Abstract
Background
Large scale association studies have found a significant association between type 2 diabetes mellitus (T2DM) and transcription factor 7-like 2 (TCF7L2) polymorphism rs7903146. However, the quality of data varies greatly, as the studies report inconsistent results in different populations. Hence, we perform this meta-analysis to give a more convincing result.
Methods
The articles, published from January 1st, 2000 to April 1st, 2017, were identified by searching in PubMed and Google Scholar. A total of 56628 participants (34232 cases and 22396 controls) were included in the meta-analysis. A total of 28 studies were divided into 4 subgroups: Caucasian (10 studies), East Asian (5 studies), South Asian (5 studies) and Others (8 studies). All the data analyses were analyzed by the R package meta.
Results
The significant association was observed by using the dominant model (OR = 1.41, CI = 1.36 - 1.47, p < 0.0001), recessive model (OR = 1.58, CI = 1.48 - 1.69, p < 0.0001), additive model(CT vs CC) (OR = 1.34, CI = 1.28-1.39, p < 0.0001), additive model(TT vs CC) (OR = 1.81, CI = 1.69-1.94, p < 0.0001)and allele model (OR = 1.35, CI = 1.31-1.39, p < 0.0001).
Conclusion
The meta-analysis suggested that rs7903146 was significantly associated with T2DM in Caucasian, East Asian, South Asian and other ethnicities.
Electronic supplementary material
The online version of this article (10.1186/s12881-018-0553-5) contains supplementary material, which is available to authorized users.
Keywords: T2DM, Polymorphism, rs7903146, Meta-analysis
Background
Diabetes is one of the largest global health emergencies in the twenty-first century. According to the International Diabetes Federation (IDF) [1], 46.5% of the adults with diabetes are undiagnosed, and 1 in 11 adults, about 415 million people, have diabetes. Every 6 s a person dies of diabetes (5.0 million deaths per year). By 2040, 1 in 10 adults, approximately 642 million people, will have diabetes. Notably, 12% of the global health expenditure, up to $673 billion, is dedicated to diabetes treatments, and the related take up most of the total expenditure.
The most prevalent form of diabetes is type 2 diabetes mellitus (T2DM), and in the developed countries up to 91% of the adults, who are being troubled by the diabetes, have T2DM. Excess body weight, physical inactivity, poor nutrition, genetics, family history of diabetes, past history of gestational diabetes and older age are risk factors that increase the rate of T2DM. Besides, T2DM is a complex disease, and and the function of the glycosylation plays a significant role [2, 3].
The SNP rs7903146(C/T) is a common variant in the gene TCF7L2, and allele T is the risk allele related to T2DM. The gene TCF7L2 is a transcription factor involved in the Wnt signaling pathway, and acts as a critical component of Wnt signalling and action [4–6]. The TCF7L2 gene product, a high mobility group box-containing transcription factor previously implicated in blood glucose homeostasis, is considered to act through the regulation of proglucagon gene expression in enteroendocrine cells via the Wnt signaling pathway [7]. In human islets, TCF7L2 expression associates positively with insulin gene expression [8, 9].
To address the genetic variations of T2DM, many scholars devoted themselves to the related research [10–16]. The common Pro12Ala polymorphism rs1801282 in PPAR γ, the E23K variant rs5219 in KCNJ11, the polymorphism of the 5-HT2C receptor rs3813929 and the VKORC1 polymorphism rs9923231 were found to be associated with T2DM [17–20]. In 2006, Grant SF, et al. [7] confirmed a strongly significant association between susceptibility related to T2DM and common variants in transcription factor 7-like 2 (TCF7L2) in Icelandic subjects, and the result was the same with case-control method in Danish cohort and U.S. cohort. In 2006, Cauchi et al. [21] reported that the T-allele of the single nucleotide polymorphism (SNP) rs7903146 increased the risk of T2DM in the French population with 2367 cases and 2499 controls.The same results were shown by Horikoshi, Yu and Barra in case of the Japanese population, African American population and Brasilia [22–24]. However, Zheng et al. [25] found no association between rs7903146 and T2DM in the Chinese population.
The quality of the data varies greatly, is one of the reasons that the studies report inconsistent results, and the small sample size is another reason. The statistical efficiency can be improved after combining some samples together. The collected data in the control group was tested by the Hardy-Weinberg Equilibrium (HWE) in view of the quality of data. Therefore, we conducted a meta-analysis of published studies involving rs7903146 and T2DM to achieve a more comprehensive result. Finally, a total of 28 studies from 26 single studies [4, 22–46] were collected to reevaluate the association between rs7903146 and T2DM.
Methods
Search strategy
The articles, published from January 1st, 2000 to April 1st, 2017, were identified by searching the keywords “rs7903146” and “type 2 diabetes mellitus” in PubMed and Google Scholar. The selected articles were written in English.
Study selection criteria
We selected studies according to the following criteria: (1) The study was designed based on the case-control method. (2) The study evaluated the association between rs7903146 and T2DM. (3) The number of genotypes in case-controls groups was provided for calculating Odds Ratios (ORs). (4) The control group meets HWE. Besides, the p value of HWE was calculated by R program HWE version 1.2 [47]. If p < 0.05, the article was preserved, otherwise the article was removed.
Data extraction
We extracted the following information from each study: (1) the first author of each article; (2) the publication year of each article; (3) the population of the study; (4) the ethnicity of individuals in each study; (5) the number of the rs7903146 genotypes both in cases and controls; (6) p value of HWE in the control group. We used R package meta to analyze the data. We also referred to some other methods [48–51] to conduct the meta-analysis.
Choice of genetic model
The rs7903146 has two alleles: C and T. We analyzed the association between rs7903146 and T2DM by using the dominant model (TT+CT versus CC), recessive model (TT versus CC+CT), additive model (CT versus CC), additive model(TT versus CC) and allele model (T versus C), respectively [52].
Heterogeneity test
Odds Ratios and 95% confidence intervals (CIs) were calculated to assess the association between rs7903146 and T2DM. The two quantities, Cochran’s Q and I2, were adopted to evaluate the heterogeneity in different kinds of ethnic groups. Q approximately follows a chi square distribution with k-1 degrees of freedom (where k is the number of studies), and the p value can be used to measure the significance level of the heterogeneity. The value of I2, ranging from 0 to 100%, is calculated according to the formula, which is I 2 = (Q-(K-1))/Q*100%. The low, moderate, and high heterogeneity were labelled by I2 levels of 25%, 50% and 75%, respectively. If I2 is less than 50%, or p is more than 0.10, the fixed effect model is used, otherwise the random effect model is adopted.
