Abstract
Cadmium (Cd), a ubiquitous environmental and occupational pollutant, acts as a metalloestrogen to induce cell proliferation. It is suggested that Cd may also contribute to the development of estrogen-related cancers like ovarian cancer which is the most lethal cancer in women. Furthermore, it was shown that melatonin has antiproliferative effect on estradiol (E2)-induced proliferation. The aim of the present study was to evaluate whether melatonin inhibits Cd-induced proliferation in ovarian cancer cell lines and also whether Cd and melatonin can modulate estrogen receptor α (ERα) expression. OVCAR3 and SKOV3 human ovarian cancer cell lines were treated with CdCl2 (1-100 nM) and melatonin (1 μM) for 48 h. Cell proliferation evaluation was carried out by bromodeoxyuridine (BrdU) incorporation assay. ERα expression was detected by western blotting method 24 h after cell treatment. The results were demonstrated that Cd increased proliferation of ovarian cancer cell lines in a dose dependent manner. Melatonin inhibited Cd-induced proliferation of OVCAR3 and SKOV3 cell lines. Moreover, CdCl2 significantly increased ERα expression in both OVCAR3 and SKOV3 cell lines compared to control. Melatonin significantly inhibited Cd inducing effect on ERα expression of OVCAR3 and SKOV3 cell. In conclusion, due to the proliferative effect on ovarian cancer cell lines, Cd could play an important role in the etiology of ovarian cancer by inducing cells ERα expression. Furthermore, melatonin has the protective role on Cd-induced cell proliferation by inhibition of ERα expression.
Keywords: Cadmium, Ovarian cancer, Melatonin, Estrogen receptor α
INTRODUCTION
Cadmium (Cd) as a ubiquitous environmental and occupational pollutant can affect human health through chronic to low-level exposure (1). Cd has been categorized as a known human carcinogen by several regulatory agencies (2). Lacking an active biochemical mechanism for elimination coupled with renal reabsorption, Cd is excreted from the body very slowly and accumulates in soft tissues for many years (1). Cd acts with a potent estrogen activity to induce breast cancer cell line proliferation, probably by forming a high-affinity complex with the hormone binding domain of the estrogen receptors (3). This metal ion interfering with estrogenic activity was defined as xenoestrogen and termed metalloestrogen. Since estrogen has some important roles in the development and progression of tissues and cells, it is suggested that metalloestrogens may also contribute to the development of estrogen-related cancers (4).
It is hypothesized that metal-induced estrogen receptor activation is a crucial step in the carcinogenic process of these cancers (5). One of the most lethal estrogen-related cancers is ovarian cancer (6). Among gynecologic cancers, the mortality of ovarian cancer is in the first place with poor prognosis and 5-year survival rate around 30% because there are no obvious early clinical symptoms and effective diagnostic approach (7). Epidemiological studies have found evidences of increased ovarian cancer risk and mortality among Cd-exposed people but its carcinogenesis mechanism in ovary is not understood (8,9).
Moreover, recent studies have demonstrated that melatonin, the main secretory product of the pineal gland and an indole hormone secreted only in darkness, has useful pharmacological and physiological properties against ovarian and breast cancer progression and metastasis (10,11).
Clinical studies have reported negative correlation between aggressiveness of ovarian cancer and serum level of melatonin (10). In normal cells, melatonin has potent antioxidant action and in many cancer types including hormone-dependent cancers, it has antiproliferative and antiangiogenic properties (10,12,13).
Studies have shown that physiological concentrations of melatonin (1 nM to 1 pM) has antiproliferative effect on estradiol (E2)-induced proliferation and invasion of breast cancer cell lines by inhibition of estrogen mediated mechanisms (11,14). While anti-estrogenic effect of melatonin at the cellular level has been proposed, the molecular basis of melatonin action remains largely unknown (15). Additionally, it has been recently reported that melatonin not only can inhibit E2-induced estrogen receptor α (ERα)-mediated transcription but also it can prevent the Cd-induced growth of breast cancer cells (11).
The aim of the present study was to evaluate whether melatonin inhibits Cd-induced proliferation in ovarian cancer cell lines and also whether Cd and melatonin can modulate ERα expression.
