Abstract
Context:
It has been observed that sex hormones may play a role in inflammatory processes and mortality of critically ill patients.
Aims:
The aim was evaluated the relationship between serum estradiol level at Intensive Care Unit (ICU) admission and mortality of critically ill patients.
Settings and Design:
This study was a prospective cohort conducted in one mixed ICU.
Subjects and Methods:
In heterogeneous group of critically ill patients admitted to the ICU, we measured serum estradiol at admission time.
Statistical Analysis Used:
The discrimination to predict mortality of serum estradiol level was assessed by the receiver-operating curve (ROC) curve and its association with mortality by logistic regression analysis.
Results:
We included 131 patients, 57.3% of which were male. The serum estradiol level measured at ICU admission was significantly higher in nonsurvivors than in survivors: 116 versus 67.2 pg/mL, respectively (P < 0.0001). The area under the ROC of serum estradiol level to predict mortality was 0.74 (P < 0.0001). Serum estradiol level ≥97.9 pg/mL had sensitivity of 60%, specificity of 90%, positive predictive value of 64%, negative predictive value of 88%, positive likelihood ratio of 6, and negative likelihood ratio of 0.44, for predicting mortality. In multivariate analysis, it had relative risk of 6.47 (P = 0.002) for ICU mortality.
Conclusions:
The serum estradiol level is elevated in critically ill patients, regardless of gender, especially in those who die. It has good discriminative capacity to predict mortality, and it is an independent risk factor for death in this group of patients.
Keywords: Critical illness, estradiol, mortality, outcome
INTRODUCTION
During acute illness, the risk of death depends mainly on the severity of the disease, previous health status of the patient, and how he/she responds to disease or injury.[1] In the past decade, some studies have evaluated the impact of gender and hormonal status on mortality of patients with different acute conditions and found that hormones may play a role that can influence the outcome of critically ill patients.[1,2,3,4,5]
During stress, gonadal synthesis of estradiol is inhibited by Class I cytokines and tumor necrosis factor alpha, whereas there is an activation of the peripheral estradiol production by enzyme aromatase in adipose tissue, regardless of gender of the patients.[1,2,3,6] Estradiol causes changes in the immune response such as inhibition of the action of T-cells and activation of B-cell and macrophages inducing the production of interleukin 2 and interferon-α, mainly.[7,8]
Different studies in animal models have shown that changes in the immune response induced by estradiol produce an inflammatory response and modify the survival.[3,5,6,7,8] In humans, some authors have shown that, in clinical settings such as trauma or surgical patients, the increase in the serum level of estradiol was associated with increased severity of disease and mortality, regardless of the gender of patients, and was proposed as a tool for predicting mortality.[1,3,5,8,9,10] All this evidence has been documented in specific groups of patients and used different cutoffs of serum estradiol levels to predict mortality. The aim of this study was to evaluate the capacity of serum estradiol level, measured at Intensive Care Unit (ICU) admission, and to predict mortality in a heterogeneous group of critically ill patients.
SUBJECTS AND METHODS
We conducted a prospective, transversal, observational, analytical, and single-center study in patients admitted to the ICU between June 1st, and December 31st, 2015. We included patients >18 years old, of both genders, and who signed informed consent to participate in the study. Patients with brain death, pregnancy, or puerperium were excluded from the study. We recorded the following demographic and clinical variables: age, sex, comorbidities, Carlson's index, Acute Physiology and Chronic Health Evaluation (APACHE II) score,[11] Sequential Organ Failure Assessment (SOFA) score,[12] patient type (medical and surgical), sepsis, vasopressors, inotropes, renal replacement therapy, mechanical ventilation, length of mechanical ventilation, tracheostomy, and length of stay in the ICU. The serum estradiol level was measured at ICU admission. For this, a sample of 3.5 cc of venous blood was taken by direct puncture or through a central venous catheter in patients who have this device. The sample was placed in a tube with ethylenediaminetetraacetic acid and centrifuged. The serum estradiol level was determined by immunofluorescence in free of hemolysis serum on a IMMULITE 2000® equipment. The main outcome was ICU mortality. The research protocol was reviewed and approved by the Local Research Committee (registration number R-2015-3501-102).
