical ventilation, Mycobacterium tuberculosis, acute respiratory failure
INTRODUCTION
Tuberculosis (TB) commonly manifests as chronic illness, but occasionally, patients with TB may present acutely and require intensive care.[1,2,3] The major indications of Intensive Care Unit (ICU) admission include respiratory failure due to tuberculous pneumonia, miliary TB or acute respiratory distress syndrome (ARDS), sepsis, and neurological deterioration due to central nervous system TB.[4,5,6] The proportion of individuals with TB requiring admission to an ICU has been reported to be <1%.[7] Whether the proportion of critically ill TB patients requiring ICU admission is higher in high TB burden country such as India remains unclear. Furthermore, the mortality in critically ill patients with TB remains high, despite the availability of effective anti-TB drugs and improvement in the standards of ICU care.[7,8] It would be useful if information regarding factors that predict mortality in critically ill patients with TB is available as it would enable risk stratification and management. The current study was planned to determine the proportion of ICU patients with active TB and investigate the factors predicting ICU mortality in these patients.
METHODS
This was a retrospective analysis of patients admitted to the respiratory ICU (RICU) between February 1, 2001, and September 30, 2016. The study protocol was approved by the Institute Ethics Committee. A consent waiver was given as the study involved the use of anonymized retrospective patient data.
All the patients admitted with a clinical diagnosis of TB, those diagnosed with TB after ICU admission, or those who had received anti-tuberculosis therapy (ATT) for <2 months at the time of admission were eligible for inclusion in the study. Active TB was diagnosed based on a composite of clinicoradiologic features consistent with TB, and the presence of smear positivity for acid-fast bacilli, a positive Xpert MTB/RIF, culture showing growth of Mycobacterium tuberculosis, or demonstration of necrotizing granulomatous inflammation on histopathological examination of biopsy samples obtained from different organs (lung, liver, spleen, pleura, lymph nodes, and kidney).
The following information was extracted from RICU database: (a) demographic profile; (b) presence of comorbid illness; (c) type of TB (pulmonary or extrapulmonary); (d) ICU severity scores including Sequential Organ Failure Assessment (SOFA) scores and Acute Physiology and Chronic Health Evaluation (APACHE II) scores; (e) type of respiratory support (oxygen supplementation and noninvasive or invasive ventilation); (f) duration of mechanical ventilation; (g) ICU length of stay (LOS) and hospital LOS; and, (h) the final outcome. Delta SOFA was calculated using the formula, SOFA maximum minus the baseline SOFA. Disseminated TB was defined as involvement of two or more noncontiguous organs. The details of some of the patients included in this study have been previously reported.[9]
All the patients were treated with standard ATT in accordance with the revised national TB control program of India. Adjuvant glucocorticoids were used in patients with tubercular meningitis, tubercular pericardial effusion, or adrenal insufficiency.
Statistical analysis
Statistical analysis was performed using the statistical software package (SPSS for Windows, version 24.0; IBM SPSS Inc.; Chicago, IL, USA). Descriptive frequencies and categorical variables were expressed as mean (standard deviation [SD]) and as percentages for the group, respectively. Differences between the means of categorical and continuous variables were compared using Chi-square test and Mann–Whitney U-test, respectively. A multivariate logistic regression analysis was performed to identify variables associated with mortality. P < 0.05 was considered statistically significant.
RESULTS
A total of 3630 patients were admitted to the RICU during the study period. Of these, 63 (1.7%) patients (mean [SD] age 37.3 [19] years, 55.6% females) had TB as an indication for ICU admission [Table 1]. None of the patients in the current study had human immunodeficiency virus (HIV) infection. Four patients were admitted to the ICU with TB in the antenatal and peripartum period. TB was clinically suspected in a majority of the patients (55/63, 87.3%) at admission to the ICU, while in the remaining patients, the diagnosis was made after admission. Disseminated TB was present in 35 patients; 28 patients had isolated pulmonary TB. Miliary TB was diagnosed in 19 patients (30.2%). TB-related ARDS was seen in 18 (28.6%) patients. Six patients with miliary shadows on chest radiograph developed ARDS.
Table 1.
