Abstract
Background
Diabetes mellitus (DM) is characterized by a decreased blood level of glutamine (Gln), which may contribute to the disturbance in the effect of insulin on skeletal muscle. Therefore, it is crucial to study how to improve the effect of insulin on skeletal muscle by increasing Gln. In the present study, we investigated the effect of Gln on the hypoglycemic action of insulin in skeletal muscle L6 cells at high glucose levels through the insulin signaling pathway and glycogen synthesis pathway.
Material/Methods
The L6 cells were cultured in and stimulated by Gln and insulin. The glutamine analogue, L-Gamma-Glutamyl-p-nitroanilide (GPNA), was used for verifying the effect of Gln. The expression of insulin signaling molecules, including phosphatidylinositol-3-kinase (PI3K), 3-phosphoinositide-dependent protein kinase-1 (PDK1), protein kinase B (AKT), protein kinase C zeta (PKCζ), and glucose transporter 4 (GLUT4), were detected by real-time PCR and Western blot analysis, GLUT4 translocation was observed by immunofluorescence staining, glycogen synthase kinase (GSK) was analyzed by Western blotting, and glucose uptake was measured by glucose oxidase method (GOD).
Results
The results demonstrated that Gln combined with insulin remarkably up-regulated PI3K and PDK1 and also increased AKT and PKCζ phosphorylation. The present study shows that Gln enhanced the impact of insulin on GLUT4 and its translocation. The results of glucose uptake and GSK phosphorylation further confirmed the hypoglycemic effect of Gln accompanied with insulin. The hypoglycemic effect of Gln was reversed by GPNA.
Conclusions
These findings suggest that Gln enhances the hypoglycemic role of insulin through the PI3K/AKT/GLUT4 signaling pathway and glycogen synthesis pathway.
MeSH Keywords: Glucose Metabolism Disorders, Glutamine, Glycogen Synthase Kinase 3, Insulin
Background
Diabetes mellitus is characterized by chronic hyperglycemia resulting from defects in insulin secretion or action [1]. Skeletal muscle is a major target of insulin in mediating glucose homeostasis, but insulin resistance leads to a reduced ability of skeletal muscle to take up and utilize glucose [2]. Chronic DM induces skeletal muscle damage and atrophy via diabetic neuropathy and the more direct effects of high glucose and low insulin on muscle cell metabolism [3], eventually reducing skeletal muscle mass and strength [4]. Therefore, it is crucial to study how to increase skeletal muscle glucose uptake, thereby reducing loss of skeletal muscle.
Gln is considered a conditionally essential amino acid [5] and plays an important role in the intermediary metabolic pathway [6]. As the precursor of peptides, proteins, neurotransmitters, and nitrogenous bases, it is used to produce energy in various organs [7], and this amino acid also maintains cell proliferation, immune function, acid-base balance, and regulation of gene expression [8]. Recent evidence suggests that DM is a chronic disease with a decreased blood level of Gln, and this alteration may contribute to disturbances of insulin secretion and action [9,10]. It was reported that Gln improved glucose tolerance, insulin sensitivity, or both in various settings, including in experimental obese animals [11], adolescents with type 1 diabetes (T1D) [12], and in critically ill polytrauma patient [13]. The literature also shows that Gln supplementation prevents the development of diabetic cardiomyopathy in diabetic rats [11] and has a protective impact on nephropathy induced by diabetes in experimental animals [14]. These previous reports suggest that Gln supplementation might become a novel therapeutic to improve glycemic control in subjects with DM [9]. It is expected that Gln might enhance the hypoglycemic effect of insulin on skeletal muscle, and the underlying microscopic metabolic mechanisms of Gln combined with insulin to improve glucose uptake need to be investigated.
Insulin is a major factor in the maintenance of the skeletal muscle glucose uptake, and its hypoglycemic effect is achieved through the PI3K/AKT/GLUT4 signaling pathway [15]. Numerous studies have confirmed that the involvement of some insulin signaling molecules, such as PI3K, AKT, PDK1, PKCζ, and GLUT4, are essential in glucose uptake and metabolism [16,17]. PI3K is activated by insulin, which subsequently stimulates AKT, PDK1, and PKCζ, in succession. The reaction of enzymatic cascade amplification ultimately leads to GLUT4 translocation, enhances glucose uptake in skeletal muscle and adipose tissue, and eventually decreases blood glucose levels [18]. It is reported that Gln supplementation of the diabetic rats significantly increased AKT phosphorylation [19], which is crucial to the role of Gln in promoting glucose uptake and attenuating diabetic muscle wasting. However, it is not clear whether Gln enhances the glucose uptake ability of insulin on the skeletal muscle mass. Thus, we investigated the hypoglycemic effect of glutamine combined with insulin in L6 cells at high glucose levels. We also discuss whether the PI3K/AKT/GLUT4 signaling pathway and glycogen synthesis pathway mediate the role of Gln at high glucose levels.
