Abstract
Several diffusion tensor imaging (DTI) studies in attention deficit hyperactivity disorder (ADHD) have shown a delay in brain white matter (WM) development. Because these studies were mainly conducted in children and adolescents, these WM abnormalities have been assumed, but not proven to progress into adulthood. To provide further insight in the natural history of WM maturation delay in ADHD, we here investigated the modulating effect of age on WM in children and adults.
120 stimulant-treatment naive male ADHD children (10–12 years of age) and adults (23–40 years of age) with ADHD (according to DSM-IV; all subtypes) were included, along with 23 age and gender matched controls. Fractional anisotropy (FA) values were compared throughout the WM by means of tract-based spatial statistics (TBSS) and in specific regions of interest (ROIs). On both TBSS and ROI analyses, we found that stimulant-treatment naive ADHD children did not differ in FA values from control children, whereas adult ADHD subjects had reduced FA values when compared to adult controls in several regions. Significant age × group interactions for whole brain FA (p = 0.015), as well as the anterior thalamic radiation (p = 0.015) suggest that ADHD affects the brain WM age-dependently.
In contrast to prior studies conducted in medicated ADHD children, we did not find WM alterations in stimulant treatment naïve children, only treatment-naïve adults. Thus, our findings suggest that the reported developmental delay in WM might appear after childhood, and that previously reported differences between ADHD children and normal developing peers could have been attributed to prior ADHD medications, and/or other factors that affect WM development, such as age and gender.
Keywords: Diffusion tensor imaging, ADHD, Age, Brain development: ADHD medications
Highlights
-
•
In medication naïve children with ADHD maturation of white matter was not delayed, only in adults.
-
•
Thus, developmental delay in ADHD seems to appear after childhood, in adolescence.
-
•
Discrepancy with prior studies likely underlie factors that affect WM development, such as medication status, age and gender.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder, characterized by excessive levels of inattention and/or hyperactivity and impulsivity, arising in childhood and often persisting during adolescence and adulthood (Biederman et al., 2000). Although behavioral and cognitive aspects of ADHD are quite well understood, a neurobiological substrate of ADHD has not yet been found. However, evidence is accumulating that abnormalities in brain structure may play an important in the pathophysiology of ADHD. For instance, imaging studies assessing gray matter thickness have shown cortical changes and maturation delay in specific areas in the prefrontal cortex in pediatric and adolescent ADHD subjects, which normalized following ADHD medications (Shaw et al., 2006, Shaw et al., 2007, Sowell et al., 2003, Yang et al., 2015). However, the role of altered development of brain white matter (WM) in ADHD is still relatively underexplored (van Ewijk et al., 2012), although reported WM volume reductions in ADHD patients are larger than for gray matter (Castellanos et al., 2002).
Diffusion tensor imaging (DTI) is an ideal tool to study WM development non-invasively. It is a magnetic resonance imaging (MRI) modality that assesses the micro-structural features of WM and microfiber pathways by measuring the diffusional motion of water molecules. Fractional anisotropy (FA) is a normalized measure, which quantifies the directional anisotropy of diffusion. Typical maturation of brain WM continues into adulthood (Kochunov et al., 2012) in trajectories such as the anterior thalamic radiations (ATR) and the corpus callosum (CC) in which FA continues to increase until adulthood (Barnea-Goraly et al., 2005, Klingberg et al., 1999, Yap et al., 2013). There is accumulating evidence that WM maturation in ADHD subjects is disturbed, especially in the frontostriatal and corticocortical tracts such as the superior longitudinal fasciculus (SLF) (Durston, 2008, van Ewijk et al., 2012). However, whereas studies in children and adolescents with ADHD have shown a reduction in overall WM volume compared to normal controls (Castellanos et al., 2002), a trend toward an overall increase in WM volume in adults with ADHD has been demonstrated (Seidman et al., 2006). Possible reasons for this discrepancy could be the sample heterogeneity between the studies, the medication status of the investigated patients and the different diffusion imaging parameters between studies. Indeed, particularly medication status seems to affect WM, as Castellanos et al. reported significantly higher WM volumes in medicated pediatric subjects with ADHD compared to (older) unmedicated patients and controls (− 8.9% and 11.65%, respectively) (Castellanos et al., 2002). In line with these clinical observations, in an experimental study in rats increased FA values of the corpus callosum of adolescent (+ 9.2%), but not adult, animals following stimulant treatment with methylphenidate (MPH) were found (van der Marel et al., 2014). Interestingly, juvenile MPH treatment has been shown to upregulate striatal genes involved in axonal myelination (Adriani et al., 2006), which may underlie these reported changes in WM.
