Abstract
Finding products with antiapoptotic activities has been one of the approaches for the treatment of neurodegenerative disorders. Serum/glucose deprivation (SGD) has been used as a model for the investigation of the molecular mechanisms of neuronal ischemia. Recent studies indicated that glucosamine (GlcN) and N-acetyl glucosamine (GlcNAc) have many pharmacological effects including antioxidant activities. The present study aimed to investigate the protective effects of GlcN and GlcNAc against SGD-induced PC12 cells injury. The PC12 cells were pretreated with GlcN and GlcNAc for 2 h, and then exposed to SGD for 6, 12 and 24 h. Cell viability was evaluated by MTT assay. The level of intracellular reactive oxygen species (ROS) was determined by flow cytometry using 2’,7’- dichlorofluorescin diacetate (DCFH-DA) as a probe. SGD condition caused a significant reduction in cell survival after 6, 12, and 24 h (P < 0.001). Pretreatment with GlcN and GlcNAc (0.6-20 mM) increased cell viability following SGD insult. A significant increase in cell apoptosis was observed in cells under SGD condition after 12 h (P < 0.001). Pretreatment with GlcN and GlcNAc (5-20 mM) decreased apoptosis following SGD condition after 12 h. SGD resulted in a significant increase in intracellular ROS production after 12 h. Pretreatment with both amino sugars at concentrations of 10 to 20 mM could reverse the ROS increment. Results indicated that GlcN and GlcNAc had a cytoprotective property against SGD-induced cell death via anti-apoptosis and antioxidant activities, suggesting that these aminosugers have the potential to be used as novel therapeutic agents for neurodegenerative disorders.
Keywords: Glucosamine, N-acetyl glucosamine, PC12, Serum/glucose deprivation, Apoptosis
INTRODUCTION
In spite of major progress in the prevention and treatment of cerebral ischemia, stroke still remains one of the most important leading causes of death in people under the age of 65 years (1). Since the fundamental pathophysiology of the stroke is the decrease of glucose, O2 and other nutrients toward neurons, serum/glucose deprivation (SGD) can be used as a suitable in vitro model to evaluate the stroke process (2). Designing the neuroprotective agents can be performed with an effective in vitro model such as SGD which could correctly describe the molecular mechanism of brain injury during cerebral ischemia (3,4). PC12 rat pheochromocytoma cell line has been widely used as a useful model to investigate the SGD condition and to evaluate the mechanistic pathways underlying neural injury (5,6). Glucosamine (GlcN) and its acetylated derivative, N-acetyl glucosamine (GlcNAc), are naturally occurring amino sugars and prominent precursors of glycoproteins, proteoglycans and glycosaminoglycans.
They have a broad spectrum of biochemical and pharmacological functions mainly due to their antioxidant and anti-inflammatory activities (7,8,9,10). GlcN and GlcNAc are commonly used as dietary supplements for the prevention and/or treatment of many diseases including osteoarthritis (OA) (11).
Scientific reports have well shown the antioxidant, strong chelating effect on ferrous ions, protection of macromolecules such as protein, lipid, and deoxyribose from oxidative damage induced by hydroxyl radicals and free radical scavenging activities of GlcN (12,13). It has also some other different pharmacological effects such as anti-inflammatory, immunomodulatory, anti-carcinogenic, cardio, chondro, liver and cytoprotective properties and also wound healing activity (14,15,16,17,18).
In addition, recently the neuroprotective effect of GlcN in rat brain ischemia/reperfusion injury and its beneficial effect on spatial learning and scopolamine-induced memory impairment has been suggested (8,19).
GlcNAc has also been found to possess several pharmacological activities such as joint damage prevention, chondroprotective activity, inflammatory bowel disease (IBD) treatment (20,21) and wound healing enhancement (22,23,24).
It can also inhibit superoxide release from human polymorphonuclear (PMN) leukocytes and acts as anti-inflammatory agent (25). Furthermore, GlcNAc can improve skin hydration, and also reduce melanin formation and the appearance of facial hyperpigmentation via up-regulation of epidermal turnover genes and antioxidant-related genes, and the downregulation of some melanosome transport involving genes (7,26).