Meta-analysis and subgroup analysis
After the heterogeneity test, we used the R package meta to perform the experiment with the fixed effect model [53].
Publication bias analysis and sensitivity analysis
Begg’s test [54] and Egger’s test [55] were selected for testing the publication bias. When a two-tailed value is less than 0.05, the publication bias is significant.
Results
Literature search
A flow diagram for the study selection process was shown in Fig. 1. A total of 355 articles were identified by the search strategy, abd 28 studies from 26 articles were left. The detailed information about the search strategy was displayed in Additional file 1: Table S1.
Fig. 1.
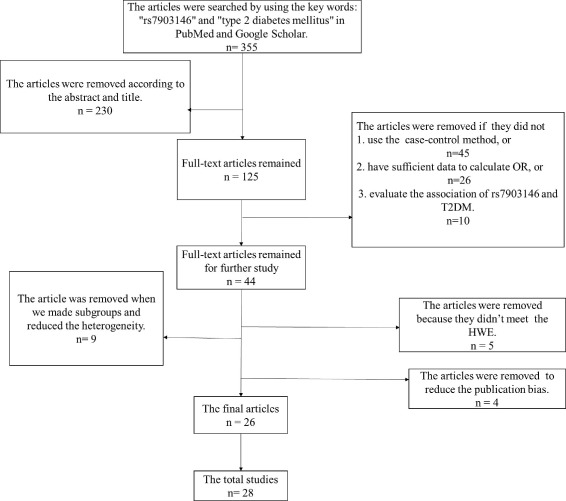
The flow chart of collecting articles for analyzing the association. And a total of 355 articles were identified by the search strategy. Firstly, a total of 230 articles were removed according to the title and abstract, and 45 articles were removed as the studies did not use case-control method, and 26 articles were removed as the studies did not have sufficient data to calculate OR, and 10 articles were excluded as they did not evaluate the association between rs7903146 and T2DM. After that 44 articles remained. Then, 5 articles were excluded as the control groups didn’t meet the Hardy-Weinberg Equilibrium (HWE), 9 articles were excluded when we made subgroup analyses and reduced the heterogeneity, and 4 articles were excluded as some LADA or type 1 diabetes patients were included in the case groups. Finally 28 studies from 26 articles were left
Study characteristics
As shown in Table 1, a total of 56628 participants (34232 cases and 22396 controls) of 28 studies from 26 articles were included in this meta-analysis. The studies were divided into Caucasian (10 studies) [4, 22, 29–36], East Asian (5 studies) [23, 25, 37–39], South Asian (5 studies) [42–46] and Others (Arab (2 studies) [26, 27], Black African (3 studies) [22, 28, 29] and Brazilian (3 studies) [24, 40, 41]) subgroups. The collected data, performed with the R package meta in this meta-analysis, was displayed in Additional file 1: Table S2.
Table 1.
The primary characteristics of the 28 studies
| T2DM | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Population | Ethnicity | CC | CT | TT | CC | CT | TT | HWE |
| Ezzidi et al. | 2009 | Arabic Tunisian | Arab | 250 | 396 | 217 | 181 | 235 | 95 | 0.227155 |
| Saadi et al. | 2008 | Arab | Arab | 30 | 54 | 11 | 71 | 94 | 23 | 0.388992 |
| Humphries et al. | 2006 | Afro-Caribbean | Black African | 141 | 136 | 30 | 161 | 124 | 26 | 0.75859 |
| Yu et al. | 2009 | African American | Black African | 255 | 212 | 48 | 1156 | 921 | 165 | 0.31807 |
| Danquah et al. | 2013 | Ghanaian | Black African | 273 | 323 | 78 | 182 | 165 | 28 | 0.257132 |
| Yu et al. | 2009 | USA Caucasian | Caucasian | 430 | 392 | 101 | 4295 | 3391 | 693 | 0.515248 |
| Groves et al. | 2006 | English | Caucasian | 771 | 960 | 270 | 1175 | 1084 | 217 | 0.944175 |
| Humphries et al. | 2006 | European | Caucasian | 601 | 665 | 193 | 1295 | 1001 | 197 | 0.854011 |
| Cauchi et al. | 2006 | Austrian | Caucasian | 200 | 208 | 78 | 555 | 432 | 88 | 0.759981 |
| Dahlgren et al. | 2007 | Swedish | Caucasian | 67 | 83 | 18 | 496 | 327 | 62 | 0.421344 |
| Mayans et al. | 2007 | Swedish | Caucasian | 452 | 318 | 54 | 532 | 253 | 35 | 0.480907 |
| Van et al. | 2007 | Dutch | Caucasian | 203 | 221 | 72 | 459 | 365 | 83 | 0.396927 |
| Kimber et al. | 2007 | English | Caucasian | 1405 | 1459 | 361 | 1714 | 1329 | 248 | 0.662991 |
| De Silva et al. | 2007 | English | Caucasian | 420 | 507 | 161 | 1032 | 887 | 180 | 0.58617 |
| Vcelak et al. | 2012 | Czech | Caucasian | 148 | 156 | 43 | 205 | 147 | 24 | 0.730572 |
| Hayashi et al. | 2007 | Japanese | East Asian | 1450 | 165 | 4 | 980 | 85 | 2 | 0.91209 |
| Horikoshi et al. | 2007 | Japanese | East Asian | 165 | 22 | 2 | 251 | 21 | 0 | 0.507848 |
| Kazuaki et al. | 2008 | Japanese | East Asian | 1921 | 228 | 5 | 1696 | 137 | 1 | 0.29539 |
| Yasuharu et al. | 2009 | Japanese | East Asian | 434 | 45 | 2 | 372 | 26 | 0 | 0.50056 |
| Zheng et al. | 2011 | Chinese | East Asian | 202 | 24 | 1 | 139 | 13 | 0 | 0.581813 |
| Marquezine et al. | 2007 | Brazilian | Brazilian | 45 | 54 | 13 | 564 | 603 | 128 | 0.070107 |
| Barra et al. | 2013 | Brazilian | Brazilian | 55 | 49 | 6 | 58 | 40 | 11 | 0.304112 |
| Assmann et al. | 2014 | Brazilian | Brazilian | 382 | 415 | 156 | 261 | 215 | 59 | 0.147418 |
| Bodhini et al. | 2007 | Asian Indian | South Asian | 462 | 455 | 114 | 555 | 391 | 92 | 0.531352 |
| Chandak et al. | 2007 | Indian | South Asian | 391 | 423 | 141 | 205 | 160 | 34 | 0.726021 |
| Rees et al. | 2008 | UK South Asian | South Asian | 352 | 360 | 116 | 222 | 166 | 44 | 0.12238 |
| Gupta et al. | 2010 | Indian | South Asian | 55 | 96 | 44 | 62 | 78 | 21 | 0.64658 |
| Hussain et al. | 2014 | Indian | South Asian | 25 | 36 | 7 | 43 | 35 | 4 | 0.349985 |
A total of 56628 participants (34,232 cases and 22,396 controls) of 28 studies from 26 articles were included in the study. The name of the first author, the publication year of, the population of the study, the ethnicity of the study, the genotypes of the case -control group and the P value of HWE. If the p value of HWE in control group met the selection criteria (P > 0.05), it would be preserved, otherwise the data would be removed
Heterogeneity test
According to the genotypes shown in Table1, a total of 28 studies were analyzed by the dominant model, recessive model, additive model and allele model, respectively. The heterogeneity of all subgroups was shown in Table 2. According to the data displayed in Table 2, we didn’t get the significant heterogeneity in the dominant model (p = 0.39 and I2 = 5.00%), recessive model (p = 0.33 and I2 = 9%), additive model (CT vs CC: p = 0.76 and I2 = 0.00%), additive model (TT vs CC: p = 0.15 and I2 = 22%) and allele model (p = 0.08 and I2 = 29%). As the p value was more than 0.1, we selected the fixed effect model.
