Abstract
Although exercise is an effective way to decrease the risk of developing Alzheimer's disease, the biological basis for such benefits from the different exercise modes remains elusive. The present study thus aimed (i) to investigate the effects of acute aerobic or resistance exercise on neurocognitive performances and molecular markers when performing a cognitive task involving executive functioning in older adults with amnestic mild cognitive impairment (aMCI), and (ii) to explore relationships of acute exercise-induced neurocognitive changes with changes in circulating levels of neuroprotective growth factors (e.g., BDNF, IGF-1, VEGF, and FGF-2, collectively termed ‘exerkines’), elicited by different acute exercise modes. Sixty-six older adults with aMCI were recruited and randomly assigned to an aerobic exercise (AE) group, a resistance exercise (RE) group, or a non-exercise-intervention (control) group. The behavioral [i.e., accuracy rate (AR) and reaction time (RT)] and electrophysiological [i.e., event-related potential (ERP) P3 latency and amplitude collected from the Fz, Cz, and Pz electrodes] indices were simultaneously measured when participants performed a Flanker task at baseline and after either an acute bout of 30 min of moderate-intensity AE, RE or a control period. Blood samples were taken at three time points, one at baseline (T1) and two after an acute exercise intervention (T2 and T3: before and after cognitive task test, respectively). The results showed that the acute AE and RE not only improved behavioral (i.e., RTs) performance but also increased the ERP P3 amplitudes in the older adults with aMCI. Serum FGF-2 levels did not change with acute aerobic or resistance exercise. However, an acute bout of aerobic exercise significantly increased serum levels of BDNF and IGF-1 and tended to increase serum levels of VEGF in elderly aMCI individuals. Acute resistance exercise increased only serum IGF-1 levels. However, the exercise-induced elevated levels of these molecular markers returned almost to baseline levels in T3 (about 20 min after acute exercise). In addition, changes in the levels of neurotrophic and angiogenic factors were not correlated with changes in RTs and P3 amplitudes. The present findings of changes in neuroprotective growth factors and neurocognitive performances through acute AE or RE suggest that molecular and neural prerequisites for exercise-dependent plasticity are preserved in elderly aMCI individuals. However, the distinct pattern of changes in circulating molecular biomarkers induced by two different exercise modes in aMCI elderly individuals and the potentially interactive mechanisms of the effects of BDNF, IGF-1, and VEGF on amyloid-β provide a basis for future long-term exercise intervention to investigate whether AE relative to RE might be more effective in prevention/treatment of an early stage neurodegenerative disease.
Keywords: Mild cognitive impairment, Cognition, Neurotrophin, Exercise
Highlights
-
•
Different exercise modes can induce distinct changes in serum levels of molecular biomarkers.
-
•
Aerobic and resistance exercise produce similar impacts on neurocognitive performance.
-
•
Exercise may attenuate the risks of cognitive impairment via divergent biologic pathways.
-
•
Older adults with MCI exhibit molecular and neural plasticity in response to exercise.
-
•
Physical exercise could reduce the risk of dementia/AD in the long term.
1. Introduction
Mild cognitive impairment (MCI) is a clinical syndrome which is proposed as a transitional stage from normal aging to dementia (Espinosa et al., 2013), with the high-risk rate of developing to dementia being about 10 to 54% within one year, and to Alzheimer's disease (AD) about 10–15% per year (Morris et al., 2001, Petersen et al., 1999). Despite an increased risk of developing dementia/AD, cognition can be preserved or even improved in MCI (Amieva et al., 2004, Fisk et al., 2003). Strategies for prevention and/or delay of progression of MCI into dementia/AD thus need to be examined and implemented, since no curative treatment for this neurodegenerative disease currently exists.
The potential mechanisms of age-associated declines in cognitive functions can be attributed to sedentary lifestyle (Kimura et al., 2013), and/or a reduced ability to secrete neurotrophic [e.g., insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor (BDNF)] (Carro et al., 2002, Sonntag et al., 2005, Wolf et al., 2006) and angiogenic factors [e.g., vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2)] (Wolf et al., 2006). The earliest, prodromal stages of dementia (i.e., MCI) are accompanied not only by apparently progressive degeneration of the hippocampus, but also by age-related attenuated neuropil and loss of synaptic connections (Morra et al., 2009). Exercise-induced neuroprotective growth (e.g., BDNF and IGF-1) and pro-angiogenic (e.g., VEGF and FGF-2) factors, collectively called exerkines, have been shown to promote neuronal survival, development, differentiation, proliferation and growth (Bibel and Barde, 2000, Park and Poo, 2013), and to mediate functional and structural changes in the hippocampus (Cotman et al., 2007, Maass et al., 2016, Wolf et al., 2006). Circulating exerkines are necessary for exercise-induced adult hippocampal neurogenesis (Fabel et al., 2003, Wolf et al., 2006). Previous studies demonstrated that decreased expression of the BDNF gene in the hippocampus may contribute to the progression of cell death in AD (Phillips et al., 1991). The parietal cortex and hippocampus of individuals with MCI (Peng et al., 2005) or AD (Peng et al., 2005, Phillips et al., 1991) contain less BDNF precursor and mature BDNF mRNA and protein, and circulating BDNF is reduced in AD (Laske et al., 2007, Yasutake et al., 2006). Patients with AD also showed significantly lower circulating IGF-1 (Murialdo et al., 2001), which is reported to act as a neuroprotective trophic factor in both in vivo and in vitro models of AD (Aguado-Llera et al., 2005, Carro and Torres-Aleman, 2004). Moreover, levels of IGF-1 have been found to correlate positively with hippocampal volume (Maass et al., 2016), while low serum IGF-1 is linked to increased Aβ accumulation in mutant mice, and higher serum IGF-1 increased clearance of the brain Aβ in rats (Carro et al., 2002, Arvat et al., 2000). Cerebrovascular alterations are often observed in MCI (DeCarli et al., 2004). AD has also been suggested to be an angiogenesis-dependent disorder (Vagnucci and Li, 2003). Compared to patients with healthy subjects, a significant decrease in serum VEGF was found in AD patients (Solerte et al., 2005). Likewise, reduced circulating levels of FGF-2 by middle age are likely the cause of aging of hippocampus (Shetty et al., 2005). Collectively, perturbations of the secretion of these exerkines are likely associated with the progression of AD neuropathology.
Executive functions are associated with the quality of life and ability to perform daily living tasks in patients with neurodegenerative diseases (Luks et al., 2010). Attentional control and response interference/inhibition are executive functions involved in self-monitoring to determine the appropriate behavior and goal-driven allocation of attention towards the processing of task-relevant stimuli and responses (Luks et al., 2010). Recently, older adults with MCI relative to healthy controls have been found to show deficits in attentional control and response interference/inhibition (Wang et al., 2013, Wylie et al., 2007). However, clinically, the cognitive reserve hypothesis has been established for the onset and progression of the disease (Fratiglioni et al., 2004), suggesting that a trained brain has a larger cognitive reserve to compensate for AD neuropathology than an inactive one (Wolf et al., 2006).
A large body of literature has documented that physical exercise is a promising nonpharmacological intervention to retard cognitive aging. Potential neurobiological mechanisms include exercise-induced exerkines (Cotman et al., 2007, Gomez-Pinilla et al., 1997, Tsai et al., 2015), which could be associated not only with cerebral plasticity and neurogenesis, but also with angiogenesis to make the brain more resistant to structural and functional neurodegeneration (van Praag et al., 2005, Voss et al., 2013). Indeed, Voss et al. (2013) demonstrated that exercise-induced changes in circulating growth factors (e.g., BDNF, IGF-1, and VEGF) were related to increases in temporal lobe functional brain connectivity in the elderly. Acute exercise can increase circulating BDNF, IGF-1 and VEGF in healthy persons (Bang et al., 1990, Correia et al., 2010, Rojas Vega et al., 2010, Schobersberger et al., 2000). In animal studies, FGF-2 expression can be regulated by short-term physical exercise to enhance cognitive function (Gomez-Pinilla et al., 1997, Gomez-Pinilla et al., 1998). However, consensus has not yet been reached concerning the effects of acute exercise on circulating levels of these exerkines in the elderly with pathology. Older individuals with MCI have lower physical fitness (Tsai et al., 2016a), and physical activity is strongly associated with exercise-induced systemic release of these exerkines (Wolf et al., 2006). Accordingly, more work is needed to explore the hypothesis that the amount of exerkines could be effectively increased via physical exercise in MCI individuals who could have deficiencies in their secretion.
Recent studies found that older MCI adults who regularly participated in physical exercise showed immediate recall improvement and reduced whole brain cortical atrophy (Suzuki et al., 2013), which could be associated with reduced cholesterol and increased BDNF (Suzuki et al., 2013). Likewise, Baker et al. (2010) found that a six-month aerobic exercise intervention had beneficial effects on cognition in individuals with amnestic MCI (aMCI). These studies suggest that although executive functions decrease in older MCI adults (Bennys et al., 2007, Cespón et al., 2015), they still exhibit cognitive and neural plasticity and physical exercise seems to play a protective role by attenuating the progression of cognitive impairments in MCI.
A growing number of studies confirm the beneficial effects of aerobic exercise on the behavioral performance of older MCI adults (Baker et al., 2010, ten Brinke et al., 2015). Resistance exercise is also increasingly recommended as an alternative to reduce dementia risk (Ahlskog et al., 2011, Portugal et al., 2015). However, too few studies have been specifically conducted on this to draw sufficient conclusions. In fact, both aerobic and resistance exercise can employ divergent molecular mechanisms to facilitate cognition (Cassilhas et al., 2012), indicating that the neural benefits of exercise might depend on the exercise mode adopted. The first purpose of this work was thus to examine the effects of acute aerobic or resistance exercise on the neurocognitive performance and molecular changes in older persons with aMCI. In addition, several reports over the past decade demonstrated electrophysiological impairments in aMCI patients (Bennys et al., 2007, Cid-Fernández et al., 2014, Tsai et al., 2016a), and exercise-induced peripheral growth factors are strongly associated with cognition/clearance of brain Aβ and, importantly, can be up-regulated by physical exercise (Carro et al., 2002, Fabel et al., 2003, Gomez-Pinilla et al., 1997). However, their role in acute-exercise-induced changes in neurocognitive performance in aMCI is still not clear. Therefore, the second purpose of this study was to investigate whether the circulating levels of exerkines are related to any exercise-induced neurocognitive changes. Since acute bouts of exercise could facilitate neurocognitive performance in older adults (Kamijo et al., 2009), and significantly increase circulating levels of exerkines, as mentioned above, we hypothesized that acute aerobic and resistance exercise would improve deficits of the executive functions, paralleled by an increased production of exerkines, which would be linked to changes in neurocognitive performance.
