Abstract
Background
Esophageal cancer is an aggressive disease with poor survival rates. A more patient-tailored approach based on predictive biomarkers could improve outcome. We aimed to predict radiotherapy (RT) response by imaging tumor hypoxia with 18F-FAZA PET/CT in an esophageal adenocarcinoma (EAC) mouse model. Additionally, we investigated the radiosensitizing effect of the hypoxia modifier nimorazole in vitro and in vivo.
Methods
In vitro MTS cell proliferation assays (OACM5 1.C SC1, human EAC cell line) were performed under normoxic and hypoxic (< 1%) conditions: control (100 μL PBS), nimorazole, irradiation (5, 10 or 20 Gy) with or without nimorazole. In vivo, subcutaneous xenografts were induced in nude mice (OACM5 1.C SC1). Treatment was given daily for 5 consecutive days: (A) control (600 μl NaCl 0.9% intraperitoneally (IP)) (N = 5, n = 7), (B) RT (5 Gy/d) (N = 11, n = 20), (C) combination (nimorazole (200 mg/kg/d IP) 30 min before RT) (N = 13, n = 21). N = number of mice, n = number of tumors. 18F-FAZA PET/CT was performed before treatment and tumor to background (T/B) ratios were calculated. Relative tumor growth was calculated and tumor sections were examined histologically (hypoxia, proliferation).
Results
A T/B ≥ 3.59 on pre-treatment 18F-FAZA PET/CT was predictive for worse RT response (sensitivity 92.3%, specificity 71.4%). Radiation was less effective in hypoxic tumors (T/B ≥ 3.59) compared to normoxic tumors (T/B < 3.59) (P = 0.0025). In vitro, pre-treatment with nimorazole significantly decreased hypoxic radioresistance (P < 0.01) while in vivo, nimorazole enhanced the efficacy of RT to suppress cancer cell proliferation in hypoxic tumor areas (Ki67, P = 0.064), but did not affect macroscopic tumor growth.
Conclusions
Tumor tissue hypoxia as measured with 18F-FAZA PET/CT is predictive for RT response in an EAC xenograft model. The radiosensitizing effect of nimorazole was questionable and requires further investigation.
Electronic supplementary material
The online version of this article (10.1186/s13014-018-0984-3) contains supplementary material, which is available to authorized users.
Keywords: Radioresistance, 18F-FAZA pet/CT, Tumor hypoxia, Predictive biomarker, Esophageal adenocarcinoma xenografts, Nimorazole
Background
Esophageal cancer patients are mostly diagnosed in a locally advanced stage and treated with neoadjuvant chemoradiation followed by surgery [1]. Prognosis is poor and response to treatment is highly variable [2]. Identification of predictive imaging biomarkers is an important challenge.
Tumor hypoxia is an attractive predictive factor as it has been correlated with chemoresistance, radioresistance, invasiveness, propensity to metastasize, genomic instability and worse prognosis in different solid tumors [3].
Also in esophageal cancer, hypoxia has been correlated with worse outcomes. Histologic examination of carbonic anhydrase 9 (CAIX) and hypoxia-inducible factor 1-alpha (HIF-1α), two factors that are overexpressed in hypoxic conditions, were correlated with worse outcomes and hypoxia imaging with 18F-FETNIM (fluoroerythronitroimidazole) positron emission tomography (PET) showed that tracer uptake might be predictive for treatment response in esophageal cancer [4–7].
PET-based hypoxia imaging is one of the most studied hypoxia detection methods with clinical applicability. Over the years, different tracers have been studied and have been proven to have predictive or prognostic value (18F-FMISO (fluoromisonidazole) [8], 18F-FAZA (fluoroazomycin arabinoside) [9], 18F-FETNIM [6], 18F-EF5 (pentafluoropropylacetamide) [10, 11]). Here, 18F-FAZA PET/CT was used to image tumor hypoxia and investigate its predictive potential in esophageal cancer. 18F-FAZA is a second generation 2-nitroimidazole that has been shown to be hypoxia specific and reproducible [12]. It has superior pharmacokinetics compared to 18F-FMISO, resulting in a better tumor-to-background ratio [13]. The tracer entrapment is based on a reduction of the NO2-group followed by continued reduction under hypoxic conditions and eventually covalent binding to intracellular macromolecules [14]. This non-invasive technique provides a 3-dimensional image of the hypoxia distribution and can be repeated over time, which allows follow-up [14]. 18F-FAZA PET is a promising tracer that already showed to be predictive for treatment response in preclinical models of rhabdomyosarcoma and breast carcinoma [15, 16]. Clinically, FAZA imaging has been studied in non-small cell lung cancer [17] and head and neck squamous cell cancer [18, 19], while trials are ongoing in rectal, lung, cervix, and prostate carcinoma (ClinicalTrials.gov: NCT02624115, NCT02701699, NCT01989364, NCT01567800).