MATERIALS AND METHODS
Chemicals
Culture media and growth supplements were obtained from Gibco (Germany). CdCl2 and melatonin were purchased from Sigma–Aldrich Company (Munich, Germany). Bromodeoxyuridine (BrdU) kit was purchased from Roche (Mannheim, Germany). Human antibody of glyceraldehyde-3 phosphate dehydrogenase (GAPDH) (G-9:sc-365062), mouse monoclonal IgG1 estrogen receptor α (sc-73479) and gout anti mouse IgG1-HRP (sc-2005) were purchased from Santa Cruz (USA).
Cell cultures
Two human ovarian cancer cell lines, OVCAR3 (NCBI code: C430) and SKOV3 (NCBI code: C209), were obtained from National Cell Bank of Iran (Pasture Institute, Iran). The cells were cultured in RPMI-1640 supplemented with 100 units/mL penicillin G, 100 μg/mL streptomycin and 10% fetal bovine serum (FBS, Gibco Co.) in a humidified atmosphere of 5% CO2, 95% air at 37 °C. CdCl2 and melatonin treatment were performed in medium supplemented with 5% charcoal treated 1% FBS. The stock solution was dissolved in deionized water and then sterilized by filtration and diluted by RPMI medium. The cells divided into four groups consisting of untreated cells, CdCl2-treated cells, melatonin-treated cells and CdCl2+melatonin-treated cells. Untreated cells were used as a negative control to CdCl2-treated cell and melatonin treated cell groups. CdCl2-treated cells also were used as the negative control to CdCl2+ melatonin-treated cell group.
Bromodeoxyuridine cell proliferation assay
The evaluation of cell proliferation was carried out by a BrdU kit according to the manufacturer’s protocol.
In BrdU cell proliferation assay, BrdU, a pyrimidine analog, is incorporated into replicating DNA instead of thymidine and then immunodetection of BrdU using specific monoclonal antibodies allows labeling of the cells in S phase of the cell cycle. Thus, cells were cultured with 5 × 103 cells/well density in 96-well plates and co-treated with CdCl2 (1-100 nM) and melatonin (1 μM) for 48 h. 10 μL of BrdU labeling solution was added to each well and the cells were incubated again for 4 h. After removal of the BrdU labeling solution, it was used kit’s FixDenta solution for 30 min at room temperature to fix and denaturation of cells. Then the cells should be incubated for 90 min by peroxidase-conjugated anti-BrdU antibody. After washing off the unbound anti-BrdU-POD, to develop the color reaction, substrate solution was performed for 3-5 min. Finally, it was stopped by adding 25 μL of sulfuric acid (1 M) and optical density of the samples was determined at 370 nm.
Western blot analysis
ERα expression was detected by western blotting method. 1 × 106 cells were incubated with CdCl2 (1-100 nM) for 24 h. Then the cells were vortexed with an ice-cold RIPA lysis buffer every 15 min for 2 h. The extracts were centrifuged at 14,000 rpm and 4 °C for 10 min. After determination of protein concentration of each lysate by Bradford protein assay (0.5-1 mg/mL), equal amount of protein (20 μg) of each sample was subjected to 10% sodium dodecyl sulphate-polyacrylamide (SDS) page gel electrophoresis and transferred on polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia Biotech, UK). Membranes were incubated with blocking buffer (5% non-fat dry milk in phosphate buffered saline (PBS) + 0.1% Tween 20) for 2 h at room temperature. Membranes were incubated with primary antibody containing mouse monoclonal antibody against ERα overnight at 4 °C. After washing three times with PBS with Tween 20 (PBST), membranes were incubated with secondary antibody, gout anti mouse IgG1-HRP conjugate, for 2 h at room temperature. Finally, the protein expression was detected by Bio‑Rad ECL detection reagent. GAPDH was used as an internal reference.
Statistical analysis
Data were reported as means ± SD. Statistical analysis was performed using SPSS18.0 for nonparametric test of variance between groups (ANOVA) followed by Dunnett’s test and t-test. Statistically significant difference was considered as P < 0.05.
