Abstract
Background
Lung ischemia/reperfusion injury (LIRI) is a medical problem featuring pulmonary dysfunction and damage. The present study aimed to investigate the protective effects of erythropoietin (EPO), which has been reported to be an anti-inflammatory agent, on LIRI through inhibiting the TLR-4/NF-κB signaling pathway.
Material/Methods
All rats were randomly divided into 3 groups (n=8): a control group, a vehicle+LIRI group, and an EPO+LIRI group. LIRI included 90-min ischemia and 120-min reperfusion, while RhEpo was administered (3 kU/kg) intraperitoneally 2 h before the operation. Levels of pulmonary inflammatory responses were examined by analyzing pulmonary permeability index (PPI), oxygenation index, histology, and expressions of inflammatory cytokines.
Results
Pretreatment with EPO significantly decreased lung W/D ratio, BALF leukocytes count and percentage, and PPI but increased oxygenation index compared with the LIRI group (P<0.05). More importantly, with EPO pretreatment there was less pathological damage compared with the vehicle group. Expressions of inflammatory cytokines (TNF-α, IL-6, and IL-1β) in the serum were significantly lower in the EPO group than in the LIRI group (P<0.05). In addition, gene expression and protein expression of TLR-4 and NF-κB were significantly inhibited with EPO pretreatment compared with the LIRI group (P<0.05).
Conclusions
Our study id the first to report that EPO protects lung injuries after LIRI through inhibiting the TLR4-NF-κB signaling pathway, which provides solid evidence for the use of EPO as a therapeutic agent for treating LIRI in the future.
MeSH Keywords: Erythropoietin, Reperfusion Injury, Toll-Like Receptor 4
Background
Lung ischemia/reperfusion injury (LIRI) is a critical problem in research and in clinical practice [1,2]. It is well known that LIRI can occur after lung transplant, cardiopulmonary bypass, pneumonectomy, and post-enucleation of pulmonary embolism, which can cause pulmonary dysfunction and damage [3,4]. The mechanism and clinical courses of LIRI have been well investigated. Oxygen radicals, inflammatory cytokines, and leukocytes have recently been identified to play key roles [5]. Since the mechanisms involved in LIRI are complicated and cross-linked, demonstrating an explicit mechanism could help to prevent the potentially severe complications [6].
Toll-like receptors (TLRs), a family of pattern recognition receptors, provide a first line of defense against bacterial and viral pathogens. It is also reported that TLRs are initial sites for inflammatory signaling activation in the lung during ischemia and reperfusion [7–10]. TLR-4, originally regarded as responding only to specific bacterial ligands, is now proved to be activated by many signals induced by stressed, necrotic, or injured cells [11,12]. TLR-4 has been reported to be a key modulator in a number of models of ischemia-reperfusion. More recently, TLR-4 was reported to be a key factor in the pathogenesis of LIRI, in which the activation of TLR4 of alveolar macrophages was thought to be the initial step [13]. Activation of TLR4 results in the nuclear translocation of nuclear factor-kappa B (NF-κB), an essential regulator involved in the productions of several inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β [14,15].
Erythropoietin (EPO) is a cytokine responsible for erythropoiesis. EPO has been proved to protect against inflammatory injuries [16,17]. In addition, it is reported that EPO can reduce the production of inflammatory mediators [18]. Recently, it was reported that RhEpo can reduce I/R-induced lung injuries through its anti-inflammatory effects, but the mechanism is still unclear [19].
In the present study, we assessed the protective effects on LIRI and the associated mechanism in rat models of LIRI. Particularly, we explored the protective effects of EPO on the expressions of TLR4, NF-κB, and inflammatory cytokines, as well as changes in pulmonary function and structure.
Material and Methods
Animals and groups
All experiments were conducted following the regulations for experimental animal welfare and were approved by the Committee for Animal Experiments at West China Hospital, Sichuan University. Male Sprague-Dawley (SD) rats, weighing 200–250 g, were purchased from the Animal Center of Sichuan University and were kept at a constant temperature (23±1°C) with a 12 h: 12 h light/dark cycle. Recombined human erythropoietin (RhEpo) was purchased from Shenyang Sunshine Pharmaceutical Co. Ltd. (Shenyang, China).