To our knowledge only two other structural imaging studies have been conducted in adult medication naïve ADHD patients, including one DTI study. In that DTI study, the authors concluded that because their voxel based morphometry results (that did not survive corrections for multiple comparisons) were similar to those obtained in adolescents (Makris et al., 2015), it is likely that ADHD patients reach adult life with structural brain alterations. However, the studies in children and adolescents the authors refer to (Seidman et al., 2005) were mainly conducted in medicated children and adolescents, making such comparison invalid in view of the relatively large effects of medication status on brain structure, as discussed above.
Thus, the only way to know more about the potential neurobiological role and natural history of WM development in ADHD is to directly compare medication naïve children and adults in one study, using the same diffusion imaging parameters, correction for motion artifacts, and using the same inclusion criteria. The aim of this study was to investigate the modulating effect of age on DTI parameters in ADHD male patients and matched healthy controls. If indeed ADHD patients reach adult life with structural brain alterations, we hypothesized to find reduced FA values in children and adult ADHD patients when compared to age-matched controls, particularly in the ATR and the CC, as these are late to mature, and in the SLF, one of the corticocortical tracts earlier reported to be impaired in ADHD (Durston, 2008, van Ewijk et al., 2012).
2. Materials and methods
2.1. Study design
122 subjects were included in this study: 99 ADHD stimulant-treatment naive ADHD subjects and 23 age-, and gender matched healthy controls. The study protocol was approved by the Central Committee on Research Involving Human Subjects in The Hague, and the research board of each participating center. All patients, and for the boys also both parents and their legal representatives, provided written informed consent. Patients were 50 outpatient stimulant-treatment naive boys (10–12 years of age) and 49 outpatient stimulant-treatment naive men (23–40 years of age) diagnosed with ADHD (all types). Boys were recruited from clinical programs at the Child and Adolescent Psychiatry Center Triversum (Alkmaar) and from the department of (Child and Adolescent) Psychiatry at the Bascule/AMC (Amsterdam). Adult patients were recruited from the Adult ADHD program at PsyQ, psycho-medical programs clinical programs at the PsyQ mental health facility (The Hague) and from the department of Psychiatry of the AMC (Amsterdam). They were diagnosed by an experienced psychiatrist based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, 4th edition) and the diagnosis was subsequently confirmed with a structured interview: Diagnostic Interview Schedule for Children (National Institute of Mental Health Diagnostic Interview Schedule for Children Version IV (DISC-IV) for children (Ferdinand and Van der Ende, 1998) and the Diagnostic Interview for ADHD (DIVA 2.0) (Kooij, 2013) for adults. Inclusion criteria were at least 6 of 9 symptoms of inattention or hyperactivity/impulsivity on the DISC-IV (for children) and on the DIVA 2.0 (for adults). Patients were excluded if they were diagnosed with a co-morbid axis I psychiatric disorder requiring pharmacological treatment at study entry. 1 ADHD child was excluded from the data analysis, as his DTI scan was missing due to technical problem, and 1 adult because of undisclosed prior treatment with ADHD medications, resulting in a total sample size of 120 subjects.
2.2. Imaging acquisition
All MR imaging was performed on a 3.0 T Philips MR scanner equipped with an SENSE 8-channel head coil and body coil transmission (Philips Medical Systems, Best, The Netherlands). A DTI scan was obtained with the following scan parameters: field of view: 224 × 224 mm, slice thickness: 5 mm, repetition time TR/TE: 8135/94 ms, scan time: 6 min 47 s, SENSE: 2, slices: 60, b:1000, 4* b:0, half-scan: 0.797, SPIR: 250, matrix: 112, 46 gradient directions, voxel size isotropic: 2 mm.
2.3. DTI data pre-processing
Pre-processing of the DTI data was performed using in-house developed software, written in Matlab (The MathWorks, Natick, MA). The pre-processing was executed using the HPCN-UvA Neuroscience Gateway and using resources of the Dutch e-Science Grid (Shahand et al., 2011). Head motion and deformations induced by eddy currents were corrected for by an affine registration of the Diffusion Weighted Images (DWIs) to the non-diffusion weighted image. The average of the total motion was then computed (Ling et al., 2012) using translation parameters only, and subsequently log-transformed to create motion-score for use in statistical analyses. Motion scores did not differ significantly between the ADHD and control groups. Corrupted slices in the DWIs were automatically detected and excluded. The gradient directions were corrected by the rotation component of the transformation. The DWIs were resampled isotropically. Rician noise in the DWIs was reduced by an adaptive noise filtering method (Caan et al., 2010). Diffusion tensors were estimated in a non-linear least squares sense. From the tensors, FA was computed. All subjects-data were then aligned into standard space by nonrigid registration (Andersson et al., 2007).