2. Methods
2.1. Participants
Sixty-six older adults (60 to 80 years) with amnestic MCI (aMCI) were recruited using a standardized clinical protocol from the Alzheimer's Disease Research Center, National Cheng Kung University Hospital. After baseline measurements as mentioned below, they were randomly assigned to either an aerobic exercise (AE, n = 25), a resistance exercise (RE, n = 21) or a control group (n = 20). The clinical inclusion criteria for the participants with aMCI were as follows while stratifying for age and gender (Petersen, 2004, Petersen et al., 1999, Suk et al., 2014, Tsai et al., 2016a): (1) subjective memory complaints confirmed by family members; (2) objective memory impairment for age; (3) a Clinical Dementia Rating (CDR) of 0.5; (4) Mini-Mental State Examination (MMSE) score > 24 (Folstein et al., 1975); (5) absence of significant levels of impairment in other cognitive domains (e.g., attention, language, orientation, and abstraction), as assessed by the Cognitive Abilities Screening Instrument (CASI) (Teng et al., 1994); (6) largely intact functional activities of daily living; (7) no brain abnormalities (e.g., stroke and malignant brain tumors) seen via structural MRI scans; (8) an absence of dementia; and (9) non-depressed, with a score of < 13 on the Beck Depression Inventory, 2nd edition (BDI-II) (Beck et al., 1996). Single photon emission computed tomography (SPECT) for functional examinations and computed tomography (CT) for structural brain examinations were performed when more information was needed to confirm the diagnosis. All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971), with normal or corrected-to-normal vision based on the minimal 20/20 standard, and without a history of current psychiatric illnesses, substance abuse or addiction, anti-dementia medicine, significant cerebrovascular and metabolic diseases, musculoskeletal impairment, or other significant neurological disorders. The demographic characteristics of the three groups are summarized in Table 1. Written informed consent, as approved by the Institutional Ethics Committee of National Cheng Kung University Hospital, was obtained from all the participants.
Table 1.
Demographic characteristics (Mean ± SD) of the two exercise intervention [i.e., aerobic exercise (AE) and resistance (RE)] groups and one non-exercise-intervention (control) group.
| AE (n = 25) | RE (n = 21) | Control (n = 20) | p | |
|---|---|---|---|---|
| Age (years) | 65.48 ± 7.53 | 66.05 ± 6.64 | 64.50 ± 6.95 | 0.780 |
| Gender (male/female) | 11/14 | 9/12 | 8/12 | 0.327 |
| Height (cm) | 160.60 ± 7.85 | 158.85 ± 8.51 | 159.74 ± 8.81 | 0.780 |
| Weight (kg) | 62.13 ± 13.60 | 62.11 ± 12.12 | 61.40 ± 12.98 | 0.978 |
| Body mass index (kg/m2) | 23.83 ± 3.20 | 24.48 ± 3.19 | 23.84 ± 3.06 | 0.740 |
| Education (years) | 12.20 ± 3.20 | 11.81 ± 3.43 | 11.85 ± 2.82 | 0.898 |
| Systolic pressure (mmHg) | 123.64 ± 15.09 | 129.00 ± 19.45 | 128.55 ± 17.17 | 0.502 |
| Diastolic pressure (mmHg) | 76.84 ± 10.27 | 78.76 ± 11.00 | 76.95 ± 9.99 | 0.794 |
| MMSE | 26.96 ± 1.21 | 26.76 ± 1.38 | 27.00 ± 1.59 | 0.837 |
| BDI-II | 6.80 ± 4.08 | 5.29 ± 5.08 | 7.5 ± 4.12 | 0.266 |
| Social participation | 10.08 ± 2.33 | 10.52 ± 2.71 | 11.05 ± 2.76 | 0.463 |
| Working memory span | 19.20 ± 1.78 | 20.38 ± 1.75 | 19.90 ± 2.17 | 0.113 |
| 7-day PAR (Kcal/d) | 1779.80 ± 392.18 | 1862.67 ± 455.42 | 1847.85 ± 377.09 | 0.763 |
| Grip (kg) | 28.88 ± 10.43 | 29.57 ± 10.75 | 26.98 ± 9.55 | 0.705 |
| Back scratch (cm) | − 5.00 ± 10.36 | − 4.14 ± 9.43 | 0.28 ± 8.28 | 0.158 |
| Chair sit-and-reach (cm) | 5.42 ± 11.23 | 5.19 ± 8.11 | 5.90 ± 9.44 | 0.972 |
| Arm curl (number) | 20.04 ± 6.18 | 19.24 ± 5.89 | 18.00 ± 5.84 | 0.527 |
| 8-foot up-and-go (sec) | 6.21 ± 0.95 | 6.30 ± 1.42 | 6.81 ± 1.50 | 0.267 |
| Chair stand (sec) | 17.16 ± 4.84 | 16.38 ± 4.39 | 15.60 ± 5.09 | 0.555 |
| VO2max (mL/kg/min) | 24.92 ± 5.05 | 23.56 ± 4.62 | 24.56 ± 3.43 | 0.579 |
AE: aerobic exercise; RE: resistance exercise; MMSE, mini mental state examination; BDI, Beck depression inventory.
2.2. Experimental procedure
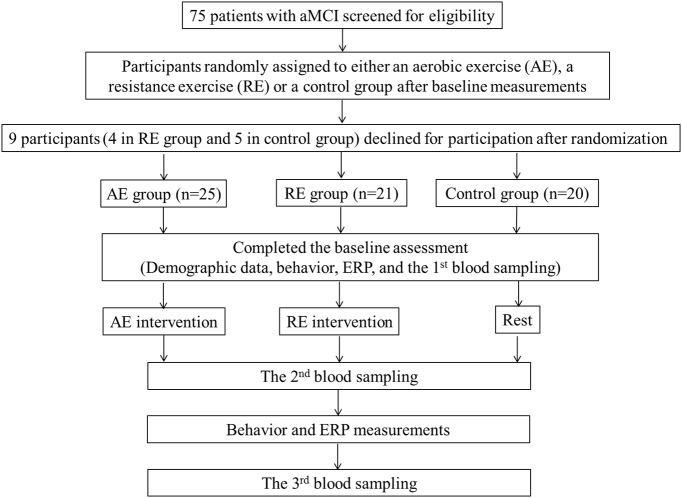
The primary interest was to investigate the effects of acute exercise on neurocognitive performance and on systemic neuroprotective signals (exerkines). The experimental procedure is shown in the flowchart presented in the Fig. 1. Two visits to the cognitive neurophysiology laboratory were required for all participants. On the first visit, the experimental procedure was explained, and an informed consent form, a medical history and demographic questionnaire, MMSE, DBI-II, social participation, and a handedness inventory were completed by the participants. Working memory span was estimated using the digit span component of the Wechsler-IV Adult intelligence test (Wechsler, 2008). To provide sufficient preactivity screening to lower potential risk factors before the acute exercise intervention, the participants' previous levels of physical activity were assessed using a seven-day physical activity recall questionnaire (7-day PAR) (Sallis et al., 1985) and Physical Activity Readiness Questionnaire (PARQ) (Thomas et al., 1992). Their height and weight were also measured to calculate their body mass index (BMI). Two certified fitness instructors then completed all assessments of functional physical fitness (Rikli and Jones, 2012). The Rockport Fitness Walking Test (Kline et al., 1987) was adopted to estimate the participants' VO2max, in which they were required to walk one mile as quickly as possible, with their heart rate (HR) being continuously recorded using a Polar heart rate (HR) monitor (RX800CX, Finland). For the RE group, one repetition maximum (1-RM) and peak muscle power for each participant were assessed, and all the participants were asked to become familiarized with the use of free weights and bodybuilding machines before the acute resistance exercise intervention.
Fig. 1.
Flowchart of the study.
Before the second visit in the same week, the participants were asked to arrive at the laboratory at about 8:30–9:30 am to control for circadian influences. They were also required to refrain from strenuous exercise for at least 24 h, and smoking, food, caffeine and alcohol intake were also prohibited for at least 12 h, since these factors could be associated with increases in P3 amplitude (Dixit et al., 2006, Geisler and Polich, 1990) and molecular makers (Wu, 2014). When arriving at the laboratory with an acoustically shielded room with dimmed lights, each participant was fitted with a Polar HR monitor (RX800CX, Finland), and was then asked to sit in an adjustable chair in front of an IBM-compatible computer with a 43-cm digital display computer screen, with the viewing distance being approximately 75 cm. An electro cap and electro-oculographic (EOG) electrodes were attached to his/her scalp and face before the cognitive test. Body temperature (BT) and resting HR (HRrest) were measured. After 20 practice trials to help the participants become familiar with the rules of the cognitive task, blood was withdrawn and then the formal cognitive test was immediately administered, with the subjects' electrophysiological signals being recorded. Both AE and RE groups then performed approximately 40 min of acute aerobic and resistance exercise, respectively. Since exercise-induced hyperthermia and tachycardia are associated with P3 modifications (Geisler and Polich, 1990), BT and HR were measured every 2 min after the acute exercise interventions. Once the participants' BT and HR had returned to within 10% of baseline levels (on average about 5 min after a bout of acute exercise), blood was immediately withdrawn from each participant, and he/she then completed the cognitive task along with electrophysiological recording. With regard to the control group, after the first cognitive test these individuals took a rest of about 45 min, during which they read magazines, and then they took the cognitive test again. Additional blood samples were immediately taken after the participants had completed the second cognitive task test (about 20 min after acute exercise).
2.3. Acute exercise prescriptions
The seat on the bicycle ergometer was adjusted according to the participant's height before the acute aerobic exercise. After a five-min warm-up, the participant performed a 30-min bout of exercise at moderate intensity, corresponding to 65–75% of the individual target heart rate reserve (HRR), as determined for each individual from the HRrest and HRmax (i.e., 220-age), and then a five-min cool down was performed.
With regard to the acute resistance exercise, after a five-min warm-up, the RE group performed an approximately 30-min bout of moderate-intensity (75% 1RM) exercise on the bodybuilding machines and free weights, and then a five-min cool down. The core resistance exercise content consisted of the following exercises in the order stated: biceps curls, triceps extensions, bench presses, leg presses, leg extensions, and vertical butterflies. The participants in the RE group performed the resistance exercise for two sets of 10 repetitions, at an average speed, with a 90-second rest between sets, and a two-minute interval between each different exercise. The warm-up and cool-down included slow-paced walking and active mobility exercises for the joints of all four limbs.