Table 2.
The result of the heterogeneity in subgroup analyses
| Subgroup | Dominant | Recessive | Additive(CT vs CC) | Allele | Additive(TT vs CC) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P | I2 | P | I2 | P | I2 | P | I2 | P | |
| Caucasian | 28.00% | 0.18 | 0.00% | 0.51 | 9.00% | 0.36 | 38.00% | 0.1 | 20.00% | 0.26 |
| East Asian | 0.00% | 0.9 | 0.00% | 0.85 | 0.00% | 0.96 | 0.00% | 0.82 | 0.00% | 0.84 |
| South Asian | 0.00% | 0.9 | 0.00% | 0.47 | 0.00% | 0.97 | 0.00% | 0.7 | 0.00% | 0.44 |
| Others | 0.00% | 0.62 | 0.00% | 0.19 | 0.00% | 0.81 | 17.00% | 0.29 | 29.00% | 0.19 |
| Total | 5.00% | 0.39 | 9.00% | 0.33 | 0.00% | 0.76 | 29.00% | 0.08 | 22.00% | 0.15 |
The I2 and P value were used to test the heterogeneity by the dominant model (TT+CT versus CC), recessive model (TT versus CC+CT), additive model (CT versus CC), additive model (TT versus CC) and allele model (T versus C), respectively
Publication bias analysis and sensitivity analysis
The publication bias was not found in all models below. The p values of Begg’s test and Egger’s test for the dominant, recessive, additive (CT vs CC), additive (TT vs CC) and allele model are 0.7821 and 0.7352, 0.3635 and 0.441, 0.6354 and 0.711, 0.4528 and 0.5199, 0.6927 and 0.5673, respectively. The results were reflected in the funnel plots Fig. 2(a-e) directly.
Fig. 2.
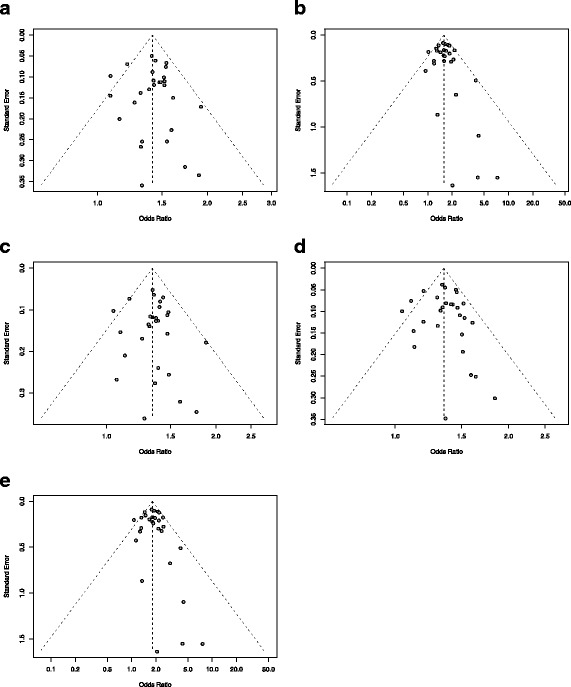
The funnel plots of publication bias in different models. The funnel plots showed the results of the publication bias analyses between rs7903146 and T2DM by using a Dominant Model, b Recessive Model, c Additive Model (CT vs CC), d Allele Model and e Additive Model (TT vs CC). The Y-axis indicated the standard error of each study, and the standard error was smaller, the effect of the meta-analysis would be better
Association between rs7903146 and type 2 diabetes mellitus
The association between rs7903146 and T2DM was shown in the forest plots: Figs. 3, 4, 5, 6 and 7 were the forest plots of the dominant model (TT+CT versus CC), recessive model (TT versus CC+CT), additive model (CT versus CC), allele model (T versus C) and additive model(TT versus CC), respectively. We made the Z test, and the result was displayed in the Table 3.
Fig. 3.
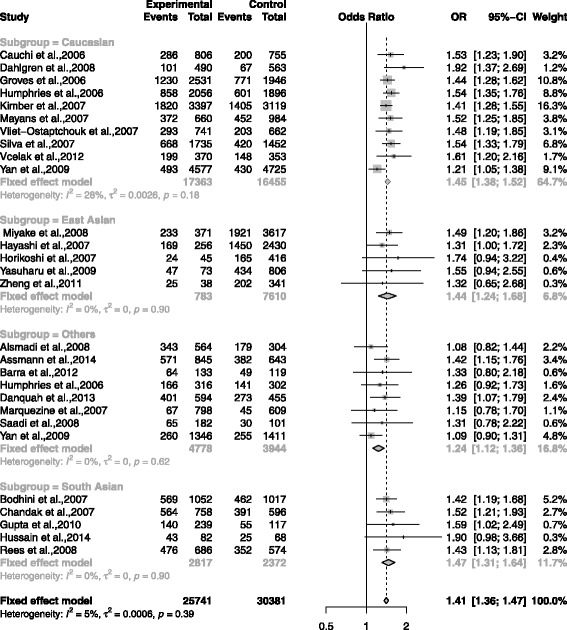
The forest plots for the meta-analysis of rs7903146 by using the dominant model. The data of CC/CT/TT was used in the dominant model (CT + TT vs CC)
Fig. 4.