Additionally, we investigated whether nimorazole could enhance radiation response in hypoxic conditions. It is a 5-nitroimidazole that mimics oxygen in the radiobiological process by promoting fixation of free radicals [20]. Nimorazole is easy applicable, has few side effects and is already part of daily practice in Denmark for HNSCC patients [21] (DAHANCA guidelines).
In summary, this study investigated the predictive value of 18F-FAZA PET/CT for hypoxia-induced radioresistance in EAC xenografts and the radiosensitizing effect of nimorazole.
Methods
Cell line
OACM5 1.C SC1 was established through in vivo selection from the parental cell line OACM5 1.C, a human esophageal adenocarcinoma (EAC) cell line, as described previously [22] and was authenticated by STR-based DNA-profiling. Cells were cultured at 37 °C in 5% CO2 humidified atmosphere in RPMI 1640 Medium supplemented with GlutaMAX™-I (Life Technologies), 10% fetal bovine serum and penicillin-streptomycin.
MTS assay
Hypoxic radioresistance and the radiosensitizing effect of nimorazole were first quantified in vitro with an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Cells (8 × 105 per T25 flask) were incubated overnight at normoxic (5% CO2 in air) or hypoxic (Anaerobic Work Station, Baker Ruskinn, 80% N2, 10% CO2, 10% H2) conditions. Treatment was given 24 h after seeding: control (100 μL PBS); nimorazole (0.2 mg/mL in PBS); RT (5, 10 or 20 Gy) with or without nimorazole. The metabolic activity of cells was analyzed 72 h post-treatment. A solution of a tetrazolium compound (MTS, CellTiter 96® Aqueous MTS Reagent Powder (Promega)) and an electron coupling reagent phenazine methosulfate (PMS) was added to each T25 flask (1 mL) and was incubated for 90 min (37 °C and 5% CO2). Absorbance was measured with Paradigm (490 nm) (SPECTRAMax Paradigm, Molecular Devices, USA). Cell viabilities were calculated relative to controls (0 Gy = 100% cell viability). (n = 3 × 2).
Animals and tumor model
Animal experiments were approved by the Animal Ethical Committee of the Ghent University (ECD 14/82) and were performed in accordance with the EU Directive 2010/63/EU. OACM5 1.C SC1 cells (3 × 106 in 100 μl of Matrigel) were injected subcutaneously in both hind legs of athymic male mice (5 weeks of age, Foxn1nu, Envigo, the Netherlands). Tumors were grown for 7 weeks. Tumors with a minimum volume of 150 mm3 were included. One day post-treatment, mice were euthanized under anesthesia by cervical dislocation. Inhalation anesthesia with isoflurane (Abbott, Belgium) was used, 5% induction, 1.5% maintenance, 0.3 L/min.
Treatment
Treatment was given daily for 5 consecutive days: (A) control (600 μl NaCl 0.9% intraperitoneally (IP)) (N = 5, n = 7), (B) RT (5 Gy/d) (N = 11, n = 20), (C) combination (nimorazole (200 mg/kg/d IP) 30 min before RT) (N = 13, n = 21). N = number of mice, n = number of tumors. Nimorazole (Adooq Bioscience LLC, USA) was dissolved in NaCl 0.9% at 10 mg/mL on the day of administration. The dosage and timing was according to previous literature [23]. Because nimorazole acts as a pure radiosensitizer at this dosage, no nimorazole monotherapy group was included (See in vitro results and [23]). Tumor nodules were measured daily with calipers and volumes were calculated according to the following formula: V = (length × width)3/2 × π/6. Relative tumor growth (RTG) was calculated as the ratio of the volume at the day of euthanasia to the volume before treatment.
Radiotherapy
RT was applied using the small animal radiation research platform (SARRP). The voltage of the X-ray source was fixed at 220 kV with a tube current of 13 mA, emitted from the 3 mm focal spot, filtered by a copper filter of 0.15 mm. For in vitro experiments, a vertical radiation beam of 10 × 10 cm2 was used. Single doses of 5, 10 or 20 Gy were administered. For in vivo experiments, a pair of parallel-opposed (anterior-posterior) radiation beams of 10 × 10 mm2 were used. Mice were anesthetized and positioned on the bed of the SARRP. Guided by lasers, the bed was moved to position tumors at the isocenter of the beam. To allow parallel-opposed beam irradiations, mice were turned around when half of the dose was given. Tumors were irradiated 5 consecutive days, 5 Gy/day.
18F-FAZA pet-CT
The radiosynthesis of 18F-FAZA was performed on a Synthra RNplus module (Synthra GmbH, Hamburg, Germany) using a fully automated procedure that was based on standard procedures [24, 25]. The precursor for the radiosynthesis, 1-(2,3-diacetyl-5-tosyl-(α-d-arabinofuranosyl)-2-nitroimidazole, was purchased from ABX GmbH (Radeberg, Germany) and all other required reagents and solvents were acquired from Sigma-Aldrich (Overijse, Belgium).