2.4. The cognitive task
The Flanker task was used to assess the participants' executive function, since older adults with MCI show a deficit when performing the cognitive task (Wang et al., 2013, Wylie et al., 2007). Such a cognitive task was programmed using E-prime software (www.pstnet.com; Psychology Software Tools, Inc.), and implemented on a computer screen positioned so that the stimuli appeared at eye level. The total width of the five arrows is 4.2 cm, and each arrow has the same height of 2.2 cm and is separated by a space of 0.05 cm. Each stimulus array was thus presented foveally, and consisted of white arrows pointing in the left or right direction against a black background. Each array consisted of a row of five arrows, a target arrow located in the center location, and two distractor stimuli (i.e., flankers) located on each side of the target arrow. The participants were required to identify the direction of the central target arrow flanked by incongruent or congruent stimulus arrays. They had to respond as quickly and accurately as possible by pressing a right/left button on a response box corresponding to the direction of the center arrow (left or right). The responses to the stimuli were registered via the response box designed to interface with E-prime software. The Flanker task consisted of three conditions: (i) a congruent condition (45%), in which all flankers point in the same direction as the target arrow, (ii) an incongruent condition (45%), in which the target arrow is flanked by arrows pointing in the opposite direction on each side, (iii) a neutral condition (10%), in which the target arrow is flanked by the “+” symbol. The cognitive task was separated into three blocks, with 100 stimuli for each block. The three conditions were placed in a random order.
A trial commenced with a three-second countdown followed 1000 ms later by the presentation of a white fixation cross in the center of the computer screen. The cross was removed after 500 ms, and replaced by a stimulus array that remained on the screen until the participant made a response. In the case of no response, the maximal inter-trial interval occurred after a period of 2 s following the stimulus array, and in such cases the computer program noted that there was a lack of response and started a new trial. If the participant responded within 2 s, the stimuli disappeared and then the screen remained blank for 750 ms until a white fixation cross appeared to signal the beginning of a new trial. Therefore, the inter-trial interval from stimuli to stimuli was variable.
2.5. Electrophysiological recording and analysis
Electroencephalographic (EEG) activity was measured through 18 Ag/AgCl sintered electrode sites (F7, F8, F3, F4, Fz, T3, T4, C3, C4, Cz, T5, T6, P3, P4, Pz, O1, O2, and Oz) embedded in an elastic electro cap (Quik-Cap, Compumedics Neuroscan, Inc., El Paso, TX, USA) according to the International 10–20 System, referenced to linked mastoid electrodes, with AFz placed on the mid-forehead serving as the ground electrode. The skin impedance was kept below 5 kΩ. To monitor possible artifacts due to eye movements, the adhesive ocular electrodes placed on the supero-lateral right canthus and below and lateral to the left eye connected to the system reference to obtain horizontal and vertical bipolar electrooculographic (i.e., HEOG and VEOG) activity for eye movements. The raw EEG signals were recorded with an A/D rate of 500 Hz/channel, a band-pass filter of 0.1–50 Hz, and a 60 Hz notch filter, and continuously written to a hard disk for off-line analysis using SCAN4.3 analysis software and amplified by a SynAmps amplifier (Compumedics Neuroscan, Inc., El Paso, TX, USA).
The ERP epoch for averaging was 900 ms, including 200 ms pre-stimulus to 700 ms post-stimulus onset. During the recording epoch, trials containing response errors and ocular artifacts were also discarded from further analysis, with a threshold of 100 μV in the VEOG, HEOG, and electromyogram being set for this. The remaining effective ERPs data was assembled across epochs according to congruent and incongruent conditions. Since executive functions involving in attentional control processes (e.g., Flanker task) are mediated by a large-scale network involving frontal and parietal systems (Miller and Cohen, 2001), the stimulus-elicited P3 component was distinguished across the three electrodes (i.e., Fz, Cz, and Pz), with correction for differences in the 200 ms pre-stimulus baseline. Latencies were defined as the time point of the maximal amplitude within the latency window for every participant. P3 mean amplitudes were calculated for the time-window between 300 and 700 ms post-stimulus. The results were equivalent for the ERP elicited by all conditions and participants.
2.6. Blood sampling and analysis
A 10-mL blood sample were obtained from the antecubital vein via an aseptic technique at three time points (T1: before the 1st cognitive task test; T2: before the 2nd cognitive task about 5 min after acute exercise; and T3: immediately after the 2nd cognitive task test) by a qualified phlebotomist. The blood samples were withdrawn for analysis of serum BDNF, IGF-1, VEGF, and FGF-2 levels. Blood samples were obtained via an indwelling catheter located in a forearm vein during the T2 and T3 time points, with sterile saline to flush the catheter to prevent clot formation. The catheter was cleared of saline prior to each sample collection. The blood samples were kept at room temperature to allow for clotting (BD Vacutainer Plus), and then centrifuged at 3000 rpm for 15 min at 4 °C (Hettich Mikro 22R, C1110). Samples were harvested, aliquoted, and stored at − 80 °C for further serum marker assays. The levels of serum BDNF, IGF-I, VEGF and FGF-2 were analyzed by Human Cytokine Antibody-Immobilized Magnetic beads (Millipore, Billerica, MA, USA). Measurements were performed on a Luminex 200 analyzer (Luminex, Austin, TX, USA). The whole procedure for the determination of the four molecular markers was performed by the same person to avoid inter-operator bias.
2.7. Data processing and statistical analysis
With respect to the behavioral performance, the participants' accuracy rates (ARs) and reaction times (RTs) were measured and analyzed. Trials with a response error or RTs shorter than 200 ms or longer than 1500 ms, which were regarded as anticipatory or delay errors, respectively, were excluded from the RT and ERP analysis. This time window was able to exclude all responses greater than two standard deviations from the mean response of each group, thereby excluding outliers that could skew the group means. The neutral condition was excluded, since the total number of trials was not sufficient to average the ERP components, and this study is concerned with the response interference/inhibition. No participant exceeded a 20% error rate. Therefore, the percentage of trials available for further ERP analysis was above 75% (i.e., at least 101 trials) in the congruent and incongruent conditions.
All independent variables for the neurocognitive (i.e., behavioral and electrophysiological) performance were separately analyzed using a mixed design, factorial, and repeated-measures analysis of variance (RM ANOVA). The ARs and mean RTs of accepted trials were submitted separately to a 3 (Group: AE vs. RE vs. Control) × 2 (Time: pre-exercise vs. post-exercise) × 2 (Condition: congruent vs. incongruent) RM–ANOVA. P3 components from ERP recordings were submitted separately to a 3 (Group: AE vs. RE vs. Control) × 2 (Time: pre-exercise vs. post-exercise) × 2 (Condition: congruent vs. incongruent) × 3 (Electrode: Fz vs. Cz vs. Pz) RM–ANOVA. All biochemical markers were submitted separately to a 3 (Group: AE vs. RE vs. control) × 3 (Time: T1 vs. T2 vs. T3) RM–ANOVA. Where a significant difference occurred, posterior comparisons of the mean values were performed by paired multiple comparisons (adjusted using the Bonferroni correction). Homogeneity and normality of variance assumptions were confirmed by Levene's and Kolmogorov-Smirnov tests, respectively. The significance levels of the F ratios were adjusted with the Greenhouse-Geisser correction for the violation of the assumption of sphericity when the degrees of freedom were more than one. The effect size, partial η2 (ηp2), was used for the group comparison to complement the significance testing, with the following conventions used to determine the magnitude of the mean effect size: < 0.08 representing a small effect, 0.08 to 0.139 a medium effect, and > 0.14 a large effect. The relations between molecular markers and the neurocognitive performances at baseline across the three groups, and the changes in the biochemical markers and the neurocognitive performances from T1 to T2 time points, were examined with the Pearson product-moment correlation. A level of p < 0.05 was accepted as statistically significant.
3. Results
3.1. Demographic characteristics
As seen in Table. 1, three groups were matched at the group level on socio-demographic variables (e.g., age range, height, weight, BMI, systolic and diastolic pressure, years of education, and social participation) (all ps > 0.05). A chi-square analysis failed to reveal any significant gender distribution difference among the three groups. The BDI-II, MMSE, and senior functional physical fitness scores also revealed non-significant differences across the three groups.
3.2. Behavioral indices
3.2.1. Accuracy rate (AR)
The RM ANOVA on the ARs only revealed significant main effects of Condition [F(1, 63) = 41.89, p < 0.001, ηp2 = 0.40]. Post-hoc analyses indicated that the ARs for the congruent condition (98.1%) were higher than those for the incongruent one (96.5%) for all three groups. Neither significant main effects of Group and Time, nor significant interactions between Group, Condition, and Time (ps > 0.30 in all cases), were obtained.
3.2.2. Reaction time (RT)
As illustrated in Fig. 2, the RM ANOVA on the RTs revealed significant main effects of Time [F(1, 63) = 4.65, p = 0.035, ηp2 = 0.07] and Condition [F(1, 63) = 244.22, p < 0.001, ηp2 = 0.80]. Post-hoc analyses indicated that the post-exercise RTs (607.10 ms) were faster than pre-exercise ones (621.11 ms) across the three groups and two conditions, and the RTs for the incongruent condition (648.67 ms) were longer than those for the incongruent one (579.54 ms) for all three groups. These main effects were superseded by the Time × Group [F(2, 63) = 4.58, p = 0.014, ηp2 = 0.13] and Group × Condition [F(2, 63) = 3.79, p = 0.028, ηp2 = 0.11] interactions. Post-hoc analyses for the Time × Group interaction indicated that the post-exercise RTs were faster than the pre-exercise ones across two conditions for the AE [pre-exercise vs. post-exercise: 625.89 ± 105.49 ms vs. 592.81 ± 71.42 ms; t(24) = 2.39, p = 0.25] and RE [pre-exercise vs. post-exercise: 622.93 ± 120.87 ms vs. 600.68 ± 117.07 ms; t(20) = 2.44, p = 0.24] groups.
Fig. 2.
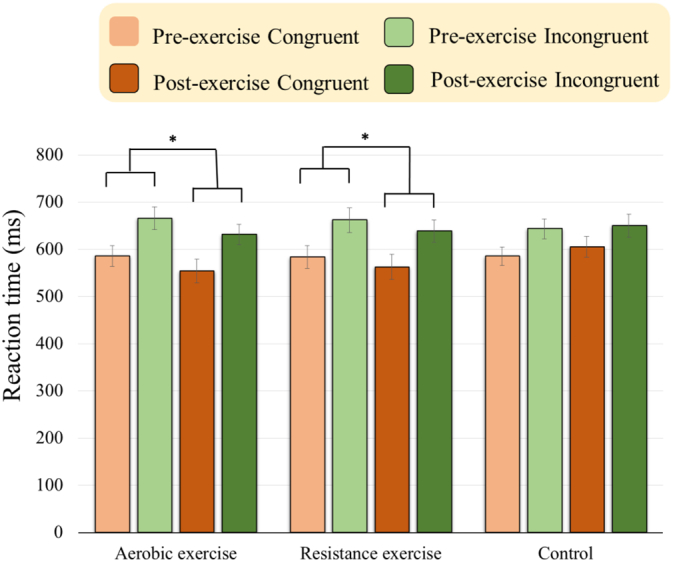
Behavioral RTs (ms) in congruent and incongruent conditions (Mean ± SE) for the aerobic and resistance exercise groups before and after an acute bout of exercise intervention and control group before and after rest. (*p < 0.05).