In addition, recent findings have revealed that the GlcNAc acts as an antioxidant and anti-apoptosis agent in human neuronal cells under hydrogen peroxide-induced oxidative stress condition. The neuroprotective effects of GlcNAc occur through the multiple mechanisms including the inhibition of the intracellular ROS generation and suppression of H2O2-induced apoptotic features by inhibiting the activation of caspase-3, poly ADP-ribose polymerase (PARP), and p38 (27,28).
Although, several studies revealed that GlcN and GlcNAc have many valuable properties correlated with their antioxidant capacity, but there is little information with respect to the neural protection effects of these amino sugars. Therefore, in this study we investigated the protective effect of GlcN and GlcNAc against the SGD-induced PC12 cells injury.
MATERIALS AND METHODS
Cell line and reagents
PC12 cell line was purchased from Pasteur Institute (Tehran, Iran). PC12 is an adhesive and spiky cell line. This was used after 3 passages. 4, 5-dimethylthiazole-2-yl, 2, 5-diphenyl tetrazolium (MTT), 2’,7’-dichloro-fluorescin diacetate (DCFH-DA) and Dulbecco’s phosphate-buffered saline (PBS) were procured from Sigma (St Louis, MO, USA).
Glucose-high Dulbecco’s modified Eagle’s medium (DMEM), glucose free DMEM, penicillin/streptomycin, and fetal bovine serum (FBS) were supplied by Gibco (Grand Island, NY). Dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany). Propidium iodide (PI), sodium citrate and Triton X-100 were obtained from Sigma (St. Louis, MO, USA).
Cell culture
PC12 cells were cultured in high glucose DMEM (4.5 g/L) supplemented with 10% FBS and 100 U/mL of penicillin/streptomycin. All cells were maintained in a humidified atmosphere (90%) containing 5% CO2 at 37 °C.
Induction of cell death by serum/glucose deprivation
For SGD-induced cytotoxicity, PC12 cells were seeded overnight and then were exposed to SGD for 6, 12, and 24 h by replacing the standard culture medium (high glucose DMEM, 4.5 g/L) with the glucose-free DMEM (0 g/L), supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (29).
Cell proliferation MTT assay
The cell viability was determined using MTT assay as described previously (30). Briefly, PC12 cells (5000/well) were seeded in a 96-well culture plate. After 24 h, the cells were pretreated with GlcN and GlcNAc (0.6-20 mM) for 2 h and then incubated subsequently for another 6, 12 and 24 h in serum and glucose free (SGD) condition. MTT solution in phosphate-buffered saline (5 mg/mL) was added to each well at final concentration of 0.05%. After 3 h, the formazan precipitate was dissolved in DMSO. The absorbances at 570 and 620 nm (background) were measured using a Stat FAX303 plate reader. All treatments were carried out in triplicate.
Cell apoptosis assay
Apoptotic cells were detected using PI staining of small DNA fragments followed by flow cytometry. It has been described that a sub-G1 peak that is reflective of DNA fragmentation can be observed following the incubation of cells in a hypotonic phosphate-citrate buffer containing a quantitative DNA-binding dye such as PI. Apoptotic cells that have lost DNA take up less stain and appear on the left side of the G1 peak in the histogram (29).
PC12 cells were seeded in a 24-well plate and after 24 h, the cells were pretreated with GlcN and GlcNAc (5-20 mM) for 2 h and then incubated for another 12 h in SGD condition. Cells were then harvested and incubated at 4 °C overnight in the dark with 750 μL of a hypotonic buffer (50 μg/mL PI in 0.1% sodium citrate with 0.1% Triton X-100). Finally, flow cytometry was carried out using a FACScan flow cytometer (Becton Dickinson). A total of 10000 events were acquired with FACS. All treatments were carried out in triplicate.
Measurement of intracellular reactive oxygen species
The intracellular reactive oxygen species (ROS) was monitored by measurement of hydrogen peroxide generation (29). PC12 cells were seeded in 24-well plates (100,000 cells/well) and incubated overnight.