18F-FAZA PET/CT was performed one day before treatment. Mice were anesthetized and 37.0±1.9 MBq of 18F-FAZA was injected in the tail vein. Three hours after injection and under anesthesia a static PET/CT was performed. The animals were positioned on a heated bed of a small animal PET/CT scanner (TriFoil Imaging, Triumph II, Northridge, CA, USA). A 30 min PET scan was acquired in list mode, with a 75-mm axial field-of-view and a 1.3-mm spatial resolution. On the same scanner and without moving the animal, a CT scan was performed. CT projection data were acquired using the following parameters: 256 projections, detector pixel size 50 μm, focal spot size 100 μm, tube voltage 50 kV, tube current 640 μA, and a field-of-view of 90 mm. The acquired PET images were reconstructed into a 200x200x64 matrix by a 2D maximum likelihood expectation maximization (MLEM) algorithm (LabPET Version 1.12.1, TriFoil Imaging®, Northridge, CA) using 50 iterations and a voxel size of 0.5 × 0.5 × 1.175 mm3 (x, y, z). CT images were analytically reconstructed using a filtered back projection reconstruction algorithm (Cobra Version 7.3.4, Exxim Computing Corporation, Pleasanton, CA) into a 256x256x512 matrix with 200 μm isotropic voxel size. Each resultant CT image is inherently co-registered with the corresponding PET scan. PET and CT images were imported into A Medical Image Data Examiner (AMIDE) [26], where tumor-to-background (T/B) ratios were calculated as the mean tumor uptake divided by the background activity. Mean tumor uptake (Bq/mL) was quantified in a volume-of-interest that was semi-automatically delineated as the activity > 40% of the maximum activity using the 3D-isocontour tool, similar to Tran et al. [27], and a sphere with radius 1.5 mm was delineated in the foreleg muscle as background tissue.
Tumor samples and histology
Consecutive 5 μm sections of FFPE tumors were prepared. H&E staining was performed and necrotic areas were excluded for further analysis. The hypoxia marker pimonidazole, administered 1 h before sacrifice (60 mg/kg, IP, Hypoxyprobe, USA), was stained with Hypoxyprobe anti-pimonidazole Ab (HP1–100 Kit)(1/50). Ki67 staining was performed with anti-Ki67 Ab ([SP6] Abcam 16,667)(1/100) and proliferation indices (fraction of Ki67+ cells/total cells) were calculated in normoxic and hypoxic regions, according to pimonidazole staining on consecutive sections (3 × 2 hotspots/tumor) (ImageJ (ImmunoRatio)). Microscopy was performed with a light microscope (ColorView I, BX43F, Olympus, Japan).
Statistical methods
Statistical analysis was performed with GraphPad Prism6 (Graphpad Software, Inc.: La Jolla, USA). Data was tested for normality (Shapiro-Wilk) and analyzed with the Mann-Whitney U test (non-parametric) or t-test (parametric). ID50 values of the MTS-assay were calculated with non-linear regression analysis (log(inhibitor) vs. normalized response). Oxygen Enhancement Ratio (OER = Radiation dose hypoxia/normoxia) and Sensitizer Enhancement Ratio (SER = Radiation dose hypoxia/hypoxia with nimorazole) were calculated. The cut-off T/B ratio to predict treatment response was determined with ROC-analysis. P-values < 0.05 were considered statistically significant and abbreviated as * = P < 0.05, ** = P < 0.01, *** = P 0.001, **** = P < 0.0001.
Results
18F-FAZA PET/CT as predictive biomarker
Forty-eight tumors were included for 18F-FAZA PET/CT (control n = 7, RT n = 20, combination n = 21) (Fig. 1a). T/B ratios were equally distributed across the treatment groups and varied from 1.17 to 5.83 with a median of 2.74. Tumors that regressed after RT (RTG < 100%) were defined radiosensitive (65%, n = 13) and tumors that continued growing (RTG > 100%), radioresistant (35%, n = 7). Pre-treatment 18F-FAZA uptake (T/B ratios) was significantly higher in radioresistant tumors than in radiosensitive tumors (P = 0.0046) (Fig. 1b), demonstrating that more hypoxic tumors are more resistant to RT than less hypoxic tumors. ROC-analysis was performed to identify a cut-off value for predicting RT response with 18F-FAZA PET/CT, which showed that a T/B of 3.59 predicted treatment response with the highest sensitivity and specificity (92.3% and 71.4% respectively, AUC 0.75). Based on pre-treatment 18F-FAZA PET/CT, tumors were divided in normoxic (T/B < 3.59) and hypoxic (T/B ≥ 3.59). Irradiation inhibited tumor growth significantly better in normoxic tumors compared to hypoxic tumors (P = 0.0025) (Fig. 1c).