SD rats were divided randomly into 3 groups (n=8): a control group, a vehicle+LIRI group, and an EPO+LIRI group. In the control group, animals just received mechanical ventilation for 3.5 h, after which we performed a thoracotomy. In the vehicle group, 2 ml of normal saline was administered by intraperitoneal injection 2 h before the operation, then rats underwent the experimental protocol of 90-min left lung ischemia and 120-min reperfusion [20]. In the EPO group, we administered RhEpo (3 kU/kg [19] diluted in 1 ml saline solution) by intraperitoneal injection 2 h before the operation, and the rest of the protocol was the same.
Collections of blood, bronchoalveolar lavage fluid (BALF), and tissue
Blood was collected from all rats and the BALF was then collected by intratracheally washing the right lungs with a total of 5 ml phosphate buffer solution (PBS). BALF was then centrifuged at 5000 rpm for 10 min. The precipitate and supernatant were collected for the next studies.
Lung tissue Wet/Dry (W/D) ratio
The rest of lung tissue was weighed immediately and recorded as the wet weight. The tissue was then dried for 48 h in a 70°C dry-box and weighed as the dry weight. W/D ratio was calculated as an indicator for pulmonary edema.
BALF leukocyte, neutropil count and percentage, and PPI
The BALF precipitate was studied for the number of leukocytes and neutrophil percentage. Protein analysis was performed in the BALF supernatant and serum. PPI was calculated as the ratio of BALF protein level to serum protein level.
Oxygenation Index
PaO2 was measured by arterial blood gas analysis, and the ratio of PaO2 to FiO2 was then calculated as the oxygenation index.
Hematoxylin & eosin (HE) staining
Lung tissues were fixed in 4% paraformaldehyde and embedded in paraffin. We made 5-μm sections and stained them with HE using a standard protocol. The slides were examined by a pulmonary pathologist who was blind to animal grouping. Each slide was given a score based on the 3 hallmarks (neutrophils, alveolar edema, and interstitial infiltrate), and the total score was calculated (ranging from 0 to 9, with 0 representing normal lung and 9 the most injured lung) [21].
Serum cytokines levels
The serum was obtained from blood samples by centrifuged at 5000 rpm for 10 min and analyzed by specific ELISA kits (R&D Systems, Minneapolis, MN) to determine the levels of cytokines TNF-α, IL-6, and IL-1β.
mRNA levels of TLR4 and NF-κB
RNA was obtained by using Trizol solution (Invitrogen, USA) and reversed with the specific kit (Invitrogen, USA). Rt-PCR was performed as follows: 95°C for 90 s, followed by 35 cycles (95°C for 5 s and 59°C for 30 s).
Primer for TLR4:
forward: 5 -TTATCCAGAGCCGTTGGTGT-3,
reverse: 5-CCCACTCGAGGTAGGTGTTT-3.
Primer for NF-κB:
forward: 5 -TTCCTGCTTACGGTGGGATT-3,
reverse: 5 -CCCCACAT CCTCTTCCTTGT-3.
Primer for GAPDH: forward:
5 -GAGACAGCCGCATCTTCTTG-3,
reverse: 5 -TGACTGTGCCGTTGAACTTG-3.
Each sample was tested 3 times.
Western blotting analysis
Protein was extracted from lung tissues with RIPA buffer. We added 50 μg of protein into SDS-PAGE. Protein was transferred to the polyvinylidene fluoride membrane and then blocked with 5% pure albumin. The membrane was incubated with TLR4 and NF-κB p65 primary antibody (Abcam, USA) overnight at 4°C. Anti-GAPDH antibody (Cell Signaling Technology, USA) was used as the loading control.
Statistical analysis
All descriptive data are expressed as mean ±SD. Statistical data were analyzed by one-way ANOVA followed by Bonferroni correction for multiplicity. All analyses were conducted using SPSS 20.0 (Chicago, USA). P<0.05 was regarded as a significant difference.