RESULTS
Effect of CdCl2 on ovarian cancer cell proliferation
To investigate Cd proliferative effect on ovarian cancer cell lines, OVCAR3 and SKOV3 cells were exposed to different concentrations of CdCl2 (1-100 nM) for 48 h. Cell proliferation was determined by BrdU incorporation assay. Before BrdU assay, MTT assay with different concentrations of CdCl2 (1 nM -100 μM) and melatonin (1 - 100 μM) was performed for 24, 48 and 72 h to select appropriate concentrations and treatment time. It was observed that (the results are not shown) CdCl2 exhibited proliferative effect at 1-100 nM while higher concentrations were cytotoxic. Melatonin at 1 μM showed inhibitory effect on Cd-induced proliferation. The best treatment time was found to be 48 h. Significant differences between viability of treated cells versus control group were not observed at 24 and 72 h treatment. Thus we selected 1-100 nM CdCl2, 1 μM melatonin and treatment time 48 h to continue other experiments.
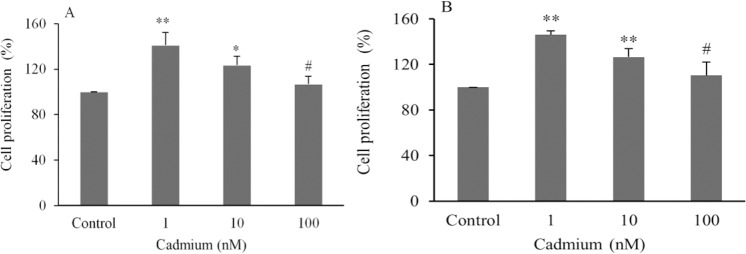
The results of BrdU assay showed that CdCl2 significantly stimulated cell proliferation in a dose dependent manner. Maximum prolifeartion was observed at lowest concentration of CdCl2 (1 nM). Proliferation was increased 7-41% in OVCAR3 (Fig. 1A) and 10-46% in SKOV3 cells (Fig. 1B). There was no statistically significant difference between 100 nM CdCl2 and control. Additionally, a significant difference was observed between highest proliferation in CdCl2 (1 nM) and lowest proliferation in 100 nM CdCl2 ; P < 0.05 (Fig. 1).
Fig. 1.
Assesment of ovarian cancer cell line proliferation in (A), OVCAR3 and (B), SKOV3 cell lines. Data are presented as mean ± SD. * and ** indicate significant difference from the control (P < 0.05 and P < 0.01, respectively); # shows significant difference with Cd (1 nM) (P < 0.05).
Effect of melatonin on Cd-induced proliferation of ovarian cancer cell lines
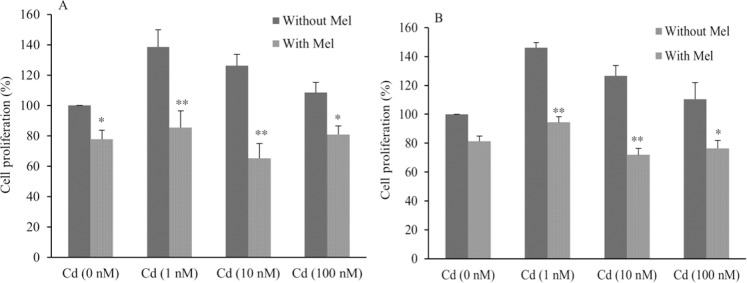
To evaluate whether melatonin can inhibit the proliferation of ovarian cancer cells induced by Cd, the cells were treated with CdCl2 (1-100 nM) in the presence or absence of melatonin for 48 h and cell proliferation was evaluated by BrdU assay. Melatonin significantly inhibited the CdCl2-induced cell proliferation compared to CdCl2-treated cells in the absence of melatonin Cell proliferation inhibition was calculated to be 38.4% at 1 nM, 48% at 10 nM, and 25.5% at 100 nM of CdCl2 in OVCAR3 cells (Fig. 2A). It was also observed that melatonin inhibited cell proliferation of SKOV3 cells as much as 35.6% at 1 nM 43% for 10 nM and 31% at 100 nM of CdCl2 (Fig. 2B). Minimum inhibitory effect of melatonin was observed in 100 nM of CdCl2 that caused the lowest proliferative effect.