Then, cells were pretreated with GlcN and GlcNAC (5-20 mM) for 2 h, afterwards they were exposed to SGD condition. After 12 h, cells were incubated with DCFH-DA (10 μM) for 30 min at 37 °C.
The medium was then transferred to a Falcon tube and the adherent cells were trypsinized and collected into the same tube. After washing twice with PBS, the intensity of DCFH-DA fluorescence and the oxidation product of DCFH-DA were determined by a FACScan flow cytometer (Becton Dickinson) at an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
Statistical analysis
One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test for multiple comparisons were used for data analysis. All results were expressed as mean ± SEM. P < 0.05 was considered statistically significant.
RESULTS
Effects of glucosamine and N-acetyl glucosamine on PC12 cell death
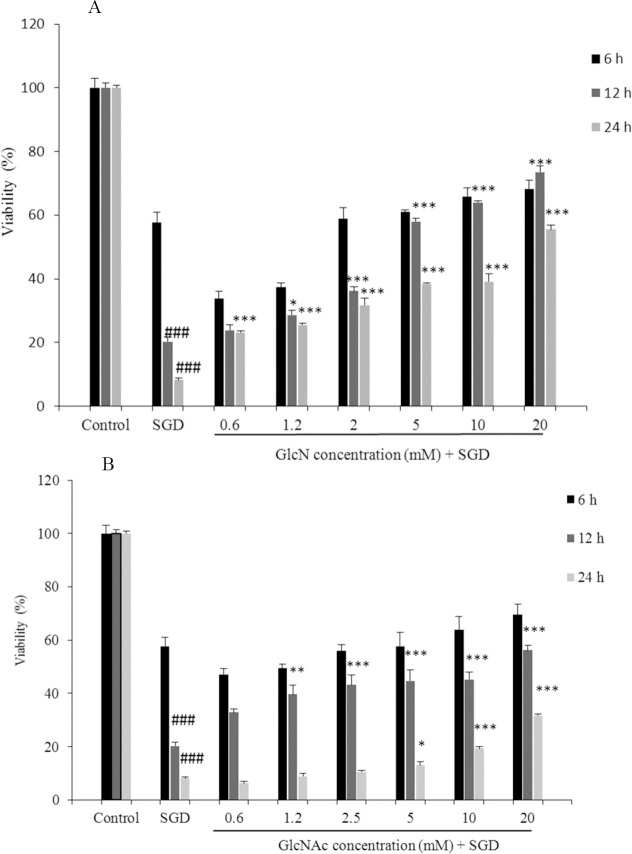
Exposure to SGD for 6, 12 and 24 h caused a significant reduction in cell viability, as compared with control group (P < 0.001). As shown in Fig. 1A, pretreatment with GlcN resulted in a time- and concentration-dependent increase in cell viability subsequent to ischemic insult after 12 and 24 h (P < 0.05 at concentration of 1.2 mM, P < 0.001 at concentrations of 2.5-20 mM after 12 h and P < 0.001 at concentrations of 0.6-20 mM after 24 h).
Fig. 1.
Effect of (A) Glucosamine and (B) N-acetyl glucosamine on PC12 cells viability exposed to serum/glucose deprivation for 6, 12 h and 24 h. The percentage cell viability (quantitated by MTT assay) was normalized against the control. ###P < 0.001 vs control. ***P < 0.001, **P < 0.01, *P < 0.05 vs serum/glucose deprived groups. Data are expressed as mean ± SEM of three separate experiments (n = 3). GlcN, glucosamine; GlcNAc, N-acetyl glucosamine; SGD, serum/glucose deprivation.
Pretreatment with GlcNAc also significantly and concentration-dependently decreased SGD-induced cell death following 12 and 24 h incubation (P < 0.01 at concentration of 1.2 mM, P < 0.001 at concentrations of 2.5-20 mM after 12 h and P < 0.05 at concentration of 5 mM and P < 0.001 at concentrations of 10-20 mM after 24 h), (Fig. 1B).
There were no significant toxic effects when PC12 cells were incubated with GlcN and GlcNAc at concentrations of 0.6 to 20 mM (Data are not shown).