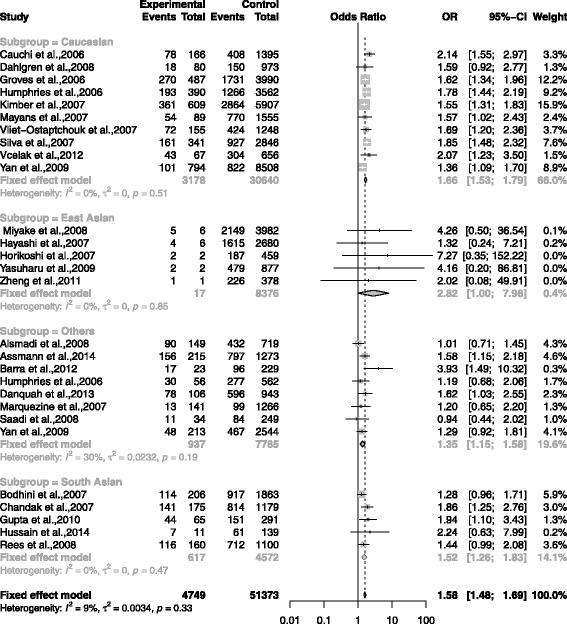
The forest plots for the meta-analysis of rs7903146 by using the recessive model. The data of CC/CT/TT was used in the recessive model (TT vs CC + CT)
Fig. 5.
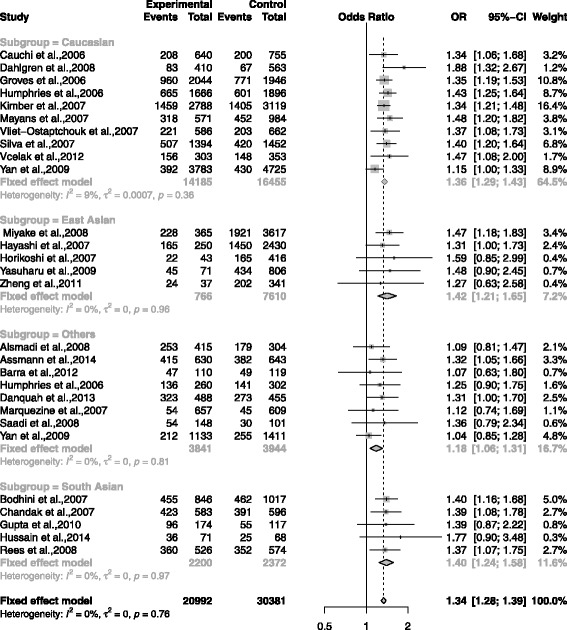
The forest plots for the meta-analysis of rs7903146 by using the additive model. The data of CC/CT/TT was used in the additive model (CT vs CC)
Fig. 6.

The forest plots for the meta-analysis of rs7903146 by using the allele model. The data of CC/CT/TT was used in the allele model (T vs C)
Fig. 7.
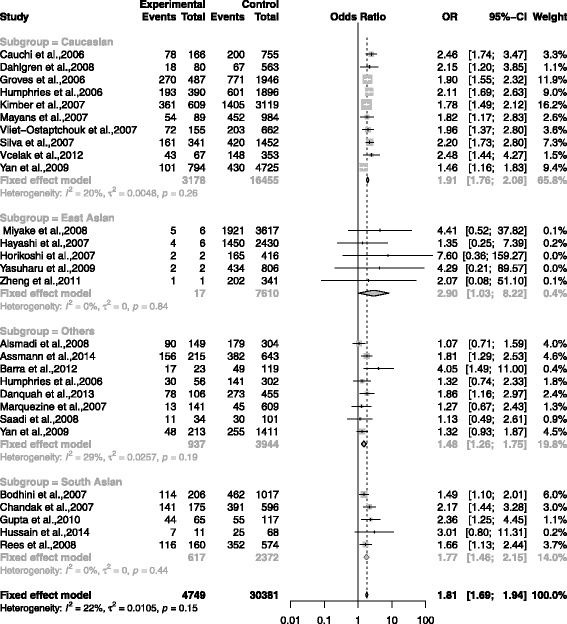
The forest plots for the meta-analysis of rs7903146 by using the additive model. The data of CC/CT/TT was used in the additive model (TT vs CC)
Table 3.
The result of the Z test in subgroup analyses
| Subgroup | Dominant | Recessive | Additive(CT vs CC) | Allele | Additive(TT vs CC) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z | P | Z | P | Z | P | Z | P | Z | P | |
| Caucasian | 14.86 | <0.0001 | 12.35 | <0.0001 | 11.67 | <0.0001 | 16.98 | <0.0001 | 15.15 | <0.0001 |
| South Asian | 4.69 | <0.0001 | 1.95 | 0.0509 | 4.42 | <0.0001 | 4.86 | <0.0001 | 2.01 | 0.0446 |
| East Asian | 6.61 | <0.0001 | 4.47 | <0.0001 | 5.45 | <0.0001 | 7.12 | <0.0001 | 5.83 | <0.0001 |
| Others | 4.17 | <0.0001 | 3.75 | 0.0002 | 3.11 | 0.0019 | 4.89 | <0.0001 | 4.65 | <0.0001 |
| Total | 17.2 | <0.0001 | 13.53 | <0.0001 | 13.73 | <0.0001 | 19.38 | <0.0001 | 13.73 | <0.0001 |
The Z test was performed with the dominant model (TT+CT versus CC), recessive model (TT versus CC+CT), additive model (CT versus CC), additive model (TT versus CC) and allele model (T versus C), respectively
In Caucasian subgroup, the results were shown as follows: dominant model (TT + CT vs CC): (OR = 1.45, CI = 1.38 - 1.52, p < 0.0001); recessive model (TT vs CC + CT): (OR = 1.66, CI = 1.53 - 1.79, p < 0.0001); additive model (CT vs CC): (OR = 1.36, CI = 1.29 - 1.43, p < 0.0001); additive model(TT vs CC): (OR = 1.91, CI = 1.76 - 2.08), p < 0.0001); allele model (T vs C): (OR = 1.37, CI = 1.32 - 1.43, p < 0.0001).