3.3. Electrophysiological indices
3.3.1. P3 latency
As shown in Fig. 3, the RM ANOVA on the P3 latency revealed significant main effects of Condition [F(1,62) = 39.62, p < 0.001, ηp2 = 0.39] and Electrode [F(2124) = 3.62, p = 0.030, ηp2 = 0.06]. Post-hoc analyses indicated that P3 latency showed significantly earlier in the congruent condition (516.23 ms) than in the incongruent condition (554.66 ms), and significantly earlier for the Fz electrode (529.41 ms) than for the Pz electrode (541.64 ms). Neither significant main effects of Group and Time, nor other significant interactions, were obtained.
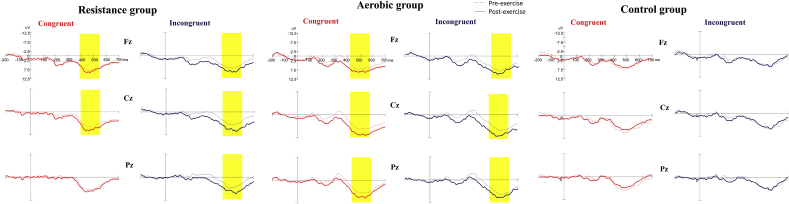
Fig. 3.
Grand averaged ERP waveforms (Fz, Cz, and Pz) in the congruent and incongruent conditions for the aerobic and resistance groups before and after an acute bout of exercise intervention and for the control group before and after rest. (Yellow marks denote significant differences between pre- and post-exercise.)
3.3.2. P3 amplitude
The RM ANOVA performed on the P3 amplitudes showed the main effects of Time [F(1,63) = 25.49, p < 0.001, ηp2 = 0.29] and Electrode [F(2126) = 26.08, p < 0.001, ηp2 = 0.16], suggesting that P3 amplitude was significantly larger post-exercise (9.47 μV) than pre-exercise (7.99 μV), and significantly larger for the Cz (9.53 μV) electrode than for the Fz (8.39 μV) and Pz (8.27 μV) electrodes. The interactions of Time × Group [F(2,63) = 7.41, p = 0.001, ηp2 = 0.19], Time × Group × Electrode [F(4126) = 2.67, p = 0.035, ηp2 = 0.08], and Time × Group × Condition × Electrode [F(4126) = 4.12, p = 0.004, ηp2 = 0.12] were also significant. Post-hoc analysis showed that the P3 amplitudes were significantly larger post-exercise than pre-exercise at all electrodes and conditions in the AE and RE groups (almost all ps < 0.007), except the post-exercise P3 amplitude (8.37 μV) relative to pre-exercise one (7.66 μV) did not achieve significance in the congruent condition at the Pz electrode in the RE group (p = 0.286).
3.4. Circulating molecular markers
3.4.1. Brain-derived neurotrophic factor (BDNF)
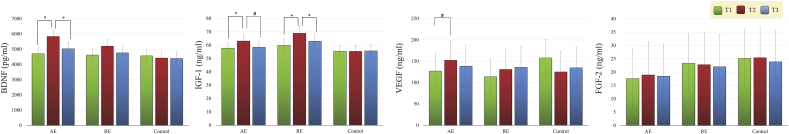
As seen in Fig. 4, there were a significant effect of Time [F(2126) = 5.23, p = 0.005, ηp2 = 0.08] and a significant effect of Group × Time [F(4126) = 2.54, p = 0.043, ηp2 = 0.08] on BDNF levels. Post-hoc analyses revealed that no significant differences in the BDNF levels were observed between three groups at the T1 and T3 time points; the BDNF levels at the T2 time point approach significance (p = 0.069) across the three groups, with higher levels in the AE group as compared to the Control group (p = 0.063); in the AE group, the BDNF level was found to increase significantly at the T2 relative to the T1 time point (T1 vs. T2: p = 0.001), and decrease significantly at the T3 relative to the T2 time point (p = 0.009).
Fig. 4.
Changes in brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF-2) (Mean ± SE) for the aerobic and resistance groups before and after an acute bout of exercise intervention and for the control group before and after rest. [Baseline: T1 (green bar); T2 (red bar): before the 2nd cognitive task; T3 (blue bar): after the 2nd cognitive task] (*p < 0.05; #p value approaches significance).
3.4.2. Insulin-like growth factor-1 (IGF-1)
There were a significant effect of Time [F(2126) = 8.32, p < 0.001, ηp2 = 0.12] and a significant Group × Time effect [F(4126) = 2.55, p = 0.042, ηp2 = 0.08] on IGF-1 levels. Post-hoc analyses revealed that no significant differences were found for the T1, T2, and T3 time points across the three groups; in the AE group, the serum IGF-1 level was found to increase significantly at T2 relative to T1 (p = 0.032), and there was an approaching significant decrease at T3 relative to T2 (p = 0.064); in the RE group, the serum IGF-1 level was found to increase significantly at the T2 relative to T1 (p = 0.005), and to decrease significantly at T3 relative to T2 (p = 0.001).
3.4.3. Vascular endothelial growth factor (VEGF)
Only the effect of the Group × Time interaction [F(4126) = 2.16, p = 0.078, ηp2 = 0.06] was marginally significant for VEGF levels. Post-hoc analyses revealed that in the AE group there was a trend towards increased levels of serum VEGF at T2 relative to T1 (p = 0.050).
3.4.4. Fibroblast growth factor 2 (FGF-2)
Neither significant main effects of Group and Time, nor a significant Group × Time interaction, were obtained.
3.4.5. Correlations between neurocognitive performances and molecular markers
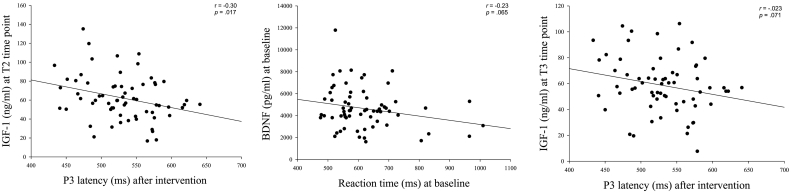
There were almost no significant correlations among the BDNF, IGF-1, VEGF, and FGF-2 levels and behavioral (i.e., ARs and RTs) and electrophysiological (i.e., P3 latency and amplitude) performances before and after acute exercise across the three groups, except for the significant correlation between P3 latency and IGF-1 levels at T2 (r = − 0.30; p = 0.017), and the approaching significant correlations between RTs and BDNF levels at baseline (r = − 0.23; p = 0.065) and P3 latency and IGF-1 levels at T3 (r = − 0.23; p = 0.071) (see Fig. 5). In addition, no significant correlations emerged among the changes in RTs and P3 amplitudes and the changes in the levels of the BDNF, IGF-1, and VEGF with acute exercise (T2 vs. T1) in the AE and RE groups.
Fig. 5.
Scatterplots of the relationship between neurocognitive performance and circulating molecular markers in the Flanker task in all participants.
4. Discussion
The present study assessed the different effects of acute aerobic and resistance exercise on neurocognitive performance and neuroprotective growth factors (i.e., exerkines) in older adults with aMCI, and investigated the relationship between changes in neurocognitive performance and exerkines induced by the two exercise modes. Although the older aMCI adults did not show significantly improved ARs, possibly due to high performance before the exercise intervention when performing the Flanker task, as seen in previous works (Luks et al., 2010, Wylie et al., 2007), acute aerobic or resistance exercise could still affect neurocognitive performance (e.g., RTs and P3 amplitude). Additionally, acute aerobic exercise significantly increased serum BDNF and IGF-1 levels (with a trend towards an increase in the VEGF levels), while acute resistance exercise only significantly increased serum IGF-1 levels in the older adults with aMCI. This suggests that the two exercise modes might have distinct effects on these neuroprotective growth factors in aMCI. However, correlations between changes in serum BDNF, IGF-1, and VEGF levels and changes in neurocognitive performance did not reach significant levels in the AE and RE groups in the present study. This could be attributed to the fact that increased levels of molecular parameters were reduced from T2 to T3 time points, or that the impact of two distinct modes of acute exercise on neurocognition was similar and the pattern of molecular changes was different.
Previous research demonstrated that older adults with MCI showed slower RTs than healthy controls, and that the greater RT cost (i.e., incongruent RTs − congruent RTs; ability in response inhibition / conflict resolution) was significantly larger in the MCI group as compared to the control group when the participants performed the Flanker task (Wang et al., 2013, Wylie et al., 2007). In the present study, the Time × Group × Condition interaction on the RTs did not achieve a significant difference, suggesting that the acute exercise intervention could not facilitate specific effects on the response inhibition/interference. However, in agreement with previous works which noted that older adults exhibited shorter RTs following the acute moderate aerobic exercise (Kamijo et al., 2009), the significant Time × Group interaction effect in the present study reflected that the RTs were significantly shortened across conditions in both AE and RE groups when performing the Flanker task after acute aerobic and resistance exercise interventions compared to pre-exercise. This suggests that both exercise modes could facilitate stimulus evaluation and response processes (response selection and execution, Doucet and Stelmack, 1999) in the older adults with aMCI. Indeed, previous studies have demonstrated that the two exercise modes could significantly facilitate behavioral performance (i.e., shorter RTs and higher ARs) when young and older adults performed cognitive tasks involving executive functioning (Barella et al., 2010, Johnson et al., 2016, Tsai et al., 2014a, Tsai et al., 2014b, Tsai et al., 2016b). Although improved behavioral performance with regard to the time efficiency of the central processing of cognitive functions was found in this work, this beneficial effect of acute exercise on response speed from baseline to immediately post-exercise is relatively transitory (Barella et al., 2010, Tomporowski, 2003), and could be attributed to increases in general physiological arousal and neural activation (Dietrich and Audiffren, 2011, Joyce et al., 2009). However, given the results of the present study, where similar effects on the speed of processing emerged in both AE and RE groups, it appears that both aerobic or resistance exercise modes could be viable approaches to enhance executive functions involving attentional control in the elderly with aMCI. In addition, the findings in this study also suggested that aerobic and resistance exercise could enhance the activities of the right hemisphere temporal-parietal junction, ventrolateral prefrontal cortex, and dorsolateral prefrontal cortex networks in older adults with aMCI, since these brain areas are associated with response speed when patients with neurodegenerative diseases (e.g., MCI and AD) perform the Flanker task (Luks et al., 2010).
