Abstract
Background
Due to the lack of strong evidence to identify the relationship between antihypertensive drugs use and the risk of prostate cancer, it was needed to do a systematic review to go into the subject.
Methods
We systematically searched PubMed, Web of Science and Embase to identify studies published, through May 2015. Two evaluators independently reviewed and selected articles involving the subject. We used the Newcastle-Ottawa Scale (NOS) to assess the quality of the studies. All extracted results to evaluate the relationship between antihypertensive drugs usage and prostate cancer risk were pool-analysed using Stata 12.0 software.
Results
A total of 12 cohort and 9 case-control studies were ultimately included in our review. Most of the studies were evaluated to be of high quality. There was no significant relationship between angiotensin converting enzyme inhibitors (ACEI) usage and the risk of prostate cancer (RR 1.07, 95% CI 0.96–1.20), according to the total pool-analysed. Use of angiotensin receptor blocker (ARB) was not associated with the risk of prostate cancer (RR 1.09, 95% CI 0.97–1.21), while use of CCB may well increase prostate cancer risk based on the total pool-analysed (RR 1.08, 95% CI 1–1.16). Moreover, subgroup analysis suggested that use of CCB clearly increased prostate cancer risk (RR 1.10, 95% CI 1.04–1.16) in terms of case-control studies. There was also no significant relationship between use of diuretic (RR 1.09, 95% CI 0.95–1.25) or antiadrenergic agents (RR 1.22, 95% CI 0.76–1.96) and prostate cancer risk.
Conclusions
There is no significant relationship between the use of antihypertensive drugs (ACEI, ARB, beta-blockers and diuretics) and prostate cancer risk, but CCB may well increase prostate cancer risk, according to existing observational studies.
Electronic supplementary material
The online version of this article (10.1186/s12894-018-0318-7) contains supplementary material, which is available to authorized users.
Keywords: Antihypertensive drugs, Prostate cancer, Meta-analysis
Background
The prevalence of hypertension is consistently high in older adults and regarded as a vital risk factor for cardiovascular diseases, congestive heart failure, and coronary heart disease [1, 2]. Antihypertensive drugs including angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), calcium-channel blockers (CCB), alpha- and beta-blockers and diuretics, were mainly used for the control of blood pressure in patients with hypertension to prevent relevant cardiovascular diseases [3]. Beneficial therapeutic effects of these drugs, on blood pressure control, have been well established in previous literature [4].
However, antihypertensive drugs and cancer risk have long been raised as concerns [5]. It was first reported that Rauwolfia derivatives increased the risk of breast cancer [6]. After that, several classes of antihypertensive agents appeared to elevate cancer risk, but the relationship between antihypertensive drug usage and increased cancer risk could not be confirmed in numerous studies due to their short follow-up and the cancer risk from hypertension itself [7]. A retrospective cohort study showed that the use of ACE inhibitors has an association with a clearly decreased risk of overall cancer [8]. Meanwhile, a meta-analysis demonstrated that there was no association between the use of ACE inhibitors or angiotensin-receptor blockers and the overall risk of cancer [9]. A large-scale, population-based cohort study proposed that there was no substantial association between the use of calcium channel blockers (CCB) and the incidence rate of cancer or cancer mortality [10]. In a study by Hershel et al. study, there was small positive association between CCB usage and risk of cancer [11].
The incidence of prostate cancer is increasing, and it is the main cause of cancer death in males in the Western countries [12]. Studies on the association between antihypertensive drug usage and prostate cancer risk remain controversial. In vitro studies, CCB enhanced apoptosis of prostate cancer cells and might have a protective effect on prostate cancer [13]. Debes et al. found that CCB significantly decreased the risk of prostate cancer, and their results varied by family history of prostate cancer [14]. However, some case-control studies did not find the this relationship [15]. For instance, a study with 1,165,781 patients did not support the association between the long-term use of CCB and prostate cancer risk [16]. Some case-control studies showed significantly increased risk between ACE inhibitor usage and prostate cancer [15, 17], while a previous meta-analysis showed that ACE inhibitors or angiotensin-receptor blockers did not affect the occurrence of cancer [18]. In a study by Perron et al., beta-blockers were associated with a reduction in prostate cancer risk, while another study by Kemppainen et al. tended to show an increased risk of prostate cancer in patients treated by beta-blockers [15, 19]. One study reported the relationship between alpha- blockers or diuretic usage and prostate cancer risk and their results were also controversial [20].
Due to there being the long-term follow-up in the observational studies, we performed a systematic review of observational studies to confirm whether the use of antihypertensive drugs was able to result in the prostate cancer in the human body.
Methods
Our review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines.
Eligibility criteria
Studies were included if they met the following points: (1) Studies were cohort or case-control studies; and (2) the relationship between the use of one or more types of antihypertensive drugs and prostate cancer was reported in the study.
Search strategy
We systematically searched PubMed, Web of Science and Embase to identify studies published through May 2015. The search terms were composed of the following: “beta blockers”, “angiotensin converting enzyme inhibitors”, “angiotensin receptor blockers”, “calcium channel blockers” “alpha blockers” “antihypertensive drugs” and “prostate cancer”. The details of the search methods are summarized in the Additional file 1. We also screened the bibliographies of relevant articles to find additional articles that met the included standard. A language limitation was not set during the search process. We did not consider animal studies when we reviewed the records.
Study selection and data extraction
Two authors independently evaluated the studies retrieved from the databases to select the studies that met the inclusion criteria. Disagreements between the two reviewers were resolved by discussion or in consultation with an arbitrator. The following information was extracted from the included articles by the two authors independently: study design, geographic region, type of medication, sex, age range, follow-up time, adjusted factors in each study (Table1). The RR (relative risk), HR (hazard ratio), OR (odds ratio), SIR (standardized incidence ratio) along with their corresponding 95%CI (confidence interval), all of which indicated the relationship between antihypertensive drugs and prostate cancer were abstracted. If there was missing information in the article, we contacted the authors via e-mail or telephone. We used data from a 2 × 2 table to recalculate crude estimates when the outcome measures were unsuitable for meta-analysis and we failed to gain the data from the authors.