In East Asian subgroup, the results were shown as follows: dominant model (TT + CT vs CC): (OR = 1.44, CI = 1.24 - 1.68, p < 0.0001); recessive model (TT vs CC + CT): (OR = 2.82, CI = 1.00 - 7.98, p = 0.0509); additive model (CT vs CC): (OR = 1.42, CI = 1.21 - 1.65, p<0.0001); additive model(TT vs CC): (OR = 1.81, CI = 1.69 - 1.94, p < 0.0001); additive model(TT vs CC): (OR = 2.90, CI = 1.03 - 8.22, p = 0.0446); allele model (T vs C): (OR = 1.37, CI = 1.32 - 1.43, p < 0.0001).
In South Asian subgroup, the results were shown as follows: dominant model (TT + CT vs CC): (OR = 1.41, CI = 1.31 - 1.64, p < 0.0001); recessive model (TT vs CC + CT): (OR = 1.52, CI = 1.26 - 1.83, p < 0.0001); additive model (CT vs CC): (OR = 1.42, CI = 1.29 - 1.43, p < 0.0001); additive model(TT vs CC): (OR = 1.81, CI = 1.69 - 1.94, p < 0.0001); additive model(TT vs CC): (OR = 1.77, CI = 1.46 - 2.15, p < 0.0001) allele model (T vs C): (OR = 1.44, CI = 1.24 - 1.67, p < 0.0001).
In Others subgroup, the results were shown as follows: dominant model (TT + CT vs CC): (OR = 1.24, CI = 1.12 - 1.36, p < 0.0001); recessive model (TT vs CC + CT): (OR = 1.35, CI = 1.15 - 1.58, p = 0.0002); additive model (CT vs CC): (OR = 1.4, CI = 1.24 - 1.58, p = 0.0019); additive model(TT vs CC): (OR = 1.48, CI = 1.26 - 1.75, p < 0.0001); allele model (T vs C): (OR = 1.37, CI = 1.25 - 1.49, p < 0.0001).
In total groups, the results were shown as follows: dominant model (TT + CT vs CC): (OR = 1.41, CI = 1.36 - 1.47, p < 0.0001); recessive model (TT vs CC + CT): (OR = 1.58, CI = 1.48 - 1.69, p < 0.0001); additive model (CT vs CC): (OR = 1.34, CI = 1.28 - 1.39, P < 0.0001); additive model(TT vs CC): (OR = 1.81, CI = 1.69 - 1.94, p < 0.0001); allele model (T vs C): (OR = 1.35, CI = 1.31 - 1.39, p < 0.0001).
Discussion
In the meta-analysis, 56628 participants (34232 cases and 22396 controls) of 28 studies from 26 articles were included. The result of the four subgroups (Caucasian, East Asian, South Asian and Others) suggested that rs7903146 was significantly associated with T2DM in all subgroups and the total groups.
We removed each one of the studies in the groups or any subgroups in the dominant, recessive, additive and allele model for testing the robustness of results, respectively. The results did not change significantly, which displayed that the conclusion was robust. The heterogeneity and publication bias were not found in our meta-analysis.
We used the keywords “rs7903146”, “type 2 diabetes” and “meta-analysis” to search in PubMed, and got nine articles [46, 56–63]. Our work was different from others. We analyzed the association between rs7903146 and T2DM in Caucasian, East Asian, South Asian and Others groups. We did not find a significant heterogeneity in all subgroup analyses, so the fixed effect model was used. We found that rs7903146 was associated with T2DM in Caucasian, East Asian, South Asian and other ethnicities significantly.
Some limitations existed in this meta-analysis. Firstly, considering the heterogeneity in all subgroup analyses, we excluded 9 articles. More articles should be added into the meta-analysis. Secondly, some of the same cases or controls may be used in different studies.
Conclusion
The meta-analysis suggested that rs7903146 was significantly associated with T2DM in Caucasian, East Asian, South Asian and other ethnicities.
Additional file
Table S1. The detailed information about the search strategy. Table S2. The collected data in the meta-analysis. (XLSX 13 kb)
Acknowledgements
The authors gratefully thanked the editors and reviewers to help improve the manuscript.
Funding
This work was supported by China Natural Science Foundation (Grant No. 11301110), Natural Science Foundation of Heilongjiang Province of China (Grant No. QC2015076, No. A2015001 and No. LC2016024), China Postdoctoral Science Foundation (Grant No. 2015T80326 and No. 2013M541346), Heilongjiang Postdoctoral Fund (Grant No. LBH-TZ0504, No. LBH-Z13058 and No. LBH-Q13072), Open Project Program of Key Laboratory of Symbolic Computation and Knowledge Engineering of Ministry of Education of Jilin University (Grant No. 93K172016K16), Open Project of State Key Laboratory of Urban Water Resource and Environment of Harbin Institute of Technology (Grant No. ES201602) and National High-Tech Research and Development Program (863) of China (No: 2015AA020101, 2015AA020108, 2014AA021505).
Availability of data and materials
All the data generated or analyzed in this study was included in this manuscript.
Abbreviations
- CIs
Confidence intervals
- HWE
Hardy-Weinberg Equilibrium
- ORs
Odds ratio
- SNP
Single necluotide polymorphism
- T2DM
Type 2 diabetes mellitus
- TCF7L2
Transcription factor 7-like 2
Authors’ contributions
WYD wrote the paper. SLJ and LX revised the paper. WYD, ZJH, LJZ and SLJ collected and selected the data, designed and performed the experiment. QHJ and ZW conducted the project. ZJH and SLJ helped interpret the results. WYD and LX developed analytical tools. All authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they had no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12881-018-0553-5) contains supplementary material, which is available to authorized users.
Contributor Information
Weiyue Ding, Email: williamding@hrbeu.edu.cn.
Li Xu, Email: xuli@hrbeu.edu.cn.
Shuilin Jin, Email: jinsl@hit.edu.cn.