Material and Methods
Cell culture and treatment
L6 cells of rats (Cell Resource Center, Peking Union Medical College) were cultivated in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Biological Industries), 100 units/ml penicillin, and 100 mg/ml streptomycin (HyClone Laboratories) at 37°C. Before stimulation with Gln and insulin, L6 cells were incubated in DMEM without FBS and Gln (Gibco) for 12 h. Cells were cultured at a high glucose concentration of 33 mmol/L in serum-free DMEM without Gln. Then, Gln (4 mmol/L, Gibco) or insulin (10−7 mmol/L, Sigma) was added to the DMEM. To determine the specific role of Gln in glucose intake, the cells were treated with the glutamine analogue, GPNA (0.5 mmol/L, Alfa Aesar), a competitive inhibitor of Gln uptake following the treatment of Gln.
Real-time PCR analysis
Total RNA was extracted from cells by using E.Z.N.A.™ HP Total RNA Kit (Omega Bio-tek), and the RNA concentration was quantified by absorbance at 260 nm. The RNA was converted to cDNA using the First-Strand cDNA Synthesis Kit (Thermo). Quantitative real-time PCR was done on a Roche light cycler 96 using SYBR Green I master mix (Takara). All assays were normalized to GAPDH level. The specific PCR primers were synthesized by the Sangon Biotechnology Company, and the sequences are shown in Table 1.
Table 1.
Primers used for real-time PCR.
| Gene | Forward (5-3′) | Reverse (5-3′) |
|---|---|---|
| PI3K | AGCAGGTACCCTGGTGATTG | AGAAGGCACAGGTCCAGAGA |
| PDK1 | TGTTCAAGAACGCAATGAGG | ATATGGGCAATCCGTAACCA |
| AKT | ACTCATTCCAGACCCACGAC | CCGGTACACCACGTTCTTCT |
| PKCζ | CGCTGGGTGTCCTTATGTTT | TGTGAGGCCTTGACAGACAG |
| GLUT4 | GCTTCTGTTGCCCTTCTGTC | TGGACGCTCTCTTTCCAACT |
| GAPDH | AGACAGCCGCATCTTCTTGT | CGTCCTCTACCATCCTCTGC |
Extraction of membrane protein
The extraction and isolation of membrane protein was performed by use of the Membrane Protein Extraction Kit (Jiangsu Keygen Biotechnology). Briefly, L6 cells were collected and washed with cold phosphate-buffered solution (PBS, Solarbio). The sample was centrifuged (4°C, 3000 rpm, 5 min) to obtain the precipitate, which was then lysed with lysis buffer. Lysate was centrifuged (4°C, 3000 rpm, 10 min) and the acquired precipitate was resuspended in extracted buffer. Finally, membrane protein was dissolved in supernatant for further analysis by centrifuging (4°C, 12 000 rpm, 10 min).
Western blotting
The cells were washed with PBS and lysed by RIPA buffer (Solarbio). Protein concentration was measured by Pierce™ BCA Protein Assay Kit (Thermo). The cell protein sample (25 μg/lane) was subjected to 10% sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore) by wet transfer. The membrane was incubated in 5% milk of TBS-Tween-20 (TBS-T) for 2 h at room temperature. Subsequently, the membrane was exposed to the desired primary antibodies of PI3K (Cell Signaling Technology, 1: 1000), PDK1 (Cell Signaling Technology, 1: 1000), AKT (Cell Signaling Technology, 1: 1000), P-AKT (Cell Signaling Technology, 1: 1000), PKCζ (Santa Cruz Biotechnology, 1: 500), P-PKCζ (Cell Signaling Technology, 1: 1000), GLUT4 (Abcam, 1: 2000), GSK3β (Cell Signaling Technology, 1: 1000), and P-GSK3β (Cell Signaling Technology, 1: 1000) at 4°C overnight and then incubated with secondary antibody anti-rabbit IgG-HRP or anti-mouse IgG-HRP (Zhongshan Biotechnology, 1: 5000) for 2 h at room temperature. β-actin (Santa Cruz Biotechnology, 1: 2000), β-tubulin (Affinity Biosciences, 1: 1000), and ATP1A1 (Affinity Biosciences, 1: 1000) were used as the control proteins. Membranes were washed with TBS-T between every step. After incubation with secondary antibody, the membrane was exposed to chemiluminescent reagent (ECL Plus, Solarbio) and image was captured using Image Quant RT-ECL equipment.