Effects of glucosamine and N-acetyl glucosamine on cell apoptosis
The results showed that exposure of PC12 cells to SGD condition, significantly increased cell apoptosis compared with control cells (P < 0.001).
A significant decrease in SGD-induced apoptosis was observed following pretreatment with high concentrations (10-20 mM) of GlcN and GlcNAc (P < 0.001 at concentrations of 5-20 mM of GlcN and GlcNAc). Results of apoptosis assay are illustrated in Figs 2A-C.
Fig. 2.
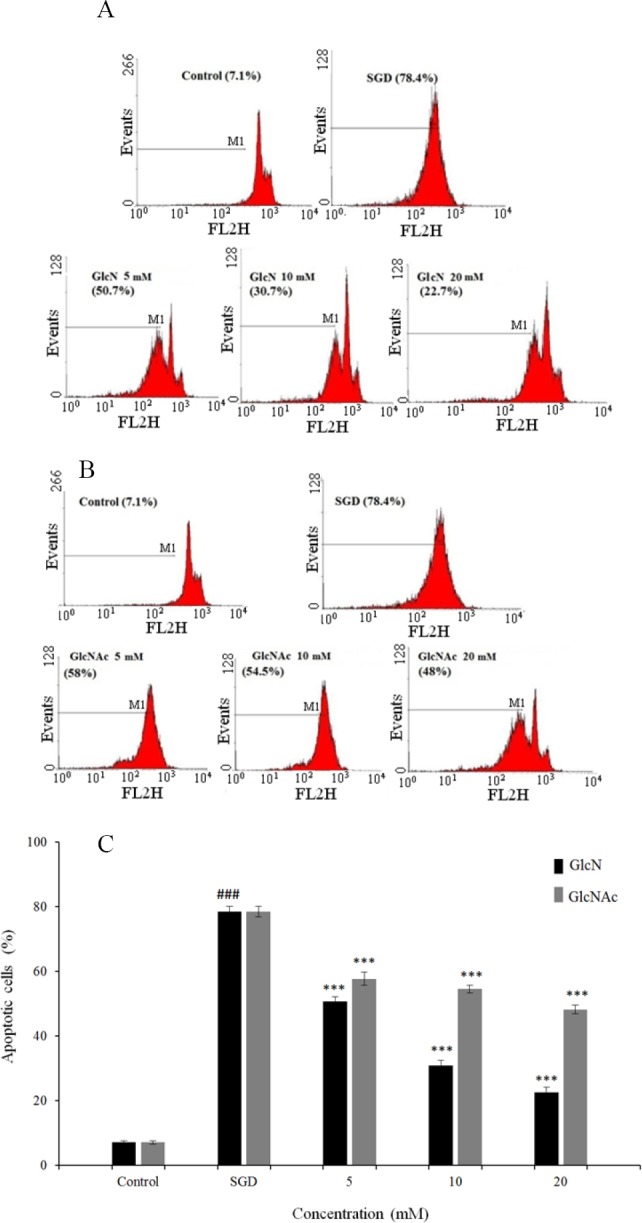
Flow cytometry histograms of propidium iodide-stained PC12 cells pretreated with (A) Glucosamine and (B) N-acetyl glucosamine for 2 h and then exposed to serum/glucose deprivation for 12 h. Sub-G1 peak as an indicative of apoptotic cells was induced in treated cells but not in the control. (C) The effects of glucosamine and N-acetyl glucosamine on apoptosis in PC12 cells using propidium iodide staining and flow cytometry. ###P < 0.001 vs control, ***P < 0.001 vs serum/glucose deprived groups. GlcN, glucosamine; GlcNAc, N-acetyl glucosamine; SGD, serum/glucose deprivation.
Effects of glucosamine and N-acetyl glucosamine on reactive oxygen species
To determine the antioxidant effects of GlcN and GlcNAc, levels of intracellular ROS were measured using DCFH-DA fluorescence staining assay in PC12 cells. ROS production was measured following the exposure of cells to stressful conditions (SGD) for 12 h with or without the pretreatment with GlcN and GlcNAc.