References
- 1.Cho NH, Whiting D, Forouhi N, Guariguata L, Hambleton I, Li R, Majeed A, Mbanya JC. Idf diabetes atlas. Brussels: International Diabetes Federation; 2015. [Google Scholar]
- 2.Yu X, Wang Y, Kristic J, Dong J, Chu X, Ge S, Wang H, Fang H, Gao Q, Liu D, et al. Profiling igg n-glycans as potential biomarker of chronological and biological ages: A community-based study in a han chinese population. Medicine. 2016;95(28). [DOI] [PMC free article] [PubMed]
- 3.Meng Q, Ge S, Yan W, Li R, Dou J, Wang H, Wang B, Ma Q, Zhou Y, Song M. Screening for potential serum-based proteomic biomarkers for human type 2 diabetes mellitus using maldi-tof ms PROTEOMICS-Clinical Applications. 2017;11(3-4). [DOI] [PubMed]
- 4.Vcelak J, Vejrakova D, Vaňkova M, Lukasova P, Bradnova O, Halkova T, Bestak J, Andělova K, Kvasnickova H, Hoskovcova P, et al. T2d risk haplotypes of the tcf7l2 gene in the czech population sample: the association with free fatty acids composition. Physiol Psychol. 2012; 61(3):229. [DOI] [PubMed]
- 5.Smith U. Tcf7l2 and type 2 diabetes—we wnt to know. Diabetologia. 2007;50(1):5–7. doi: 10.1007/s00125-006-0521-z. [DOI] [PubMed] [Google Scholar]
- 6.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, Macdougald OA. Inhibition of adipogenesis by wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 7.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, et al. Variant of transcription factor 7-like 2 (tcf7l2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 8.Lyssenko V, Lupi R, Marchetti P, Guerra SD, Orhomelander M, Almgren P, Sjogren M, Ling C, Eriksson K, Lethagen S, et al. Mechanisms by which common variants in the tcf7l2 gene increase risk of type 2 diabetes. J Clin Investig. 2007;117(8):2155–63. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osmark P, Hansson O, Jonsson AM, Ronn T, Groop L, Renstrom E. Unique splicing pattern of the tcf7l2 gene in human pancreatic islets. Diabetologia. 2009;52(5):850–4. doi: 10.1007/s00125-009-1293-z. [DOI] [PubMed] [Google Scholar]
- 10.Orahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: The end of the beginning? Science. 2005;307(5708):370–3. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 11.Manolopoulos VG, Ragia G, Tavridou A. Pharmacogenomics of oral antidiabetic medications: current data and pharmacoepigenomic perspective. Pharmacogenomics. 2011;12(8):1161–91. doi: 10.2217/pgs.11.65. [DOI] [PubMed] [Google Scholar]
- 12.Iordanidou M, Tavridou A, Petridis I, Arvanitidis KI, Christakidis D, Vargemezis V, Manolopoulos VG. The serotonin transporter promoter polymorphism (5-httlpr) is associated with type 2 diabetes. Clinica Chimica Acta. 2010;411(3):167–71. doi: 10.1016/j.cca.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Ragia G, Petridis I, Tavridou A, Christakidis D, Manolopoulos VG. Presence of cyp2c9* 3 allele increases risk for hypoglycemia in type 2 diabetic patients treated with sulfonylureas. Pharmacogenomics. 2009;10(11):1781–87. doi: 10.2217/pgs.09.96. [DOI] [PubMed] [Google Scholar]
- 14.Ragia G, Tavridou A, Petridis I, Manolopoulos VG. Association of kcnj11 e23k gene polymorphism with hypoglycemia in sulfonylurea-treated type 2 diabetic patients. Diabetes Res Clin Pract. 2012;98(1):119–24. doi: 10.1016/j.diabres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Zhao F, Mamatyusupu D, Wang Y, Fang H, Wang H, Gao Q, Dong H, Ge S, Yu X, Zhang J, et al. The uyghur population and genetic susceptibility to type 2 diabetes: potential role for variants in capn10, apm1 and fut6 genes. J Cell Mol Med. 2016;20(11):2138–47. doi: 10.1111/jcmm.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adua E, Roberts P, Sakyi SA, Yeboah FA, Dompreh A, Frimpong K, Anto EO, Wang W. Profiling of cardio-metabolic risk factors and medication utilisation among type ii diabetes patients in ghana: a prospective cohort study. Clin Transl Med. 2017;6(1):32. doi: 10.1186/s40169-017-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl M, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, et al. The common ppar|[gamma]| pro12ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26(1):76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 18.Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, et al. Large-scale association studies of variants in genes encoding the pancreatic β-cell katp channel subunits kir6. 2 (kcnj11) and sur1 (abcc8) confirm that the kcnj11 e23k variant is associated with type 2 diabetes. Diabetes. 2003;52(2):568–72. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 19.Iordanidou M, Tavridou A, Vasiliadis MV, Arvanitidis KI, Petridis J, Christakidis D, Vargemezis V, Bougioukas G, Manolopoulos VG. The-759c/t polymorphism of the 5-ht2c receptor is associated with type 2 diabetes in male and female caucasians. Pharmacogenet Genomics. 2008;18(2):153–9. doi: 10.1097/FPC.0b013e3282f4ae93. [DOI] [PubMed] [Google Scholar]
- 20.Tavridou A, Petridis I, Vasileiadis M, Ragia G, Heliopoulos I, Vargemezis V, Manolopoulos VG. Association of vkorc1- 1639 g/a polymorphism with carotid intima–media thickness in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;94(2):236–41. doi: 10.1016/j.diabres.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Cauchi S, Meyre D, Dina C, Choquet H, Samson C, Gallina S, Balkau B, Charpentier G, Pattou F, Stetsyuk V, et al. Transcription factor tcf7l2 genetic study in the french population. Diabetes. 2006;55(10):2903–8. doi: 10.2337/db06-0474. [DOI] [PubMed] [Google Scholar]
