Abstract
Early identification of children who experience developmental delays due to prenatal alcohol exposure (PAE) remains a challenge for individuals who do not exhibit facial dysmorphia. It is well-established that children with PAE may still exhibit the cognitive and behavioral difficulties, and individuals without facial dysmorphia make up the majority of individuals affected by PAE. This study employed a prospective cohort design to capture alcohol consumption patterns during pregnancy and then followed the infants to 6 months of age. Infants were assessed using magnetoencephalography to capture neurophysiological indicators of brain development and the Bayley Scales of Infant Development-III to measure behavioral development. To account for socioeconomic and family environmental factors, we employed a two-by-two design with pregnant women who were or were not using opioid maintenance therapy (OMT) and did or did not consume alcohol during pregnancy. Based on prior studies, we hypothesized that infants with PAE would exhibit broad increased spectral amplitude relative to non-PAE infants. We also hypothesized that the developmental shift from low to high frequency spectral amplitude would be delayed in infants with PAE relative to controls. Our results demonstrated broadband increased spectral amplitude, interpreted as hypersynchrony, in PAE infants with no significant interaction with OMT. Unlike prior EEG studies in neonates, our results indicate that this hypersynchrony was highly lateralized to left hemisphere and primarily focused in temporal/lateral frontal regions. Furthermore, there was a significant positive correlation between estimated number of drinks consumed during pregnancy and spectral amplitude revealing a dose-response effect of increased hypersynchrony corresponding to greater alcohol consumption. Contrary to our second hypothesis, we did not see a significant group difference in the contribution of low frequency to high frequency amplitude at 6 months of age. These results provide new evidence that hypersynchrony, previously observed in neonates prenatally exposed to high levels of alcohol, persists until 6 months of age and this measure is detectable with low to moderate exposure of alcohol with a dose-response effect. These results indicate that hypersynchrony may provide a sensitive early marker of prenatal alcohol exposure in infants up to 6 months of age.
Keywords: Prenatal alcohol exposure, Rest, Spectral amplitude, Magnetoencephalography, Infants
Highlights
-
•
6 month old infants with prenatal alcohol exposure exhibit broadband hypersynchrony.
-
•
Hypersynchrony was positively correlated with number of drinks consumed prenatally.
-
•
Distribution of low frequency to high frequency amplitude did not differ by group.
-
•
Hypersynchrony may provide an early marker of prenatal alcohol exposure in infants.
1. Introduction
Both clinical and preclinical studies have identified neurological and teratogenic effects of prenatal alcohol exposure (PAE) including neuronal loss (Ikonomidou et al., 2000), alterations in inhibitory interneurons (Skorput et al., 2015, Smiley et al., 2015), and reductions in neuronal connectivity evidenced by diffusion tensor imaging (Fan et al., 2016, Newville et al., 2017, Paolozza et al., 2017). PAE has been shown to cause persistent deficits in executive functioning into adulthood (Kodituwakku et al., 2011, Kodituwakku, 2009) which may lead to the increased likelihood for addictive behaviors and increased rates of incarceration experienced by individuals with known PAE (Streissguth et al., 2004). This is in contrast to the recent long-term follow-up study indicating that infants exposed to cocaine prenatally exhibit no measurable deficits relative to their well-matched, nonexposed peers throughout development (Hurt et al., 2009). Therefore, it is imperative that early markers of atypical brain development caused by PAE be identified.
Children with dysmorphic features caused by PAE typically can be identified in the preschool age range based on growth and dysmorphology assessments; however, there are no reliable markers to identify children affected by PAE who do not have dysmorphic features. Despite the premise that children with structural defects characteristic of Fetal Alcohol Syndrome (FAS) represent the more severe end of the FASD spectrum, children with FASD without characteristic facial features may fare worse than children with FAS (Streissguth et al., 2004). Streissguth and colleagues have attributed this discrepancy, in part, to the fact that children with dysmorphic features are more readily identified and qualify for provider services, whereas those without dysmorphic features go largely unidentified yet still experience the neurobiological effects associated with PAE.