Immunofluorescence staining
The L6 cells were washed with PBS and fixed with 4% polyformaldehyde (Solarbio) at room temperature for 30 min. Then, cells were incubated with 0.5% Triton X-100 (Solarbio) for 5 min, followed by three 5-min washes in PBS. Nonspecific protein binding was blocked by 1-h incubation with 1% bovine serum albumin. Afterwards, cells were incubated with primary antibody GLUT4 (1: 100) overnight at 4°C. The cells were then probed with Alexa Fluor 488 (Molecular Probes) conjugated secondary antibody (1: 200) for 1 h following three 5-min washes in PBS. Finally, the cells were counterstained by Fluorescent Mounting Medium with 40,6-diamidino-2-phenylindole (DAPI, Zhongshan Bio-technology) for 20 min. Images were captured and analyzed by fluorescence microscopy.
Glucose uptake assay
The L6 cells were exposed to insulin or Gln for 24 h in phenol red-free, serum-free DMEM media with 33 mmol/L glucose. Then, supernatant was collected and centrifuged at 12 000 rpm for 10 min. The residual glucose concentration in supernatant was measured via Glucose Oxidase Method (GOD, Applygen Technologies) [20]. In other words, 5 uL supernatant and 195 uL working solution were mixed at 37°C for 20 min. Absorbance was measured using a Microplate Reader at 550 nm wavelength. The glucose concentration is expressed as value (mmol/L)=(At–A0)/(Ac–A0)×C where At is absorbance of the sample; Ac is absorbance of the standard solution; and A0 is absorbance of distilled water and C is concentration of standard solution. The glucose uptake ratio was expressed using the following equation:
Statistical analysis
Data were collected from independent experiments, with 3 replicates per experiment, and analyzed with one-way ANOVA with Bonferroni post hoc test or t test in GraphPad 5 (GraphPad Software). p<0.05 was considered statistically significant. Error bars represent standard error of the mean (SEM).
Results
Gln enhances the hypoglycemic effect of insulin in association with the up-regulated expression of PI3K
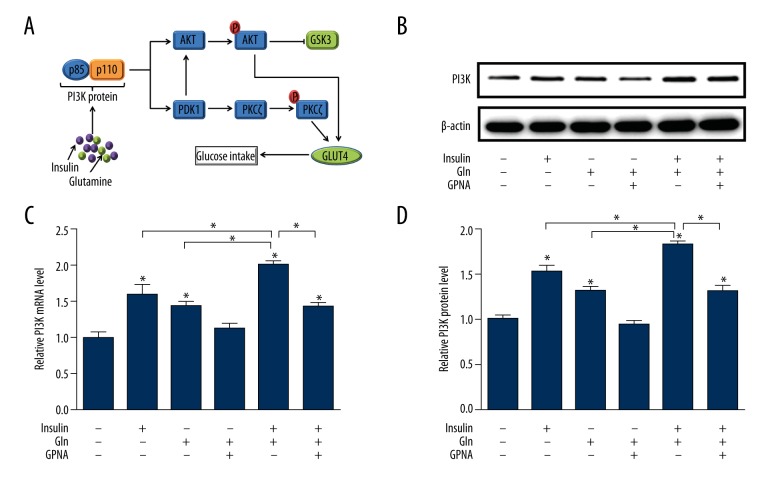
The PI3K/AKT/GLUT4 signaling pathway (Figure 1A) is a well-described signaling cascade of intracellular phosphorylation reaction that regulates cell cell-cycle progression, metabolism, proliferation, and survival [21]. Firstly, we investigated the PI3K expression of L6 cells and found that it was significantly up-regulated by Gln or insulin at a high glucose level. Moreover, the combination Gln and insulin remarkably elevated the expression of PI3K (Figure 1B–1D), while PI3K expression was decreased under Gln-deprived condition and a similar effect was observed in L6 cells treated with GPNA.
Figure 1.
The enhancement effect of Gln on the PI3K in the insulin signaling pathway. (A) The progression of the PI3K pathway. (B) Western blotting image of PI3K protein. (C) Quantification of mRNA expression of PI3K. (D) Quantification of protein expression of PI3K.