We focused our analysis on the central parts of the WM tracts, by skeletonizing a template FA-map (FA was thresholded at 0.2) (Smith et al., 2006). FA maps of all subjects were projected onto this FA skeleton. Average values were then computed over the entire WM and within the CC, the left and right ATR and the left and right SLF. These regions of interest (ROIs) were selected because their important role in executive function and the continuous development of the fibers in these tracts (Yap et al., 2013).
2.4. Statistical analyses
Independent Student t-tests were used to examine the group differences within the age groups, on continuous demographic and symptom severity variables, and Chi-square tests for categorical data. For the ROI analyses, we used univariate analysis of covariance (ANCOVA) to compare the differences in age and in diagnosis in overall mean FA values of whole brain and the selected ROIs, i.e. the CC, the ATR bilaterally, and SLF bilaterally. Demeaned age (within group) and demeaned motion-score were used as covariates. A p value (two-sided) < 0.05 was considered significant. A multiple comparison's correction using Sidak's correction was applied for the 3 selected ROI's, resulting in a corrected α of 0.017. Statistical analyses were conducted with IBM SPSS version 22.
Tract Based Spatial Statistics was used for voxel-wise group comparisons in age, group and age by group interaction and included demeaned age and motion-score as covariates. Multiple comparisons were corrected for by using permutation tests, as implemented using the Randomise software within FSL tool (http://fsl.fmrib.ox.ac.uk/fsl), employing the threshold free cluster enhancement, in which p-values < 0.05 were considered significant.
3. Results
The stimulant-treatment naive ADHD subjects and control groups were relatively well matched, except that the adult ADHD group was on average 3.5 years older than the adult controls (Table 1) and the normal developing children had a significantly higher IQ when compared to the ADHD subjects. A majority of adult ADHD patients (65%) had a history recreational drug use of mainly cannabis, followed by MDMA. The details are summarized in Table 1.
Table 1.
Demographic characteristics of the sample (n = 120).
| Male children |
Male adults |
|||||||
|---|---|---|---|---|---|---|---|---|
| ADHD subjects |
Controls |
Test statistics | ADHD subjects |
Controls |
Test statistics | |||
| n = 49 |
n = 11 |
n = 48 |
n = 12 |
|||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age, y, mean | 11.34 (0.87) | 11.36 (0.84) | t(58) = − 0.077, p = 0.939 | 28.59 (4.64) | 25.18 (1.86) | p < 0.01 | ||
| Estimated IQa | 104.62 (18.08) | 121.6 (10.9) | t(55) = − 2.849, p < 0.01 | 107.86 (7.5) | 108.08 (5.52) | p = 0.92 | ||
| Drugs of abuseb | MDMA | Cocaine | Amphetamine | Cannabis | ||||
| n | n | n | n | |||||
| No (< 1 yrs) | 31 | 38 | 38 | 22 | ||||
| Short (1–2 yrs) | 6 | 2 | 4 | 3 | ||||
| Moderate (3–4 yrs) | 4 | 2 | 3 | 10 | ||||
| Long (≥ 5 yrs) | 7 | 6 | 3 | 13 | ||||
| ADHD subtype | ||||||||
| Inattentive | 27 (55.1%) | 16 (33.3%) | ||||||
| Hyperactive/impulsive | 1 (2.0%) | 0 (0.0%) | ||||||
| Combined | 21 (42.9%) | 32 (67.3%) | ||||||
| ADHD symptom severity rating scalesc | ||||||||
| Attention (DBD-SR) | 22.35 (3.22) | |||||||
| Hyperactivity/impulsivity (DBD-SR) | 15.75 (5.67) | |||||||
| ADHD-RS | 31.46 (9.60) | |||||||
| Co-morbidity | ||||||||
| Depressive episode(s) in the pastd | 6 (12.2%) | |||||||
| Anxiety disorder in the paste | 1 (2.0%) | |||||||
| ODD/CDd | 3 (6.1%) | |||||||
For children: WISC, for adults, NART.
Classification according to Yang et al. (2015).
For children: DBD—SR, for adults ADHD-SR.