Methodological quality assessment
The quality of the observational studies was independently evaluated by two authors based on the Newcastle-Ottawa Assessment Scale(NOS) on the website (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The scale provided eight items consisting of three subscales: selection of subjects (four items), comparability of subjects (one item) and assessment of outcome/exposure (three items). The highest scores were nine for the eight items because there were two scores in the comparability of subjects. A study with greater than or equal to seven scores was considered to be of high methodological quality.
Data synthesis and analysis
Extracted RRs, HRs, ORs and SIRs and their 95% CIs that were adjusted for most confounders were pooled to compute the RR between antihypertensive drugs and prostate cancer risk [21]. The four measures of association above were expected to yield similar estimates of RR, due to the incidence of prostate cancer being generally low [22]. For a single study that reported more than one type of cancer, only the data on the risk between prostate cancer and antihypertensive drugs were extracted and then pooled. If there were studies involving multi-drug treatment and we were not familiar with the data that reported which specific drug was associated with the incidence of prostate cancer in these studies, the data of the study would not be extracted to conduct a meta-analysis. In the included studies, the data that reported the risk between a single antihypertensive drugs and the occurrence of prostate cancer will be pooled for analysis based on the single drug category respectively. The meta-analysis was performed with Stata 12.0. Since the clinical and methodological heterogeneity were known, we used a random-effects model to calculate pooled RRs and their 95%CIs. Subgroup analyses were performed according to whether they corresponded to case-control or cohort studies. Between-study heterogeneity was assessed by using Cochran’s Q statistic (significance level at p < 0.1) and by estimating I2. If I2 was more than 40%, there was significant heterogeneity among studies [23].
Results
Characteristic of included studies
Our search strategy yielded 729 records, A total of 193 and 491 records were excluded due to duplicated records and irrelevant subjects respectively. A total of 45 full-text articles were assessed and 21 studies that met our criteria were ultimately included (Fig. 1). These studies included 12 cohort studies [10, 20, 24–33], 4 nested case-control studies [11, 16, 34, 35] and 5 case-control studies [15, 17, 19, 36, 37]. The follow-up time in most cohort studies was more than 5 years. There were 11 studies that involved males only [10, 15, 19, 20, 24, 26, 28, 31, 35, 36, 38]. It was reported that the main outcomes of the included studies were adjusted for most of the confounding factors, and this information was missing in two studies (Tables 1 and 2) [10, 32].
Fig. 1.
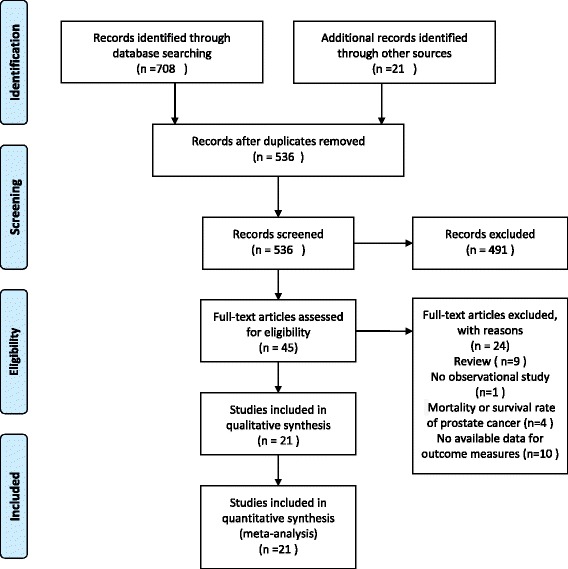
PRISMA flow diagram
Table 1.
Characteristics of cohort studies included in the meta-analysis
| Studies | Types of studies | Population and slection of cases | NO. of participants | Type of medication (reference group) | Duration of follow-up,yr | Sex (%) | Mean age (range), yr | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Pai, P. Y.et al. 2015 [20] | cohort study | Male patients with hypertension or without hypertension selected from CCHIA-NHI database | 80,299 | Diuretics, Alpha-blockersBeta-blockers, ARBsCCBs, ACEIOthers (no use of antihypertensive drugs) | 9 | Male (100) | 69.28 VS 69.31(50-) | Age, urbanization level, income, comorbidities |
| Rao, G. A. et al. 2013 [24] | cohort study | Males patients receiving drug treatment from VA of U.S.A. | 543,824 | ARBs (no use of ARBs) | 8 | Male (100) | 63.6 VS 63.6 | All 54 variables that was used to compute propensity to receive treatment |
| Bhaskaran, K. et al. 2012 [25] | cohort study | Hypertensive patients receiving drug treatment from General Practice Research Database (GPRD) of U.K. | 377,649 | ARBs (no use of ARBs) | >5 | M (52) F (48) | 64 (18–103) | Age, sex, BMI, smoking, alcohol, diabetes (with or without metformin/insulin use), hypertension, heart failure, statin use, index of multiple deprivation score, calendar year. |
| Rodriguez, C. 2009 [26] | cohort study | Males patients receiving drug treatment from the CPS-II Nutrition Cohort of U.S.A | 3031 | CCBs, Beta-blockers, ACEIs, diuretics, and other anti-hypertensives (no use of anti-hypertensive drugs) | 8 | Males (100) | NA | Age at interview, race, education, BMI in 1997, family history of prostate cancer, history of diabetes, history of PSA screening,history of heart disease or bypass surgery, and use of cholesterol-lowering drugs |
| van der Knaap, R. et al. 2008 [27] | cohort study | Eligible individuals from the Rotterdam Study started with a baseline interview between July 1989 and July 1993. | 7983 | ACEI and/or angiotensin II type 1 receptor antagonist (no use of the drugs) | 9.6 | M (38.7) F (61.3) | 70.4(50-) | Age, BMI, use of salicylates, diabetes mellitus, hypertension, and myocardial infarction. |
| Harris, A. M. et al. 2007 [28] | cohort study | Male patients receiving drug treatment seen at Lexington Veterans Affairs (VA) Hospital | 27,138 | α1-blockers (no use of α1-blockers) | >5 | Male (100) | 68 (50–89) VS 72 (46–99) | Unadjusted |
| Debes, J. D. et al. 2004 [29] | cohort study | Males from subgroup of Olmsted County Study of Urinary Symptoms and Health Status | 2115 | CCBs (no use of CCBs) | 10 | Male (100) | NA(40–79) | Age and family history of prostate cancer |
| Friis, S. et al. 2001 [30] | cohort study | Persons receiving drug treatment from Pharmacoepidemiological Prescription Research Database of North Jutland County, Denmark, | 17,897 | ACEI (no use of ACEI) | 8 | Male (50) Female (50) | 62(NA) | Adjustment for age, gender, and duration of follow-up |
| Fitzpatrick, A. L. 2001 [31] | cohort study | Individuals receiving drug treatment from chrot of the Cardiovascular Health Study (CHS) of USA | 2442 | CCBsACEIβ-blockersDiureticVasodilator (no use of antihypertensive drugs) | 5.6 | Male (100) | NA (65-) | Adjusted for age, race (black), and body mass index (BMI) |
| Sorensen, H. T. 2000 [10] | cohort study | Individuals taking CCBs from Pharmaco-Epidemiological Prescription Database of the County of North Jutland, Denmark | 23, 167 | CCBs (compared with the number expected, based on population rates from the Danish Cancer Registry) | 3.2 | Male (100) | 63.4 | NA |
| Olsen, J. H. 1997 [32] | cohort study | Individuals receiving treatment of CCBs from the County of North Jutland | 17,911 | CCBs (compared with the number expected, based on population rates from the Danish Cancer Registry) | 1.8 years | Male (49), Female (51) | NA | NA |
| Pahor, M. 1996 [33] | cohort study | Individuals aged 65 years or older living in East Boston, Massachusetts, and in the counties of Iowa and Washington in the state of Iowa from epidemiologic studies of the elderly (EPESE) in U.S. | 5052 | CCBs (no use of CCBs) | 3.6 | Male (35.7), Female (64.3) | MA (65-) | Adjusted for age, sex, ethnic origin, heart failure, number of hospital admissions, cigarette smoking, and alcohol intake. |
CCB calcium-channel blockers, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin II receptor blockers, NA not available
Table 2.