As shown in Figs 3A-C, SGD for 12 h could significantly increase the number of DCFH-positive cells illustrating an elevation of ROS production compared to the control (P < 0.001). Pretreatment with GlcN and GlcNAc (10-20 mM) resulted in a significant attenuation of ROS production subsequent to SGD (P < 0.001).
Fig. 3.
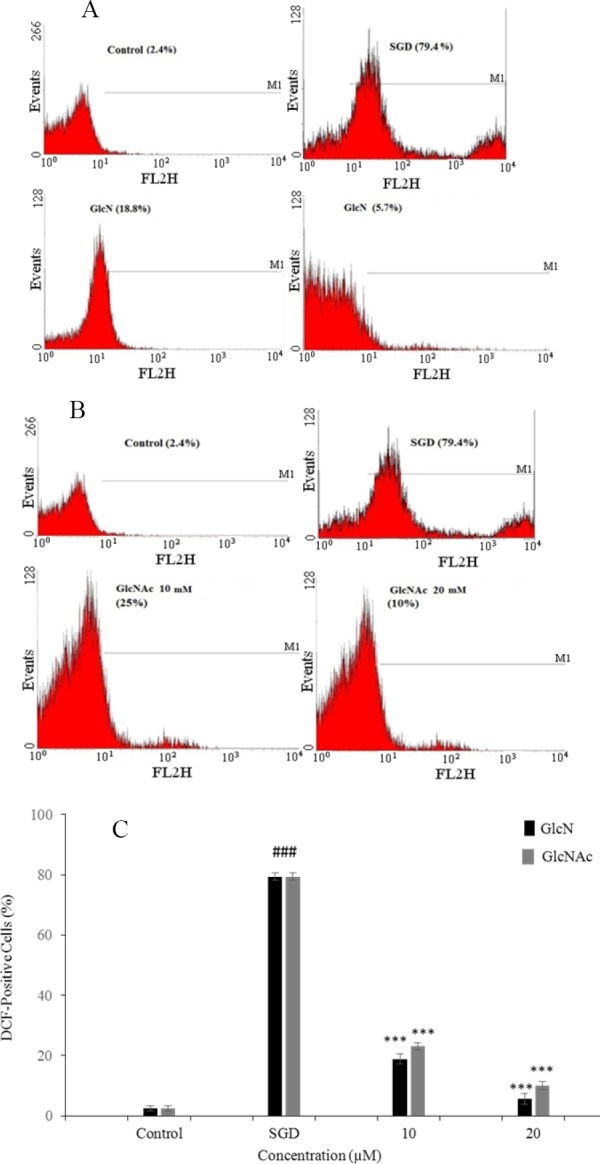
Flow cytometry histograms of reactive oxygen species production assay in PC12 cells pretreated with (A) glucosamine and (B) N-acetyl glucosamine for 2 h and then exposed to serum/glucose deprivation for 12 h. Reactive oxygen species was measured using 2’,7’-dichlorofluorescin diacetate by flow cytometric analysis. (C) The effects of glucosamine and N-acetyl glucosamine on intracellular reactive oxygen species production in PC12 cells using 2’,7’-dichlorofluorescin diacetate and flow cytometry. ###P < 0.001 vs control, ***P < 0.001 vs SGD. GlcN, glucosamine; GlcNAc, N-acetyl glucosamine; SGD, serum/glucose deprivation.
DISCUSSION
Ischemic stroke is the third leading cause of mortality and disability in industrialized countries. Currently, therapeutic choices for the treatment of stroke are limited. Therefore, extensive efforts are being made to identify new neuroprotective agents with anti-apoptotic activities (31). PC12 rat pheochromocytoma cell line has been generally utilized as an in vitro model to study the SGD condition and other alterations in neural tissue (32). Cell apoptosis and oxidative stress are the characteristic features of many neurodegenerative diseases such as ischemic stroke (33).