Early EEG studies in infants born to women with alcohol use disorders identified hypersynchrony evidenced by higher spectral amplitude across the physiological frequency bands in infants with heavy prenatal alcohol exposure relative to age-matched controls (Havlicek et al., 1977). This finding was replicated by Chernick et al. (1983) who identified increased spectral amplitude in delta, theta, alpha and beta frequency bands spanning 2–17 Hz in neonates exposed to alcohol relative to neonates of abstaining mothers. Ioffe et al. (1984) confirmed that this hypersynchrony persisted in infants 4–6 weeks of age indicating that this abnormal spectral pattern was not due to withdrawal effects. These early studies were focused primarily on women with alcohol use disorders with heavy alcohol consumption throughout pregnancy. Despite these early EEG findings suggesting that neurophysiological measures are sensitive to PAE, more recent follow-up studies were not performed. To the best of our knowledge no previous magnetoencephalography (MEG) studies have examined neurophysiological alterations in PAE infants.
Prior EEG/MEG studies have also mapped out the developmental pattern across childhood of neural oscillations during rest (Clarke et al., 2001, Somsen et al., 1997). These results reveal a pattern that leads to decreasing low frequency amplitude and increasing high frequency amplitude with increasing age. This is attributed to the initial establishment of long-range connectivity within the brain followed by local specialization within cortical areas, consistent with the findings indicating that low-frequency oscillations facilitate long-range coordination of activity across the brain whereas high frequency oscillations are associated with local processing (Siegel et al., 2012). Previous studies indicate that various environmental stressors, related to institutional care in Romanian orphanages, lead to an altered developmental pattern such that children who were placed in foster care at a younger age had greater alpha band power than those who remained institutionalized (Marshall et al., 2008). We also found that infants whose mothers scored higher on a posttraumatic stress disorder (PTSD) severity scale demonstrated a delayed developmental pattern with greater low frequency amplitude relative to infants whose mothers did not have PTSD (Sanjuan et al., 2016). Due to the known teratogenic effects of PAE, we further hypothesized that infants with PAE would similarly show a delayed developmental pattern relative to controls.
Assessment of infant brain connectivity using resting state functional magnetic resonance imaging (rsfMRI) has expanded rapidly over the last 10 years demonstrating that infant brains are highly connected locally and later develop long-range connectivity patterns that resemble adults by two years of age (Graham et al., 2015, Grayson and Fair, 2017). Importantly, rsfMRI connectivity among adults has demonstrated that reliable networks are observed across individuals and that these connectivity patterns are altered in multiple clinical disorders (e.g. (Li et al., 2017, Mwansisya et al., 2017, Takamura and Hanakawa, 2017)). Furthermore, reliable connectivity measures can be assessed during sleep making rsfMRI feasible in infants (Gao et al., 2016). However, changes in sleep state are still considered to confound rsfMRI connectivity results, and collection during the awake state may lead to more reliable results across development. Despite this growth in knowledge in infant rsfMRI, few studies have examined the effects of PAE on infant brain connectivity. Salzwedel et al. (2015) examined prenatal drug exposure in neonates and determined that cocaine was the primary moderator of functional connectivity despite co-exposure with alcohol in approximately one third of the drug exposed participants. Therefore, little is known about the effects of prenatal alcohol exposure on infant resting connectivity based on fMRI studies, to date.
The current prospective study identified women during pregnancy, and eligible infants were followed until 6 months of age, at which time they were assessed using MEG and developmental testing. We hypothesized that infants with PAE would exhibit hypersynchrony, operationally defined as increased spectral amplitude across the physiological frequency bands, relative to infants without PAE. We further hypothesized a delayed shift in low to high frequency amplitude in PAE infants relative to controls.