Characteristics of case-control studies included in the meta-analysis
| Studies | Types of studies | Case selection | NO. of participants | Collection of medication data (period) | Age of cases, yr., mean (range) | Sex of cases, % | Type of drugs (reference group) | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Hallas, J. 2012 [17] | Case control | Review of data from the Danish Cancer Registry (DCR), the Danish National Registry of Patients (DNRP),the Prescription Database of the DanishMedicines Agency and the Danish Person Registry (2000–2005). | 149, 417 | Review of electronic medical records (1995 until cancer diagnosis) | 69.4 | Male (47.7), Female (52.3) | Use of ARBs or ACEI (never-use of the durgs) | (1)chronic obstructive pulmonary disease (COPD) as a crude marker of heavy smoking; (2) inflammatory bowel disease; (3) a modified Charlson Index that contains 19 categories of comorbidity and each category has an associated weight based on the adjusted risk of 1 year mortality; (4) non-steroidal antiinflammatory drugs (NSAIDs) or hig dose aspirin, oestrogen hormone therapy, oral contraceptives, finasteride or statins. |
| Azoulay, L. 2012 [39] | Nested case-control | Review of data from General Practice Research Database (GPRD)in U.K. (1995–2010) | 1,165,781 | Review of computerized medical records (1995 until cancer diagnosis) | 72.4 | Male (52.7), Female (47.3) | use of ARBs or ACEIs or CCBs or alpha-blockers(use of Diuretics and/or beta-blockers) | Excessive alcohol use, body mass index, smoking, diabetes, previous cancer, and ever of aspirin, statins, and NSAIDs. In addition,cholecystectomy, inflammatory bowel disease and history of polyps for colorectal cancer; benign prostatic hyperplasia, 5-alpha reductase inhibitors, and number of PSA tests for prostate cancer; oophorectomy, use of hormone replacement therapy, and prior use of oral contraceptives for breast cancer. |
| Kemppainen, K. J. 2011 [15] | Case control | Review of data from the Finnish Cancer Registry (1995–2002) | 25,029 | Review of the prescription database of the Social Insurance Institution of Finland (1995 until cancer diagnosis) | NA | Males (100) | use of ARBs or ACEIs or CCBs or alpha-blockers or beta-blockers or diuretics (Nonusers of any antihypertensive medication) | Adjusted for age, place of residence, and use of cholesterol-lowering drugs, antidiabetic drugs, finasteride, or alpha-blockers. |
| Assimes, T. L. 2008 [34] | Nested case-control | Review of computerized database files of Saskatchewan Health (1980–2003) | 11,697 | Review of the linkable databases including the world’s oldest electronic prescription database (1978 until cancer diagnosis) | 71.8 | Male (53.2) Female (46.8) | Use of β-blockers or CCBs or RAS inhibitors and never use of thiazide diuretics (use of thiazide diuretics and never use of β-blockers or CCBs or RAS inhibitors) | Adjusted for age, all measured comorbid conditions, and exposure to all other classes of antihypertensive not of interest except for potassium sparing diuretics. |
| Ronquist, G. 2004 [35] | Nested case-control | Review of the General Practice Research Database (GPRD) in U.K. (1995–1999) | 243,331 | Review of computerized medical records (1995 untilcancer diagnosis) | 50–79 | Males (100) | Use of diuretics, beta-blockers, ACE-inhibitors, CCBs, alpha-blockers and other antihypertensives (no use) | Adjusted for age, calendar year, prostatism and and other variables. |
| Perron, L. 2004 [19] | case-control | Review of the source population in Quebec cancer registry (1993–1995) | 13,326 | Review of computerized medical records (1981 untilcancer diagnosis) | 75.7 | Males(100) | Use of CCBs or ACEIs or beta-blockers or thiazidic diuretics and similars or others inlclusing vasodilatators and centrally acting adrenocep-tor antagonists. (no use) | Adjusted for age, recent medical contacts, and Aspirin use |
| Vezina, R. M. 1998 [36] | case-control | Monthly contact with the tumor registrar and review of Massachusetts Cancer Registry for males less than 70 years of age diagnosed with prostate cancers in Massachusetts (1992–1995) | 2617 | Telephone interview (lifetime until cancer diagnosis) | 64 | Males(100) | Use of CCBs or beta-blockers or ACEIs or Thiazides or others (no use) | Age; race; level of education; family history of prostate cancer; dietary fat intake; BMI; alcohol, tobacco, and coffee use; urologic symptoms; and physician visits 2 years previously. |
| Rosenberg, L. 1998 [37] | case-control | Interviewed patients aged 40 to 69 years in Boston, Mass, New York, NY, Philadelphia, Pa,and Baltimore, Md (1976–1996) | 16,005 | Interview with standard questionnaires by trained nurse (lifetime until cancer diagnosis) | 56(40–69) | Males (41) Females (59) | Use of CCBs or beta-blockers or ACEIs (no use) | Age, BMI, interview year, annual visits to a physician 2 yr. before admission, smoking amount(pack year) for all cancers, and other additional risk factors for regressions for each cancer site) |
| Jick, H.1997 [11] | Nested case-control | Review of all hypertensive patients on the General Practice Research Database (GPRD) who were current users of beta-blockers only, ACEIs only, or CCBs only (with or without diuretics) and who had a first-time diagnosis of any cancer recorded in 1995. | 2196 | Review of computerized medical records (1987 until cancer diagnosis) | 71.6 (NA) | Males (49.6) Females (50.4) | Use of CCBs (use of beta-blockers) | Smoking, BMI, change of medication, duration of hypertension, and diuretic use |
CCB calcium-channel blockers, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin II receptor blockers, NA not available
Quality of included studies
The results of the quality assessment for the included studies are summarized in Tables 3 and 4. Quality scores for cohort studies ranged between 5 and 9, and those for case-control studies ranged between 7 and 9. Five studies showed that their outcomes of interest were not present at the start of the study. Thirteen studies gained two scores in the section of comparability due to their well the control of confounding factors [15, 17, 24–27, 31, 33, 34–37, 39]. There was only one study whose ascertainment of exposure was deruved from self-report [26]. The duration of follow-up in two studies was less than 5 years [10, 32]. The non-response rate was low in the included cohort studies but the scores for this item were lacking in the case-control studies.