For adults: MINI Plus 5.0.
For children: NIMH DISC-IV.
3.1. ROI analyses
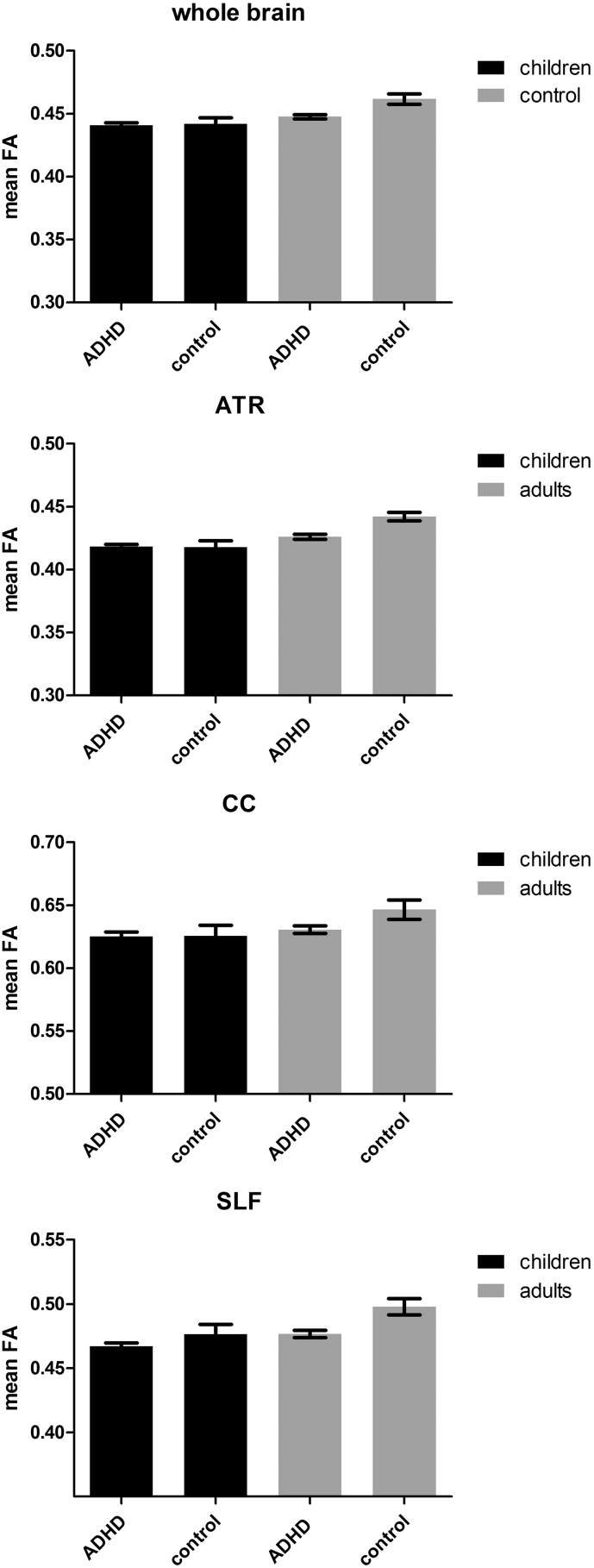
We found a significant age × diagnosis interaction (F = 6.09, df = 1112, p = 0.015) for whole brain FA. Post-hoc tests showed that whole brain FA was significantly lower in control children when compared to control adults (F = 7.39, df = 1,19, p = 0.001), whereas whole brain FA did not differ between ADHD children and adults (F = 2.09, df = 1,91, p = 0.08) (Fig. 1). Adult ADHD subjects had significantly lower overall FA when compared to adult control subjects (F = 13.44, df = 1,55, p < 0.01), whereas such an effect was absent in the children (F = 0.0002, df = 1,55, p = 0.97). In the ATR and CC we also found a significant age × diagnosis interaction (F = 6.14, df = 1112, p = 0.015, F = 4.07, df = 1112, p = 0.046, respectively). However, after applying Sidak's correction for multiple comparison's the interaction effect in the CC was no longer significant (p > 0.017). Post-hoc tests showed significantly lower FA in children when compared to adults: in both healthy controls (F = 9.64, df = 1,19, p < 0.01) as well as the ADHD group (F = 6.35, df = 1,91, p = 0.01). However, in the CC we only found an age effect in controls (controls F = 4.54, df = 1,19, p = 0.046; ADHD: F = 0.025, df = 1,91, p = 0.62, respectively). Interestingly, in the adult ADHD group, FA values in the ATR as well as the CC were significantly lower than in the control group (F = 12.09, df = 1,55, p < 0.01; F = 7.82, df = 1,55, p < 0.01, respectively), whereas no such difference was observed between the two children groups (F < 0.01,df = 1,55, p = 0.98; F = 0.07,df = 1,55, p = 0.79, respectively). However, we did not find a significant interaction effect in the SLF (F = 1.81, df = 1112, p = 0.18). Instead, we found a main effect of age (F = 6.47, df = 1113, p = 0.012) and of clinical group (F = 9.31, df = 1113, p < 0.01).