- 22.Yan Y, North KE, Ballantyne CM, Brancati FL, Chambless LE, Franceschini N, Heiss G, Kottgen A, Pankow JS, Selvin E, et al. Transcription factor 7-like 2 (tcf7l2) polymorphism and context-specific risk of type 2 diabetes in african american and caucasian adults the atherosclerosis risk in communities study. Diabetes. 2009;58(1):285–9. doi: 10.2337/db08-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikoshi M, Hara K, Ito C, Nagai R, Froguel P, Kadowaki T. A genetic variation of the transcription factor 7-like 2 gene is associated with risk of type 2 diabetes in the japanese population. Diabetologia. 2007;50(4):747–51. doi: 10.1007/s00125-006-0588-6. [DOI] [PubMed] [Google Scholar]
- 24.Barra GB, Dutra LAS, Watanabe S, Costa PGG, Cruz PSMD, Azevedo MF, Amato AA. Association of the rs7903146 single nucleotide polymorphism at the transcription factor 7-like 2 (tcf7l2) locus with type 2 diabetes in brazilian subjects. Arq Bras Endocrinol E Metabologia. 2012;56(8):479–84. doi: 10.1590/S0004-27302012000800003. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Ren W, Zhang S, Liu J, Li S, Li J, Yang P, He J, Su S, Li P. Association of type 2 diabetes susceptibility genes (tcf7l2, slc30a8, pcsk1 and pcsk2) and proinsulin conversion in a chinese population. Mol Biol Rep. 2011;39(1):17–23. doi: 10.1007/s11033-011-0705-6. [DOI] [PubMed] [Google Scholar]
- 26.Saadi H, Nagelkerke N, Carruthers SG. Association of tcf7l2 polymorphism with diabetes mellitus, metabolic syndrome, and markers of beta cell function and insulin resistance in a population-based sample of emirati subjects. Diabetes Res Clin Pract. 2008;80(3):392–398. doi: 10.1016/j.diabres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Ezzidi I, Mtiraoui N, Cauchi S, Vaillant E, Dechaume A, Chaieb M, Kacem M, Almawi WY, Froguel P, Mahjoub T, et al. Contribution of type 2 diabetes associated loci in the arabic population from tunisia: a case-control study. BMC Med Genet. 2009;10(1):33–33. doi: 10.1186/1471-2350-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danquah I, Othmer T, Frank LK, Beduaddo G, Schulze MB, Mockenhaupt FP. The tcf7l2 rs7903146 (t) allele is associated with type 2 diabetes in urban ghana: a hospital-based case–control study. BMC Med Genet. 2013;14(1):96–96. doi: 10.1186/1471-2350-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphries SE, Gable DR, Cooper JA, Ireland H, Stephens JW, Hurel SJ, Li KW, Palmen J, Miller MA, Cappuccio FP, et al. Common variants in the tcf7l2 gene and predisposition to type 2 diabetes in uk european whites, indian asians and afro-caribbean men and women. J Mol Med. 2006;84(12):1005–1014. doi: 10.1007/s00109-006-0108-7. [DOI] [PubMed] [Google Scholar]
- 30.Groves CJ, Zeggini E, Minton JAL, Frayling TM, Weedon MN, Rayner NW, Hitman GA, Walker M, Wiltshire S, Hattersley AT, et al. Association analysis of 6,736 u.k. subjects provides replication and confirms tcf7l2 as a type 2 diabetes susceptibility gene with a substantial effect on individual risk. Diabetes. 2006;55(9):2640–44. doi: 10.2337/db06-0355. [DOI] [PubMed] [Google Scholar]
- 31.Cauchi S, Achhab YE, Choquet H, Dina C, Krempler F, Weitgasser R, Nejjari C, Patsch W, Chikri M, Meyre D, et al. Tcf7l2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J Mol Med. 2007;85(7):777–82. doi: 10.1007/s00109-007-0203-4. [DOI] [PubMed] [Google Scholar]
- 32.Van Vlietostaptchouk JV, Shirisverdlov R, Zhernakova A, Strengman E, Van Haeften TW, Hofker MH, Wijmenga C. Association of variants of transcription factor 7-like 2 (tcf7l2) with susceptibility to type 2 diabetes in the dutch breda cohort. Diabetologia. 2007;50(1):59–62. doi: 10.1007/s00125-006-0477-z. [DOI] [PubMed] [Google Scholar]
- 33.Mayans S, Lackovic K, Lindgren P, Ruikka K, Agren A, Eliasson M, Holmberg D. Tcf7l2 polymorphisms are associated with type 2 diabetes in northern sweden. Eur J Hum Genet. 2007;15(3):342–6. doi: 10.1038/sj.ejhg.5201773. [DOI] [PubMed] [Google Scholar]
- 34.Kimber CH, Doney AS, Pearson ER, Mccarthy MI, Hattersley AT, Leese GP, Morris AD, Palmer CNA. Tcf7l2 in the go-darts study: evidence for a gene dose effect on both diabetes susceptibility and control of glucose levels. Diabetologia. 2007;50(6):1186–91. doi: 10.1007/s00125-007-0661-9. [DOI] [PubMed] [Google Scholar]
- 35.De Silva NMG, Steele AM, Shields BM, Knight B, Parnell K, Weedon MN, Hattersley AT, Frayling TM. The transcription factor 7-like 2 (tcf7l2) gene is associated with type 2 diabetes in uk community-based cases, but the risk allele frequency is reduced compared with uk cases selected for genetic studies. Diabet Med. 2007;24(10):1067–72. doi: 10.1111/j.1464-5491.2007.02253.x. [DOI] [PubMed] [Google Scholar]
- 36.Dahlgren A, Zethelius B, Jensevik K., Syvänen A-C, Berne C. Variants of the tcf7l2 gene are associated with beta cell dysfunction and confer an increased risk of type 2 diabetes mellitus in the ulsam cohort of swedish elderly men. Diabetologia. 2007;50(9):1852. doi: 10.1007/s00125-007-0746-5. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T, Iwamoto Y, Kaku K, Hirose H, Maeda S. Replication study for the association of tcf7l2 with susceptibility to type 2 diabetes in a japanese population. Diabetologia. 2007;50(5):980–4. doi: 10.1007/s00125-007-0618-z. [DOI] [PubMed] [Google Scholar]
- 38.Miyake K, Horikawa Y, Hara K, Yasuda K, Osawa H, Furuta H, Hirota Y, Yamagata K, Hinokio Y, Oka Y, et al. Association of tcf7l2 polymorphisms with susceptibility to type 2 diabetes in 4,087 japanese subjects. J Hum Genet. 2007;53(2):174–180. doi: 10.1007/s10038-007-0231-5. [DOI] [PubMed] [Google Scholar]