Table 3.
Assessment of the methodologic quality of the cohort studies included in meta-analysis
| Studies | Slection | Comparability | Outcome | Total scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 1 | 2 | 3 | ||
| Pai, P. Y.et al. 2015 [20] | + | + | + | + | + | + | + | + | 8 | |
| Rao, G. A. et al. 2013 [24] | + | + | + | + | + | + | + | + | + | 9 |
| Bhaskaran, K. et al. 2012 [25] | + | + | + | + | + | + | + | + | + | 9 |
| Rodriguez, C. 2009 [26] | + | + | + | + | + | + | + | + | 8 | |
| van der Knaap, R. et al. 2008 [27] | + | + | + | + | + | + | + | + | + | 9 |
| Harris, A. M. et al. 2007 [28] | + | + | + | + | + | 5 | ||||
| Debes, J. D. et al. 2004 [29] | + | + | + | + | + | + | + | + | 8 | |
| Friis, S. et al. 2001 [30] | + | + | + | + | + | + | + | 7 | ||
| Fitzpatrick, A. L. 2001 [31] | + | + | + | + | + | + | + | + | + | 9 |
| Sorensen, H. T. 2000 [10] | + | + | + | + | + | 5 | ||||
| Olsen, J. H. 1997 [32] | + | + | + | + | + | 5 | ||||
| Pahor, M. 1996 [33] | + | + | + | + | + | + | + | + | + | 9 |
+: the article gain 1 score in the item
Table 4.
Assessment of the methodologic quality of the case-control studies included in meta-analysis
| Studies | Slection | Comparability | Exposure | Total scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 1 | 2 | 3 | ||
| Hallas, J. 2012 [17] | + | + | + | + | + | + | + | + | + | 9 |
| Azoulay, L. 2012 [39] | + | + | + | + | + | + | + | + | 8 | |
| Kemppainen, K. J. 2011 [15] | + | + | + | + | + | + | + | 7 | ||
| Assimes, T. L. 2008 [34] | + | + | + | + | + | + | + | + | 8 | |
| Ronquist, G. 2004 [35] | + | + | + | + | + | + | + | + | 8 | |
| Perron, L. 2004 [19] | + | + | + | + | + | + | + | 7 | ||
| Vezina, R. M. 1998 [36] | + | + | + | + | + | + | + | + | 8 | |
| Rosenberg, L. 1998 [37] | + | + | + | + | + | + | + | + | + | 9 |
| Jick, H. 1997 [11] | + | + | + | + | + | + | + | 7 | ||
+: the article gain 1 score in the item
ACEI and prostate cancer risk
There were ten studies that reported the relationship between the use of ACE inhibitors and the risk of prostate cancer [15–17, 19, 26, 30, 31, 35–37]. We found no significant association between ACE inhibitor usage and the risk of prostate cancer in the meta-analysis of the ten studies (RR1.07, 95% CI0.96–1.20). However, obvious clear heterogeneity existed among these studies (I2 = 86%). Subgroup analysis also showed no significant relationship between the use of ACE inhibitor and the risk of prostate cancer according to the poolanalysis of cohort studies (RR0.92, 95% CI0.77–1.11) and case-control studies (RR1.11, 95% CI0.98–1.26) (Fig. 2).
Fig. 2.
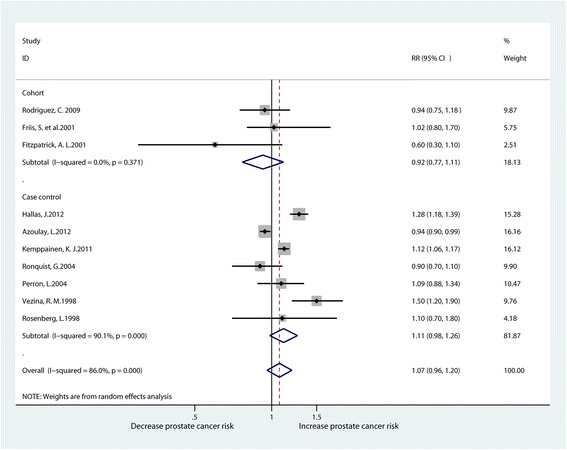
Forest plot for ACEI use and prostate cancer risk (RR relative risk, CI confidence interval)
ARB and prostate cancer risk
Five studies reported the association between ARB usage and the risk of prostate cancer [15–17, 24, 25]. There was no significant relationship between ARB usage and the risk of prostate cancer according to the pool-analysis of all studies (RR1.09, 95% CI0.97–1.21). Subgroup analysis also suggested no significant connection between the use of ARB and the risk of prostate cancer according to the pooled-analysis of cohort studies (RR1.00, 95% CI0.83–1.20) and case-control studies (RR1.16, 95% CI0.98–1.38). However, heterogeneity among these studies was high (I2 = 83.7%) (Fig. 3).