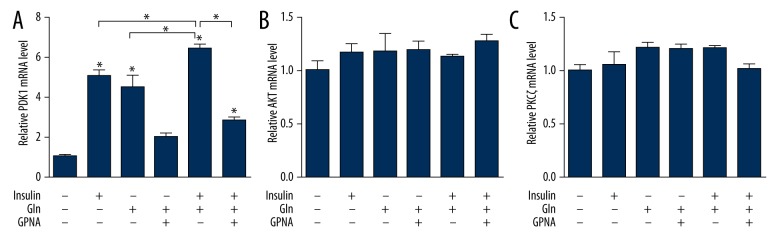
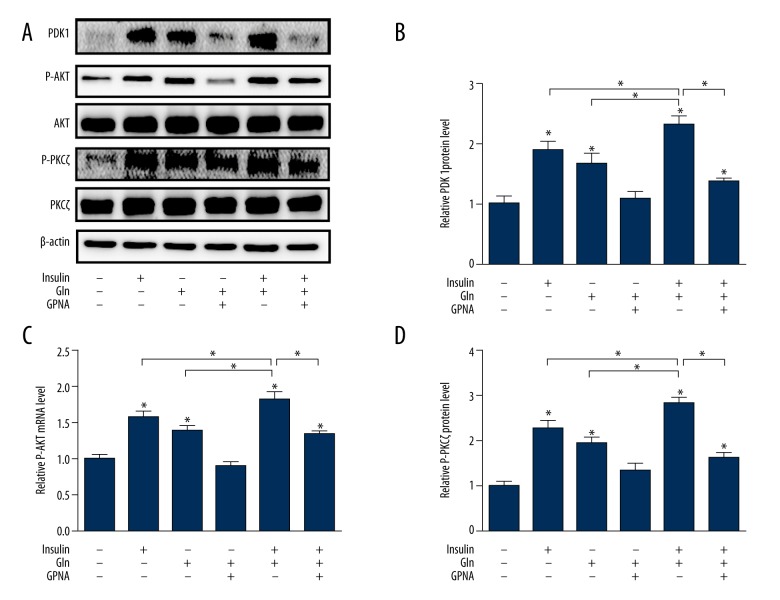
Effect of Gln combined with insulin on PDK1/AKT/PKCζ signaling pathway
In the present study, the PDK1 expression was obviously abated in depletion of Gln, while the effect was reversed with Gln or insulin supplementation. Importantly, the results also demonstrated that PDK1 expression was stronger in the combined effect of Gln and insulin. However, the enhancement role of Gln was remarkably reversed by GPNA (Figures 2A, 3A, 3B). The meaningful influence of Gln or insulin on AKT and PKCζ was to promote the protein phosphorylation (Figure 3A, 3C, 3D). There was no evidence that the mRNA (Figure 2B, 2C) and protein expressions (Figure 3A, 3C, 3D) of AKT and PKCζ were changed by the Gln or insulin. This study demonstrated that exogenous Gln increased AKT and PKCζ phosphorylation, while the effect of Gln on AKT and PKCζ phosphorylation was more pronounced in the presence of insulin. The inhibitory effect of GPNA on AKT and PKCζ phosphorylation is shown in Figure 3C and 3D.
Figure 2.
Effect of Gln and insulin on PDK1/AKT/PKCζ signaling pathway at gene level. (A) Quantification of mRNA expression of PDK1. (B) Quantification of mRNA expression of AKT. (C) Quantification of mRNA expression of PKCζ.
Figure 3.
Effect of Gln and insulin on PDK1/AKT/PKCζ signaling pathway at protein level. (A) Western blotting images of PDK1, P-AKT, AKT, P-PKCζ, and PKCζ. (B) Quantification of protein expression of PDK1. (C) Quantification of protein expression of P-AKT. (D) Quantification of protein expression of P-PKCζ.
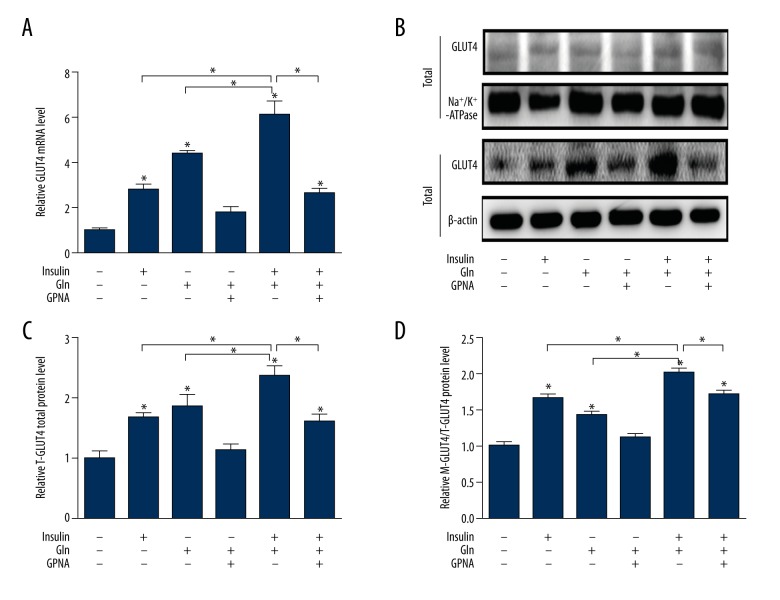
Effect of Gln and insulin on the expression of GLUT4
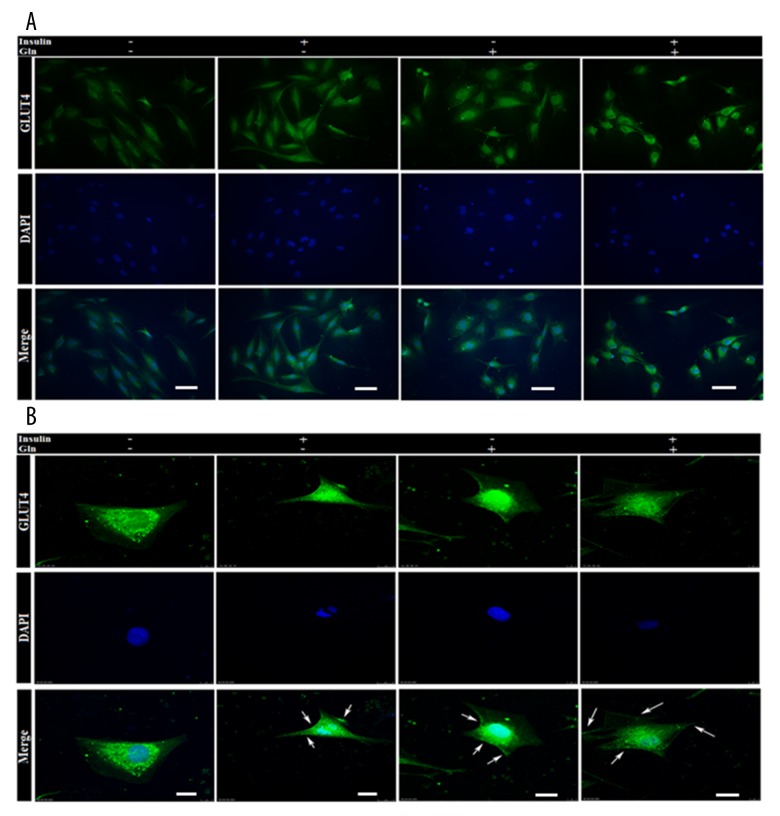