Fig. 2.
The effect of melatonin on ovarian cancer cell proliferation in (A), OVCAR3 and (B), SKOV3 cell lines. * and ** show significant differences from corresponding treated cells in the absence of melatonin (P < 0.05 and P < 0.01, respectively). (Mel), melatonin; (Cd), CdCl2.
Effect of melatonin on Cd-induced ERα expression in ovarian cancer cell lines
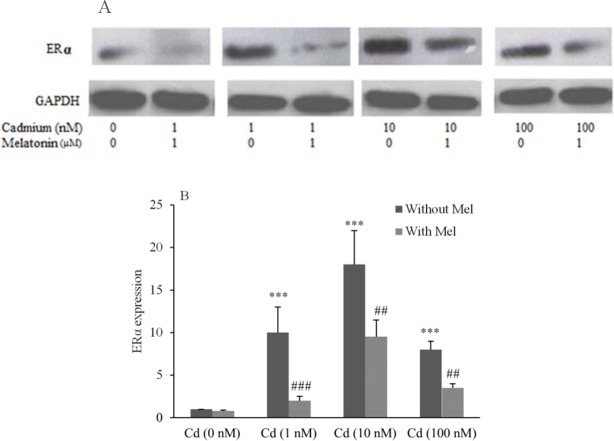
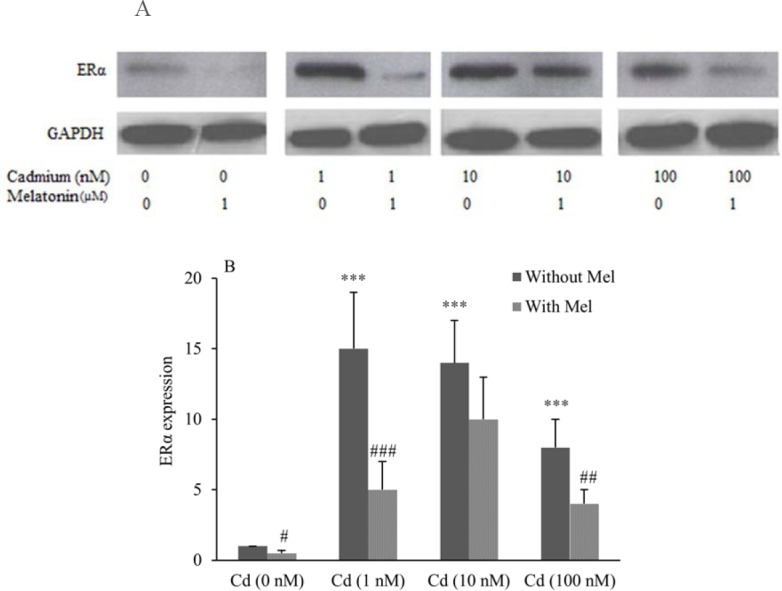
To determine whether Cd can modulate ERα expression, cell lines were incubated for 24 h with 1-100 nM CdCl2. ERα expression was measured using western blot analysis by ERα monoclonal antibody. Data analysis demonstrated that Cd significantly increased ERα expression in both OVCAR3 (Figs. 3A and 3B) and SKOV3 (Figs. 4A and 4B) cell lines compared to control.
Fig. 3.
Modulation of estrogen receptor α (ERα) expression in OVCAR3 cells treated with CdCl2 and assessment of inhibitory effect of melatonin. (A), evaluation of ERα expression performed using Western blotting technique. (B), Quantitative analysis of relative level of ERα expression performed by image j software. *** shows significant differences vs. Cd (0 nM) without melatonin (P < 0.001). #, ##, and ### show significant differences between the cells treated with melatonin and corresponding cells without melatonin (P < 0.05, P < 0.01 and P < 0.001, respectively). (Mel), melatonin; (Cd), CdCl2.
Fig. 4.
Modulation of estrogen receptor α (ERα) expression in SKOV3 cells treated with CdCl2 and assessment of inhibitory effect of melatonin. (A), evaluation of ERα expression performed using Western blotting technique. (B), Quantitative analysis of relative level of ERα expression performed by image j software. *** shows significant differences vs. Cd (0 nM) without melatonin (P < 0.001). ##, and ### show significant differences between the cells treated with melatonin and corresponding cells without melatonin (P < 0.01 and P < 0.001, respectively). Mel, melatonin; Cd, CdCl2.