Fig. 1.
ROI FA analyses.
FA values in whole brain WM, ATR CC and SLF. Displays mean ± standard error of the mean.
3.2. TBSS analyses
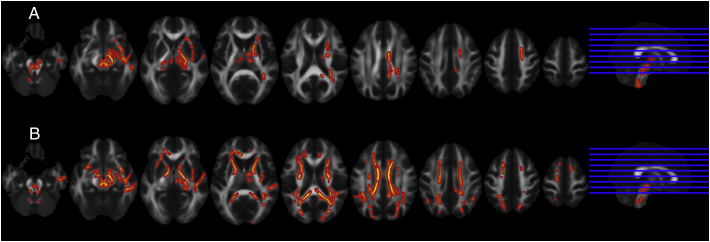
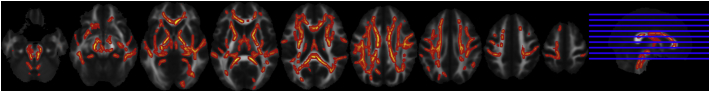
As expected, FA values were widespread significantly lower in control children vs. adults including the corona radiate (CR), the CC and ATR (Fig. 2, panel B). However, in ADHD children when compared to ADHD adults, we found less pronounced differences (Fig. 2, panel A), only in the ATR. In addition, we observed significantly lower FA values in adult ADHD patients in regions including the SLF, CC and ATR bilaterally (p < 0.05) compared to the control subjects (Fig. 3), whereas such an effect was absent in the children with ADHD when compared to the control children. No significant age × group interaction effect was found.
Fig. 2.
TBSS analysis in children versus adults in ADHD (panel A) and control subjects (panel B).
a) Significant lower FA values are showed (p < 0.05 corrected) in ADHD children when compared to ADHD adults in the ATR.
b) Significant lower FA values are showed (p < 0.05 corrected) in control children when compared to control adults in the CC and also the ATR.
Fig. 3.
TBSS analysis in ADHD subjects versus control subjects.
Significantly lower FA values (p < 0.05 corrected) in adult ADHD subjects when compared to control adult subjects in the SLF, CC and the ATR trajectories.
We did not find a statistically significant relationship between FA values and extent of ADHD symptom severity nor co-morbidity, nor did exclusion of these subjects affect our results.
4. Discussion
The aim of this study was to investigate the modulating effect of age on WM development in stimulant naïve ADHD male subjects and controls. We found that stimulant-treatment naive adult ADHD subjects had reduced FA values when compared to age-matched control subjects in several brain regions, whereas stimulant-treatment naive ADHD children did not differ in FA values from the control children. Finally, the significant age × group interaction lend further support to our observation that ADHD affects the brain WM age-dependently: reduced FA values in stimulant-treatment naive ADHD subjects were only visible in adults but not in children with ADHD.
In line with the existing literature, we found significantly lower FA values in healthy children compared to the healthy adults, most prominently in the ATR. Although a greater increase in FA is observed from childhood to adolescence compared to from adolescence to adulthood, it is well established that FA increases from 10–12 years up to 40 years of age in all WM tracts including continuous development in the ATR (Lebel et al., 2012, Yap et al., 2013), as we report here. Consistent with previous studies we found the strongest age effects between normal developing and ADHD subjects (Asato et al., 2010, Simmonds et al., 2014) in the ATR and CC. The ATR connects the anterior and the midline nuclear group of the thalamus with the prefrontal and orbitofrontal cortices (Schmahmann and Pandya, 2009), both key regions for executive function like reward processing and decision making (O'Doherty et al., 2003, Paus et al., 2008). It has been suggested that abnormal development of the ATR may underlie the protracted development of these functions in ADHD.