Previous studies have proposed that the ERP P3 component is sensitive enough to identify an early stage of cognitive impairment in the elderly with MCI (Bennys et al., 2007, Jiang et al., 2015, Tsai et al., 2016a). Even though Wang et al., 2013 found that older adults with MCI showed longer P3 latencies than healthy controls when performing the Flanker task, and Kamijo et al., 2009 found that the P3 latency following a bout of light or moderate aerobic exercise was shorter than during the baseline session in older adults when performing the Flanker task, acute bouts of exercise, regardless of aerobic or resistance mode, failed to modulate the P3 latency in the present study. This suggests that the time required to detect and process a stimulus in the environment could not be facilitated in the older adults with aMCI. By contrast, acute aerobic and resistance exercise enlarged the P3 amplitude in the older adults with aMCI when performing the Flanker task, implying that neuronal activity could be facilitated by the two exercise modes in the preclinical dementia/AD disease stage. Similar to the general non-selective effects of acute bouts of exercise on the RTs, the changes in P3 amplitudes were also observed across task conditions in the present study, which could be attributed to an increase in the arousal levels or modification of humoral functioning (e.g., catecholamine) (Barella et al., 2010, Dietrich and Audiffren, 2011, Joyce et al., 2009, Tsai et al., 2014a, Tsai et al., 2016b), which further induce more resources to be made available for stimulus-driven attention and motor readiness. It is worth noting that chronic exercise intervention can potentiate long-term potentiation (van van Praag et al., 1999), increase the expression of neuroplasticity-related transcription factors (e.g., cAMP) (Shen et al., 2001), gene products, (e.g., synapsin I and synaptophysin) (Vaynman et al., 2004, Vaynman et al., 2006) and synaptic plasticity genes (Stranahan et al., 2010), and enhance hippocampal dendritic length and dendritic spine complexity (Redila and Christie, 2006). Further studies with long-term exercise interventions are necessary to strengthen the current findings and determine the exact mechanisms of aerobic and resistance exercise on the neurocognitive facilitation seen in the elderly with aMCI.
BDNF, a member of the neurotrophin family of factors, supports neural growth and survival and synaptic plasticity, and is highly concentrated in the hippocampus and cortex (Cowansage et al., 2010, Gottmann et al., 2009, Lipsky and Marini, 2007). Lower serum levels of BDNF are associated with smaller hippocampal volumes (Szeszko et al., 2005) and a decline in executive functions in MCI (Shimada et al., 2014), partly supporting the finding that an approaching significant correlation emerges between RTs and BDNF levels in older adults with aMCI when performing the Flanker task at baseline. Patients with AD whose cognitive abilities are rapidly declining have significantly lower serum BDNF concentrations than those with a slow cognitive decline (Laske et al., 2011). Previous animal studies have also demonstrated that physical exercise can increase BDNF expression in the hippocampus and cortical regions (Aguiar et al., 2011, Uysal et al., 2015). Importantly, peripheral serum BDNF levels might serve as a proxy for cortical levels, since significant links between serum and cortical BDNF levels were found in a rodent study (Karege et al., 2002). Increases in BDNF have been related to exercise-induced positive effects on cognitive functions (Griffin et al., 2011). In line with most investigations that have examined young adults (Ferris et al., 2007, Griffin et al., 2011) and healthy older adults (Coelho et al., 2014), acute aerobic exercise was found to significantly increase circulating BDNF levels in older adults with aMCI in the present study. Importantly, even AD patients can increase plasma BDNF levels via acute aerobic exercise (Coelho et al., 2014). The previous and present findings suggest that although there are decreased levels of BDNF precursor (pro-BDNF) and mature BDNF in the cortex and hippocampus of older adults in the preclinical stages of AD (Peng et al., 2005) and in those with AD (Ferrer et al., 1999), which could constitute a lack of trophic support and contribute to cognitive impairment (Coelho et al., 2014), older adults with such a neurodegenerative disease could increase their circulating BDNF levels via aerobic exercise. However, although the effects of resistance exercise on BDNF have been demonstrated in the elderly (Coelho et al., 2012), in the present study, acute resistance exercise did not induce a significant elevation in circulating serum BDNF levels. The results of this work are in accordance with earlier studies investigating the effects of acute resistance exercise on circulating BDNF levels in young adults (Correia et al., 2010, Goekint et al., 2010). Moreover, although Rojas Vega et al., 2010 found that serum BDNF levels increased after acute resistance exercise, the neuroprotective growth factor did not show a significant difference from pre-exercise values in the young adults.
Systemic IGF-1 in adults increases neuronal excitability and modulates neurogenesis, synaptic density and plasticity, and neurotransmission (Fernandez and Torres-Alemán, 2012). In addition, another study also found that IGF-1 is involved in vascular remodeling and maintenance (Lopez-Lopez et al., 2004). Therefore, age-related reductions in the levels of serum and brain IGF-1 have been associated with decreased cerebral vascular density and blood flow (Sonntag et al., 1997), and are a potential mechanism that may influence cognitive function in the elderly (Sonntag et al., 2005), partly supporting the significant correlation between P3 latency and IGF-1 levels at the T2 time point that was observed in the older adults with aMCI in the present study. These findings also support the idea that a decrease in the level of IGF-1 with aging is a well-established risk factor for neurodegenerative diseases (Busiguina et al., 2000). Physical exercise can increase peripheral IGF-1 levels to mediate neurogenesis and prevent brain damage through increased uptake of such a growth factor by the brain (Cotman et al., 2007). Rojas Vega et al., 2010 found that either low or high acute resistance exercise increases the level of IGF-1 in young adults. Likewise, previous studies also demonstrated that acute aerobic exercise can significantly increase circulating IGF-1 levels in young adults (Bang et al., 1990). However, Bang et al., 1990 found that serum IGF-1 levels could be significantly increased after acute aerobic exercise only in healthy individuals but not in patients with GH deficiency. In the present study, we found that both acute aerobic and resistance exercises could significantly increase serum IGF-1 levels in the older adults with aMCI, suggesting that both physical exercise modes could be effective to improve reduced circulating IGF-1 levels in aMCI (Baker et al., 2010) and AD (Murialdo et al., 2001). In addition, such a neuroprotective hormone could, probably by enhancing transport of Aβ carrier proteins (e.g., albumin and transthyretin) into the brain, reduce brain (e.g., hippocampus, and parietal and pyriform cortices) Aβ load (Carro et al., 2002). For example, in animal studies, although over 18-month-old aging rats showed increased levels of Aβ in the brain as compared to the younger ones (Vaucher et al., 2001), the Aβ levels of the aging rats were reduced back to those seen in young rats in the hippocampus and cortex after treatment with IGF-1 (Carro et al., 2002). Similarly, transgenic mice with a 60% decrease in serum IGF-1 levels showed prematurely increased brain Aβ levels. After one month of treatment with IGF-1, the brain Aβ levels were significantly reduced (Carro et al., 2002). These studies with normal aging or transgenic animals may support the idea that IGF-1 modulates endogenous/transgenic Aβ clearance in the brain. It is worth noting that, relative to aerobic exercise, only long-term resistance exercise could significantly increase basal serum levels of IGF-1 in the elderly (Vale et al., 2009). The resistance exercise mode might also have potential effects to reduce brain Aβ load (Carro et al., 2002) and increase/preserve hippocampal volume (Maass et al., 2016) in older adults with aMCI.
VEGF produced in central and peripheral areas is expressed in multiple cells (e.g., endothelial cells and platelets) and tissues (smooth and skeletal muscle) (Gavin et al., 2004). VEGF not only promotes the formation and growth of blood vessels (Adams and Alitalo, 2007), but also, operating as an intermediate mechanism, promotes neurogenesis and synaptogenesis through neural cell differentiation, growth, and regeneration (Ruiz de Almodovar et al., 2009), suggesting that this biomarker can sustain widespread angiogenic and neurotrophic actions. In effect, VEGF can counteract the negative effects of oligemia in AD (Jin et al., 2000), and VEGF activity may be associated with AD neuropathology and brain areas linked to cognitive functions. VEGF deficiency determines neural degeneration with a reduction in neural blood flow. The reduction in VEGF synthesis could be directly linked to the specific molecular effects of Aß1–42 (Solerte et al., 2005). VEGF liberation through exercise could be due to alterations in the cardiovascular and hemodynamic components, with an increase in shear stress and tension on the blood vessels (Prior et al., 2003), and expression of VEGF mRNA and protein due to hypoxia (Prior et al., 2003). The amount of hypoxia-inducible protein can increase in the periphery after acute exercise (Schobersberger et al., 2000) and cross the blood-brain barrier to mediate angiogenesis (Cotman et al., 2007), which has been associated with cognitive facilitation (Adams and Alitalo, 2007). Therefore, VEGF seems to be associated with the vascular benefits of physical exercise, developing the role of an important moderator in the process of angiogenesis (Cotman et al., 2007, Fabel et al., 2003). In the present study, there was a trend towards increased levels of serum VEGF through acute aerobic exercise in the older adults with aMCI, which was in accordance with previous research. For example, through muscle biopsy, Gavin et al. (2004) found that amount of VEGF protein decreased after acute aerobic exercise in young men, reflecting the secretion of VEGF from the skeletal muscle into the circulation after exercise, and suggesting that circulating VEGF levels could be increased by acute aerobic exercise. Similarly, Kraus et al., 2004 also found acute aerobic exercise could increase plasma VEGF levels in sedentary and endurance-trained men. Two previous studies also verified that an acute bout of aerobic exercise can increase VEGF concentrations in the elderly with artery pathology (Adams et al., 2004, Sandri et al., 2011). However, the present study failed to find that acute resistance exercise could increase serum VEGF levels in older adults with aMCI, partly in line with Rojas Vega et al., 2010 findings that acute resistance exercise of both low and high intensity could not increase VEGF levels in young adults. Thus far, to the best of our knowledge, no studies have been conducted to investigate the effects of acute exercise on VEGF in healthy older adults or in the elderly with MCI. Therefore, the present findings extend current knowledge with regard to whether aerobic or resistance exercise could be more effective to increase the serum VEGF levels in older adults with aMCI.