Results
Effects of EPO on pulmonary damage
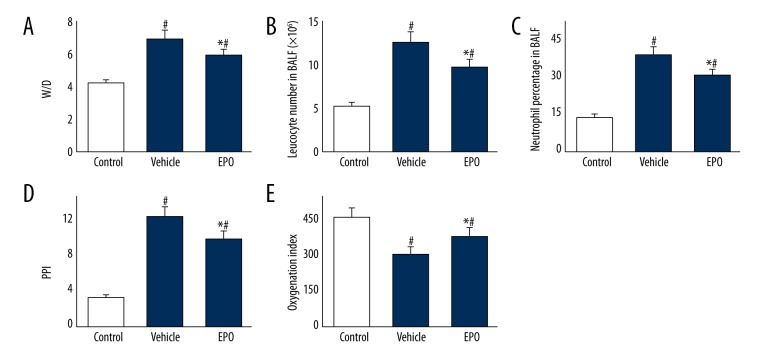
As shown in Figure 1, the W/D ratio was significantly higher in the vehicle group than in the control group (P<0.05). BALF leukocyte count and neutrophil ratio were also increased in the vehicle group, and PPI in the vehicle group was remarkably elevated compared with the control group. Compared to the vehicle group, those changes in the EPO group were remarkably decreased (P<0.05, respectively). The oxygenation index was much higher in the control group than in the vehicle group (P<0.05). Interestingly, the oxygenation index in the EPO group was better than in the vehicle group (P<0.05).
Figure 1.
(A–E) The effects of EPO on lung tissue W/D, BALF leukocyte count, BALF neutrophil ratio, PPI, and oxygenation index. Comparison of lung tissue W/D, BALF leukocyte count, BALF neutrophil ratio, PPI, and oxygenation index among all groups. Lung tissue, BALF, and arterial blood were collected immediately after the IR procedure was completed. Data are expressed as means ±SD and were analyzed by ANOVA followed by Bonferroni correction for multiplicity where appropriate. n=8 for each group. # P<0.05 compared with control group; * P<0.05 compared with vehicle group.
Effects of EPO on lung pathology
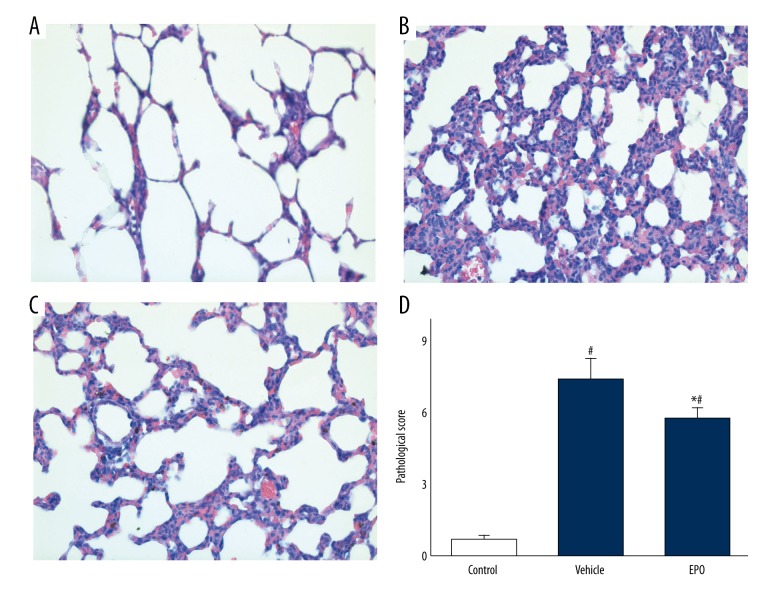
To study the effects of EPO on LIRI, we analyzed pathological changes (Figure 2). In the control group (Figure 2A), the pulmonary alveoli and interstitium was intact and no signs of pulmonary edema and structural damages were observed. In the vehicle group (Figure 2B), significant pulmonary edema and structural damages were identified. Excessive inflammatory cells were infiltrated into the lung tissue and increased the thickness of the interstitium. These pathological changes in the EPO group (Figure 2C) were improved. Pathological scores in the vehicle group were remarkably higher than in the control group (P<0.05), but were significantly (P<0.05) reduced in the EPO group (Figure 2D).
Figure 2.
(A–D) Pathologic changes and scores of lung tissues were examined by H&E staining. H&E staining of lung tissue sections from different groups (scale bars: 100 μm, magnification ×100). Data are expressed as means ±SD and were analyzed by ANOVA followed by Bonferroni correction for multiplicity where appropriate. n=8 for each group. # P<0.05 compared with control group; * P<0.05 compared with vehicle group.