Fig. 3.
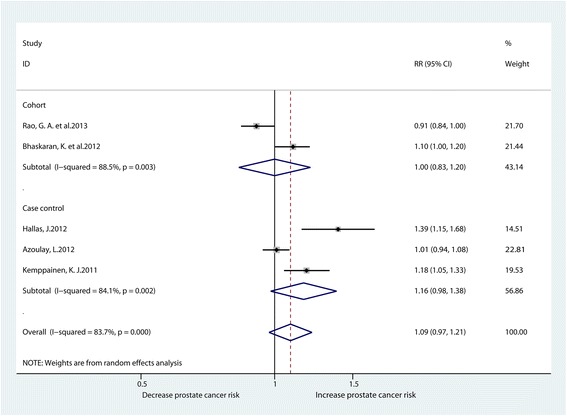
Forest plot for ARB use and prostate cancer risk (RR relative risk, CI confidence interval)
CCB and prostate cancer risk
A total of 14 studies reported the connection between CCB usage and the risk of prostate cancer [10, 11, 15, 16, 19, 26, 31–35, 36–38]. There appeared to be a significant association between CCB usage and the risk of prostate cancer, according to the meta-analysis of all studies (RR1.08, 95% CI1.00–1.16). There was considerable heterogeneity existing among these studies (I2 = 57.4%). Subgroup analysis also indicated that without significant relationship between CCB usage and the risk of prostate cancer in terms of cohort studies (RR0.93, 95% CI0.71–1.21) but there was clear association between the use of CCB and the risk of prostate cancer according to the pool-analysis of case-control studies (RR1.10, 95% CI1.04–1.16) (Fig. 4).
Fig. 4.
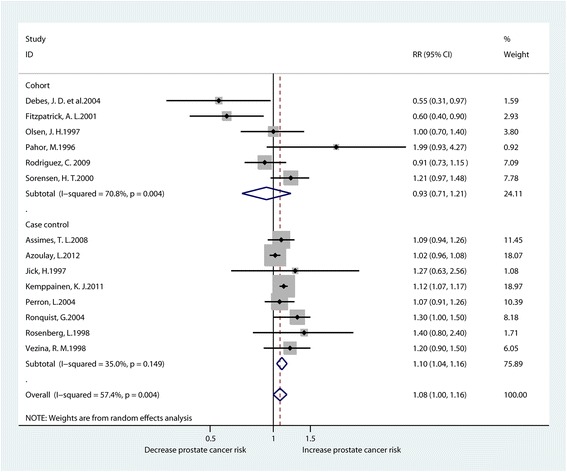
Forest plot for CCB use and prostate cancer risk (RR relative risk, CI confidence interval)
Beta-blockers and prostate cancer risk
There were 8 studies involving the subject of the relationship between beta-blocker usage and the risk of prostate cancer [15, 19, 26, 31, 34–37]. The meta-analysis of all studies suggested that there was no significant association between beta-blocker usage and the risk of prostate cancer (RR0.91, 95% CI0.81–1.02). We also did not find a significant connection between beta-blocker usage and the risk of prostate cancer, according to the pooled analysis of cohort (RR0.85, 95% CI0.69–1.04) or case-control studies (RR0.92, 95% CI0.81–1.05). There was significant heterogeneity existing among these studies (I2 = 70.1%) (Fig. 5).
Fig. 5.
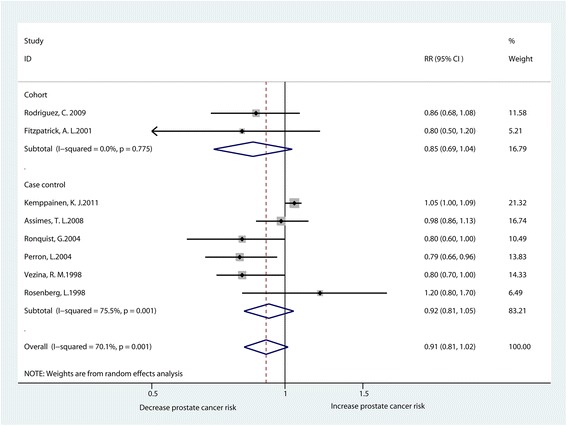
Forest plot for beta-blockers use and prostate cancer risk (RR relative risk, CI confidence interval)
Diuretics or antiadrenergic drugs and prostate risk
There were 5 and 4 studies involving the association between the use of diuretics [15, 31, 36, 35] and antiadrenergic drugs [15, 16, 28, 31, 35] and the risk of prostate cancer, respectively. We did not find significant association between the use of antiadrenergic drugs and the risk of prostate cancer (RR1.22, 95% CI0.76–1.96). The relationship between the use of diuretics and the risk of prostate cancer was demonstrated as not significant(RR1.09, 95% CI0.95–1.25). Significant associations between antiadrenergic drug usage and the risk of prostate cancer were found according to the cohort studies (RR0.71, 95% CI0.57–0.90) (Fig. 6).
Fig. 6.
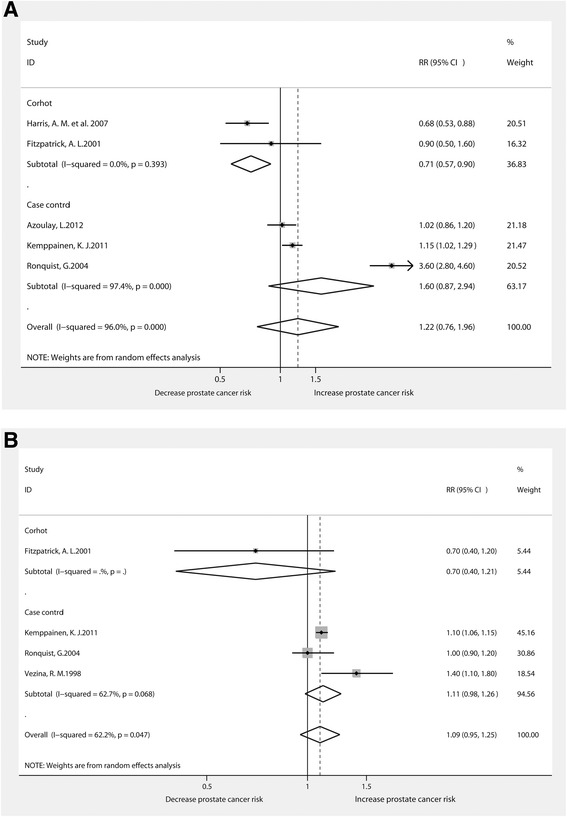
Forest plot for use of antiadrenergic agents or diuretic and prostate cancer risk: a antiadrenergic agents and b diuretic. RR relative risk, CI confidence interval
Discussion
To the best of our knowledge, this is first meta-analysis to investigate the relationship between the use of antihypertensive drugs and the risk of prostate cancer. Our main findings suggested that there was no significant association between the use of ACE inhibitors, ARB, beta-blockers, diuretics or antiadrenergic drugs and the risk of prostate cancer. However, CCB usage appeared to be associated with the risk of prostate cancer. Moreover, that considerable heterogeneity existed among the studies resulted in the reduction of evidence levels.