Interestingly, we did not observe significant differences in FA values between medication naïve ADHD children compared to normal developing control children, which is in contrast to the existing literature. All six ROI studies in children conducted so far (see review by van Ewijk et al., 2012) reported decreased FA values in ADHD children when compared to control in several cluster areas. Silk et al. (2009) even found increased FA values in prefrontal-temporal regions of the left hemisphere and parietal-occipital regions of male ADHD subjects when compared to healthy controls. TBSS studies also reported reductions in FA in ADHD children when compared to adults (Nagel et al., 2011). However, these studies typically involved a mixture of medicated children. As pointed out previously, not only differences in medication status, but also diagnostic methods, ADHD subtypes, and matching criteria (e.g. gender, IQ) likely explain the discrepancies between the studies reported so far. In contrast, in the current study we accounted for most of these differences. To our knowledge, we are the first to report on FA values in stimulant-treatment naive ADHD children. As discussed above, in rats greatly affects FA values age-dependently, increasing FA values in the CC of adolescent rats, but not adult rats (van der Marel et al., 2014). Indeed, Castellanos et al. (2002) has previously shown that stimulant medication results in brain WM volumes similar to typically developing children. Thus, medication status could be an important confounder explaining the discrepancy between our study and previous studies.
In adult ADHD patients, we found widespread reductions in FA in the splenium and body of the CC, the anterior and superior regions of the CR, the SLF and the ATR bilaterally. These findings are consistent with several earlier studies in adults (Cortese et al., 2013, Dramsdahl et al., 2012, Konrad et al., 2010, Onnink et al., 2015, van Ewijk et al., 2014). To our knowledge, the study by Konrad et al. (2010) is the only other DTI study in a large sample of stimulant-treatment naïve male ADHD patients. In line with our study, they found, using voxel based analysis, significantly lower FA values in the right anterior cingulum bundle and in the bilaterally in orbitofrontal WM structures including parts of the ATR and CC. However, additionally they found significantly higher FA values in temporal structures. Indeed, more voxel based analysis studies not only show decreases, but also increases in FA in several brain areas, mostly regions with a larger number of fiber crossings in specific structures, such as the fronto-temporal region. Fiber crossings are known to result in a decrease of FA (Beaulieu, 2002) and therefore, underdevelopment of crossing fibers in ADHD patients could result in a relative increase in FA in these regions.
Our study is the first to investigate the modulating effect of age. We found significant age × group interaction effects in the ATR, suggesting that ADHD affects this brain region age-dependently. The absence of an interaction effect in other regions may be attributed to a Type II error. Alterations in the ATR and other tracts have been associated with executive cognitive deficits and sensorimotor and oculomotor function deficits. For example, the SLF is involved in attention processing and executive functioning (Makris et al., 2008). Also, a recent study by Cortese et al. shows that decreases in FA might be an enduring trait of ADHD (Cortese et al., 2013). This disruption of maturation in ADHD is in accordance with the literature which suggests that ADHD is a developmental disorder that progresses over the years (Mous et al., 2014). Our findings suggest that the delay in WM maturation may only be visible later in childhood or in adolescence. Alternatively, childhood-onset ADHD is not the same as adult onset ADHD, as recently suggested by Moffitt et al. (2015). In that four decade longitudinal study, 90% of adult ADHD cases lacked a history of childhood ADHD. Although in our study all patients met DSM-IV criteria for ADHD (which requires an onset of symptoms before age of 7 years), retrospective recall of childhood symptoms can be questionable in the adult subjects.
We included a relatively large sample of ADHD subjects in this study compared to other DTI studies. However, as we included a restricted age range and only male subjects, our findings have limited generalizability to other ages, and the female gender. For example, the age range covering adolescence and late adulthood that is missing in this study (12 − 23) would be interesting to study. Hopefully large cohort studies such as ENIGMA (http://enigma.ini.usc.edu/ongoing/enigma-adhd-working-group) or NEUROIMAGE (http://www.neuroimage.nl) might be able to answer some of these questions, such as the effect of medication use. In addition, our control groups are relatively small and subsequent studies would benefit from a balanced design in terms of statistical power. Our findings therefore need to be replicated in a longitudinal study, with different ages and both genders. Some of the adult ADHD subjects had been exposed to recreational drugs, which is in a way inherent to having ADHD, mainly cannabis. This is relevant, as particularly cocaine has been shown to affect FA values (Kaag et al., 2017). However, only few (6/48) of our ADHD patients had used cocaine for a long time.