Statistical analysis
Descriptive statistics are used for data presentation. Numeric variables with normal distribution are expressed as a mean ± standard deviation and those with free distribution are expressed as medians with interquartile range (IQR). The distribution of the data was determined by the Kolmogorov–Smirnov's test. Nominal variables are expressed as a percentage. Quantitative variables were compared using the Student's t-test or Mann–Whitney's U-test, as appropriate. Nominal variables were compared using the Chi-square test or the Fisher's exact test. The discriminative capacity of serum estradiol level for predicting mortality was evaluated by the area under the receiver-operating curve (ROC) curve. The identification of serum estradiol level cutoff associated with mortality was performed by ROC curve. The serum estradiol level with the highest sensitivity and specificity for discriminating mortality was identified using Youden's index. The positive predictive value and negative predictive value (NPV) were calculated using the appropriate serum estradiol level cutoff. The association between serum estradiol level and mortality was assessed by multivariate logistic regression analysis. In all cases, P < 0.05 was considered statistically significant. The data analysis was performed using the Statistical Package for the Social Sciences version 20.0 for Windows (SPSS Statistics 20.0 for Windows, Armonk, NY, USA).
RESULTS
One hundred thirty-one patients were included, 75 (57.3%) were males and 56 (42.7%) were females. The mean age was 48.9 ± 17.2 years. There were 70 (53.4%) patients admitted by surgical conditions and 46 (35.1%) patients were septic. At least one comorbidity was present in 108 (82.4%) patients, of which the most frequent was hypertension. The overall ICU mortality rate was 22.9% (n = 30).
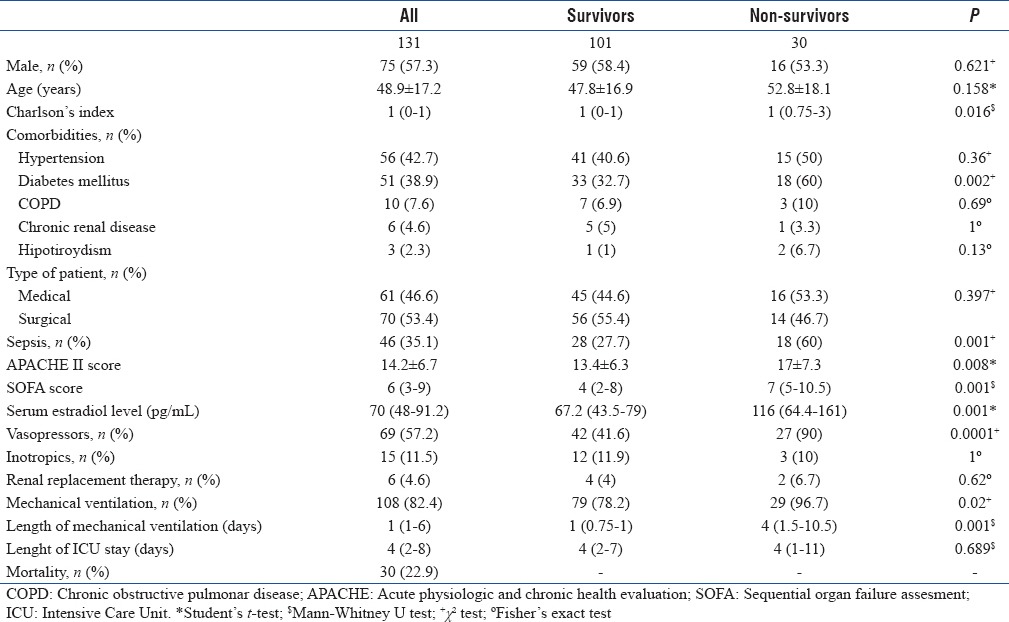
Patients were divided for comparison per the clinical condition at ICU discharge. In nonsurvivors, a higher proportion of subjects had diabetes mellitus (P = 0.002) and sepsis (P = 0.001), higher Charlson's index (P = 0.01), and higher APACHE II (P = 0.02) and SOFA (P = 0.001) scores at ICU admission. In this group, the use of vasopressors (P < 0.0001) and mechanical ventilation (P = 0.02) were more frequent, and the length of mechanical ventilation was higher (P = 0.001). Other results are shown in Table 1.