Age-related memory impairment can be attributed to age-related changes in the hippocampus. Such conditions have been correlated with dramatically decreased neurogenesis in the dentate gyrus during senescence due to reduced levels of FGF-2 (also VEGF and IGF-1) in the hippocampus (Shetty et al., 2005). FGF-2 is believed to afford neuroprotection by acting against glutamate induced neurotoxicity (Mattson et al., 1993), interfering with a number of signaling pathways (e.g., including maintenance of calcium homeostasis), and strengthening the anti-apoptotic pathways (Alzheimer and Werner, 2002). Decreased levels of FGF-2 may also increase the vulnerability of the aging hippocampus to different types of neuronal loss/damage (Shetty et al., 2005). Indeed, intracerebroventricular or subcutaneous injections of FGF-2 can significantly enhance neurogenesis in the dentate gyrus in the aged brain (Jin et al., 2003). FGF-2 has been proposed to participate in exercise-induced increases in the number of new hippocampal neurons (van Praag et al., 1999). In animal studies, Gomez-Pinilla et al., 1997 found increases in FGF-2 mRNA levels in the hippocampal but not in the caudal and middle regions of the cerebral cortex or striatum following variable periods of exercise, reaching a peak by the 4th night and returning to about normal levels by the 7th night of wheel-running. However, in the present study, the level of FGF-2 did not seem to be increased via acute aerobic or resistance exercise in the older adults with aMCI. Still, it is worth noting that the levels of FGF-2 are abnormally elevated in AD brains, especially in the limbic area (Cummings et al., 1993), which induces up-regulation of the expression of tau and inhibits neuronal differentiation in hippocampal progenitor cells (Tatebayashi et al., 1999). This gives rise to the possibility that the high FGF-2 levels seen with AD might be inhibitory for neurogenesis, as FGF-2 was significantly decreased after long-term aerobic exercise in amyloid precursor protein (APP)-23 mice (Wolf et al., 2006).
Some cross-sectional studies found that, after adjusting for multiple covariates, circulating BDNF was significantly associated with cognitive test scores in the elderly (Komulainen et al., 2008, Swardfager et al., 2011). Similarly, circulating IGF-1 levels in seniors or patients with AD revealed a significant, positive association with cognitive functions (Murialdo et al., 2001). However, in the present study, the changes in neurocognitive performance (e.g., RTs and P3 amplitudes) were not significantly correlated with the acute-exercise-induced changes of serum BDNF, IGF-1, and VEGF levels in the older adults with aMCI. There are two possible explanations to account for this. First, the beneficial effects of acute aerobic and resistance exercise on neurocognitive performance might be explained in terms of heightened arousal, due to exercise-induced temporary changes in the reallocation of mental resources and metabolic rate (Audiffren, 2009). Second, although the positive effects of exercise on the levels of some neuroprotective growth factors in older adults with aMCI were found in the present study, the effects were only transient. Indeed, Tsai et al., 2014b found that, even though the serum levels of neurotrophic factors (e.g., GH and IGF-1) were significantly increased via acute resistance exercise in young adults, these increased levels fell significantly within 20 min after exercise. Similarly, although Adams et al. (2004) found that significant VEGF increases remained for up to 24 h after the end of acute aerobic interventions in the elderly with peripheral arterial occlusive disease, in line with Gavin et al., 2004 findings, the increased serum VEGF levels induced by acute aerobic exercise showed a decreased trend in the present study, suggesting that skeletal muscle may take up VEGF from the circulation (Hiscock et al., 2003). Therefore, although one physical exercise session, with either aerobic or resistance exercise, could promote increases in BDNF, IGF-1, or VEGF levels, the transient nature of these effects could explain the lack of correlations between the changes in neurocognitive performance and those of the neurocognitive growth factors.
Both BDNF and IGF-1 are widely expressed in the human adult brain (Ahlskog et al., 2011, Murer et al., 2001). BDNF and IGF-1 insufficiencies have been proposed as risk factors for AD (Laske et al., 2007, Torres-Aleman, 2008, Yasutake et al., 2006), while an enhanced level of peripheral IGF-I is thought to mediate the induction of hippocampal BDNF (Cotman et al., 2007). The acute-exercise-mediated effect on the brain via IGF-1 might be involve in the increased hippocampal expression of BDNF and increased c-fos activation labeling through the brain (Carro et al., 2000). The interactive effects of BDNF and IGF-1 are thus considered as the key factors in the beneficial effects of exercise on cognitive functions (Cotman et al., 2007). In addition, IGF-I induces VEGF expression and secretion by a paracrine mechanism, indicating that VEGF secretion is involved in the IGF-1 pathway (Gruden et al., 2003). Exercise-dependent stimulation of hippocampal neurogenesis and angiogenesis seems to be regulated by the interactive effects of IGF-1 and VEGF (Cotman et al., 2007). These interactive mechanisms of BDNF, IGF-1, and VEGF and these biomarkers are strongly associated with the regulation of Aβ deposits (Adlard et al., 2005, Arvat et al., 2000, Carro et al., 2002, Solerte et al., 2005), suggesting that aerobic exercise could have more effects for patients with AD-related pathology, since the serum levels of the three growth factors could be induced by acute aerobic exercise, while acute resistance exercise could only induce the IGF-1 levels.
4.1. Limitations
There are some limitations to this study, which must be addressed in future work. First, since the brain levels of neurotrophic and angiogenesis factors cannot easily be obtained in humans, the circulating levels in the present study may or may not reflect what is actually going on within the brain. Second, since the increased level of circulating serum BDNF and VEGF in the present study could be attributed to the large contribution of platelet-derived BDNF and VEGF (Fujimura et al., 2002, Maloney et al., 1998), the sources of the two biomarkers used in the measurements (i.e., serum vs. plasma) might be an issue worth exploring in the future. However, a significant correlation between serum and plasma VEGF protein has been documented (Kraus et al., 2004), and the VEGF findings in the present study still provide important information about exercise in relation to aMCI. Third, since VEGF could be co-accumulated with Aβ deposits in AD brains, which results in impaired VEGF availability in the central nervous system (CNS) and contributes to vascular dysfunction and neurodegeneration (Yang et al., 2004), Chiappelli et al., 2006 found that the levels of plasma VEGF were higher in AD patients and the controls, and were much higher in AD patients with a fast cognitive decline and the APOE ɛ4 allele, which might reflect the progressive VEGF deficiency in the AD brain. Therefore, whether the increased serum VEGF levels through aerobic exercise in the older adults with aMCI found in the present study are a positive or negative effect needs further explanation, and to some extent some speculation. Further work is also needed to clarify the relation between exercise and VEGF in the MCI subgroups (e.g., MCI with or without the APOE ɛ4 allele). Lastly, although acute aerobic and resistance exercise could temporarily increase the levels of some neuroprotective growth factors and enhance neurocognitive performance in the older adults with aMCI in this study, which suggests that exercise is an effective way to improve neural growth and activity, how long these parameters could be changed during the baseline conditions needs further examination, possibly via long-term exercise intervention.
5. Conclusions
The present study finds that changes in neuroprotective growth factors and neurocognitive performances after acute exercise among older adults with aMCI and different exercise (e.g., aerobic and resistance) modes seem to have distinct effects on exerkines, which suggests that the cognitive neuroprotective effects of exercise, regardless of aerobic or resistance, may attenuate the risks of neurocognitive impairment and slow dementia in older adults with aMCI via plausibly divergent biologic pathways. Most importantly, the individuals at this stage still exhibit molecular and neural plasticity in response to exercise. Although the elderly with MCI showed impaired neurocognitive performance and reduced circulating levels of the neuroprotective growth factors, the present findings might reflect that physical exercise could regulate expression of some growth factors (e.g., BDNF, IGF-1 and VEGF), and retard these negative issues and reduce the risk of dementia/AD in the long term. However, compared to resistance exercise, aerobic exercise would be more effective for increasing exerkine levels. Although the transient improvements shown in the present work need further long-term exercise intervention studies to elucidate the substantially facilitative effects and the association between different exercise modes and neurocognitive/biochemical changes in aMCI, the implications of this study could provide a neural and molecular basis for the improvements in the deficits of cognitive function associated with active lifestyles, and guide the development of behavioral strategies to successful aging in older adults with aMCI.
Acknowledgments
Acknowledgments
The authors are grateful to the participants and their family caregivers who gave their precious time to participate in this research and facilitate the work reported here. The research reported in this publication was a Taiwan-Slovak Joint Research Cooperation project, and was supported by the National Science Council in Taiwan under grant number NSC 103-2923-H-006-001-MY3 to Dr. Tsai and Dr. Pai, and by the Slovak Academy of Sciences in Slovakia under grant number SAS/NSC JRP 2013/17 to Dr. Ukropcová and Dr. Ukropec.
Contributor Information
Chia-Liang Tsai, Email: andytsai@mail.ncku.edu.tw.
Ming-Chyi Pai, Email: pair@mail.ncku.edu.tw.