Fig. 1.
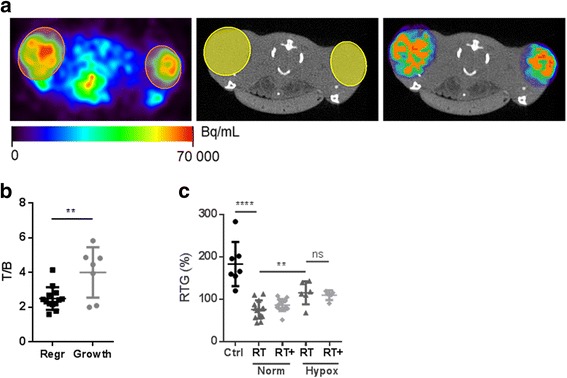
Predictive value of 18F-FAZA PET/CT in EAC xenografts. (a) Transverse slices at the level of the hind legs with mice in prone position. PET/CT acquired 3 h after tracer injection. Left: 18F-FAZA PET images with subcutaneous EAC tumors delineated spherically (orange). Middle: corresponding CT images. Right: Overlay 18F-FAZA PET/CT. The PET data exterior to the ROI’s was erased. Orange = ROI > 40% isocontour. High 18F-FAZA uptake was also seen in the urinary bladder due to renal excretion of the tracer. (b) Pre-treatment 18F-FAZA uptake of RT treated tumors. Regr = tumors that regressed (radiosensitive), Growth = tumors that continued growing (radioresistant). T/B ratios (single values, mean, SD, t-test). (c) Control (Ctrl); Radiotherapy (RT); Combination (RT+). Hypoxia status was defined by 18F-FAZA PET/CT: T/B < 3.59 = normoxic; T/B ≥ 3.59 = hypoxic. RTG of EAC xenografts (single values, mean, SD, t-test)
Hypoxic radioresistance and radiosensitizing effect of nimorazole
First, radiosensitizing effects of nimorazole were investigated in vitro in the OACM5 1.C SC1 cell line (Fig. 2a-b). As expected, RT was less efficient under hypoxic conditions, illustrated by an upwards movement of the dose-response curve (OERD50 = 2.82). Pretreatment with nimorazole radiosensitized hypoxic tumor cells (SERD50 = 1.51). Nimorazole had no effect on RT efficacy in normoxic conditions and acted as a pure radiosensitizer without intrinsic cytotoxic effect (Additional file 1: Figure S1).
Fig. 2.
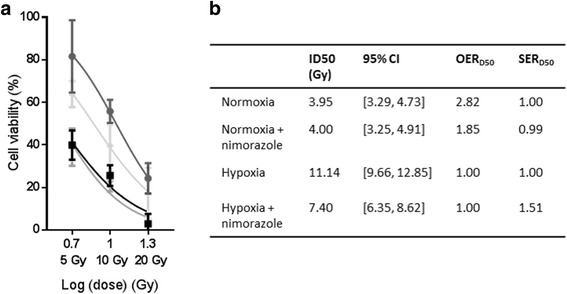
In vitro effects of nimorazole on radiotherapy (RT) response. (a)  Normoxia;
Normoxia;  Hypoxia;
Hypoxia;  Normoxia + nimorazole;
Normoxia + nimorazole;  Hypoxia + nimorazole. Dose-response curve of MTS-assay with RT doses (x-axis, logarithmic) and cell viabilities (y-axis, mean, SD, non-linear regression fitted curve) relative to controls (0 Gy, cell viability = 100%). (b) ID50 = Radiation Dose (RD) to inhibit 50% of the cell viability; OERD50 = Oxygen Enhancement Ratio (RD hypoxia/normoxia); SERD50 = Sensitizer Enhancement Ratio (RD hypoxia/hypoxia with nimorazole)
Hypoxia + nimorazole. Dose-response curve of MTS-assay with RT doses (x-axis, logarithmic) and cell viabilities (y-axis, mean, SD, non-linear regression fitted curve) relative to controls (0 Gy, cell viability = 100%). (b) ID50 = Radiation Dose (RD) to inhibit 50% of the cell viability; OERD50 = Oxygen Enhancement Ratio (RD hypoxia/normoxia); SERD50 = Sensitizer Enhancement Ratio (RD hypoxia/hypoxia with nimorazole)
Second, nimorazole was investigated in vivo. Histologic examination of EAC xenografts showed that hypoxic tumor areas were resistant to RT with significantly higher proliferation indices than in normoxic areas (P = 0.0025) (Fig. 3a, b). Pre-treatment with nimorazole radiosensitized hypoxic cancer cells with a trend to decrease proliferation indices (P = 0.064). Evaluation of the effect of nimorazole on tumor growth control showed that it had no effect in less hypoxic tumors (T/B < 3.59) (Fig. 1c). Further, opposite to the in vitro and histological results where nimorazole increased radiosensitivity in hypoxic conditions, nimorazole did not seem to improve tumor growth control in hypoxic tumors (T/B ≥ 3.59). (Fig. 1c).