Stimulative effect of CdCl2 on ERα expression was determined to be 15 fold for 1 nM, 14 fold for 10 nM and 8 fold for 100 nM in OVCAR3. Furthermore, in SKOV3 cells, Cd induced ERα expression 10 fold for 1 nM, 18 fold for 10 nM and 8 fold for 100 nM compared to control.
It was observed that when the cells were co-treated with CdCl2 and melatonin (1 μM), melatonin could inhibit Cd inducing effect on ERα expression of cells after 24 h. Melatonin decreased ERα expression of OVCAR3 cells by (3 fold) for 1 nM, (1.4 fold) for 10 nM and (2 fold) for 100 nM (Figs. 3B).
In SKOV3 cells, the results also demonstrated that melatonin significantly decreased ERα expression by (5 fold) for 1 nM, (1.89 fold) for 10 nM and (2.6 fold) for 100 nM (Figs. 4B).
DISCUSSION
Cd has been considered as an important harmful environmental pollutant which has role in tumorigenesis and carcinogenesis especially in estrogen target tissues because of its estrogen mimicking effect. It is identified as the best qualified of the metalloestrogens (16). Moreover, previous studies have demonstrated that melatonin has anti-carcinogenic and oncostatic activity on cancer cells (17,18,19,20). Some proposed mechanisms to explain protective oncostatic activity of melatonin are focused on anti-oxidative properties, anti-proliferation effect, induction of apoptosis, enhancement of immune system activity, anti-inflammatory and anti-angiogenic function in many cancer types including hormone-dependent cancers (10,17,18). The pharmacological and physiological properties of melatonin indicate that it can inhibit progression and metastasis of ovarian cancer cells (10). Furthermore, some experimental in vitro and in vivo studies have shown that melatonin inhibits the growth of hormone-related tumors by decreasing estrogen receptor expression (17), also its protective effects on Cd-induced breast cancer cell proliferation point to a possible role as a preventive factor for Cd effects (21).
Therefore, the first aim of our study was to clarify whether Cd has proliferative effect and if melatonin has oncostatic activity on ovarian cancer cell lines. Secondly we investigated the oncostatic effect of melatonin on inhibition of Cd proliferative effect and its possible underlying mechanism. CdCl2 exhibited proliferative activity at 1-10 nM and oncostatic concentration of melatonin was found to be 1 μM. We observed that oncostatic concentration of melatonin inhibited proliferative effect of CdCl2 through inhibition of ERα expression.
In the present study, our data indicated that Cd induced the proliferation of ovarian cancer cell lines. Cell proliferation was increased in a Cd-dose dependent manner where lowest concentration of CdCl2 caused highest proliferation. Few studies have reported the effect of Cd on ovarian cancer and only a few epidemiological studies indicating controversial data (8,22,23). Our study evaluated the effects and mechanisms of Cd on proliferation of ovarian cancer cell lines. Animal and cellular studies have shown that Cd exposure can induce tumorigenesis and increase proliferation of mammary and uterus cells (16,24,25,26). Jiang, et al showed that Cd induced significant cell proliferation at low concentrations (27). Hao, et al. demonstrated that Cd stimulated cell proliferation in a dose-dependent manner in breast cancer cell line (28). Huff, et al. showed that nanomolar concentrations of CdCl2 have dose dependent proliferative effect in some types of lung cancer cell lines (29). Khojastehfar, et al. reported that nanomolar concentration of Cd increased MCF7 cell proliferation and higher concentrations induced cell apoptosis (30).
Recent studies have proposed that melatonin has an oncostatic role on hormone-dependent tumors (10,11,17). Our results also demonstrated that melatonin has oncostatic activity on ovarian cancer cell lines. In parallel to our study, Shen, et al. were observed that melatonin (400 and 800 μM) suppressed ovarian cancer cell line growth (12). Some evidences presented that melatonin may be beneficial in ovarian cancer treatment (10). Since Cd treatment stimulated growth of ovarian cancer cell line, we encouraged to evaluate the ability of melatonin as an anti-proliferative agent in Cd-induced cell proliferation.