Prostate cancer is the most frequently diagnosed cancer diagnosed and the main causes of cancer death in older men in Western countries. Antihypertensive drugs such as ACE inhibitors and ARB are widely used for the management of hypertension and the prevention of cardiovascular disease events in high-risk persons [4, 40, 41]. Patients usually have long-term use of two or more different types of antihypertensive drugs to control their blood pressure [42]. The long-term ingestion of antihypertensive drugs can lead to adverse effects in patients, such as hypercholesterolemia, diabetes mellitus, chronic renal disease, and other cardiovascular diseases [43]. In addition, discussion of the connection between the use of antihypertensive drugs and the risk of cancer has consistently been a hot topic since the first relevant study was raised. We know that the older men are exposed to the condition of frequent use of antihypertensive drugs, and there is a high incidence of prostate cancer in this population [12, 44].
The topic of the relationship between the use of antihypertensive drugs and the risk of prostate cancer remain controversial, especially in the use of CCB and ACE inhibitors or ARB. The findings of a case-control study by Vezina, RM et al. suggested that there was no association between the use of CCB and the risk of prostate cancer in men younger than the age of 70 [36]. Debes, JD et al. found that there was an inverse association between prostate cancer and the use of CCB, and the result varied according to a family history of prostate cancer [14]. Loughlin KR reviewed relevant literature and thought that CCB had a protective effect on the development of prostate cancer on a basic science level, although the association in clinical practice has been controversial [45].
ACEI and ARB have been successfully used as potent antihypertensive drugs for a long period of time, and some literatures have suggested that these drugs could serve as new anticancer drugs [46]. The increased expression of AngII type 1 receptor (AT1R) mRNA was found in prostate cancer tissues compared with expression levels in the normal human prostate [47]. According to the data from basic science, ACE inhibitors or ARB might have a protective role in cancer [48]. A meta-analysis of randomized controlled trials (RCT) suggested that ARB are associated with an increased risk of new cancer diagnoses, while another meta-analysis of observational studies did not find significant associations between the use of ACE inhibitors or ARB and the risk of cancer, noting that the previous meta-analysis of RCT had a short duration of follow-up [9, 18]. In a case-control study, Hallas J et al. found significantly elevated OR for prostate cancer (OR 1.28, 95% CI 1.18, 1.39) in the patients using ACE inhibitors [17]. A cohort study by Rodriguez, C et al. indicated that there was no significant relationship between the use of ACE inhibitors and the risk of prostate cancer [26].
A meta-analysis suggested that the use of BB was associated with reduced specific mortality among patients with prostate cancer [49]. However, the relationship between BB usage and the risk of prostate cancer lacks consistent evidence. Perron, L et al. found that the long-term use of BB might prevent prostate cancer (OR 0.79, 95% CI 0.66, 0.96) [19]. However, Kemppainen, KJ et al. found that beta-blockers were associated with a marginally elevated risk of prostate cancer (OR 1.05, 95% CI 1.00,1.09) [15]. Fewer studies have paid attention to the relationship between the use of diuretics or antiadrenergic drugs and prostate risk compared with other types of antihypertensive drugs and their use also had different results [15, 20].
The present systematic review and meta-analysis provided a summary analysis of previous relevant studies which could yield a conclusion characterized by compromise. According to the pooled analyses of cohort studies, we generally did not find significant associations between the use of antihypertensive drugs and the risk of prostate cancer. Nevertheless, CCB usage might contribute to the higher risk of prostate cancer, based on findings from the case-control studies. Moreover, antiadrenergic drugs had a protective effect on the development of prostate cancer, according to the meta-analysis of two cohort studies. As we know, there was longer duration of follow-up in observational studies and we only reviewed these studies to explore whether the long-term use of antihypertensive drugs affected the incidence of prostate cancer in natural population. In the present review, most of the studies had a follow-up time of at least five years, and we had a larger sample to analyse than any previous studies. The main confounding factors, such as age, BMI, and race were adjusted for in most of the studies. Although we included studies with samples containing females, the relative risk was calculated under an adjustment for sex. Moreover, most of the studies were assessed with a high quality of methodology. As a result, our pooled analyses provided a conclusion that was more closer to the truth.
Our studies also had some limitations. First, our evidence grade was compromised by the considerable heterogeneity, which might be caused by various factors, including study design, race, follow-up time, social background. Second, small sample were analysed on the association between diuretics or antiadrenergic drugs and prostate risk, and the results should be cautiously considered. Third, we did not detect the publication bias, although it might exist among these studies. Finally, we did not conduct a dose-response meta-analysis due to the lack of relevant data in the included studies.
Conclusions
We reviewed 21 observational studies and found that there was no significant association between use of antihypertensive drugs and the risk of prostate cancer. Nevertheless, CCB usage might contribute to the higher risk of prostate cancer based on findings from the case-control studies. Unfortunately, the high heterogeneity downgraded the evidence level and more well-designed studies with large samples are needed.