To determine whether the hypoglycemic effect of Gln and insulin was associated with regulation of GLUT4, we analyzed the mRNA and protein expression of GLUT4 in L6 cells by real-time PCR, Western blotting, and immunofluorescence staining. The data showed a significant GLUT4 mRNA decrease of L6 cells when the Gln was not provided at a high glucose level, and we found the better effect of improvement of Gln than insulin on the GLUT4 mRNA expression (Figure 4A). The use of Gln combined with insulin at a high glucose level produced the best enhancement of GLUT4 mRNA expression (Figure 4A), but we found that GLUT4 mRNA expression was suppressed in L6 cells treated with GPNA. The results demonstrated that both total GLUT4 (T-GLUT4) protein and translocation GLUT4 (M-GLUT4) protein in L6 cell membranes were down-regulated in the absence of Gln. The T-GLUT4 and M-GLUT4 protein expressions were noticeably up-regulated by Gln and insulin, while the role of insulin without Gln in promoting GLUT4 protein translocation to the cell membrane was even more pronounced, concomitant with changes in the PI3K/AKT signaling pathway. Importantly, the use of Gln combined with insulin at a high glucose level produced the best enhancement of both T-GLUT4 and m-GLUT4 protein expression (Figure 4B–4D), but the enhancement effect was suppressed by GPNA. To determine if Gln plus insulin affects GLUT4 translocation, the distribution of GLUT4 in the membrane region of L6 cells was examined using immunofluorescent staining. The results showed that GLUT4 translocated from the cytosol to the cell periphery upon Gln administration, as well as insulin stimulation (Figure 5).
Figure 4.
Effect of Gln and insulin on GLUT4 at gene and protein levels. (A) Quantification of mRNA expression of GLUT4. (B) Western blotting images of GLUT4 both in total and in cell membrane. (C) Quantification of protein expression of GLUT4 in total. (D) Quantification of protein expression of GLUT4 in cell membrane.
Figure 5.
Immunofluorescence images of GLUT4. (A) The expression of total GLUT4 in L6 cells was determined by immunofluorescence staining. The scale bar represents 50 μm. (B) The expression of GLUT4 in cytosol and on membrane was assessed by confocal microscopy. The scale bar represents 10 μm.