The Effects of EPO on Levels of TNF-α, IL-6, and IL-1β
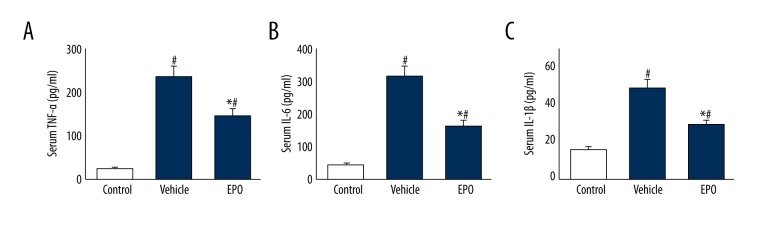
As shown in Figure 3, serum TNF-α, t IL-6, and IL-1β levels in the vehicle group were remarkably elevated compared with those in the control group (P<0.05), while a remarkable decrease was found with EPO treatment (P<0.05).
Figure 3.
The effects of EPO on serum TNF-α, IL-6, and IL-1β. Comparison of serum TNF-α, IL-6, and IL-1β levels among all groups. Data are expressed as means ±SD and were analyzed by ANOVA followed by Bonferroni correction for multiplicity where appropriate. n=8 for each group. # P<0.05 compared with control group; * P<0.05 compared with vehicle group.
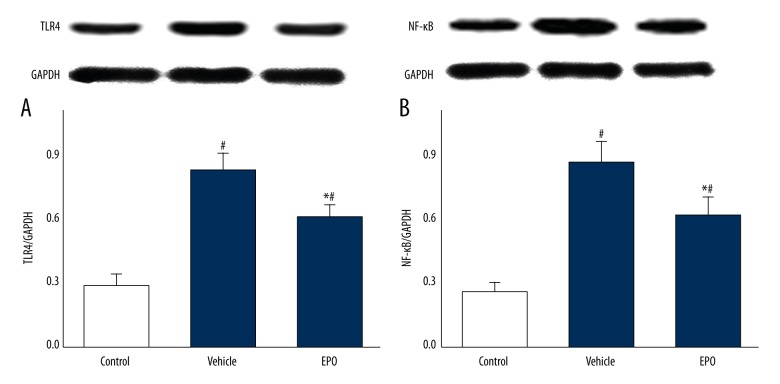
The Effects of EPO on Expressions of TLR4 and NF-κB
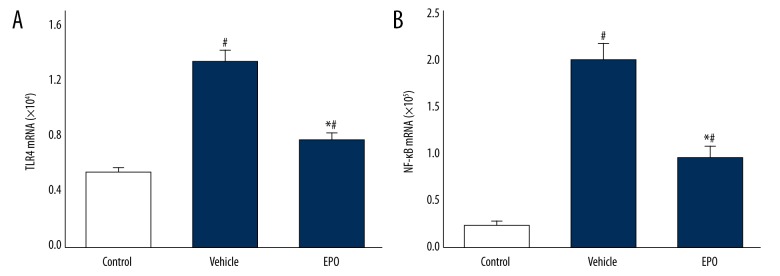
Figures 4 and 5 show mRNA and protein expressions of TLR4 and NF-κB. Compared to the control group, mRNA and protein expressions of TLR4 and NF-κB in the vehicle group were significantly increased (P<0.05), and those in the EPO group were significantly decreased compared with the vehicle-treated LIRI group (P<0.05).
Figure 4.
(A, B) The effects of EPO on gene expression of TLR4 and NF-κB. Comparison of mRNA levels of TLR4 and NF-κB in lung tissue among all groups. Data are expressed as means ±SD and were analyzed by ANOVA followed by Bonferroni correction for multiplicity where appropriate. n=8 for each group. # P<0.05 compared with control group; * P<0.05 compared with vehicle group.
Figure 5.
(A, B) The effects of EPO on protein expression of TLR4 and NF-κB. Comparison of protein levels of TLR4 and NF-κB in lung tissue by Western blotting among all groups. Data are expressed as means ±SD and were analyzed by ANOVA followed by Bonferroni correction for multiplicity where appropriate. n=8 for each group. # P<0.05 compared with control group; * P<0.05 compared with vehicle group.