References
- Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Adams V., Lenk K., Linke A., Lenz D., Erbs S., Sandri M., Tarnok A., Gielen S., Emmrich F., Schuler G., Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise induced ischemia. Arterioscler. Thromb. Vasc. Biol. 2004;24:684–690. doi: 10.1161/01.ATV.0000124104.23702.a0. [DOI] [PubMed] [Google Scholar]
- Adlard P.A., Perreau V.M., Pop V., Cotman C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J. Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado-Llera D., Arilla-Ferreiro E., Campos-Barros A., Puebla-Jimenez L., Barrios V. Protective effects of insulin-like growth factor-I on the somatostatinergic system in the temporal cortex of beta-amyloid-treated rats. J. Neurochem. 2005;92:607–615. doi: 10.1111/j.1471-4159.2004.02889.x. [DOI] [PubMed] [Google Scholar]
- Aguiar A.S., Castro A.A., Moreira E.L., Glaser V., Santos A.R.S., Tasca C.I., Latini A., Prediger R.D.S. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 2011;132:560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ahlskog J.E., Geda Y.E., Graff-Radford N.R., Petersen R.C. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin. Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer C., Werner S. Fibroblast growth factors and neuroprotection. Adv. Exp. Med. Biol. 2002;513:335–351. doi: 10.1007/978-1-4615-0123-7_12. [DOI] [PubMed] [Google Scholar]
- Amieva H., Letenneur L., Dartigues J.F., Rouch-Leroyer I., Sourgen C., D'Alchée-Birée F., Dib M., Barberger-Gateau P., Orgogozo J.M., Fabrigoule C. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement. Geriatr. Cogn. Disord. 2004;18:87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- Arvat E., Broglio F., Ghigo E. Insulin-like growth factor I: implications in aging. Drugs Aging. 2000;16:29–40. doi: 10.2165/00002512-200016010-00003. [DOI] [PubMed] [Google Scholar]
- Audiffren M. Acute exercise and physiological functions: a cognitive-energetics approach. In: McMorris T., Tomporowski P.D., editors. Exercise and Cognitive Function. John Wiley & Sons; Hoboken, N.J: 2009. pp. 3–39. [Google Scholar]
- Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A., Plymate S.R., Fishel M.A., Watson G.S., Cholerton B.A., Duncan G.E., Mehta P.D., Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang P., Brandt J., Degerblad M., Enberg G., Kaijser L., Thorén M., Hall K. Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur. J. Clin. Investig. 1990;20:285–292. doi: 10.1111/j.1365-2362.1990.tb01857.x. [DOI] [PubMed] [Google Scholar]
- Barella L.A., Etnier J.L., Chang Y.K. The immediate and delayed effects of an acute bout of exercise on cognitive performance of healthy older adults. J. Aging Phys. Act. 2010;18:87–98. doi: 10.1123/japa.18.1.87. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. The Psychological Corporation; San Antonio, T.X: 1996. BDI-II: 2nd Edition Manual. [Google Scholar]
- Bennys K., Portet F., Touchon J., Rondouin G. Diagnostic value of event-related evoked potentials N200 and P300 subcomponents in early diagnosis of Alzheimer's disease and mild cognitive impairment. J. Clin. Neurophysiol. 2007;24:405–412. doi: 10.1097/WNP.0b013e31815068d5. [DOI] [PubMed] [Google Scholar]
- Bibel M., Barde Y.A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Busiguina S., Fernandez A.M., Barrios V., Clark R., Tolbert D.L., Berciano J., Torres-Aleman I. Neurodegeneration is associated to changes in serum insulin-like growth factors. Neurobiol. Dis. 2000;7:657–665. doi: 10.1006/nbdi.2000.0311. [DOI] [PubMed] [Google Scholar]
- Carro E., Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer's disease. Eur. J. Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Carro E., Nunez A., Busiguina S., Torres-Aleman I. Circulating insulin-like growth factor I mediate effects of exercise on the brain. J. Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E., Trejo J.L., Gomez-Isla T., LeRoith D., Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat. Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- Cassilhas R.C., Lee K.S., Fernandes J., Oliveira M.G., Tufik S., Meeusen R., de Mello M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Cespón J., Galdo-Álvarez S., Pereiro A.X., Díaz F. Differences between mild cognitive impairment subtypes as indicated by event-related potential correlates of cognitive and motor processes in a Simon task. J. Alzheimers Dis. 2015;43:631–647. doi: 10.3233/JAD-132774. [DOI] [PubMed] [Google Scholar]
- Chiappelli M., Borroni B., Archetti S., Calabrese E., Corsi M.M., Franceschi M., Padovani A., Licastro F. VEGF gene and phenotype relation with Alzheimer's disease and mild cognitive impairment. Rejuvenation Res. 2006;9:485–493. doi: 10.1089/rej.2006.9.485. [DOI] [PubMed] [Google Scholar]
- Cid-Fernández S., Lindín M., Díaz F. Effects of amnestic mild cognitive impairment on N2 and P3 Go/NoGo ERP components. J. Alzheimers Dis. 2014;38:295–306. doi: 10.3233/JAD-130677. [DOI] [PubMed] [Google Scholar]
- Coelho F.M., Pereira D.S., Lustosa L.P., Silva J.P., Dias J.M., Dias R.C., Queiroz B.Z., Teixeira A.L., Teixeira M.M., Pereira L.S. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012;54:415–420. doi: 10.1016/j.archger.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Coelho F.G., Vital T.M., Stein A.M., Arantes F.J., Rueda A.V., Camarini R., Teodorov E., Santos-Galduróz R.F. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J. Alzheimers Dis. 2014;39:401–408. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- Correia P.R., Pansani A., Machado F., Andrade M., Silva A.C., Scorza F.A., Cavalheiro E.A., Arida R.M. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clin. (Sao Paulo) 2010;65:1123–1126. doi: 10.1590/S1807-59322010001100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cowansage K.K., LeDoux J.E., Monfils M.H. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Cummings B.J., Su J.H., Cotman C.W. Neuritic involvement within bFGF immunopositive plaques of Alzheimer's disease. Exp. Neurol. 1993;124:315–325. doi: 10.1006/exnr.1993.1202. [DOI] [PubMed] [Google Scholar]
- DeCarli C., Mungas D., Harvey D., Reed B., Weiner M., Chui H., Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Audiffren M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci. Biobehav. Rev. 2011;35:1305–1325. doi: 10.1016/j.neubiorev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Dixit A., Vaney N., Tandon O.P. Evaluation of cognitive brain functions in caffeine users: a P3 evoked potential study. Indian J. Physiol. Pharmacol. 2006;50:175–180. [PubMed] [Google Scholar]
- Doucet C., Stelmack R.M. The effect of response execution on P3 latency, reaction time, and movement time. Psychophysiology. 1999;36:351–363. doi: 10.1017/s0048577299980563. [DOI] [PubMed] [Google Scholar]
- Espinosa A., Alegret M., Valero S., Vinyes-Junque G., Hernandez I., Mauleon A., Rosende-Roca M., Ruiz A., López O., Tárraga L., Boada M. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J. Alzheimers Dis. 2013;34:769–780. doi: 10.3233/JAD-122002. [DOI] [PubMed] [Google Scholar]
- Fabel K., Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C.J., Palmer T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fernandez A.M., Torres-Alemán I. Themany faces of insulin-like peptide signaling in the brain. Nat. Rev. Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Marin C., Rey M.J., Ribalta T., Goutan E., Blanco R., Tolosa E., Martí E. BDNF and full-length and truncated TrkB expression in Alzheimer's disease. Implications in therapeutic strategies. J. Neuropathol. Exp. Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Ferris L.T., Williams J.S., Shen C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Fisk J.D., Merry H.R., Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L., Paillard-Borg S., Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Fujimura H., Altar C.A., Chen R., Nakamura T., Nakahashi T., Kambayashi J., Sun B., Tandon N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- Gavin T.P., Robinson C.B., Yeager R.C., England J.A., Nifong L.W., Hickner R.C. Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J. Appl. Physiol. 2004;96:19–24. doi: 10.1152/japplphysiol.00748.2003. [DOI] [PubMed] [Google Scholar]
- Geisler M.W., Polich J. P300 and time of day: circadian rhythms, food intake, and body temperature. Biol. Psychol. 1990;31:117–136. doi: 10.1016/0301-0511(90)90012-l. [DOI] [PubMed] [Google Scholar]
- Goekint M., De Pauw K., Roelands B., Njemini R., Bautmans I., Mets T., Meeusen R. Strength training does not influence serum brain-derived neurotrophic factor. Eur. J. Appl. Physiol. 2010;110:285–293. doi: 10.1007/s00421-010-1461-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F., Dao L., So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F., So V., Kesslak J.P. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- Gottmann K., Mittmann T., Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp. Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Griffin É.W., Mullally S., Foley C., Warmington S.A., O'Mara S.M., Kelly A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Gruden G., Araf S., Zonca S., Burt D., Thomas S., Gnudi L., Viberti G.C. IGF-I induces vascular endothelial growth factor in human mesangial cells via a Src-dependent mechanism. Kidney Int. 2003;63:1249–1255. doi: 10.1046/j.1523-1755.2003.00857.x. [DOI] [PubMed] [Google Scholar]
- Hiscock N.J., Fischer C.P., Pilegaard H., Pedersen B.K. Vascular endothelial growth factor mRNA expression and a–v balance in response to prolonged, submaximal exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1759–H1763. doi: 10.1152/ajpheart.00150.2003. [DOI] [PubMed] [Google Scholar]
- Jiang S., Qu C., Wang F., Liu Y., Qiao Z., Qiu X., Yang X., Yang Y. Using event-related potential P300 as an electrophysiological marker for differential diagnosis and to predict the progression of mild cognitive impairment: a meta-analysis. Neurol. Sci. 2015;36:1105–1112. doi: 10.1007/s10072-015-2099-z. [DOI] [PubMed] [Google Scholar]
- Jin K.L., Mao X.O., Greenberg D.A. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Sun Y., Xie L., Batteur S., Mao X.O., Smelick C., Logvinova A., Greenberg D.A. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Johnson L., Addamo P.K., Selva Raj I., Borkoles E., Wyckelsma V., Cyarto E., Polman R.C. An acute bout of exercise improves the cognitive performance of older adults. J. Aging Phys. Act. 2016;24:591–598. doi: 10.1123/japa.2015-0097. [DOI] [PubMed] [Google Scholar]
- Joyce J., Graydon J., McMorris T., Davranche K. The time course effect of moderate intensity exercise on response execution and response inhibition. Brain Cogn. 2009;71:14–19. doi: 10.1016/j.bandc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Hayashi Y., Sakai T., Yahiro T., Tanaka K., Nishihira Y. Acute effects of aerobic exercise on cognitive function in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2009;64:356–363. doi: 10.1093/geronb/gbp030. [DOI] [PubMed] [Google Scholar]
- Karege F., Schwald M., Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kimura K., Yasunaga A., Wang L.Q. Correlation between moderate daily physical activity and neurocognitive variability in healthy elderly people. Arch. Gerontol. Geriatr. 2013;56:109–117. doi: 10.1016/j.archger.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Kline G.M., Porcari J.P., Hintermeister R., Freedson P.S., Ward A., McCarron R.F., Ross J., Rippe J.M. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med. Sci. Sports Exerc. 1987;19:253–259. [PubMed] [Google Scholar]
- Komulainen P., Pedersen M., Hanninen T., Bruunsgaard H., Lakka T.A., Kivipelto M., Hassinen M., Rauramaa T.H., Pedersen B.K., Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA study. Neurobiol. Learn. Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Kraus R.M., Stallings H.W., Yeager R.C., Gavin T.P. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J. Appl. Physiol. 2004;96:1445–1450. doi: 10.1152/japplphysiol.01031.2003. [DOI] [PubMed] [Google Scholar]