Fig. 3.
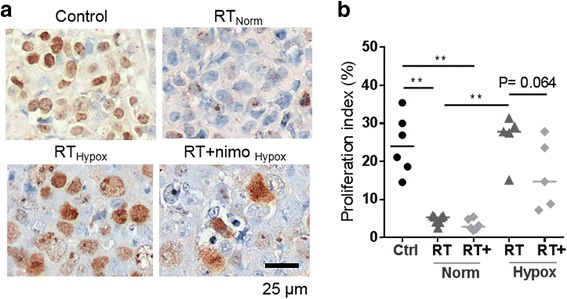
Effect of nimorazole on cancer cell proliferation in vivo. (a) Representative pictures of Ki67 stained tumor sections of each treatment group. Normoxic and hypoxic tumor areas were based on pimonidazole staining of consecutive sections. (b) Control (Ctrl); Radiotherapy (RT); Combination (RT+). Cancer cell proliferation indices from Ki67 staining (single values, median). Norm (normoxia) and Hypox (hypoxia) were based on pimonidazole staining of consecutive sections. (Mann-Whitney test)
Discussion
This study investigated the predictive value of 18F-FAZA PET/CT for hypoxia-induced radioresistance and the radiosensitizing effect of nimorazole in an EAC model in mice. We showed that pre-treatment 18F-FAZA PET/CT could identify more and less hypoxic tumors, which was related with radiation response. We identified a T/B of ≥3.59 that predicted radioresistance with a sensitivity of 92.3% and specificity of 71.4%. Further, nimorazole clearly decreased hypoxia-induced radioresistance in the OACM5 1.C SC1 cell line in vitro and in the EAC xenografts (histologically). Moreover, this was the first study investigating 18F-FAZA PET in esophageal cancer. We focused on esophageal adenocarcinoma because it has become the main subtype in patients in the United States and Northern and Western Europe [28].
It is difficult to compare the T/B ratios of this study with others, because no consensus exists for quantifying 18F-FAZA uptake. Some studies quantify tracer uptake as percentage of the totally injected activity (%ID/g or SUV (standardized uptake values) if standardized to the animals’ weight). However, because FAZA is excreted in urine and feces, tracer activity at the moment of the scan can vary substantially between animals, making %ID/g or SUV parameters rather unreliable. Here, 18F-FAZA uptake was quantified relative to a reference non-hypoxic tissue (tumor to background ratio), according to a method used by Tran et al. [27] and was found feasible. For the future, it will be a challenge to use a uniform parameter.
Here, nimorazole was shown to have a SERD50 of 1.51 in hypoxic conditions in vitro, which is in accordance to previous literature [23, 29]. Also histologically, nimorazole increased radiation response in hypoxic tumor areas (Ki67 staining). The lack of its effect on tumor growth control could be for the following reasons. First, tumors were harvested one day post-treatment to evaluate the histological effects of radiation/nimorazole. This was rather early to analyze the total effect on tumor growth and we believe a longer follow-up could result in more significant differences. Second, single RT doses were used in vitro, whereas in vivo, more clinically relevant doses (5 × 5 Gy) were used. As it is known that fractionation causes tumor cell reoxygenation, the RT regimen itself could have radiosensitizing effects, minimizing the effect of nimorazole [30, 31]. Whether nimorazole will have a sensitizing effect in a clinical radiation regimen (23 × 1.8 Gy according to the recent CROSS trial [1]), is to be investigated.
As 18F-FAZA PET/CT has already been proven to be safe in the clinical setting, these results encourage a subsequent clinical trial where the predictive value of 18F-FAZA PET/CT is investigated in EAC patients. This could lead to a more patient-tailored approach. For example, if a tumor is predicted to show a good response, it seems to be worth to administer neoadjuvant treatment before surgery. Meanwhile, if a tumor is predicted to be resistant to neoadjuvant treatment, it could be better to perform the surgical resection earlier or to modify the neoadjuvant treatment and decrease radioresistance, like modifications to the RT regimen itself (e.g. dose-painting [32]) or addition of a hypoxia modifier (e.g. nimorazole [33]). Still, tumor hypoxia is distributed heterogeneously in space and over time [3]. For sure, repeating 18F-FAZA PET/CT scans will be needed to reevaluate tumor’s hypoxia status and indications for radiosensitizers.