2. Materials and methods
2.1. Participants
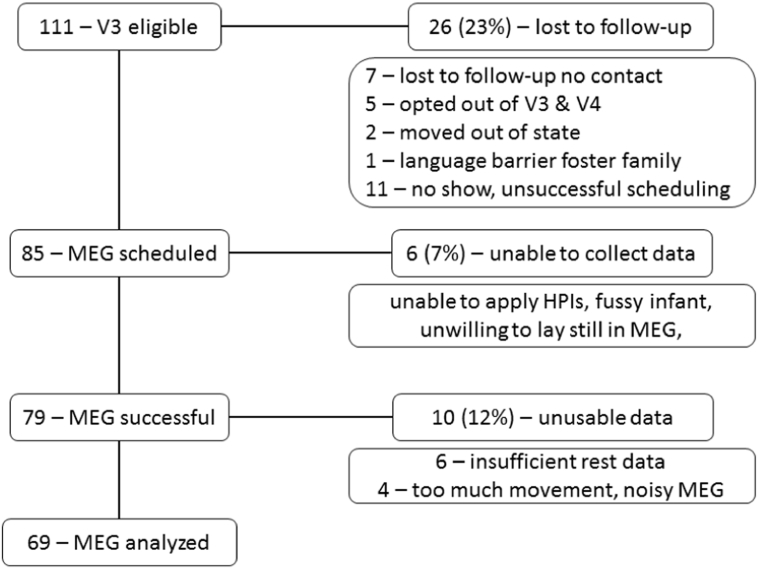
Women were recruited into the ENRICH study (Bakhireva et al., 2015) from multiple clinics at the University of New Mexico Health Sciences Center (UNM-HSC). After screening, the study was described to pregnant women and written informed consent, approved by the UNM-HSC Institutional Review Board, was obtained prior to any study procedures. Four groups of pregnant women were recruited into the study: 1. Controls - with no reported prenatal alcohol consumption, no tobacco use, and no lifetime exposure to illicit drugs. 2. Controls + OMT - socioeconomic controls who were being treated with opioid maintenance therapy (OMT; methadone or buprenorphine) but reported no prenatal alcohol consumption [confirmed by a comprehensive battery of ethanol biomarkers - (Bakhireva et al., 2015)]. 3. PAE - women recruited from general obstetrics clinics with acknowledged prenatal alcohol consumption and no illicit drug use, and 4. PAE + OMT - women being treated with OMT who also acknowledged prenatal alcohol consumption. Women were included in the PAE groups if they reported risky drinking (AUDIT-C score ≥ 3 and at least one binge drinking episode after the last menstrual period [LMP] or average consumption of ≥ 3drinks/week after LMP) at their prenatal visit. They remained in this group if follow-up blood/urine tests from the woman or newborn infant indicated positive alcohol biomarkers (maternal gamma-glutamyltranspeptidase (GGT), carbohydrate-deficient transferrin (%dCDT), phosphatidylethanol (PEth), urine ethyl sulfate (uEtS), and urine ethyl glucuronide (uEtG) and infant PEth from dry blood spot) [see detailed study design described previously in (Bakhireva et al., 2015)]. Briefly, women for each group were tested for alcohol biomarkers at both visit 1 (prenatal visit: average gestational age 24.6 ± 0.9 weeks) and visit 2 (biospecimens collected at admission for labor & delivery; interview completed within 24 h of giving birth). Women who did not test positive for at least one alcohol biomarker were excluded from the alcohol groups. Women in the control groups who did not divulge alcohol consumption during pregnancy but tested positive on alcohol biomarkers were either excluded or reclassified (3 PAE + OMT, 1 PAE) as alcohol-exposed infants. Exposure to stimulants (cocaine, methamphetamines) were exclusions to study participation and drug screens were obtained at both visit 1 and visit 2 in all participants. Infants who still qualified (N = 111) after the maternal drug screen and alcohol biomarker testing were scheduled for 6-month neuroimaging/neurodevelopmental assessment. The 6-month assessment was conducted based on age adjusted for prematurity (< 37 weeks gestational age). The reasons for data loss between the 111 eligible at V3 and the 69 included in this report are outlined in Fig. 1. The participants reported are based on individuals whose MEG had been fully analyzed and had completed visit V3 between August 5, 2012 and August 15, 2017.