Our results showed that melatonin prevented inducing role of Cd in ovarian cell proliferation. In confirmation of our research, Alonso-Gonzalez, et al. demonstrated that melatonin has the protective effects on Cd-induced proliferation of mice mammary glands and uterus (21). Martínez-Campa, et al. have also described that melatonin enforced anti-proliferative effects on MCF7 cells triggered by Cd (11). Furthermore, some studies have reported the effect and action mechanism of melatonin on Cd proliferative effect. For instance, Martinez-Campa, et al. claimed that melatonin down-regulates hTERT expression induced by Cd in MCF-7 cells (31), and also Alonso-Gonzalez, et al. exhibited that melatonin modulates the expression of metallothioneins induced by Cd in MCF-7, MDA-MB-231 and HeLa cells (32). Sánchez-Barceló EJ, et al. suggested that melatonin has an important role in the prevention and treatment of hormone-dependent mammary cancer through three anti-estrogenic mechanisms including estrogen receptor interfering, inhibition of estrogen synthesis and decreasing circulating levels of estradiol (33).
It is obvious that the expression of steroid hormone receptors, specially ERα, is very important in etiology, incidence, pathology and survival rate of hormone related cancers and it has been investigated as prognostic biomarker for endometriosis and ovarian cancer (34). Additionally, Cd as a xenoesterogen stimulates ERα in breast cancer cells and mimics the action of estrogen in these cells by binding to the receptor and mediating receptor activation (16,35). Ronchett, et al. reported that nanomolar concentration of Cd up-regulated mRNA expression of ERα in anterior pituitary cells (36). Huff, et al. claimed that 100 nM CdCl2 and E2 increased ERα expression (29). Our data demonstrated that Cd significantly stimulated ERα expression in both OVCAR3 and SKOV3 cell lines. Additinally, melatonin with a potent inhibitory effect reduced the expression of ERα in control and Cd-treated samples. These data indicate that melatonin can act as a modifier to Cd activity on ovarian cancer cell proliferation mediated by ERα. In support of our findings, Martinez-Campa, et al. showed that ERα transcription are stimulated by Cd (1 μM) and melatonin is a specific inhibitor of ERα in MCF7cell (11). Alonso-Gonzalez, et al. demonstrated that Cd exhibited estrogenic effects on mammary glands and melatonin (10 μM) inhibited these Cd effects in ovariectomized mice (21). Liu, et al. observed that in ovarian follicles of chicken, melatonin decreased ERα expression (37). The results of Hill, et al. determined that melatonin modulated ERα expression in MCF-7 cells (38). Finally, according to our results and other references, it is possible that anti-estrogenic action of melatonin through inhibition of ERα expression can inhibit estrogenic effects of Cd which can have role in ovarian cancer etiology (21).
CONCLUSION
In conclusion, because of proliferative effect of Cd on ovarian cancer cell lines, it could play an important role in the etiology of ovarian cancer by inducing ERα expression. Furthermore, melatonin has the protective effects on Cd-induced ovarian cancer cell proliferation. Not only can melatonin inhibit Cd-induced proliferation of ovarian cancer cells but also it decreases Cd-induced ERα expression. These results suggest a possible role of melatonin as a preventive agent for Cd-induced ovarian cancer.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Ph.D thesis (No. 394160) submitted by Negar Ataei which was financially supported by the Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112(10):1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68(1):167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Morales P, Saceda M, Kenney N, Kim N, Salomon DS, Gottardis MM, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem. 1994;269(24):16896–16901. [PubMed] [Google Scholar]
- 4.Darbre PD. Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006;26(3):191–197. doi: 10.1002/jat.1135. [DOI] [PubMed] [Google Scholar]
- 5.Safe S. Cadmium’s disguise dupes the estrogen receptor. Nat Med. 2003;9:1000–1001. doi: 10.1038/nm0803-1000. [DOI] [PubMed] [Google Scholar]