Some limitations have to be taken in consideration regarding the present study. First, one tumor model (subcutaneous xenografts) was investigated with one tumor type (EAC), which limits conclusions and future clinical trials to this tumor type. The subcutaneous model was chosen because a previous study with orthotopic esophageal tumors localized at the distal esophagus was not feasible. Tumors could not be delineated due to background tracer activity in the liver (hepatic metabolization of FAZA). We believe visualization in patients will be better because of larger structures and higher soft tissue resolution on human CT scans. Further, this should not be a problem in esophageal squamous cell carcinomas that are typically located in the thoracic part of the esophagus. We believe that repeating the study at an orthotopic site is of little interest at the moment. By demonstrating the predictive value of 18F-FAZA in esophageal adenocarcinoma xenografts, we believe the next step should be a clinical study instead of another preclinical experiment. Second, because the cut-off T/B was defined retrospectively, the predictive value should ideally be confirmed in a prospective experiment. Third, other modification methods than nimorazole (e.g. dose-painting or carbogen breathing) could have been included to compare effects.
Tumor hypoxia is a long-known problem within oncology with little impact in the daily clinic. This is partially because hypoxia detection methods have not reached the routine clinical work-up of cancer patients. To continue enhancing patients’ outcomes and minimizing useless treatments, we are convinced a patient-tailored approach is required where tumor hypoxia will be one of the guiding biomarkers.
Conclusions
This study showed that pre-treatment 18F-FAZA PET/CT is predictive for radiotherapy response in esophageal adenocarcinoma xenografts and encourages a subsequent clinical trial where the predictive value of 18F-FAZA PET/CT is investigated in esophageal adenocarcinoma patients. The benefit of the hypoxia modifier nimorazole was modest and asks for further investigation.
Additional file
Figure S1. In vitro effect of nimorazole monotherapy. Ctrl = control; Nimo = nimorazole; Norm = normoxia; Hypox = hypoxia. Y-axis shows absorbance, analyzed 72 h after treatment. No significant difference was observed between cells treated with PBS (control) or treated with nimorazole, under hypoxic or normoxic conditions (t-test). (DOCX 20 kb)
Acknowledgements
The authors would like to thank the Department of Clinical Chemistry, Microbiology and Immunology (Prof. Geert Leroux-Roels and Leen Van Simaey) and the Department of Basic Medical Sciences (Prof. A. Vral and Leen Pieters) of the Ghent University, Jan Courtyn, Natacha Rosseel, Evelien Dierick and Glenn Wagemans for the professional technical assistance. W. Ceelen is a Senior clinical investigator from the Fund for Scientific Research–Flanders (FWO).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC
Area under the curve
- CAIX
Carbonic anhydrase
- EAC
Esophageal adenocarcinoma
- EF5
Pentafluoropropylacetamide
- FAZA
Fluoroazomycin arabinoside
- FETNIM
Fluoroerythronitroimidazole
- FFPE
Formalin-fixed paraffin-embeded
- FMISO
Fluoromisonidazole
- HIF
Hypoxia-inducible factor
- HNSCC
Head and neck squamous cell carcinoma
- IP
Intraperitoneal
- OER
Oxygen enhancement ratio
- RT
Radiotherapy
- SER
Sensitizer enhancement ratio
- T/B
Tumor to background
Authors’ contributions
EM: Contributed to the conception and design of the study. Contributed to the acquirement, analyzation and interpretation of data. Contributed to drafting the manuscript and approved the final content of the manuscript. EDV: Contributed to the conception and design of the study. Contributed to the acquirement, analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. BD: Contributed to the conception and design of the study. Contributed to the acquirement, analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. CV: Contributed to the conception and design of the study. Contributed to the acquirement, analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. KK: Contributed to the conception and design of the study. Contributed to the acquirement, analyzation and interpretation of data. Approved the final content of the manuscript. FDV: Contributed to conception and design of the study. Contributed to the analyzation and interpretation of data. Approved the final content of the manuscript. IG: Contributed to the conception and design of the study. Contributed to the analyzation and interpretation of data. Contributed to critically revising the manuscript. Approved the final content of the manuscript. BB: Contributed to the conception and design of the study. Contributed to the analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. ODW: Contributed to the conception and design of the study. Contributed to the analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. Approved the final content of the manuscript. WC: Contributed to the conception and design of the study. Contributed to the analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. PP: Contributed to the conception and design of the study. Contributed to the analyzation and interpretation of data. Contributed to critically revising the manuscript and enhancing its intellectual content. Approved the final content of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Animal experiments were approved by the Animal Ethical Committee of the Ghent University (ECD 14/82).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13014-018-0984-3) contains supplementary material, which is available to authorized users.
Contributor Information
Elodie Melsens, Phone: +32 (0)9 332 12 95, Email: Elodie.Melsens@ugent.be.