Baseline characteristics of the study population
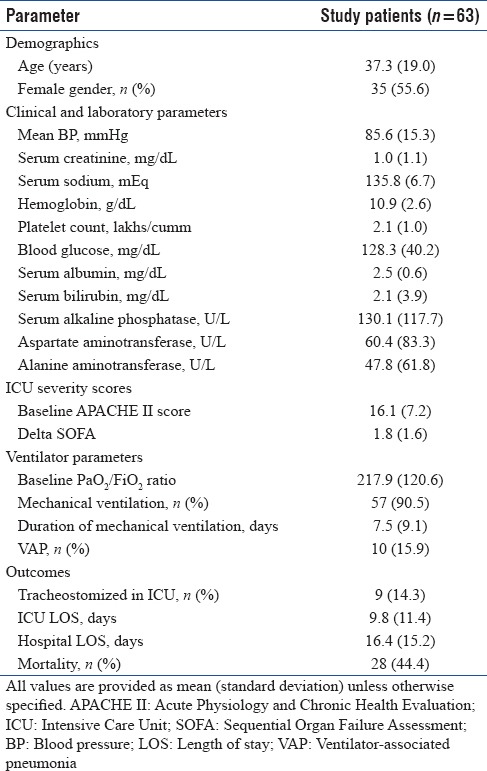
Five patients developed ATT-induced hepatitis that needed treatment modification. Systemic glucocorticoids were administered in 18 patients (15 with tubercular meningitis, 2 for adrenal insufficiency, and 1 for pericardial effusion).
Fifty-seven patients were mechanically ventilated (56, invasive; 1, noninvasive) for a mean (SD) duration of 7.5 (9.1) days. There were 28 (44.4%) deaths while 9 patients required tracheostomy for weaning. The cause of death was severe sepsis (n = 16), raised intracranial tension (n = 7), and refractory hypoxemia (n = 5). Ventilator-associated complications were seen in 14 patients. Ten patients developed ventilator-associated pneumonia (VAP), and four patients developed pneumothorax that required intercostal tube drainage. In two patients with hydrocephalus, a ventricular drain was placed.
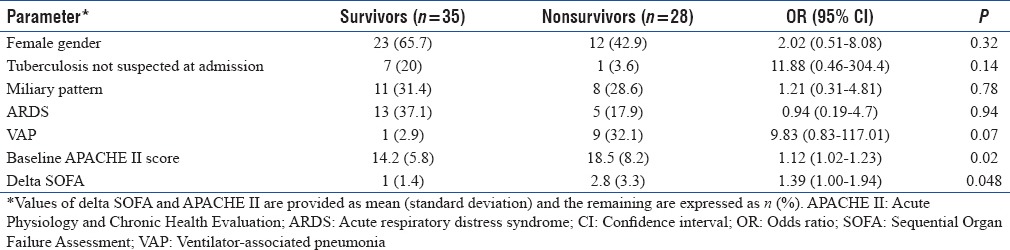
Non survivors had high baseline APACHE II score and high delta SOFA score (new organ dysfuntion). Patients who died also had a higher incidence of VAP and vasopressor-requiring shock [Table 2]. The presence of miliary opacities on chest radiograph was not associated with an increased mortality. On a multivariate logistic regression analysis, baseline APACHE II and delta SOFA were the independent predictors of mortality after adjusting for the presence of ARDS, miliary TB, VAP, and other variables [Table 3].
Table 2.
Characteristics of Intensive Care Unit (ICU) survivors and nonsurvivors with tuberculosis
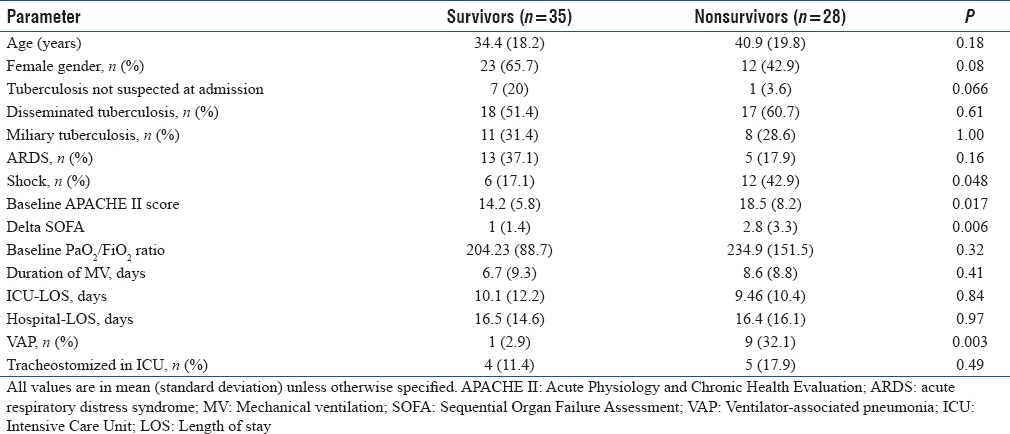
Table 3.