Fig. 1.
Tracking of study participants. The number of participants is listed at each stage of the V3 visit on the left. The number of participants excluded and the reason for exclusion is presented on the right.
2.2. Developmental assessment
Developmental assessment of infants was performed using the Bayley Scales of Infant Development-III (Bayley-III). Assessment was performed at a separate visit from the MEG to minimize patient's burden. The average time between developmental and MEG visits was 5 days, and there were no group differences as to which assessment (MEG versus BSID) came first (p > 0.2). The Bayley-III cognitive, language and motor scales were administered. The language scale consists of receptive and expressive subscales and the motor scale consists of fine motor and gross motor subscales. The test is well standardized and subtest scores were converted to a scaled score with a mean of 10 and standard deviation of 3. For the purpose of this analysis we compared Bayley-III cognitive, fine motor, and gross motor composite scores with MEG spectral measures.
2.3. Sensorimotor mu rhythm task
During MEG measurements, infants participated in an interactive play paradigm designed to engage and disengage the sensorimotor mu rhythm similar to our previous study (Berchicci et al., 2011). The task included three different segments of data collection: 1. infant rest – infant rests during MEG data acquisition, 2. infant squeeze – infant squeezes a pipette to identify activation of the sensorimotor system and suppression of the mu rhythm, 3. investigator squeeze – infant observes investigator squeeze. The MEG session was videotaped, and the video was synchronized to the MEG data via a light visible in the video and a simultaneous trigger recorded with the MEG data.
2.4. MEG data collection
The infant was first fitted with an EGI 124 channel EEG system (EEG data not reported here). MEG/EEG data collection then occurred simultaneously. The MEG head position indicator coils were attached to the EEG sensor net with permanent clips. This avoided the need to attach the coils to the face of the infant and ensured that coils remained within the MEG helmet for head position monitoring throughout data collection. Bipolar EEG channels were attached to each clavicle to measure the electrocardiogram (ECG) during data collection. Also, bipolar EEG channels were attached to the infant forearms to capture infant squeezing activity (one electrode was attached to the elbow bone and one electrode was placed on the belly of the forearm muscle). EMG bipolar leads were placed on both the left and right forearms of the infant. Finally, an EMG bipolar electrode pair was placed on the investigator forearm that was used to perform the investigator squeeze to confirm investigator activity. After electrode impedances were checked, the Polhemus 3D tracking device was used to identify infant fiducial points (nasion, left and right preauricular points), MEG coil location, and EEG sensor array in addition to extra points to outline infant head shape.
MEG data were collected using the Elekta Neuromag Vector View 306 channel biomagnetometer (Elekta Oy, Helsinki, Finland). Data acquisition rate for both the MEG and EEG data were 1000 Hz with 0.03–330 Hz anti-aliasing filter. All infants were measured in supine position with the left hemisphere positioned close to the dewar to optimize signal strength from left hemisphere similar to our previous study (Berchicci et al., 2011) and the investigator and parent were seated on the left and right side of the infant, respectively. When the child complied, data collection began with an infant rest period of 2 min. However, some infants required interaction to settle into the MEG environment in which case the play paradigm was begun immediately. The play paradigm consisted of the infants being offered a pipette to squeeze. The pipette was connected through water-filled plastic tubing to a pressure transducer which was connected to the MEG A/D channels to indicate when the infant or investigator squeezed the pipette relative to the MEG data. The investigator also wore an auditory insert during data collection. This insert presented the investigator with a regularly spaced auditory tone (every 4 s) to cue squeezing. This helped to regulate both infant and investigator squeezes to ensure sufficient time between squeezes for sensorimotor mu rhythm rebound prior to the next squeeze. While squeezes did not happen on every tone, the tone minimized the phenomenon of increased frequency with self-paced squeezing. A second investigator outside of the shielded room monitored infant rest, infant squeezes and investigator squeezes to ensure sufficient numbers of stimuli for each condition (minimum of 30 good epochs per condition) were obtained. The parent was engaged with the child, as needed, to calm the child throughout the task. Data collection time ranged from 10 to 20 min depending on infant behavior (infant fussing, etc.). The video-recording and physiological monitoring channels (infant EMG and investigator EMG) were used to identify behavior during MEG data collection.