Antioxidants are beneficial for ischemic brain injury treatment due to the reduction of oxidative damage and scavenging free radicals (34). In the present study, the protective effects of GlcN and GlcNAc against SGD-induced cell death were investigated in PC12 cells for the first time. Results showed that these amino sugars have no cytotoxicity on PC12 cells at used concentrations (up to 20 mM). Moreover, we observed that about 50% of cell loss was taking place under SGD condition after 12 and 24 h, which is in agreement with findings of previous studies (29,35). Furthermore, data showed that pretreatment with GlcN and GlcNAc significantly increased cell survival and decreased cell apoptosis under SGD condition in a concentration-dependent fashion.
In the current study, pretreatment with GlcN and GlcNAc significantly decreased SGD-induced ROS production. These data confirmed the inhibitory effect of GlcN and GlcNAc on intracellular ROS generation which showed that the neuroprotective activity of these aminosugers may be mediated through their antioxidant activities.
Recent studies have reported that GlcN and GlcNAc have several pharmacological properties including antiapoptotic, antioxidant and cytoprotective effects. Yan, et al. investigated antioxidative and immunostimulating properties of GlcN in vitro and in vivo. They reported that GlcN has strong antioxidant activities as manifested by excellent chelating effect on ferrous ions and protection of macromolecules including protein, lipid, and deoxyribose from oxidative damage induced by hydroxyl radicals (10).
A study conducted by Jamialahmadi, et al. also has shown the antioxidant and scavenging ability of GlcN hydrochloride. The results of the aforementioned study showed that GlcN can efficiently suppress lipid/protein peroxidation and protect erythrocytes against oxidative damage induced by free radicals. Thus, GlcN could be recommended as a pharmaceutical supplement to alleviate oxidative stress (36).
In another research, Jamialahmadi, et al. reported that GlcN at concentrations of 2.5-40 mM exhibited a potent antigenotoxic effect and can protect human lymphocyte against DNA damage induced by hydrogen peroxide. In contrast to GlcN, its N-acetylated analog only indicated a slight antigenotoxic effect at concentration of 40 mM. GlcN protection activity might be related to the presence of 2’-NH2 functional moiety in its chemical backbone (18).
In addition, recent findings have revealed that the GlcN at concentration of 5 mM protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and enhanced mitochondrial Bcl-2 translocation (17). Moreover, the neuroprotective effects of GlcN and GlcNAc have also been indicated in some studies. Hwang, et al. reported that GlcN exerts a neuroprotective effect through suppression of inflammation in rat brain ischemia/reperfusion injury (8). Recently, Choi, et al. also showed that the GlcNAc dose-dependently decreased ROS production and inhibited H2O2-induced apoptosis in human neuronal cells (27).
CONCLUSION
The results of this study confirmed that GlcN and GlcNac have protective effects against SGD-induced cytotoxicity in PC12 cells. These effects act possibly through their antioxidant and anti-apoptotic properties. However, more detailed studies are essential to elucidate the probable underlying mechanisms of these beneficial effects.
ACKNOWLEDGMENTS
This work was financially supported by a research grant No. 931050 from the Vice Chancellery of Research of Mashhad University of Medical Sciences, Mashhad, I.R. Iran.