Risk factors predicting mortality in patients of active tuberculosis admitted to respiratory Intensive Care Unit (multivariate logistic regression analysis)
DISCUSSION
The results of this study indicate that TB is an uncommon cause of admission to ICU even in a high TB burden country Critically ill patients with TB had a high ICU mortality (44.1%). Baseline APACHE II score and delta SOFA score were the independent predictors of ICU mortality after adjusting for the presence of ARDS, miliary TB, and other covariates.
Despite an increase in the global incidence of TB, it remains an uncommon indication for ICU admission.[10] Even in the current study, the proportion of patients with active TB was only 1.7% of all ICU admissions. Early detection, availability of effective ATT, and assurance of high compliance to ATT under directly observed therapy could explain the lower prevalence of TB in the ICU. The low proportion of TB in the current study could also be due to the fact that not all patients get admitted to the ICU due to limited bed availability. It is also possible that TB was not suspected in few of our patients similar to the previous studies.[2,11,12] The mortality among critically ill TB patients was high similar to previous studies.[8,13,14] The high mortality could reflect a referral bias with the sickest of patients getting admitted to ICU. Another likely factor for high mortality could be the unpredictable pharmacokinetics and pharmacodynamics of ATT in the critically ill patients.[1] Unlike antibacterial agents that result in immediate control of bacterial infection, the antitubercular drugs take time for their effect. Finally, infection with M. tuberculosis per se suppresses monocyte expression causing a state of immunosuppression, thereby increasing the risk for secondary infections.[8] This was seen in the current study where the incidence of VAP was higher amongst the nonsurvivors.
In the current study, higher ICU severity scores at baseline predicted mortality on a multivariate logistic regression analysis, similar to previous studies.[8,10] However, unlike previous studies, the presence of ARDS or miliary TB did not predict mortality.[15,16,17] This is likely due to the difference in the geographical profile and presence of comorbid illnesses. In the current study, patients were younger and were less likely to have comorbid illnesses. Furthermore, most of the patients with TB-related ARDS in a previous study were managed with noninvasive ventilation.[17] The use of noninvasive ventilation in ARDS is associated with a high mortality.[18]
What are the clinical implications of the current study? TB is a communicable infectious disease with high secondary attack rates, especially those with cavitation. It is likely that health-care workers involved in the care of critically ill patients with TB are at an increased risk of infection. Ideally, critically ill patients with TB should be managed in isolation units with the facility of negative pressure rooms, requisite air exchanges, and high-efficiency particulate air filtration. However, in resource-constrained settings, this is not usually available. Certain measures could reduce the chances of transmission of infection in the ICU setting. Those involved in the care of these patients should use personal protective equipment that includes N-95 respirators, eye protective gear, disposable protective gowns, and gloves. For the management of airways such as suctioning, a closed inline suction equipment should be used. A bacterial filter at the expiratory end of the ventilator tubing could reduce the bacterial load in the expired air. Finally, after the patient is discharged from the unit, the surface of ventilator, monitors, walls, and floor should be cleaned with a disinfectant such as EcoShield®, and the unit should be disinfected (chemical fogging) before accepting a new patient in the unit. Those who are routinely involved in the care of critically ill patients with TB should be monitored routinely for any symptoms of TB (fever, weight loss, chronic cough, and others).
Finally, our study has a few limitations. This was a retrospective single-center study with a possibility of selection and a referral bias. The study did not include patients with HIV or multidrug-resistant TB; thus, these results are not applicable to this population. Furthermore, we do not have the follow-up information and the long-term outcomes of the patients after hospital discharge. However, this study provides a real-world scenario of critically ill patients with TB from a developing country.