- Laske C., Stransky E., Leyhe T., Eschweiler G.W., Maetzler W., Wittorf A., Soekadar S., Richartz E., Koehler N., Bartels M., Buchkremer G., Schott K. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J. Psychiatr. Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Laske C., Stellos K., Hoffmann N., Stransky E., Straten G., Eschweiler G.W., Leyhe T. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int. J. Neuropsychopharmacol. 2011;14:399–404. doi: 10.1017/S1461145710001008. [DOI] [PubMed] [Google Scholar]
- Lipsky R.H., Marini A.M. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann. N. Y. Acad. Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C., LeRoith D., Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks T.L., Oliveira M., Possin K.L., Bird A., Miller B.L., Weiner M.W., Kramer J.H. Atrophy in two attention networks is associated with performance on a Flanker task in neurodegenerative disease. Neuropsychologia. 2010;48:165–170. doi: 10.1016/j.neuropsychologia.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A., Düzel S., Brigadski T., Goerke M., Becke A., Sobieray U., Neumann K., Lӧvdén M., Lindenberger U., Bäckman L., Braun-Dullaeus R., Ahrens D., Heinze H.J., Müller N.G., Lessmann V., Sendtner M., Düzel E. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage. 2016;131:142–154. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- Maloney J.P., Silliman C.C., Ambruso D.R., Wang J., Tuder R.M., Voelkel N.F. In vitro release of vascular endothelial growth factor during platelet aggregation. Am. J. Physiol. Heart Circ. Physiol. 1998;275:H1054–H1061. doi: 10.1152/ajpheart.1998.275.3.H1054. [DOI] [PubMed] [Google Scholar]
- Mattson M.P., Kumar K.N., Wang H., Cheng B., Michaelis E.K. Basic FGF regulates the expression of a functional 71 kDa NMDA receptor protein that mediates calcium influx and neurotoxicity in hippocampal neurons. J. Neurosci. 1993;13:4575–4588. doi: 10.1523/JNEUROSCI.13-11-04575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morra J.H., Tu Z., Apostolova L.G., Green A.E., Avedissian C., Madsen S.K., Parikshak N., Toga A.W., Jack C.R., Jr., Schuff N., Weiner M.W., Thompson P.M., Alzheimer's Disease Neuroimaging Initiative Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. NeuroImage. 2009;45:S3–S15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C., Storandt M., Miller P., McKeel Jr D.W., Price J.L., Rubin E.H., Berg L. Mild cognitive impairment represents early-stage Alzheimer's disease. Arch. Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Murer M.G., Yan Q., Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Murialdo G., Barreca A., Nobili F., Rollero A., Timossi G., Gianelli M.V., Copello F., Rodriguez G., Polleri A. Relationships between cortisol, dehydroepiandrosterone sulphate and insulin-like growth factor-I system in dementia. J. Endocrinol. Investig. 2001;24:139–146. doi: 10.1007/BF03343833. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park H., Poo M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Peng S., Wuu J., Mufson E.J., Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J. Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Phillips H.S., Hains J.M., Armanini M., Laramee G.R., Johnson S.A., Winslow J.W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Portugal E.M., Vasconcelos P.G., Souza R., Lattari E., Monteiro-Junior R.S., Machado S., Deslandes A.C. Aging process, cognitive decline and Alzheimer's disease: can strength training modulate these responses? CNS Neurol. Disord. Drug Targets. 2015;14:1209–1213. doi: 10.2174/1871527315666151111121749. [DOI] [PubMed] [Google Scholar]
- Prior B.M., Lloyd P.G., Yang H.T., Terjung R.L. Exercise-induced vascular remodeling. Exerc. Sport Sci. Rev. 2003;31:26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Redila V.A., Christie B.R. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Rikli R.E., Jones C.J. 2nd ed. Human Kinetics; United States: 2012. Senior Fitness Test Manual. [Google Scholar]
- Rojas Vega S., Knicker A., Hollmann W., Bloch W., Strüder H.K. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab. Res. 2010;42:982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C., Lambrechts D., Mazzone M., Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Sallis J.F., Haskell W.L., Woo d P.D., Fortmann S.P., Rogers T., Blair S.N., Paffenbarger R.S.Jr. Physical activity assessment methodology in the five-city project. Am. J. Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- Sandri M., Beck E.B., Adams V., Gielen S., Lenk K., Höllriegel R., Mangner N., Linke A., Erbs S., Möbius-Winkler S., Scheinert D., Hambrecht R., Schuler G. Maximal exercise, limb ischemia, and endothelial progenitor cells. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18:55–64. doi: 10.1097/HJR.0b013e32833ba654. [DOI] [PubMed] [Google Scholar]
- Schobersberger W., Hobisch-Hagen P., Fries D., Wiedermann F., Rieder-Scharinger J., Villiger B., Frey W., Herold M., Fuchs D., Jelkmann W. Increase in immune activation, vascular endothelial growth factor and erythropoietin after an ultramarathon run at moderate altitude. Immunobiology. 2000;201:611–620. doi: 10.1016/S0171-2985(00)80078-9. [DOI] [PubMed] [Google Scholar]
- Shen H., Tong L., Balazs R., Cotman C.W. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Shetty A.K., Hattiangady B., Shetty G.A. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Lee S., Park H., Suzuki T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014;6:69. doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solerte S.B., Ferrari E., Cuzzoni G., Locatelli E., Giustina A., Zamboni M., Schifino N., Rondanelli M., Gazzaruso C., Fioravanti M. Decreased release of the angiogenic peptide vascular endothelial growth factor in Alzheimer's disease: recovering effect with insulin and DHEA sulfate. Dement. Geriatr. Cogn. Disord. 2005;19:1–10. doi: 10.1159/000080963. [DOI] [PubMed] [Google Scholar]
- Sonntag W.E., Lynch C.D., Cooney P.T., Hutchins P.M. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Sonntag W.E., Ramsey M., Carter C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Stranahan A.M., Lee K., Becker K.G., Zhang Y., Maudsley S., Martin B., Cutler R.G., Mattson M.P. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol. Aging. 2010;31:1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk H.I., Lee S.W., Shen D., Alzheimer's Disease Neuroimaging Initiative Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. NeuroImage. 2014;101:569–582. doi: 10.1016/j.neuroimage.2014.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Ito K., Shimokata H., Washimi Y., Endo H., Kato T. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W., Herrmann N., Marzolini S., Saleem M., Shammi P., Oh P.I., Albert P.R., Daigle M., Kiss A., Lanctôt K.L. Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients with coronary artery disease. Brain Behav. Immun. 2011;25:1264–1271. doi: 10.1016/j.bbi.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko P.R., Lipsky R., Mentschel C., Robinson D., Gunduz-Bruce H., Sevy S., Ashtari M., Napolitano B., Bilder R.M., Kane J.M., Goldman D., Malhotra A.K. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Tatebayashi Y., Iqbal K., Grundke-Iqbal I. Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J. Neurosci. 1999;19:5245–5254. doi: 10.1523/JNEUROSCI.19-13-05245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brinke L.F., Bolandzadeh N., Nagamatsu L.S., Hsu C.L., Davis J.C., Miran-Khan K., Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Sports Med. 2015;49:248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E.L., Hasegawa K., Homma A., Imai Y., Larson E., Graves A., Sugimoto K., Yamaguchi T., Sasaki H., Chiu D. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- Thomas S., Reading J., Shephard R.J. Revision of the physical activity readiness questionnaire (PAR-Q) Can. J. Sport Sci. 1992;17:3338–3345. [PubMed] [Google Scholar]
- Tomporowski P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Torres-Aleman I. Mouse models of Alzheimer's dementia: current concepts and new trends. Endocrinology. 2008;149:5952–5957. doi: 10.1210/en.2008-0905. [DOI] [PubMed] [Google Scholar]
- Tsai C.L., Chen F.C., Pan C.Y., Wang C.H., Huang T.H., Chen T.C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF level. Psychoneuroendocrinology. 2014;41:121–131. doi: 10.1016/j.psyneuen.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Tsai C.L., Wang C.H., Pan C.Y., Chen F.C., Huang T.H., Chou F.Y. Executive function and endocrinological responses to acute resistance exercise. Front. Behav. Neurosci. 2014;8:262. doi: 10.3389/fnbeh.2014.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.L., Wang C.H., Pan C.Y., Chen F.C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015;9:23. doi: 10.3389/fnbeh.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.L., Pai M.C., Ukropec J., Ukropcová B. The role of physical fitness in the neurocognitive performance of task switching in older persons with mild cognitive impairment. J. Alzheimers Dis. 2016;53:143–159. doi: 10.3233/JAD-151093. [DOI] [PubMed] [Google Scholar]
- Tsai C.L., Pan C.Y., Chen F.C., Wang C.H., Chou F.Y. Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp. Physiol. 2016;101:836–850. doi: 10.1113/EP085682. [DOI] [PubMed] [Google Scholar]
- Uysal N., Kiray M., Sisman A.R., Camsari U.M., Gencoglu C., Baykara B., Cetinkaya C., Aksu I. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2015;90:55–68. doi: 10.3109/10520295.2014.946968. [DOI] [PubMed] [Google Scholar]
- Vagnucci A.H.Jr., Li W.W. Alzheimer's disease and angiogenesis. Lancet. 2003;361:605–608. doi: 10.1016/S0140-6736(03)12521-4. [DOI] [PubMed] [Google Scholar]
- Vale R.G., de Oliveira R.D., Pernambuco C.S., de Meneses Y.P., Novaes Jda S., de Andrade Ade F. Effects of muscle strength and aerobic training on basal serum levels of IGF-1 and cortisol in elderly women. Arch. Gerontol. Geriatr. 2009;49:343–347. doi: 10.1016/j.archger.2008.11.011. [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B.R., Sejnowski T.J., Gage F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucher E., Aumont N., Pearson D., Rowe W., Poirier J., Kar S. Amyloid β peptide levels and its effects on hippocampal acetylcholine release in aged, cognitively impaired and unimpaired rats. J. Chem. Neuroanat. 2001;21:323–329. doi: 10.1016/s0891-0618(01)00120-x. [DOI] [PubMed] [Google Scholar]
- Vaynman S., Ying Z., Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J. Neurosci. Res. 2004;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Vaynman S.S., Ying Z., Yin D., Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Voss M.W., Erickson K.I., Prakash R.S., Chaddock L., Kim J.S., Alves H., Szabo A., Phillips S.M., Wójcicki T.R., Mailey E.L., Olson E.A., Gothe N., Vieira-Potter V.J., Martin S.A., Pence B.D., Cook M.D., Woods J.A., McAuley E., Kramer A.F. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhang X., Liu Y., Liu S., Zhou B., Zhang Z., Yao H., Zhang X., Jiang T. Perceptual and response interference in Alzheimer's disease and mild cognitive impairment. Clin. Neurophysiol. 2013;124:2389–2396. doi: 10.1016/j.clinph.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson; San Antonio, Texas: 2008. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) Technical and Interpretive Manual. [Google Scholar]
- Wolf S.A., Kronenberg G., Lehmann K., Blankenship A., Overall R., Staufenbiel M., Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol. Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Wu B.H. Dose effects of caffeine ingestion on acute hormonal responses to resistance exercise. J. Sports Med. Phys. Fitness. 2014;55:1242–1251. [PubMed] [Google Scholar]
- Wylie S.A., Ridderinkhof K.R., Eckerle M.K., Manning C.A. Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia. 2007;45:1408–1419. doi: 10.1016/j.neuropsychologia.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Yang S.P., Bae D.G., Kang H.J., Gwag B.J., Gho Y.S., Chae C.B. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer's disease. Neurobiol. Aging. 2004;25:283–290. doi: 10.1016/S0197-4580(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Yasutake C., Kuroda K., Yanagawa T., Okamura T., Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer's disease and vascular dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]