Discussion
The present study is the first to demonstrate that EPO can attenuate the expressions of TLR4 and NF-κB in lung tissues in an LIRI rat model. Additionally, we found that EPO inhibited the releasing of serum TNF-α, IL-1β, and IL-6 in the LIRI rat model. These results show that EPO exerts its protective effects on LIRI through inhibiting the TLR4/NF-κB signaling pathway.
The TLRs family is essential in innate immunity and many inflammatory responses [22]. In all TLRs, TLR4 has been extensively studied and is considered to play important roles in mediating inflammatory responses. Activation of TLR4-mediated inflammatory signaling pathways resulted in the translocation of NF-κB, subsequently leading to production of many pro-inflammatory mediators and induction of inflammatory reactions [23]. It is reported that immune and inflammatory responses play essential roles in LIRI [24]. Accumulating evidence also shows that the TLR4-NF-κB signaling pathway induces inflammatory reactions and consequently intensifies LIRI [25]. Moreover, recent reports show that TLR-4 is critical in the development of LIRI, and its activation in the alveolar macrophage may be the first step [13]. In the present study, we found that the expressions of TLR4, NF-κB, and inflammatory mediators were elevated at the end of the experiment, demonstrating that TLR4 initiated the releasing of pro-inflammatory cytokines by activating the NF-κB signaling pathway in LIRI. Thus, targeting the modulation of TLR4 and NF-κB expression might be a promising therapeutic strategy for inhibiting inflammatory cascades in LIRI.
The pharmacological roles of EPO have been largely extended from a traditionally hematopoietic hormone to a multi-function cytokine. The beneficial effects of EPO on IR-caused injuries in the heart [26], kidney [27], and brain [27,28] have been revealed. However, only a few of reports showed that pretreatment with EPO attenuates LIRI [19,29]. In this study, we investigated the damaged lung structure in a rat IRI model, showing the damaged alveoli wall, dilated capillaries, and infiltrated inflammatory cells in the interstitium by HE staining. Treatment with EPO resulted in less structural damage and infiltration of neutrophils, lower PPI, and increased oxygenation index, indicating the protective effect of EPO in LIRI.
It was reported that EPO regulates TLR4-mediated inflammatory responses and NF-κB and PI3K signaling pathways to protect against hypoxic injury [30,31]. Through activating these critical signaling components, EPO can modulate many downstream inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [31], regulating oxidative stress, apoptosis, inflammatory damage, and regeneration. Our results demonstrate that the structural and functional presentations of lung injury were relieved and the mRNA and protein levels of TLR4 and NF-κB were significantly decreased by treatment with EPO. In addition, after the administration of EPO, the levels of TNF-α, IL-1β, and IL-6 in serum were significantly reduced, revealing that EPO inhibited inflammatory damage through down-regulating the TLR4/NF-κB signaling pathway, thereby protecting against the effects of LIRI.
Conclusions
In summary, our results indicate that EPO exerts protective effects on lung injuries after LIRI through inhibiting the TLR4-NF-κB signaling pathway and decreasing production of inflammatory cytokines (TNF-α, IL-6, and IL-1β). The present study provides solid support for the use of EPO as a therapeutic agent in treating LIRI.