- 6.Hajiahmadi S, Panjehpour M, Aghaei M, Mousavi S. Molecular expression of adenosine receptors in OVCAR-3, Caov-4 and SKOV-3 human ovarian cancer cell lines. Res Pharm Sci. 2015;10(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- 7.Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51(3):72–76. doi: 10.4103/0019-509X.154049. [DOI] [PubMed] [Google Scholar]
- 8.Adams SV, Quraishi SM, Shafer MM, Passarelli MN, Freney EP, Chlebowski RT, et al. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women’s Health Initiative. Environ Health Perspect. 2014;122(6):594–600. doi: 10.1289/ehp.1307054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams SV, Passarelli MN, Newcomb PA. Cadmium exposure and cancer mortality in the third national health and nutrition examination survey cohort. Occup Environ Med. 2012;69(2):153–156. doi: 10.1136/oemed-2011-100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuffa LG de A, Reiter RJ, Lupi Júnior LA. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38(10):945–952. doi: 10.1093/carcin/bgx054. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Campa C, Alonso-González C, Mediavilla MD, Cos S, González A, Ramos S, et al. Melatonin inhibits both ER alpha activation and breast cancer cell proliferation induced by a metalloestrogen, cadmium. J Pineal Res. 2006;40(4):291–296. doi: 10.1111/j.1600-079X.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 12.Shen CJ, Chang CC, Chen YT, Lai CS, Hsu YC. Melatonin suppresses the growth of ovarian cancer cell lines (OVCAR-429 and PA-1) and potentiates the effect of G1 arrest by targeting CDKS. Int J Mol Sci. 2016;17(2) doi: 10.3390/ijms17020176. pii: E176. doi: 10.3390/ijms17020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futagami M, Sato S, Sakamoto T, Yokoyama Y, Saito Y. Effects of melatonin on the proliferation and cis-diamminedichloroplatinum (CDDP) sensitivity of cultured human ovarian cancer cells. Gynecol Oncol. 2001;82(3):544–549. doi: 10.1006/gyno.2001.6330. [DOI] [PubMed] [Google Scholar]
- 14.Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58(19):4383–4390. [PubMed] [Google Scholar]
- 15.del Río B, García Pedrero JM, Martínez-Campa C, Zuazua P, Lazo PS, Ramos S. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279(37):38294–38302. doi: 10.1074/jbc.M403140200. [DOI] [PubMed] [Google Scholar]
- 16.Siewit CL, Gengler B, Vegas E, Puckett R, Louie MC. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERαlpha and c-Jun. Mol Endocrinol. 2010;24(5):981–992. doi: 10.1210/me.2009-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukowska A. Anticarcinogenic role of melatonin-potential mechanisms. Med Pr. 2011;62(4):425–434. [PubMed] [Google Scholar]
- 18.Ma Z, Yang Y, Fan C, Han J, Wang D, Di S, et al. Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget. 2016;7(29):46768–46784. doi: 10.18632/oncotarget.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh CM, Su SC, Lin CW, Yang WE, Chien MH, Reiter RJ, et al. Melatonin as a potential inhibitory agent in head and neck cancer. Oncotarget. 2017;8(52):90545–90556. doi: 10.18632/oncotarget.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta A, Kaur G. Potential role of melatonin in prevention and treatment of oral carcinoma. Indian J Dent. 2014;5(2):86–91. doi: 10.4103/0975-962X.135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso-González C, González A, Mazarrasa O, Güezmes A, Sánchez-Mateos S, Martínez-Campa C, et al. Melatonin prevents the estrogenic effects of sub-chronic administration of cadmium on mice mammary glands and uterus. J Pineal Res. 2007;42(4):403–410. doi: 10.1111/j.1600-079X.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen KT, Halkjær J, Sørensen M, Meliker JR, McElroy JA, Tjønneland A, et al. Dietary cadmium intake and risk of breast, endometrial and ovarian cancer in Danish postmenopausal women: a prospective cohort study. PloS One. 2014;9(6):1008–1015. doi: 10.1371/journal.pone.0100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julin B, Wolk A, Åkesson A. Dietary cadmium exposure and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Br J Cancer. 