2.5. MEG data processing
MEG data were preprocessed using Maxfilter 2.1 with MaxMove and each of the datasets was transformed to a standard head position to allow for inter-subject comparisons within a fixed head position. The default head position was determined based on obtaining the average head position across subjects within this study and transforming the MEG data to this averaged head position. This helped to minimize the distance that the head position was transformed for each individual subject, which can lead to noise amplification. After Maxfilter, a cardiac signal space projector (SSP) was generated using Graph since Maxfilter does not reliably remove cardiac artifact from infant MEG signal (Uusitalo and Ilmoniemi, 1997). The SSP was applied to the continuous data to allow for spectral analysis of the artifact-free epochs. After preprocessing, the MEG data were coded for each of the conditions (rest, active, observe). The overall procedure is illustrated in Fig. 2.
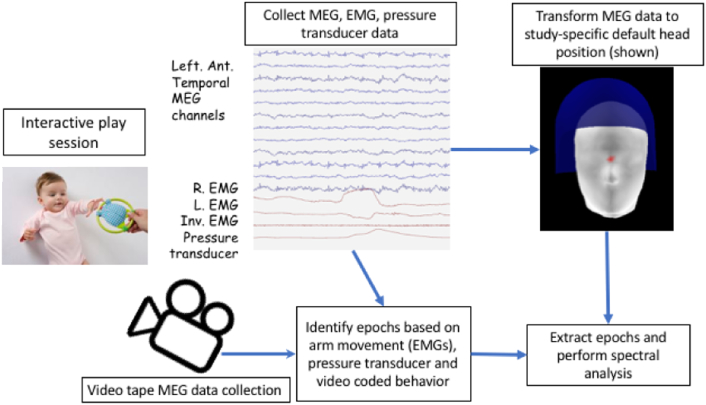
Fig. 2.
Diagram of data collection and analysis methods. During MEG data collection, the infant participated in an interactive play paradigm where they were offered a pipette to grasp, which was connected to a pressure transducer, the infant was also encouraged to observe the investigator squeezing the same pipette, and finally the infant was encouraged to rest throughout data collection. EMG from infant left and right arm (R. EMG & L. EMG) as well as EMG from the investigator “squeeze” arm (Inv. EMG) were collected in conjunction with the pressure transducer using the synchronously collected bipolar EEG channels and the A/D channels that are collected simultaneously with the MEG data. In this example, the R. EMG shows movement and a small increase in pressure on the pressure transducer channel (top and bottom red lines) indicating an infant squeeze event. At the same time, the MEG data collection is recorded via a video camera to capture infant behavior. After data collection, the MEG and video data are coded simultaneously to identify periods of rest. The MEG data for each infant is transformed to the same position relative to the sensor array using the Maxfilter movement compensation algorithm. Finally, the rest epochs were extracted and spectral analysis was performed on 2 second epochs.
Video coding of behavior occurred in conjunction with the MEG coding of the EMG and data quality. The goal was to identify periods of rest (infant resting arms and hands quietly during data collection), periods of infant squeezing (monitor infant arm EMGs and video), and periods of investigator squeezing while the infant observes the investigator (infant must be attending the investigator, while resting quietly). By monitoring the quality of the MEG data, infant EMGs, investigator EMG, pipette pressure signal, and the synchronized video, we were able to reliably identify 2-second intervals of noise-free MEG activity for each of these conditions. To address our specific hypotheses, we focused on only the MEG rest condition to compare to the previously published rest studies.