Elly De Vlieghere, Email: Elly.devlieghere@ugent.be.
Benedicte Descamps, Email: Benedicte.descamps@ugent.be.
Christian Vanhove, Email: Christian.vanhove@ugent.be.
Ken Kersemans, Email: Ken.kersemans@ugent.be.
Filip De Vos, Email: FilipX.devos@ugent.be.
Ingeborg Goethals, Email: Ingeborg.goethals@uzgent.be.
Boudewijn Brans, Email: Boudewijn.brans@ugent.be.
Olivier De Wever, Email: Olivier.dewever@ugent.be.
Wim Ceelen, Email: Wim.ceelen@ugent.be.
Piet Pattyn, Email: Piet.pattyn@ugent.be.
References
- 1.Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 2.Staal EFWC, Aleman BMP, Boot H, van Velthuysen MLF, van Tinteren H, van Sandick JW. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg. 2010;97(10):1482–1496. doi: 10.1002/bjs.7175. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 4.Ping W, Sun W, Zu YK, Chen WS, Fu XN. Clinicopathological and prognostic significance of hypoxia-inducible factor-1 alpha in esophageal squamous cell carcinoma: a meta-analysis. Tumor Biol. 2014;35(5):4401–4409. doi: 10.1007/s13277-013-1579-0. [DOI] [PubMed] [Google Scholar]
- 5.Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, Haustermans K, et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg. 2006;243(3):334–340. doi: 10.1097/01.sla.0000201452.09591.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue J, Yang Y, Cabrera AR, Sun X, Zhao S, Xie P, et al. Measuring tumor hypoxia with 18F-FETNIM PET in esophageal squamous cell carcinoma: a pilot clinical study. Dis Esophagus. 2012;25(1):54–61. doi: 10.1111/j.1442-2050.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 7.Peerlings J, Van De Voorde L, Mitea C, Larue R, Yaromina A, Sandeleanu S, et al. Hypoxia and hypoxia response-associated molecular markers in esophageal cancer: a systematic review. Methods. 2017; [DOI] [PubMed]
- 8.De Bruycker S, Vangestel C, Van den Wyngaert T, Wyffels L, Wouters A, Pauwels P, et al. Baseline [F-18]FMISO mu PET as a predictive biomarker for response to HIF-1 alpha inhibition combined with 5-FU chemotherapy in a human colorectal cancer xenograft model. Mol Imaging Biol. 2016;18(4):606–616. doi: 10.1007/s11307-015-0926-5. [DOI] [PubMed] [Google Scholar]
- 9.Beck R, Roper B, Carlsen JM, Huisman MC, Lebschi JA, Andratschke N, et al. Pretreatment F-18-FAZA PET predicts success of hypoxia-directed radiochemotherapy using tirapazamine. J Nucl Med. 2007;48(6):973–980. doi: 10.2967/jnumed.106.038570. [DOI] [PubMed] [Google Scholar]
- 10.Ali R, Apte S, Vilalta M, Subbarayan M, Miao Z, Chin FT, et al. 18F-EF5 PET Is predictive of response to fractionated radiotherapy in preclinical tumor models. PLoS One. 2015;10(10):e0139425. 10.1371/journal.pone.0139425. [DOI] [PMC free article] [PubMed]
- 11.Komar G, Lehtio K, Seppanen M, Eskola O, Levola H, Lindholm P, et al. Prognostic value of tumour blood flow, [F-18]EF5 and [F-18]FDG PET/CT imaging in patients with head and neck cancer treated with radiochemotherapy. Eur J Nucl Med Mol Imaging. 2014;41(11):2042–2050. doi: 10.1007/s00259-014-2818-3. [DOI] [PubMed] [Google Scholar]
- 12.Busk M, Mortensen LS, Nordsmark M, Overgaard J, Jakobsen S, Hansen KV, et al. PET hypoxia imaging with FAZA: reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur J Nucl Med Mol Imaging. 2013;40(2):186–197. doi: 10.1007/s00259-012-2258-x. [DOI] [PubMed] [Google Scholar]
- 13.Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D, et al. Imaging of tumor hypoxia with [I-124] IAZA in comparison with [F-18] FMISO and [F-18]FAZA - first small animal PET results. J Pharm Pharm Sci. 2007;10(2):203–211. [PubMed] [Google Scholar]
- 14.Lopci E, Grassi I, Chiti A, Nanni C, Cicoria G, Toschi L, et al. PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am J Nucl Med Mol Imaging. 2014;4(4):365–384. [PMC free article] [PubMed] [Google Scholar]