Additional file
Search strategy for relevant studies. (DOCX 12 kb)
Acknowledgments
Not applicable
Funding
The innovation program of Critical illness (Shaanxi administration of traditional Chinese medicine) no.2015-JDM.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitors
- ARB
Angiotensin II receptor blockers
- CCB
Calcium-channel blockers
- NOS
Newcastle-Ottawa Scale
- RR
Relative risk
- SMD
Standardized mean difference
Authors’ contributions
LC: Project development, articles search, review, data extract, statistical analysis, and manuscript writing and revising; SZ: Project development, data extract, statistical analysis and articles search; CMJ: articles search, review, statistical analysis and manuscript writing; WH: review, data extract, statistical analysis, and manuscript writing and revising; LTW: data extract, statistical analysis, and manuscript writing and revising; YQL: data extract, and statistical analysis, and manuscript writing and revising; WW: articles search, review and pictures production; ZL: Project development and pictures production; JM: Project development, and manuscript writing and revising; All authors have read and approve of the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
All authors declare that they have no conflict of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12894-018-0318-7) contains supplementary material, which is available to authorized users.
Contributor Information
Liang Cao, Email: axbbzl@sina.com.
Sha Zhang, Email: osrqmq@sina.com.
Cheng-ming Jia, Email: wnyvsk@sina.com.
Wei He, Email: aecbin@sina.com.
Lei-tao Wu, Email: swwtes@sina.com.
Ying-qi Li, Email: miuqyb@sina.com.
Wen Wang, Email: xtpldz@sina.com.
Zhe Li, Email: lizhetcm@163.com.
Jing Ma, Email: jingma@fmmu.edu.cn.
References
- 1.Vokonas PS, Kannel WB, Cupples LA. Epidemiology and risk of hypertension in the elderly: the Framingham study. Journal Hypertens Suppl. 1988;6:S3–S9. [PubMed] [Google Scholar]
- 2.Redmond N, Booth JN, 3rd, Tanner RM, Diaz KM, Abdalla M, Sims M, et al. Prevalence of masked hypertension and its association with subclinical cardiovascular disease in African Americans: results from the Jackson heart study. J Am Heart Assoc. 2016;5:e002284. doi: 10.1161/JAHA.115.002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs--overview and meta-analyses. J Hypertens. 2015;33:195–211. doi: 10.1097/HJH.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 4.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 5.Aromaa A, Hakama M, Hakulinen T, Saxen E, Teppo L, Ida lan-Heikkila J. Breast cancer and use of rauwolfia and other antihypertensive agents in hypertensive patients: a nationwide case-control study in Finland. Int J Cancer. 1976;18:727–738. doi: 10.1002/ijc.2910180603. [DOI] [PubMed] [Google Scholar]
- 6.Stanford JL, Martin EJ, Brinton LA, Hoover RN. Rauwolfia use and breast cancer: a case-control study. J Natl Cancer Inst. 1986;76:817–822. [PubMed] [Google Scholar]
- 7.Dyer AR, Stamler J, Berkson DM, Lindberg HA, Stevens E. High blood-pressure: a risk factor for cancer mortality? Lancet. 1975;1:1051–1056. doi: 10.1016/S0140-6736(75)91826-7. [DOI] [PubMed] [Google Scholar]
- 8.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 9.Yoon C, Yang HS, Jeon I, Chang Y, Park SM. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. Can Med Assoc J. 2011;183:E1073–E1E84. doi: 10.1503/cmaj.101497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen HT, Olsen JH, Mellemkjaer L, Marie A, Steffensen FH, McLaughlin JK, et al. Cancer risk and mortality in users of calcium channel blockers. A cohort study. Cancer. 2000;89:165–170. doi: 10.1002/1097-0142(20000701)89:1<165::AID-CNCR21>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997;349:525–528. doi: 10.1016/S0140-6736(97)80084-0. [DOI] [PubMed] [Google Scholar]
- 12.Dijkman GA, Debruyne FM. Epidemiology of prostate cancer. Eur Urol. 1996;30:281–295. doi: 10.1159/000474185. [DOI] [PubMed] [Google Scholar]
- 13.Jan CR, Lee KC, Chou KJ, Cheng JS, Wang JL, Lo YK, et al. Fendiline, an anti-anginal drug, increases intracellular Ca2+ in PC3 human prostate cancer cells. Cancer Chemother Pharmacol. 2001;48:37–41. doi: 10.1007/s002800000262. [DOI] [PubMed] [Google Scholar]
- 14.Debes JD, Roberts RO, Jacobson DJ, Girman CJ, Lieber MM, Tindall DJ, et al. Inverse association between prostate cancer and the use of calcium channel blockers. Cancer Epidem Biomar. 2004;13:255–259. doi: 10.1158/1055-9965.EPI-03-0093. [DOI] [PubMed] [Google Scholar]
- 15.Kemppainen KJ, Tammela TL, Auvinen A, Murtola TJ. The associationbetween anti-hypertensive drug use and incidence of prostate cancer in Finland: a population-based case–control study. Cancer Causes Control. 2011;22:1445–1452. doi: 10.1007/s10552-011-9819-3. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One. 2012;7:e50893. doi: 10.1371/journal.pone.0050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallas J, Christensen R, Andersen M, Friis S, Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol. 2012;74:180–188. doi: 10.1111/j.1365-2125.2012.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sipahi I, Chou J, Mishra P, Debanne SM, Simon DI, Fang JC. Meta-analysis of randomized controlled trials on effect of Angiotensin-converting enzyme inhibitors on cancer risk. Am J Cardiol. 2011;108:294–301. doi: 10.1016/j.amjcard.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 20.Pai PY, Hsieh VC, Wang CB, Wu HC, Liang WM, Chang YJ, et al. Long term antihypertensive drug use and prostate cancer risk: a 9-year population-based cohort analysis. Int J Cardiol. 2015;193:1–7. doi: 10.1016/j.ijcard.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 22.Siristatidis C, Sergentanis TN, Kanavidis P, Trivella M, Sotiraki M, Mavromatis I, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer--a systematic review and meta-analysis. Hum Reprod Update. 2013;19:105–123. doi: 10.1093/humupd/dms051. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPTGS. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011]. The Cochrane collaboration. 2011. [Google Scholar]