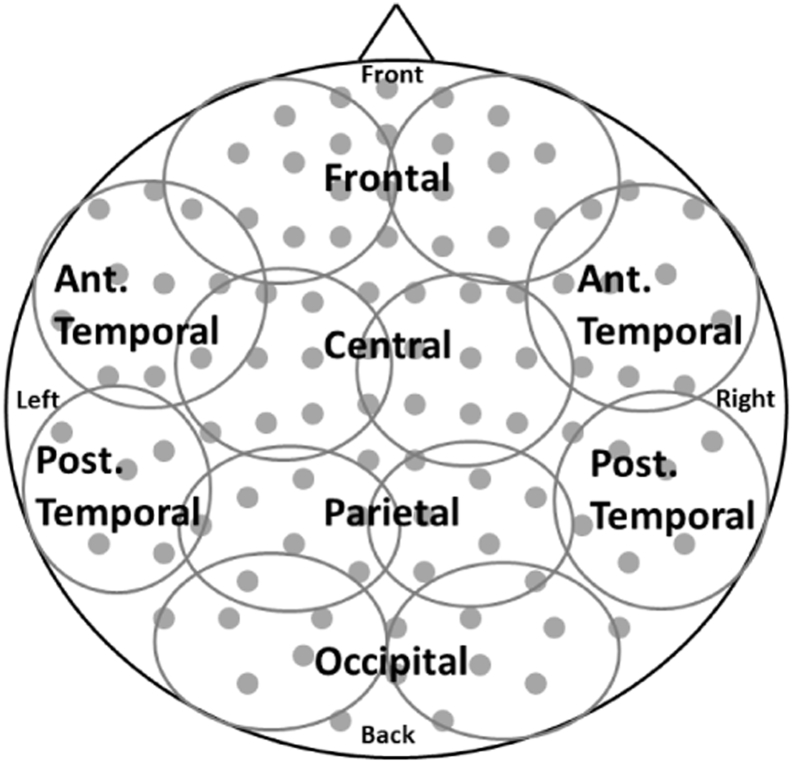
After coding, multiple 2-second epochs of rest were extracted from the continuous MEG file using MNE (http://martinos.org/mne/stable/index.html) for each infant. Of the available epochs, we arbitrarily chose at most 25 epochs per condition to estimate spectral amplitude in each participant to limit the variability in number of epochs across participants. If > 25 epochs were available for the rest condition, the 25 epochs were randomly chosen from the available epochs for each participant using the randomize feature in Matlab. Amplitude spectra were calculated using custom Matlab scripts using the absolute value of the FFT command with a specified length of the transform window set to 2048. With a 2 second epoch length each epoch contains 2000 data points, the fft function automatically pads the remaining 48 cells with zeros. The mean amplitude spectrum across epochs was obtained for each sensor. Then, mean spectral amplitude within region (Fig. 3) and frequency band was calculated. The frequency bands were: delta – 1–4 Hz, theta – 5–7 Hz, alpha – 8–13 Hz, beta – 14–28 Hz, gamma 31–58 Hz. To examine the second hypothesis we combined the low (delta and theta) and high (beta and gamma) frequency spectral amplitudes to obtain a low frequency to high frequency ratio (LFHFR).
Fig. 3.
Diagram of regions. The sensor channels were divided evenly into the following regions for both left and right hemispheres (frontal, anterior and posterior temporal, central, parietal and occipital). The top-down view depicts the sensor array with approximate channel groupings with left/right representing channels over left/right hemisphere, anterior channels shown at the top, and posterior channels shown at the bottom.
2.6. Statistical analysis
MEG and developmental measures were compared statistically using IBM SPSS version 20. The generalized linear mixed model ANOVA was performed with age at scan and total SES included in the analysis as covariates in the model. When appropriate, Greenhouse-Geisser correction was used. Correlations between spectral amplitude and Bayley-III scores were performed using Spearman's correlation. Sex by group differences were compared using the chi-square test. Multiple comparisons of the correlations were corrected using FDR correction (Benjamini and Hochberg, 1995).