Effect of Gln and insulin on glycogen synthesis pathway and glucose uptake
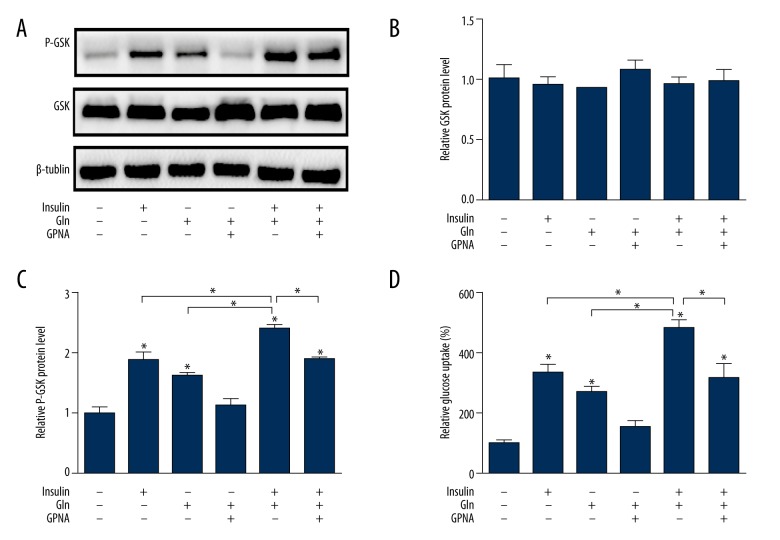
We showed there was no effect on GSK protein expression in the presence of glutamine and insulin (Figure 6A, 6B). However, the treatment without Gln and insulin led to a GSK phosphorylation decrease, and the GSK phosphorylation was enhanced following Gln or insulin supplementation (Figure 6C). These results also suggest that Gln with insulin together worked best in GSK phosphorylation, but the enhancement effect was suppressed by GPNA. The glucose uptake was higher after insulin intervention than after Gln. Further, the combination of Gln and insulin more significantly elevated glucose uptake compared with Gln deficiency. However, their effect on glucose uptake was reduced by GPNA (Figure 6D).
Figure 6.
Effect of Gln and insulin on glycogen synthesis pathway and glucose uptake. (A) Western blotting images of GSK/P-GSK. (B) Quantification of protein expression of GSK. (C) Quantification of protein expression of P-GSK. (D) Effect of Gln and insulin on glucose uptake.
Discussion
It has been suggested that Gln improves glucose tolerance and insulin sensitivity [22], and has an acute glucose-lowering effect after exercise in adolescents with longstanding T1D [12]. Therefore, we investigated the hypoglycemic effect of Gln in L6 cells at high glucose levels, showing that the underlying mechanism might be that Gln combined with insulin activates the signaling pathway of PI3K/AKT/GLUT4. Our results also suggest that Gln combined with insulin enhances glycogen synthesis and glucose uptake. Suppressing Gln uptake with GPNA had a similar inhibition effect on the PI3K/AKT/GLUT4 signaling pathway, glycogen synthesis pathway, and glucose uptake, compared with Gln deficiency.
Gln is the most abundant free amino acid in the circulation [23] and pool in skeletal muscle [24]. Because Gln is a major carbon donor for gluconeogenesis [25] and glycogen synthesis [26], it contributes to the regulation of protein and energy homeostasis [19]. However, a reduction of Gln was observed in the plasma and skeletal muscle of diabetics [8,9]. It was reported that the metabolic sequelae of increased liver gluconeogenesis and hyperglycemia caused skeletal muscle Gln reduction [27]. In the present study, we found that supplementary Gln could increase glucose uptake in L6 cells at high glucose levels, which was consistent with a rat study [28]. We also discussed the related mechanisms in the enhanced role of Gln in glucose uptake.
The PI3K/AKT signaling pathway plays an important role in the metabolic function of insulin, which regulates the absorption and metabolism of the glucose and glycogen synthesis [29,30]. PI3K is an intracellular phosphatidyl inositol kinase and its primary effector in the downstream signaling network is AKT [31]. Insulin can activate phosphorylation of AKT via PI3K [32]. The up-regulated phosphorylation of AKT increased GLUT4 transmembrane to transport glucose, accelerating glycometabolism [33]. GLUT4 plays an explicit role in regulating glucose homeostasis through translocation and activation in skeletal muscle, triggered by the insulin-dependent PI3K/AKT pathway [34]. In addition to insulin, the oral administration of supplemental Gln results in sufficient plasma and intramuscular concentrations of the amino acid to alter the activity and/or expression of the signaling molecules in the direction of increased protein synthesis and decreased catabolism [19]. Therefore, it is reasonable to assume that Gln combined with insulin alters the activity or expression of the signaling molecules of PI3K/AKT/GLUT4 in the direction of glucose uptake on L6 cells at high glucose levels.