REFERENCES
- 1.Amantea D, Marrone MC, Nistico R, Federici M, Bagetta G, Bernardi G, et al. Oxidative stress in stroke pathophysiology validation of hydrogen peroxide metabolism as a pharmacological target to afford neuroprotection. Int Rev Neurobiol. 2005;85:363–374. doi: 10.1016/S0074-7742(09)85025-3. [DOI] [PubMed] [Google Scholar]
- 2.Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis. 2000;5(2):99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- 3.Chu LF, Wang WT, Ghanta VK, Lin CH, Chiang YY, Hsueh CM. Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-kB signaling pathway. Brain Res. 2008;1239:24–35. doi: 10.1016/j.brainres.2008.08.087. [DOI] [PubMed] [Google Scholar]
- 4.Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25(2):154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res. 2001;64(6):654–660. doi: 10.1002/jnr.1119. [DOI] [PubMed] [Google Scholar]
- 6.Woronowicz A, Amith SR, Davis VW, Jayanth P, De Vusser K, Laroy W, et al. Trypanosome trans-sialidase mediates neuroprotection against oxidative stress, serum/glucose deprivation, and hypoxia-induced neurite retraction in Trk-expressing PC12 cells. Glycobiol. 2007;17(7):725–734. doi: 10.1093/glycob/cwm034. [DOI] [PubMed] [Google Scholar]
- 7.Bissett DL, Robinson LR, Raleigh PS, Miyamoto K, Hakozaki T, Li J, et al. Reduction in the appearance of facial hyperpigmentation by topical N-Acetyl glucosamine. J Cosmet Dermatol. 2007;6:20–26. doi: 10.1111/j.1473-2165.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 8.Hwang YP, Kim HG, Han EH, Choi JH, Park BH, Jung KH, et al. N-acetylglucosamine suppress collagenases activation in ultraviolet B-irradiated human dermal fibroblasts: Involvement of calcium ions and mitogen-activated protein kinases. J Dermatol Sci. 2011;63:93–103. doi: 10.1016/j.jdermsci.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Rudert WA, Harnaha J, Wright M, Machen J, Lakomy R, et al. Immunosuppressive effects of glucosamine. J Biol Chem. 2002;277(42):39343–39349. doi: 10.1074/jbc.M204924200. [DOI] [PubMed] [Google Scholar]
- 10.Yan Y, Wanshun L, Baoqin H, Changhong W, Chenwei F, Bing L, et al. The antioxidative and immunostimulating properties of D-glucosamine. Int immunopharmacol. 2007;7:29–35. doi: 10.1016/j.intimp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Salazar J, Bello L, Chavez M, Anez R, Rojas J, Bermudez V. Glucosamine for osteoarthritis: biological effects, clinical efficacy, and safety on glucose metabolism. Arthritis. 2014;2014:432463. doi: 10.1155/2014/432463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing R, Liu S, Guo Z, Yu H, Li C, Ji X, et al. The antioxidant activity of glucosamine hydrochloride in vitro. Bioorg Med Chem. 2006;14(6):1706–1709. doi: 10.1016/j.bmc.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Mendis E, Kim MM, Rajapakse N, Kim SK. Sulfated glucosamine inhibits oxidation of biomolecules in cells via a mechanism involving intracellular free radical scavenging. Eur J. Pharmacol. 2008;579(1-3):74–85. doi: 10.1016/j.ejphar.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309–315. doi: 10.1111/j.1473-2165.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 15.Forchhammer L, Thorn M, Met O, Gad M, Weidner M, Claesson MH. Immunobiological effects of glucosamine in vitro. Scand J Immunol. 2003;58(4):404–411. doi: 10.1046/j.1365-3083.2003.01313.x. [DOI] [PubMed] [Google Scholar]
- 16.Chesnokov V, Gong B, Sun C, Itakura K. Anticancer activity of glucosamine through inhibition of N-linked glycosylation. Cancer Cell Int. 2014;14:45. doi: 10.1186/1475-2867-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008;294(6):C1509–C20. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamialahmadi K, Soltani F, Nabavi Fard M, Behravan J, Mosaffa F. Assessment of protective effects of glucosamine and N-acetyl glucosamine against DNA damage induced by hydrogen peroxide in human lymphocytes. Drug Chem Toxicol. 2014;37(4):427–432. doi: 10.3109/01480545.2013.878951. [DOI] [PubMed] [Google Scholar]