- 39.Tabara Y, Osawa H, Kawamoto R. Replication study of candidate genes associated with type 2 diabetes based on genome-wide screening. Diabetes. 2009;58(2):493–498. doi: 10.2337/db07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquezine GF, Pereira AC, Sousa AB, Mill JG, Hueb W, Krieger JE. Tcf7l2 variant genotypes and type 2 diabetes risk in brazil: significant association, but not a significant tool for risk stratification in the general population. BMC Med Genet. 2008;9(1):106–106. doi: 10.1186/1471-2350-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assmann TS, Duarte GCK, Rheinheimer J, Cruz LA, Canani LH, Crispim D. The tcf7l2 rs7903146 (c/t) polymorphism is associated with risk to type 2 diabetes mellitus in southern-brazil. Arq Bras Endocrinol E Metabologia. 2014;58(9):918–25. doi: 10.1590/0004-2730000003510. [DOI] [PubMed] [Google Scholar]
- 42.Chandak GR, Janipalli CS, Bhaskar S, Kulkarni SR, Mohankrishna P, Hattersley AT, Frayling TM, Yajnik CS. Common variants in the tcf7l2 gene are strongly associated with type 2 diabetes mellitus in the indian population. Diabetologia. 2007;50(1):63–67. doi: 10.1007/s00125-006-0502-2. [DOI] [PubMed] [Google Scholar]
- 43.Bodhini D, Radha V, Dhar M, Narayani N, Mohan V. The rs12255372(g/t) and rs7903146(c/t) polymorphisms of the tcf7l2 gene are associated with type 2 diabetes mellitus in asian indians. Metab Clin Exp. 2007;56(9):1174–8. doi: 10.1016/j.metabol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Rees SD, Bellary S, Britten AC, Ohare JP, Kumar S, Barnett AH, Kelly MA. Common variants of the tcf7l2 gene are associated with increased risk of type 2 diabetes mellitus in a uk-resident south asian population. BMC Med Genet. 2008;9(1):8–8. doi: 10.1186/1471-2350-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta V, Khadgawat R, Ng HKT, Kumar S, Aggarwal A, Rao VR, Sachdeva MP. A validation study of type 2 diabetes-related variants of the tcf7l2, hhex, kcnj11, and adipoq genes in one endogamous ethnic group of north india. Ann Hum Genet. 2010;74(4):361–8. doi: 10.1111/j.1469-1809.2010.00580.x. [DOI] [PubMed] [Google Scholar]
- 46.Hussain H, Ramachandran V, Ravi S. Tcf7l2 rs7903146 polymorphism and diabetic nephropathy association is not independent of type 2 diabetes—a study in a south indian population and meta-analysis. Endokrynologia Polska. 2014;65(4):298–305. doi: 10.5603/EP.2014.0041. [DOI] [PubMed] [Google Scholar]
- 47.Guo SW, Thompson EA. Performing the exact test of hardy-weinberg proportion for multiple alleles. Biometrics. 1992:361–372. [PubMed]
- 48.Han Z, Jiang Q, Zhang T, Wu X, Ma R, Wang J, Bai Y, Wang R, Tan R, Wang Y. Analyzing large-scale samples confirms the association between the rs1051730 polymorphism and lung cancer susceptibility. Sci Rep. 2015;5:15642. doi: 10.1038/srep15642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Wang W, Liu G, Yu Y, Liao M. Association of single nucleotide polymorphism rs3803662 with the risk of breast cancer. Sci Rep. 2016;6:29008. doi: 10.1038/srep29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, Von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, et al. Strengthening the reporting of genetic association studies (strega): an extension of the strobe statement. Hum Genet. 2009;125(2):131–51. doi: 10.1007/s00439-008-0592-7. [DOI] [PubMed] [Google Scholar]
- 51.Lewis CM. Genetic association studies: design, analysis and interpretation. Brief Bioinform. 2002;3(2):146–53. doi: 10.1093/bib/3.2.146. [DOI] [PubMed] [Google Scholar]
- 52.Zhao F, Song M, Wang Y, Wang W. Genetic model. J Cell Mol Med. 2016;20(4):765–765. doi: 10.1111/jcmm.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 55.Egger M, Smith GD, Schneider M, Minder CE. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan Y, Yan L, Liu XY, Zhu X, Wang S, Chen L. Correlation of the tcf7l2 (rs7903146) polymorphism with an enhanced risk of type 2 diabetes mellitus: a meta-analysis. Genet Mol Res. 2016;15(3). [DOI] [PubMed]
- 57.Peng S, Zhu Y, Lu B, Xu F, Li X, Lai M. Tcf7l2 gene polymorphisms and type 2 diabetes risk: a comprehensive and updated meta-analysis involving 121 174 subjects. Mutagenesis. 2013;28(1):25–37. doi: 10.1093/mutage/ges048. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Xie CG, An Y, Zhang XX, Wu W. Meta-analysis of the association between the rs7903146 polymorphism at the tcf7l2 locus and type 2 diabetes mellitus susceptibility. Genet Mol Res. 2015;14(4):16856. doi: 10.4238/2015.December.14.12. [DOI] [PubMed] [Google Scholar]
- 59.Ding Y, Hu Z, Yuan S, Xie P, Liu Q. Association between transcription factor 7-like 2 rs7903146 polymorphism and diabetic retinopathy in type 2 diabetes mellitus: A meta-analysis. Diabetes Vasc Dis Res. 2015;12(6):436–444. doi: 10.1177/1479164115598274. [DOI] [PubMed] [Google Scholar]
- 60.Lin Y, Li P, Cai L, Zhang B, Tang X, Zhang X, Li Y, Xian Y, Yang Y, Wang L, et al. Association study of genetic variants in eight genes/loci with type 2 diabetes in a han chinese population. BMC Med Genet. 2010;11(1):97–97. doi: 10.1186/1471-2350-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dou H, Ma E, Yin L, Jin Y, Wang H. The association between gene polymorphism of tcf7l2 and type 2 diabetes in chinese han population: a meta-analysis. PLoS ONE. 2013;8(3). [DOI] [PMC free article] [PubMed]
- 62.Zhang B, Li W, Zhu M, Xu Y. Association of tcf7l2 gene polymorphisms with type 2 diabetes mellitus in han chinese population: a meta-analysis. Gene. 2013;512(1):76–81. doi: 10.1016/j.gene.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Hu F, Feng T, Zhao J, Yin L, Li L, Wang Y, Wang Q, Hu D. Meta-analysis of associations between tcf7l2 polymorphisms and risk of type 2 diabetes mellitus in the chinese population. BMC Med Genet. 2013;14(1):8–8. doi: 10.1186/1471-2350-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The detailed information about the search strategy. Table S2. The collected data in the meta-analysis. (XLSX 13 kb)
Data Availability Statement
All the data generated or analyzed in this study was included in this manuscript.