- 24.Rao GA, Mann JR, Bottai M, Uemura H, Burch JB, Bennett CL, et al. Angiotensin receptor blockers and risk of prostate cancer among United States veterans. J Clin Pharmacol. 2013;53:773–778. doi: 10.1002/jcph.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK general practice research database. BMJ. 2012;344:e2697. doi: 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez C, Jacobs EJ, Deka A, Patel AV, Bain EB, Thun MJ, et al. Use of blood-pressure-lowering medication and risk of prostate cancer in the cancer prevention study II nutrition cohort. Cancer Causes Control. 2009;20:671–679. doi: 10.1007/s10552-008-9280-0. [DOI] [PubMed] [Google Scholar]
- 27.van der Knaap R, Siemes C, Coebergh JW, van Duijn CM, Hofman A, Stricker BH. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: the Rotterdam study. Cancer. 2008;112:748–757. doi: 10.1002/cncr.23215. [DOI] [PubMed] [Google Scholar]
- 28.Harris AM, Warner BW, Wilson JM, Becker A, Rowland RG, Conner W, et al. Effect of alpha1-adrenoceptor antagonist exposure on prostate cancer incidence: an observational cohort study. J Urol. 2007;178:2176–2180. doi: 10.1016/j.juro.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debes JD, Roberts RO, Jacobson DJ, Girman CJ, Lieber MM, Tindall DJ, et al. Inverse association between prostate cancer and the use of calcium channel blockers. Cancer Epidemiol, Biomarkers Prev. 2004;13:255–259. doi: 10.1158/1055-9965.EPI-03-0093. [DOI] [PubMed] [Google Scholar]
- 30.Friis S, Sorensen HT, Mellemkjaer L, McLaughlin JK, Nielsen GL, Blot WJ, et al. Angiotensin-converting enzyme inhibitors and the risk of cancer: a population-based cohort study in Denmark. Cancer. 2001;92:2462–2470. doi: 10.1002/1097-0142(20011101)92:9<2462::AID-CNCR1596>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick AL, Daling JR, Furberg CD, Kronmal RA, Weissfeld JL. Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann Epidemiol. 2001;11:534–542. doi: 10.1016/S1047-2797(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 32.Olsen JH, Sorensen HT, Friis S, McLaughlin JK, Steffensen FH, Nielsen GL, et al. Cancer risk in users of calcium channel blockers. Hypertension. 1997;29:1091–1094. doi: 10.1161/01.HYP.29.5.1091. [DOI] [PubMed] [Google Scholar]
- 33.Pahor M, Guralnik JM, Ferrucci L, Corti MC, Salive ME, Cerhan JR, et al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet. 1996;348:493–497. doi: 10.1016/S0140-6736(96)04277-8. [DOI] [PubMed] [Google Scholar]
- 34.Assimes TL, Elstein E, Langleben A, Suissa S. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf. 2008;17:1039–1049. doi: 10.1002/pds.1656. [DOI] [PubMed] [Google Scholar]
- 35.Ronquist G, Garcia Rodriguez LA, Ruigomez A, Johansson S, Wallander MA, Frithz G, et al. Association between Captopril, other antihypertensive drugs and risk of prostate cancer. Prostate. 2004;58:50–56. doi: 10.1002/pros.10294. [DOI] [PubMed] [Google Scholar]
- 36.Vezina RM, Lesko SM, Rosenberg L, Shapiro S. Calcium channel blocker use and the risk of prostate cancer. Am J Hypertens. 1998;11:1420–1425. doi: 10.1016/S0895-7061(98)00176-9. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg L, Rao RS, Palmer JR, Strom BL, Stolley PD, Zauber AG, et al. Calcium channel blockers and the risk of cancer. JAMA. 1998;279:1000–1004. doi: 10.1001/jama.279.13.1000. [DOI] [PubMed] [Google Scholar]
- 38.Debes JD, Roberts RO, Jacobson DJ, Girman CJ, Lieber MM, Tindall DJ, et al. Inverse association between prostate cancer and the use of Calcium Channel blockers. Cancer Epidemio Biomark Prev. 2004;13:255–259. doi: 10.1158/1055-9965.EPI-03-0093. [DOI] [PubMed] [Google Scholar]
- 39.Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of Angiotensin receptor blockers and the risk of cancer. PLoS One 2012;7:e50893. [DOI] [PMC free article] [PubMed]
- 40.Kario K, Saito I, Kushiro T, Teramukai S, Ishikawa Y, Mori Y, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension. 2014;64:989–996. doi: 10.1161/HYPERTENSIONAHA.114.04262. [DOI] [PubMed] [Google Scholar]
- 41.Heerspink HJ, Gao P, de Zeeuw D, Clase C, Dagenais GR, Sleight P, et al. The effect of ramipril and telmisartan on serum potassium and its association with cardiovascular and renal events: results from the ONTARGET trial. Eur J Prev Cardiol. 2014;21:299–309. doi: 10.1177/2047487313510678. [DOI] [PubMed] [Google Scholar]
- 42.Richards TR, Tobe SW. Combining other antihypertensive drugs with beta-blockers in hypertension: a focus on safety and tolerability. Can J Cardiol. 2014;30:S42–S46. doi: 10.1016/j.cjca.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Karagiannis A, Tziomalos K, Anagnostis P, Gossios TD, Florentin M, Athyros VG, et al. The effect of antihypertensive agents on insulin sensitivity, lipids and haemostasis. Curr Vasc Pharmacol. 2010;8:792–803. doi: 10.2174/157016110793563906. [DOI] [PubMed] [Google Scholar]
- 44.Landahl S, Bengtsson C, Sigurdsson JA, Svanborg A, Svardsudd K. Age-related changes in blood pressure. Hypertension. 1986;8:1044–1049. doi: 10.1161/01.HYP.8.11.1044. [DOI] [PubMed] [Google Scholar]
- 45.Loughlin KR. Calcium channel blockers and prostate cancer. Urol Oncol. 2014;32:537–538. doi: 10.1016/j.urolonc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Lindberg H, Nielsen D, Jensen BV, Eriksen J, Skovsgaard T. Angiotensin converting enzyme inhibitors for cancer treatment? Acta Oncol. 2004;43:142–152. doi: 10.1080/02841860310022346. [DOI] [PubMed] [Google Scholar]
- 47.Uemura H, Ishiguro H, Nakaigawa N, Nagashima Y, Miyoshi Y, Fujinami K, et al. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Mol Cancer Ther. 2003;2:1139–1147. [PubMed] [Google Scholar]
- 48.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrin Met. 2005;16:293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Lu H, Liu X, Guo F, Tan S, Wang G, Liu H, et al. Impact of beta-blockers on prostate cancer mortality: a meta-analysis of 16,825 patients. OncoTargets Ther. 2015;8:985–990. doi: 10.2147/OTT.S78836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy for relevant studies. (DOCX 12 kb)
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