- 19.Jamialahmadi K, Sadeghnia HR, Mohammadi G, Kazemabad AM, Hosseini M. Glucosamine alleviates scopolamine induced spatial learning and memory deficits in rats. Pathophysiology. 2013;20(4):263–267. doi: 10.1016/j.pathophys.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Salvatore S, Heuschkel R, Tomlin S, Davies S, Edwards S, Walker-Smith JA, et al. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14(12):1567–1579. doi: 10.1046/j.1365-2036.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- 21.Selvan T, Rajiah K, Nainar MS, Mathew EM. A clinical study on glucosamine sulfate versus combination of glucosamine sulfate and NSAIDs in mild to moderate knee osteoarthritis. Sci World J. 2012;2012:902676. doi: 10.1100/2012/902676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JK, Shen CR, Liu CL. N-acetylglucosamine: production and applications. Mar Drugs. 2010;8:2493–251. doi: 10.3390/md8092493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shikhman AR, Amiel D, D’Lima D, Hwang SB, Hu C, Xu A. Chondroprotective activity of N -acetylglucosamine in rabbits with experimental osteoarthritis. Ann Rheum Dis. 2005;64:89–94. doi: 10.1136/ard.2003.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayo T, Sakai S, Inoue S. Synergestic effect of N-acetylglucosamine and retinoids on hyaluronan production in human keratinocytes. Skin Pharmacol Physiol. 2004;17:77–83. doi: 10.1159/000076017. [DOI] [PubMed] [Google Scholar]
- 25.Kamel M, Alnahdi M. Inhibition of superoxide anion release from human polymorphonuclear leukocytes by N-acetylgalactosamine and N-acetylglucosamine. Clin Rheumatol. 1992;11:254–260. doi: 10.1007/BF02207968. [DOI] [PubMed] [Google Scholar]
- 26.Malaekeh-Nikouei B, Golmohammadzadeh S, Salmani-Chamanabad S, Mosallaei N, Jamialahmadi K. Preparation, characterization, and moisturizing effect of liposomes containing glucosamine and N-acetyl glucosamine. J Cosmet Dermatol. 2013;12(2):96–102. doi: 10.1111/jocd.12034. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Chung M, Kweon Park J, Park Y. Neuroprotective effects of N-acetylglucosamine against hydrogen peroxide-induced apoptosis in human neuronal SK-N-SH cells by inhibiting the activation of caspase-3, PARP, and p38. Food Sci Biotechnol. 2013;22(3):853–858. [Google Scholar]
- 28.Azam MS, Kim EJ, Yang HS, Kim JK. High antioxidant and DNA protection activities of N-acetylglucosamine (GlcNAc) and chitobiose produced by exolytic chitinase from Bacillus cereus EW5. Springerplus. 2014;3:354. doi: 10.1186/2193-1801-3-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC 12 cells death. Cell Mol Neurobiol. 2010;30(4):591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarvmeili N, Jafarian-Dehkordi A, Zolfaghari B. Cytotoxic effects of Pinus eldarica essential oil and extracts on HeLa and MCF-7 cell lines. Res Pharm Sci. 2016;11(6):476–483. doi: 10.4103/1735-5362.194887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 32.Arabsolghar R, Saberzadeh J, Khodaei F, Abbasi Borojeni R, Khorsand M, Rashedinia M. The protective effect of sodium benzoate on aluminum toxicity in PC12 cell line. Res Pharm Sci. 2017;12(5):391–400. doi: 10.4103/1735-5362.213984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forouzanfar F, Afkhami Goli A, Asadpour E, Ghorbani A, Sadeghnia HR. Protective Effect of Punica granatum L. against serum/glucose deprivation-induced PC 12 cells injury. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/716730. 716730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakhtiari E, Hosseini A, Mousavi SH. Protective effect of Hibiscus sabdariffa against serum/glucose deprivation-induced PC12 cells injury. Avicenna J Phytomed. 2015;5(3):231–237. [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenz L, Dang J, Misiak M, Tameh Abolfazl A, Beyer C, Kipp M. Combined 17beta-oestradiol and progesterone treatment prevents neuronal cell injury in cortical but not midbrain neurons or neuroblastoma cells. J Neuroendocrinol. 2009;21(10):841–849. doi: 10.1111/j.1365-2826.2009.01903.x. [DOI] [PubMed] [Google Scholar]
- 36.Jamialahmadi K, Arasteh O, Riahi MM, Mehri S, Riahi-Zanjani B, Karimi G. Protective Effects of glucosamine hydrochloride against free radical-induced erythrocytes damage. Environ Toxicol Pharmacol. 2014;38:212–219. doi: 10.1016/j.etap.2014.05.018. [DOI] [PubMed] [Google Scholar]