Although we employed adequate data preprocessing to reduce image distortions inherent to the acquisition technique, as well as artifacts caused by head movement, we cannot exclude at least to some extent, false positive findings due to artifacts. For instance, DTI suffers serious limitations in regions of crossing fibers because traditional tensor techniques cannot represent multiple, independent intra-voxel orientations. Also, the acquisition used in this study was limited to a single b = 1000 s/mm2 value. In future studies a more advanced protocol using multiple (at least two) b-values would allow to distinguish additional WM details, for example, fiber crossings, which cannot be reliably estimated otherwise.
In conclusion, we here present for the first time DTI cross-sectional data in medication naïve children and adults with ADHD. In contrast to prior studies in which most subjects were medicated, or medication history was not taken into account (van Ewijk et al., 2012), we found no evidence for alterations in WM microstructure in children, only adults. Although we tried to adequately correct for most potential confounders, our DTI acquisition protocol suffered from a limited b value, and therefore should be replicated in a longitudinal study with multiple b values. These observations suggest that the previously reported differences between ADHD children and normal developing peers could have been attributed to prior ADHD medications, or other factors that affect WM microstructure, such as age and gender. In line with the literature, we found that ADHD patients reach adult life with structural WM alterations. Yet our data indicate that the start of these structural alterations are not yet visible in childhood, but later, most likely during adolescence, when WM tracts have undergone more profound remodeling. Our findings stress the need for future longitudinal studies in adolescents to provide further insight in the natural history of WM maturation delay in ADHD.
Funding
This work was supported by faculty resources of the Academic Medical Center (an AMC Fellowship grant awarded to Prof. L. Reneman grant nr. 11.25.017) and 11.32050.26 ERA-NET PRIOMEDCHILD FP 6 (EU) (ZoNMW:11.32050.26).
References
- Adriani W., Leo D., Guarino M., Natoli A., Di Consiglio E., De Angelis G., Traina E., Testai E., Perrone-Capano C., Laviola G. Short-term effects of adolescent methylphenidate exposure on brain striatal gene expression and sexual/endocrine parameters in male rats. Ann. N. Y. Acad. Sci. 2006;1074:52–73. doi: 10.1196/annals.1369.005. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. Non-linear registration aka spatial normalisation FMRIB technial report TR07JA2. In Pract. 2007;22 [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A., Dant C.C., Reiss A.L. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Biederman J., Mick E., Faraone S.V. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am. J. Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Caan M.W.A., Khedoe G., Poot D., den Dekker A., Olabarriaga S., Grimbergen K., van Vliet L., Vos F. Adaptive noise filtering for accurate and precise diffusion estimation in fiber crossings. Med. Image Comput. Comput. Assist. Interv. 2010;13:167–174. doi: 10.1007/978-3-642-15705-9_21. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S., Blumenthal J.D., James R.S., Ebens C.L., Walter J.M., Zijdenbos A., Evans A.C., Giedd J.N., Rapoport J.L. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Cortese S., Imperati D., Zhou J., Proal E., Klein R.G., Mannuzza S., Ramos-Olazagasti M.A., Milham M.P., Kelly C., Castellanos F.X. White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74:591–598. doi: 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramsdahl M., Westerhausen R., Haavik J., Hugdahl K., Plessen K.J. Adults with attention-deficit/hyperactivity disorder - a diffusion-tensor imaging study of the corpus callosum. Psychiatry Res. 2012;201:168–173. doi: 10.1016/j.pscychresns.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Durston S. Converging methods in studying attention-deficit/hyperactivity disorder: what can we learn from neuroimaging and genetics? Dev. Psychopathol. 2008;20:1133–1143. doi: 10.1017/S0954579408000539. [DOI] [PubMed] [Google Scholar]
- van Ewijk H., Heslenfeld D.J., Zwiers M.P., Buitelaar J.K., Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- van Ewijk H., Heslenfeld D.J., Zwiers M.P., Faraone S.V., Luman M., Hartman C. a, Hoekstra P.J., Franke B., Buitelaar J.K., Oosterlaan J. Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:790–799. doi: 10.1016/j.jaac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Ferdinand R.F., Van der Ende J. Netherlands Erasmus Univ. Dep. Child Adolesc. Psychiatry; Rotterdam: 1998. Diagnostic Interview Schedule for Children (DISC-IV) [Dutch Translation NIMH-DISC-IV] [Google Scholar]