The results suggest that Gln combined with insulin increased the expression of PI3K, PDK1, and GLUT4 in L6 cells at high glucose levels, and promoted AKT and PKCζ phosphorylation along with GLUT4 translocation. Therefore, Gln combined with insulin could give help convey glucose from outside cells to inside cells, and resulted in a significant increase of glucose uptake. We also showed that Gln combined with insulin had a marked effect on making glucose in further metabolism by the glycogen synthesis pathway. The total GSK protein level was unaltered but phosphorylation of GSK was significantly increased through Gln and insulin, suggesting that GSK was inhibited and the synthesis of glycogen was increased. Nevertheless, the defective GSK phosphorylation and ultimately the decreased glycogen synthesis might have a relationship with impaired PI3K/AKT activation, consistent with the data reported by Mangala et al. [35]. We found that GPNA, which is one of the Gln analogs suppressing Gln uptake [36], reversed the enhancement effect of Gln combined with insulin on glucose uptake. The results suggest that GPNA decreased the expression of PI3K, PDK1, and GLUT4 in L6 cells, and inhibited AKT and PKCζ phosphorylation, as well as GLUT4 translocation.
Recent research has revealed that the role of Gln in diabetes goes beyond traditional metabolic functions of amino acids. New discoveries of Gln function have been reported, including the role of enhanced endothelial progenitor cell mobilization in diabetic mice [37], differential effects in calcitonin gene-related peptide-ergic, nitrergic, and vasoactive intestinal polypeptide-ergic myenteric innervation in diabetic rats [38], as well as the effect on adhesion molecule expression and oxidative stress in mice with diabetes [39]. Similarly, our results show that Gln has a beneficial hypoglycemic effect on L6 cells through the PI3K/AKT/GLUT4 signaling pathway, and the hypoglycemic effect of insulin was enhanced in the presence of Gln.
Conclusions
Our study indicated that Gln and insulin increased glucose uptake of L6 cells at high glucose levels, which might have resulted from the activation of the PI3K/AKT/GLUT4 signaling pathway and glycogen synthesis pathway. Our findings suggest that supplementation with Gln helps insulin to reduce glucose levels and is a novel and low-cost treatment for diabetes.
Acknowledgements
We thank the Department of Life Sciences, Nankai University for providing technical assistance. We also thank Dr. Wang for modification of our manuscript.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (Grant 81671835)
Conflicts of interest
None.
References
- 1.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 2.Kahn CR. Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes. Exp Diabesity Res. 2003;4:169–82. doi: 10.1155/EDR.2003.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros C, Frederico MJ, Daluz G, et al. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J Cell Physiol. 2011;226:666–74. doi: 10.1002/jcp.22387. [DOI] [PubMed] [Google Scholar]
- 4.Fortes MAS, Scervino MVM, Marzuca-Nassr GN, et al. Hypertrophy stimulation at the onset of type I diabetes maintains the soleus but not the EDL muscle mass in Wistar rats. Front Physiol. 2017;8:830. doi: 10.3389/fphys.2017.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newsholme P, Procopio J, Lima MM, et al. Glutamine and glutamate – their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 6.Wu G. Amino acids: Metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 7.Darosa CV, Azevedo SC, Bazotte RB, et al. Supplementation with L-Glutamine and L-Alanyl-L-Glutamine changes biochemical parameters and jejunum morphophysiology in type 1 diabetic Wistar rats. PLoS One. 2015;10:e0143005. doi: 10.1371/journal.pone.0143005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newsholme P, Abdulkader F, Rebelato E, et al. Amino acids and diabetes: Implications for endocrine, metabolic and immune function. Front Biosci. 2011;16:315–39. doi: 10.2741/3690. [DOI] [PubMed] [Google Scholar]
- 9.Menge BA, Schrader H, Ritter PR, et al. Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul Pept. 2010;160:75–80. doi: 10.1016/j.regpep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Tessari P, Cecchet D, Cosma A, et al. Insulin resistance of amino acid and protein metabolism in type 2 diabetes. Clin Nutr. 2011;30:267–72. doi: 10.1016/j.clnu.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Badole SL, Jangam GB, Chaudhari SM, et al. L-glutamine supplementation prevents the development of experimental diabetic cardiomyopathy in streptozotocin-nicotinamide induced diabetic rats. PLoS One. 2014;9:e92697. doi: 10.1371/journal.pone.0092697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Santiago L, Mauras N, Hossain J, et al. Does oral glutamine improve insulin sensitivity in adolescents with type 1 diabetes? Nutrition. 2017;34:1–6. doi: 10.1016/j.nut.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grintescu IM, Luca Vasiliu I, Cucereanu Badica I, et al. The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients – A randomized-controlled clinical study. Clin Nutr. 2015;34:377–82. doi: 10.1016/j.clnu.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Sadar S, Kaspate D, Vyawahare N. Protective effect of L-glutamine against diabetes-induced nephropathy in experimental animal: Role of KIM-1, NGAL, TGF-beta1, and collagen-1. Ren Fail. 2016;38:1483–95. doi: 10.1080/0886022X.2016.1227918. [DOI] [PubMed] [Google Scholar]