Table 1.
Demographic and clinical characteristics of studied population
In the survivors, the serum estradiol level had a median of 67.2 (IQR: 43.45–79) pg/mL, whereas in nonsurvivors was 116 (IQR 64.4–161) pg/mL. This difference was statistically significant (P < 0.0001). There was no significant difference in mortality according to gender (21.3% in man vs. 25% in women, P = 0.62). In addition, we found a higher serum estradiol level in septic patients (median 82.6 [IQR 69.23–145.25] pg/mL), compared to nonseptic patients (median 58.1 [IQR 43.1–77.4] pg/mL), this difference was statistically significant (P < 0.0001). Other variables such as gender, age of women (older or younger than 45 years), and type of patient (medical or surgical) were not statistically different between groups.
Discriminative capacity for predicting mortality of serum estradiol level is shown in Figure 1. The area under the ROC curve was 0.74 (95% confidence interval [CI] 0.63–0.86) and P < 0.0001. The best serum estradiol level cutoff for predicting mortality was 97.9 pg/mL. It had sensitivity of 60%, specificity of 90%, positive predictive value of 64%, NPV of 88%, positive likelihood ratio of 6, and negative likelihood ratio of 0.44.
Figure 1.
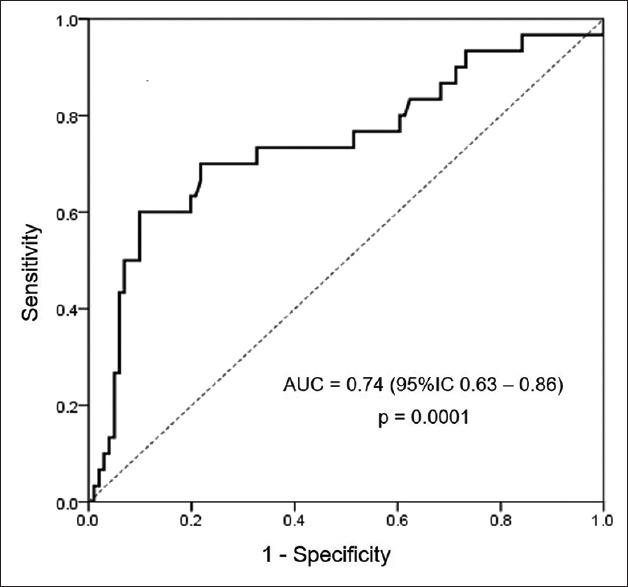
Receiver-operating curve for predicting mortality of serum estradiol level in critically ill patients
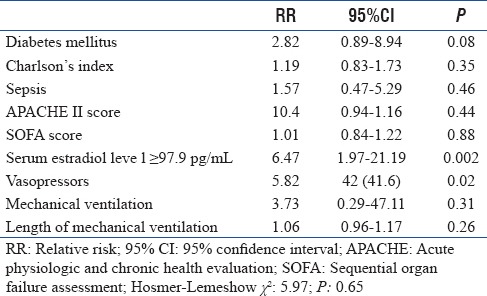
In the multivariate logistic regression analysis, serum estradiol level ≥97.9 pg/mL (relative risk [RR]: 6.47, 95% CI: 1.97–21.19, P = 0.002) and use of vasopressors (RR: 5.82, 95% CI: 1.38–24.63, P = 0.02) were identified as independent risk factors for death in our patients [Table 2].
Table 2.
Multivariate logistic regression analysis to indentify independent risk factors for death in the studied population
DISCUSSION
This study evaluated the capacity of serum estradiol level (measured at ICU admission) to predict mortality in a heterogeneous cohort of critically ill patients. We found that the serum estradiol level at ICU admission was higher in patients who die and that had no significant differences between genders. We also found that serum estradiol level is independently associated with ICU mortality, that it has good capacity to identify patients at increased risk for death, and that the cutoff of 97.9 pg/mL had the best sensitivity and specificity to predict mortality.