CONCLUSION
TB was an uncommon cause of ICU admission, even in a high burden country like India. Critically ill patients with TB admitted to ICU had high mortality. The severity of ICU illness and the occurrence of new organ dysfunction rather than the presence of ARDS or miliary TB were the predictors of mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Penner C, Roberts D, Kunimoto D, Manfreda J, Long R. Tuberculosis as a primary cause of respiratory failure requiring mechanical ventilation. Am J Respir Crit Care Med. 1995;151:867–72. doi: 10.1164/ajrccm/151.3_Pt_1.867. [DOI] [PubMed] [Google Scholar]
- 2.Silva DR, Menegotto DM, Schulz LF, Gazzana MB, Dalcin PT. Mortality among patients with tuberculosis requiring intensive care: A retrospective cohort study. BMC Infect Dis. 2010;10:54. doi: 10.1186/1471-2334-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkema CA, Irusen EM, Taljaard JJ, Koegelenberg CF. Tuberculosis in the Intensive Care Unit: A prospective observational study. Int J Tuberc Lung Dis. 2014;18:824–30. doi: 10.5588/ijtld.13.0044. [DOI] [PubMed] [Google Scholar]
- 4.Piqueras AR, Marruecos L, Artigas A, Rodriguez C. Miliary tuberculosis and adult respiratory distress syndrome. Intensive Care Med. 1987;13:175–82. doi: 10.1007/BF00254701. [DOI] [PubMed] [Google Scholar]
- 5.Verdon R, Chevret S, Laissy JP, Wolff M. Tuberculous meningitis in adults: Review of 48 cases. Clin Infect Dis. 1996;22:982–8. doi: 10.1093/clinids/22.6.982. [DOI] [PubMed] [Google Scholar]
- 6.Puri MM, Kumar S, Prakash B, Lokender K, Jaiswal A, Behera D, et al. Tuberculosis pneumonia as a primary cause of respiratory failure – Report of two cases. Indian J Tuberc. 2010;57:41–7. [PubMed] [Google Scholar]
- 7.Agarwal MK, Muthuswamy PP, Banner AS, Shah RS, Addington WW. Respiratory failure in pulmonary tuberculosis. Chest. 1977;72:605–9. doi: 10.1378/chest.72.5.605. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Kim H, Kim WJ, Lee SJ, Hong Y, Lee HY, et al. Mortality and predictors in pulmonary tuberculosis with respiratory failure requiring mechanical ventilation. Int J Tuberc Lung Dis. 2016;20:524–9. doi: 10.5588/ijtld.15.0690. [DOI] [PubMed] [Google Scholar]
- 9.Muthu V, Dhooria S, Aggarwal AN, Behera D, Sehgal IS, Agarwal R, et al. Acute respiratory distress syndrome due to tuberculosis in a respiratory ICU over a 16-year period. Crit Care Med. 2017;45:e1087–90. doi: 10.1097/CCM.0000000000002479. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SK, Mohan A, Banga A, Saha PK, Guntupalli KK. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis. 2006;10:429–35. [PubMed] [Google Scholar]
- 11.Mahmoud ES, Baharoon SA, Alsafi E, Al-Jahdaly H. Acute respiratory distress syndrome complicating community-acquired pneumonia secondary to Mycobacterium tuberculosis in a tertiary care center in Saudi Arabia. Saudi Med J. 2016;37:973–8. doi: 10.15537/smj.2016.9.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zahar JR, Azoulay E, Klement E, De Lassence A, Lucet JC, Regnier B, et al. Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensive Care Med. 2001;27:513–20. doi: 10.1007/s001340000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frame RN, Johnson MC, Eichenhorn MS, Bower GC, Popovich J., Jr Active tuberculosis in the medical Intensive Care Unit: A 15-year retrospective analysis. Crit Care Med. 1987;15:1012–4. doi: 10.1097/00003246-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Park YB, Kim YS, Kang SB, Shin JW, Park IW, et al. Miliary tuberculosis and acute respiratory distress syndrome. Int J Tuberc Lung Dis. 2003;7:359–64. [PubMed] [Google Scholar]
- 15.Erbes R, Oettel K, Raffenberg M, Mauch H, Schmidt-Ioanas M, Lode H, et al. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur Respir J. 2006;27:1223–8. doi: 10.1183/09031936.06.00088105. [DOI] [PubMed] [Google Scholar]
- 16.Valade S, Raskine L, Aout M, Malissin I, Brun P, Deye N, et al. Tuberculosis in the Intensive Care Unit: A retrospective descriptive cohort study with determination of a predictive fatality score. Can J Infect Dis Med Microbiol. 2012;23:173–8. doi: 10.1155/2012/361292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng W, Yu M, Ma H, Hu LA, Chen G, Wang Y, et al. Predictors and outcome of patients with acute respiratory distress syndrome caused by miliary tuberculosis: A retrospective study in Chongqing, China. BMC Infect Dis. 2012;12:121. doi: 10.1186/1471-2334-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: A proportion meta-analysis. Respir Care. 2010;55:1653–60. [PubMed] [Google Scholar]