- Kaag A.M., van Wingen G.A., Caan M.W.A., Homberg J.R., van den Brink W., Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict. Biol. 2017;22(4):1048–1056. doi: 10.1111/adb.12375. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Vaidya C.J., Gabrieli J.D., Moseley M.E., Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kochunov P., Williamson D.E., Lancaster J., Fox P., Cornell J., Blangero J., Glahn D.C. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol. Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A., Dielentheis T.F., El Masri D., Bayerl M., Fehr C., Gesierich T., Vucurevic G., Stoeter P., Winterer G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur. J. Neurosci. 2010;31:912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Kooij J.J.S. Adult ADHD Diagnostic Assess. Treat. 3rd ed. 2013. Adult ADHD: diagnostic assessment and treatment (3rd ed.) [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Ling J., Merideth F., Caprihan A., Pena A., Teshiba T., Mayer A.R. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum. Brain Mapp. 2012;33:50–62. doi: 10.1002/hbm.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Buka S.L., Biederman J., Papadimitriou G.M., Hodge S.M., Valera E.M., Brown A.B., Bush G., Monuteaux M.C., Caviness V.S., Kennedy D.N., Seidman L.J. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb. Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Makris N., Liang L., Biederman J., Valera E.M., Brown A.B., Petty C., Spencer T.J., Faraone S.V., Seidman L.J. Toward defining the neural substrates of ADHD: a controlled structural MRI study in medication-naive adults. J. Atten. Disord. 2015;19:944–953. doi: 10.1177/1087054713506041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel K., Klomp A., Meerhoff G.F., Schipper P., Lucassen P.J., Homberg J.R., Dijkhuizen R.M., Reneman L. Long-term oral methylphenidate treatment in adolescent and adult rats: differential effects on brain morphology and function. Neuropsychopharmacology. 2014;39:263–273. doi: 10.1038/npp.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Houts R., Asherson P., Belsky D.W., Corcoran D.L., Hammerle M., Harrington H., Hogan S., Meier M.H., Polanczyk G.V., Poulton R., Ramrakha S., Sugden K., Williams B., Rohde L.A., Caspi A. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am. J. Psychiatry. 2015;172:967–977. doi: 10.1176/appi.ajp.2015.14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mous S.E., Muetzel R.L., El Marroun H., Polderman T.J.C., van der Lugt A., Jaddoe V.W., Hofman A., Verhulst F.C., Tiemeier H., Posthuma D., White T. Cortical thickness and inattention/hyperactivity symptoms in young children: a population-based study. Psychol. Med. 2014:1–11. doi: 10.1017/S0033291714000877. [DOI] [PubMed] [Google Scholar]
- Nagel B.J., Bathula D., Herting M., Schmitt C., Kroenke C.D., Fair D., Nigg J.T. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:283–292. doi: 10.1016/j.jaac.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J., Critchley H., Deichmann R., Dolan R.J. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J. Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnink a.M.H., Zwiers M.P., Hoogman M., Mostert J.C., Dammers J., Kan C.C., Vasquez A.A., Schene A.H., Buitelaar J., Franke B. Deviant white matter structure in adults with attention-deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;63:14–22. doi: 10.1016/j.pnpbp.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D. Oxford University Press; USA: 2009. Fiber Pathways of the Brain. [Google Scholar]
- Seidman L.J., Valera E.M., Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Valera E.M., Makris N., Monuteaux M.C., Boriel D.L., Kelkar K., Kennedy D.N., Caviness V.S., Bush G., Aleardi M., Faraone S.V., Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol. Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Shahand S., Santcroos M., Mohammed Y., Korkhov V., Luyf A., van Kampen A.H.C., Olabarriaga S.D. Heal. Vol. 2011. 2011. Front-ends to biomedical data analysis on grids. [Google Scholar]
- Shaw P., Lerch J., Greenstein D., Sharp W., Clasen L., Evans A., Giedd J., Castellanos F.X., Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P., Eckstrand K., Sharp W., Blumenthal J., Lerch J.P., Greenstein D., Clasen L., Evans A., Giedd J., Rapoport J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk T.J., Vance A., Rinehart N., Bradshaw J.L., Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum. Brain Mapp. 2009;30:2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D.J., Hallquist M.N., Asato M., Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W., Peterson B.S. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Yang X.-R., Carrey N., Bernier D., MacMaster F.P. Cortical thickness in young treatment-naive children with ADHD. J. Atten. Disord. 2015;19:925–930. doi: 10.1177/1087054712455501. [DOI] [PubMed] [Google Scholar]
- Yap Q.J., Teh I., Fusar-Poli P., Sum M.Y., Kuswanto C., Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J. Neural Transm. 2013;120:1369–1395. doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]