Footnotes
Source of support: Departmental sources
References
- 1.Langer F, Schramm R, Bauer M, et al. Cytokine response to pulmonary thromboendarterectomy. Chest. 2004;126(1):135–41. doi: 10.1378/chest.126.1.135. [DOI] [PubMed] [Google Scholar]
- 2.Grichnik KP, D’Amico TA. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Semin Cardiothorac Vasc Anesth. 2004;8(4):317–34. doi: 10.1177/108925320400800405. [DOI] [PubMed] [Google Scholar]
- 3.Ng CS, Wan S, Yim AP, Arifi AA, et al. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121(4):1269–77. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 4.van der Kaaij NP, Kluin J, Haitsma JJ. Ischemia of the lung causes extensive long-term pulmonary injury: An experimental study. Respir Res. 2008;9:28. doi: 10.1186/1465-9921-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 6.Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36(3):205–14. doi: 10.1007/s00595-005-3124-2. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Li Y, Vodovotz Y, et al. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L738–46. doi: 10.1152/ajplung.00280.2005. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi ST, Zhang X, Aberg E, et al. Inducible activation of TLR4 confers resistance to hyperoxia-induced pulmonary apoptosis. J Immunol. 2006;176(8):4950–58. doi: 10.4049/jimmunol.176.8.4950. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Shan P, Qureshi S, et al. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175(8):4834–38. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- 10.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–79. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim JJ, Yang D. Alarmins: Chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Merry HE, Phelan P, Doak MR, et al. Role of toll-like receptor-4 in lung ischemia-reperfusion injury. Ann Thorac Surg. 2015;99(4):1193–99. doi: 10.1016/j.athoracsur.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 15.Barakat W, Safwet N, El-Maraghy NN, Zakaria MN, et al. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur J Pharmacol. 2014;724:43–50. doi: 10.1016/j.ejphar.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Gassmann M, Heinicke K, Soliz J, Ogunshola OO, et al. Non-erythroid functions of erythropoietin. Adv Exp Med Biol. 2003;543:323–30. doi: 10.1007/978-1-4419-8997-0_22. [DOI] [PubMed] [Google Scholar]
- 17.Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83(11):673–86. doi: 10.1007/s00277-004-0911-6. [DOI] [PubMed] [Google Scholar]
- 18.Agnello D, Bigini P, Villa P, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952(1):128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Dong G, Liu H, et al. Erythropoietin attenuates ischemia-reperfusion induced lung injury by inhibiting tumor necrosis factor-alpha and matrix metalloproteinase-9 expression. Eur J Pharmacol. 2009;602(2–3):406–12. doi: 10.1016/j.ejphar.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Waldow T, Alexiou K, Witt W, et al. Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia. Transpl Int. 2005;18(2):198–205. doi: 10.1111/j.1432-2277.2004.00005.x. [DOI] [PubMed] [Google Scholar]
- 21.Maxey TS, Enelow RI, Gaston B, et al. Tumor necrosis factor-alpha from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2004;127(2):541–47. doi: 10.1016/j.jtcvs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Kong Y, Le Y. Toll-like receptors in inflammation of the central nervous system. Int Immunopharmacol. 2011;11(10):1407–14. doi: 10.1016/j.intimp.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90(4):417–27. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash A, Sundar SV, Zhu YG, et al. Lung ischemia-reperfusion is a sterile inflammatory process influenced by commensal microbiota in mice. Shock. 2015;44(3):272–79. doi: 10.1097/SHK.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Q, Wu J, Lin Z, et al. Resolvin D1 Alleviates the Lung Ischemia Reperfusion Injury via Complement, Immunoglobulin, TLR4, and Inflammatory Factors in Rats. Inflammation. 2016;39(4):1319–33. doi: 10.1007/s10753-016-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiss K, Csonka C, Pálóczi J, et al. Novel, selective EPO receptor ligands lacking erythropoietic activity reduce infarct size in acute myocardial infarction in rats. Pharmacol Res. 2016;113(Pt A):62–70. doi: 10.1016/j.phrs.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Zou YR, Zhang J, Wang J, et al. Erythropoietin receptor activation protects the kidney from ischemia/reperfusion-induced apoptosis by activating ERK/p53 signal pathway. Transplant Proc. 2016;48(1):217–21. doi: 10.1016/j.transproceed.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Wang R, Wu X, et al. Erythropoietin delivered via intra-arterial infusion reduces endoplasmic reticulum stress in brain microvessels of rats following cerebral ischemia and reperfusion. J Neuroimmune Pharmacol. 2015;10(1):153–61. doi: 10.1007/s11481-014-9571-z. [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Ren B, Zhu J, et al. Pretreatment with recombined human erythropoietin attenuates ischemia-reperfusion-induced lung injury in rats. Eur J Cardiothorac Surg. 2006;29(6):902–7. doi: 10.1016/j.ejcts.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Rocchetta F, Solini S, Mister M, et al. Erythropoietin enhances immunostimulatory properties of immature dendritic cells. Clin Exp Immunol. 2011;165(2):202–10. doi: 10.1111/j.1365-2249.2011.04417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang L, Zhang N, Dong N, et al. Erythropoietin protects rat brain injury from carbon monoxide poisoning by inhibiting toll-like receptor 4/NF-kappa B-dependent inflammatory responses. Inflammation. 2016;39(2):561–68. doi: 10.1007/s10753-015-0280-4. [DOI] [PubMed] [Google Scholar]