May et al.[10] found that serum estradiol level >100 pg/mL in surgical patients with trauma was associated with increased mortality. Dossett et al.[9] reported that a serum estradiol level >50 pg/mL had a good discriminative capacity to predict mortality in patients with trauma. Kauffmann et al.[3] found that serum estradiol level >67 pg/mL in surgical patients with trauma had good discriminative capacity to predict mortality, with area under the ROC curve of 0.64 (95% CI 0.59–0.68), and that it was associated with mortality, with RR 1.3 (95% CI 1.1–1.5). In addition, Kauffmann et al.[3] did serial measurements of serum estradiol level and found that, those patients who haved changes from baseline, have an increased risk for death. Finally, Lu et al.[5] found in patients with acute pancreatitis that nonsurvivors had a significantly higher serum estradiol level than survivors (206 vs. 39 pg/mL, respectively).
The results of our study coincide with others previously published by other researchers. However, those studies were performed in patients with specific conditions. In our study, we included a heterogeneous population with medical and surgical conditions. In addition, in our population, septic patients had a significantly higher serum estradiol level than nonseptic patients.
Some authors[1,3,13] have described an impact of gender on the outcomes in critically ill patients, placing the female gender as a risk factor for increased mortality and other complications. However, we do not find differences in mortality between genders, as reported in various studies.[1,9,10]
The strengths of this study include its prospective nature, its conduction in a heterogeneous population, and its confirmation that the increase in the serum estradiol level at ICU admission is associated with increased risk for death, regardless of the gender of the patients, the diagnosis, and the severity of the illness.
The study has some limitations: it was performed in a single center, the sample size is limited, we measured the serum estradiol level only at UCI admission, and we do not follow survivors after ICU discharge.
CONCLUSIONS
The serum estradiol level is increased in critically ill patients, regardless of gender, especially in those who die. It has good discriminative capacity to predict mortality and it is an independent risk factor for death in this group of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dossett LA, Swenson BR, Evans HL, Bonatti H, Sawyer RG, May AK, et al. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg Infect (Larchmt) 2008;9:41–8. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, et al. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit Care. 2013;17:R50. doi: 10.1186/cc12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauffmann RM, Norris PR, Jenkins JM, Dupont WD, Torres RE, Blume JD, et al. Trends in estradiol during critical illness are associated with mortality independent of admission estradiol. J Am Coll Surg. 2011;212:703–12. doi: 10.1016/j.jamcollsurg.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romo H, Amaral AC, Vincent JL. Effect of patient sex on Intensive Care Unit survival. Arch Intern Med. 2004;164:61–5. doi: 10.1001/archinte.164.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Lu CW, Liu LC, Hsieh YC, Yang LH, Chen RJ, Hsieh CH, et al. Increased admission serum estradiol level is correlated with high mortality in patients with severe acute pancreatitis. J Gastroenterol. 2013;48:374–81. doi: 10.1007/s00535-012-0636-6. [DOI] [PubMed] [Google Scholar]
- 6.Feng JY, Liu KT, Abraham E, Chen CY, Tsai PY, Chen YC, et al. Serum estradiol levels predict survival and acute kidney injury in patients with septic shock – A prospective study. PLoS One. 2014;9:e97967. doi: 10.1371/journal.pone.0097967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expressionin human monocyte-derived dendritic cells. Blood. 2004;104:1404–10. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 8.Dossett LA, Swenson BR, Heffernan D, Bonatti H, Metzger R, Sawyer RG, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008;64:580–5. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May AK, Dossett LA, Norris PR, Hansen EN, Dorsett RC, Popovsky KA, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36:62–8. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Luyt CE, Trouillet JL, Nieszkowska A, Chastre J. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. 2009;37:2506–11. doi: 10.1097/CCM.0b013e3181a569df. [DOI] [PubMed] [Google Scholar]
- 13.Heffernan DS, Dossett LA, Lightfoot MA, Fremont RD, Ware LB, Sawyer RG, et al. Gender and acute respiratory distress syndrome in critically injured adults: A prospective study. J Trauma. 2011;71:878–83. doi: 10.1097/TA.0b013e31822c0d31. [DOI] [PMC free article] [PubMed] [Google Scholar]