- 15.Tran LB, Bol A, Labar D, Cao-Pham TT, Jordan B, Gregoire V, et al. Predictive value of (18)F-FAZA PET imaging for guiding the association of radiotherapy with nimorazole: a preclinical study. Radiother Oncol. 2015;114(2):189–194. doi: 10.1016/j.radonc.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Mortensen LS, Busk M, Nordsmark M, Jakobsen S, Theil J, Overgaard J, et al. Accessing radiation response using hypoxia PET imaging and oxygen sensitive electrodes: a preclinical study. Radiother Oncol. 2011;99(3):418–423. doi: 10.1016/j.radonc.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Saga T, Inubushi M, Koizumi M, Yoshikawa K, Zhang MR, Tanimoto K, et al. Prognostic value of F-18-fluoroazomycin arabinoside PET/CT in patients with advanced non-small-cell lung cancer. Cancer Sci. 2015;106(11):1554–1560. doi: 10.1111/cas.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saga T, Inubushi M, Koizumi M, Yoshikawa K, Zhang MR, Obata T, et al. Prognostic value of PET/CT with F-18-fluoroazomycin arabinoside for patients with head and neck squamous cell carcinomas receiving chemoradiotherapy. Ann Nucl Med. 2016;30(3):217–224. doi: 10.1007/s12149-015-1048-5. [DOI] [PubMed] [Google Scholar]
- 19.Servagi-Vernat S, Differding S, Hanin FX, Labar D, Bol A, Lee JA, et al. A prospective clinical study of (18) F-FAZA PET-CT hypoxia imaging in head and neck squamous cell carcinoma before and during radiation therapy. Eur J Nucl Med Mol Imaging. 2014;41(8):1544–1552. doi: 10.1007/s00259-014-2730-x. [DOI] [PubMed] [Google Scholar]
- 20.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol. 2007;19(6):397–417. doi: 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma, results of the Danish head and neck cancer study (DAHANCA) protocol 5-85. Radiother Oncol. 1998;46(2):135–146. doi: 10.1016/S0167-8140(97)00220-X. [DOI] [PubMed] [Google Scholar]
- 22.Melsens E, De Vlieghere E, Descamps B, Vanhove C, De Wever O, Ceelen W, et al. Improved xenograft efficiency of esophageal adenocarcinoma cell lines through in vivo selection. Oncol Rep. 2017;38(1):71–81. doi: 10.3892/or.2017.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overgaard J, Overgaard M, Nielsen OS, Pedersen AK, Timothy AR. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br J Cancer. 1982;46(6):904–911. doi: 10.1038/bjc.1982.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, et al. Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Appl Radiat Isot. 2005;62(6):897–901. doi: 10.1016/j.apradiso.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K, Furutsuka K, Takei M, Muto M, Nakao R, Aki H, et al. High-yield automated synthesis of [18F]fluoroazomycin arabinoside ([18F]FAZA) for hypoxia-specific tumor imaging. Appl Radiat Isot. 2011;69(7):1007–1013. doi: 10.1016/j.apradiso.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2(3):131–137. doi: 10.1162/153535003322556877. [DOI] [PubMed] [Google Scholar]
- 27.Tran LB, Bol A, Labar D, Karroum O, Bol V, Jordan B, et al. Potential role of hypoxia imaging using (18)F-FAZA PET to guide hypoxia-driven interventions (carbogen breathing or dose escalation) in radiation therapy. Radiother Oncol. 2014;113(2):204–209. doi: 10.1016/j.radonc.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Castro C, Bosetti C, Malvezzi M, Bertuccio P, Levi F, Negri E, et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015. Ann Oncol. 2014;25(1):283–290. doi: 10.1093/annonc/mdt486. [DOI] [PubMed] [Google Scholar]
- 29.Sugie C, Shibamoto Y, Ito M, Ogino H, Suzuki H, Uto Y, et al. Reevaluation of the radiosensitizing effects of sanazole and nimorazole in vitro and in vivo. J Radiat Res (Tokyo) 2005;46(4):453–459. doi: 10.1269/jrr.46.453. [DOI] [PubMed] [Google Scholar]
- 30.Carlson DJ, Keall PJ, Loo BW, Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol. 2011;79(4):1188–1195. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng WL, Huang Q, Liu X, Zimmerman M, Li F, Li CY. Molecular mechanisms involved in tumor repopulation after radiotherapy. Transl Cancer Res. 2013;2(5):442–448. doi: 10.3978/j.issn.2218-676X.2013.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supiot S, Lisbona A, Paris F, Azria D, Fenoglietto P. "dose-painting": myth or reality? Cancer Radiother. 2010;14(6–7):554–562. doi: 10.1016/j.canrad.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25(26):4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. In vitro effect of nimorazole monotherapy. Ctrl = control; Nimo = nimorazole; Norm = normoxia; Hypox = hypoxia. Y-axis shows absorbance, analyzed 72 h after treatment. No significant difference was observed between cells treated with PBS (control) or treated with nimorazole, under hypoxic or normoxic conditions (t-test). (DOCX 20 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