- 15.Satoh T. Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int J Mol Sci. 2014;15:18677–92. doi: 10.3390/ijms151018677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima T, Arai T, Ariga-Nedachi M, et al. Insulin receptor substrates form high-molecular-mass complexes that modulate their availability to insulin/insulin-like growth factor-I receptor tyrosine kinases. Biochem Biophys Res Commun. 2011;404:767–73. doi: 10.1016/j.bbrc.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Pereira RO, O’Neill BT, et al. Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake independent of mTORC1 and GLUT4 translocation. Mol Endocrinol. 2013;27:172–84. doi: 10.1210/me.2012-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–89. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 19.Lambertucci AC, Lambertucci RH, Hirabara SM, et al. Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS One. 2012;7:e50390. doi: 10.1371/journal.pone.0050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen X, Zhou N, Mi L, et al. Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des Devel Ther. 2017;11:313–24. doi: 10.2147/DDDT.S127010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabbe T, Welham MJ, Ward SG. The PI3K inhibitor arsenal: Choose your weapon! Trends Biochem Sci. 2007;32:450–56. doi: 10.1016/j.tibs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 23.Wernerman J. Clinical use of glutamine supplementation. J Nutr. 2008;138:2040s–44s. doi: 10.1093/jn/138.10.2040S. [DOI] [PubMed] [Google Scholar]
- 24.Savarese DM, Savy G, Vahdat L, et al. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev. 2003;29:501–13. doi: 10.1016/s0305-7372(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 25.Hankard RG, Haymond MW, Darmaun D. Role of glucose in the regulation of glutamine metabolism in health and in type 1 insulin-dependent diabetes. Am J Physiol Endocrinol Metab. 2000;279:E608–13. doi: 10.1152/ajpendo.2000.279.3.E608. [DOI] [PubMed] [Google Scholar]
- 26.Varnier M, Leese GP, Thompson J, Rennie MJ. Stimulatory effect of glutamine on glycogen accumulation in human skeletal muscle. Am J Physiol. 1995;269:E309–15. doi: 10.1152/ajpendo.1995.269.2.E309. [DOI] [PubMed] [Google Scholar]
- 27.Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT. Amino acid metabolism in the Zucker diabetic fatty rat: Effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol. 2004;82:506–14. doi: 10.1139/y04-067. [DOI] [PubMed] [Google Scholar]
- 28.Mansour A, Mohajeri-Tehrani MR, Qorbani M, et al. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition. 2015;31:119–26. doi: 10.1016/j.nut.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S, Ren D, Li J, et al. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia. 2014;95:58–64. doi: 10.1016/j.fitote.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Vareda PM, Saldanha LL, Camaforte NA, et al. Myrcia bella Leaf extract presents hypoglycemic activity via PI3k/Akt insulin signaling pathway. Evid Based Complement Alternat Med. 2014;2014:543606. doi: 10.1155/2014/543606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 32.Gao YF, Zhang MN, Wang TX, et al. Hypoglycemic effect of D-chiro-inositol in type 2 diabetes mellitus rats through the PI3K/Akt signaling pathway. Mol Cell Endocrinol. 2016;433:26–34. doi: 10.1016/j.mce.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi GR, Jothi G, Antony PJ, et al. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARgamma in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. 2014;745:201–16. doi: 10.1016/j.ejphar.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 35.Viswanathan MP, Mullainadhan V, Chinnaiyan M, Karundevi B. Effects of DEHP and its metabolite MEHP on insulin signalling and proteins involved in GLUT4 translocation in cultured L6 myotubes. Toxicology. 2017;386:60–71. doi: 10.1016/j.tox.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–84. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su ST, Yeh CL, Hou YC, et al. Dietary glutamine supplementation enhances endothelial progenitor cell mobilization in streptozotocin-induced diabetic mice subjected to limb ischemia. J Nutr Biochem. 2017;40:86–94. doi: 10.1016/j.jnutbio.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Pereira RV, Linden DR, Miranda-Neto MH, Zanoni JN. Differential effects in CGRPergic, nitrergic, and VIPergic myenteric innervation in diabetic rats supplemented with 2% L-glutamine. An Acad Bras Cienc. 2016;88(Suppl 1):609–22. doi: 10.1590/0001-3765201620150228. [DOI] [PubMed] [Google Scholar]
- 39.Tsai PH, Liu JJ, Chiu WC, et al. Effects of dietary glutamine on adhesion molecule expression and oxidative stress in mice with streptozotocin-induced type 1 diabetes. Clin Nutr. 2011;30:124–29. doi: 10.1016/j.clnu.2010.07.005. [DOI] [PubMed] [Google Scholar]