2011;105(3):441–444. doi: 10.1038/bjc.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brama M, Gnessi L, Basciani S, Cerulli N, Politi L, Spera G, et al. Cadmium induces mitogenic signaling in breast cancer cell by an ERα-dependent mechanism. Mol Cell Endocrinol. 2007;264(1-2):102–108. doi: 10.1016/j.mce.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Yang J, Wang J, Xia P, Xu Y, Jia H, et al. Comparative studies on the increase of uterine weight and related mechanisms of cadmium and p-nonylphenol. Toxicology. 2007;241(1-2):84–91. doi: 10.1016/j.tox.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Yu L, Moore AB, Kissling GE, Waalkes MP, Dixon D. Cadmium and proliferation in human uterine leiomyoma cells: evidence of a role for EGFR/MAPK pathways but not classical estrogen receptor pathways. Environ Health Perspect. 2015;123(4):331–336. doi: 10.1289/ehp.1408234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang G, Duan W, Xu L, Song S, Zhu C, Wu L. Biphasic effect of cadmium on cell proliferation in human embryo lung fibroblast cells and its molecular mechanism. Toxicol In Vitro. 2009;23(6):973–978. doi: 10.1016/j.tiv.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Hao C, Hao W, Wei X, Xing L, Jiang J, Shang L. The role of MAPK in the biphasic dose-response phenomenon induced by cadmium and mercury in HEK293 cells. Toxicol In Vitro. 2009;23(4):660–666. doi: 10.1016/j.tiv.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Huff MO, Todd SL, Smith AL, Elpers JT, Smith AP, Murphy RD, et al. Arsenite and cadmium activate mapk/erk via membrane estrogen receptors and g-protein coupled estrogen receptor signaling in human lung adenocarcinoma cells. Toxicol Sci. 2016;152(1):62–71. doi: 10.1093/toxsci/kfw064. [DOI] [PubMed] [Google Scholar]
- 30.Khojastehfar A, Aghaei M, Gharagozloo M, Panjehpour M. Cadmium induces reactive oxygen species-dependent apoptosis in MCF-7 human breast cancer cell line. Toxicol Mech Methods. 2015;25(1):48–55. doi: 10.3109/15376516.2014.985353. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Campa CM, Alonso-González C, Mediavilla MD, Cos S, González A, Sanchez-Barcelo EJ. Melatonin down-regulates hTERT expression induced by either natural estrogens (17β- estradiol) or metalloestrogens (cadmium) in MCF-7 human breast cancer cells. Cancer Lett. 2008;268(2):272–277. doi: 10.1016/j.canlet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Gonzalez C, Mediavilla D, Martinez-Campa C, Gonzalez A, Cos S, Sanchez-Barcelo EJ. Melatonin modulates the cadmium-induced expression of MT-2 and MT-1 metallothioneins in three lines of human tumor cells (MCF-7, MDA-MB-231 and HeLa) Toxicol Lett. 2008;181(3):190–195. doi: 10.1016/j.toxlet.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Barceló EJ, Cos S, Mediavilla D, Martínez-Campa C, González A, Alonso-González C. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005 May;38(4):217–222. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 34.Sieh W, Köbel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14(9):853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Z, Song X, Shaikh ZA. Cadmium promotes the proliferation of triple-negative breast cancer cells through EGFR-mediated cell cycle regulation. Toxicol Appl Pharmacol. 2015;289(1):98–108. doi: 10.1016/j.taap.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronchetti SA, Miler EA, Duvilanski BH, Cabilla JP. Cadmium mimics estrogen-driven cell proliferation and prolactin secretion from anterior pituitary cells. PLoS One. 2013;8(11):e81101. doi: 10.1371/journal.pone.0081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Li D, Gilbert ER, Xiao Q, Zhao X, Wang Y, et al. Effect of monochromatic light on expression of estrogen receptor (ER) and progesterone receptor (PR) in ovarian follicles of chicken. PloS One. 2015;10(12):0144102. doi: 10.1371/journal.pone.0144102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill SM, Collins A, Kiefer TL. The modulation of oestrogen receptor-alpha activity by melatonin in MCF-7 human breast cancer cells. Eur J Cancer. 2000;36:117–118. doi: 10.1016/s0959-8049(00)00273-2. [DOI] [PubMed] [Google Scholar]