3. Results
The demographics of the study population are presented in Table 1. The mean age of women upon enrollment was 27.8 ± 0.7 years. Although maternal age range was relatively narrow, there was a significant PAE group effect (p = 0.041) and interaction between PAE and OMT (p = 0.046) with PAE + OMT representing the oldest group and healthy controls representing the youngest group at study recruitment. Women were recruited into the study and data collected at visit 1 on average at 24.6 ± 0.9 weeks gestational age equivalent to the 2nd trimester; women in the OMT groups were on average recruited at a later gestational age than women without OMT. There was no significant difference in infant age (adjusted for prematurity) at the 6 month assessment with infants being measured at between 5 and 8 months with a mean age of 6.8 ± 0.14 months. The mean gestational age at birth was within the normal range (> 38 weeks) with a mean gestational age of 38.6 ± 0.27 weeks (32% < 38 weeks, minimum GA 31.1 weeks). Mean birthweight was 3018 ± 78.8 g. There was a significant main effect of PAE on gestational age at birth and birth weight – as expected based on the known risk factor for premature birth in alcohol consuming women and the relation between gestational age at birth and birthweight. Due to the known effects of prematurity on brain development, this was examined as a covariate in the models. There were no significant effects of PAE or OMT on any Bayley-III scores. The scaled scores were reported for the Bayley-III representing a normed mean of 10 for each subscale and 20 for the composite motor and language scores. The mean SES score was 32.0 ± 1.5 with a range of scores from 5 to 58 and a possible score range of 0–66. Therefore, the study represented individuals across the full spectrum of SES. The average total number of trials captured for estimation of spectral amplitude across groups was 16.5 ± 0.9 with no significant group effects or interactions (p's > 0.069). There was no significant difference in amount of alcohol consumed (after transforming to log(Number of drinks)) between the PAE and PAE + OMT groups. Finally, it is important to note that with the exception of two alcohol-consuming women, PAE was below 1.5 drinks per day averaged across pregnancy demonstrating that PAE was moderate-to-low in most cases in this study.
Table 1.
Participant demographics and Bayley Scales of Infant Development (Bayley-III).
| Controls N = 28 |
Controls + OMT N = 14 |
PAE N = 12 |
PAE + OMT N = 15 |
p-Value | |
|---|---|---|---|---|---|
| Maternal age visit 1 (years) | 26.0 ± 1.0 | 27.7 ± 1.5 | 32.1 ± 1.6 | 27.7 ± 1.4 | 0.041 |
| Gestational age visit 1 | 25.6 ± 1.4 | 20.3 ± 2.0 | 27.6 ± 2.1 | 24.3 ± 1.9 | 0.051 |
| Infant age at assessment (months) | 6.92 ± 0.23 | 6.63 ± 0.33 | 6.46 ± 0.33 | 6.92 ± 0.30 | 0.701 |
| Sex (F/M) | 14/14 | 6/8 | 9/3 | 8/7 | 0.39 |
| Gestational age at birth (weeks) | 39.27 ± 0.41 | 38.86 ± 0.58 | 37.45 ± 0.63 | 38.13 ± 0.56 | 0.019 |
| Birth weight (grams) | 3314 ± 115 | 2976 ± 163 | 2677 ± 176 | 2778 ± 158 | 0.010 |
| Total SESa | 34.4 ± 2.3 | 26.3 ± 3.3 | 33.7 ± 3.3 | 31.3 ± 3.2 | 0.484 |
| Bayley cognitive | 9.88 ± 0.35 | 10.33 ± 0.51 | 10.75 ± 0.51 | 10.13 ± 0.45 | 0.471 |
| Bayley language | 19.48 ± 0.49 | 20.67 ± 0.71 | 20.75 ± 0.71 | 20.40 ± 0.63 | 0.461 |
| Receptive | 8.48 ± 0.34 | 9.58 ± 0.49 | 9.33 ± 0.49 | 9.40 ± 0.44 | 0.432 |
| Expressive | 9.76 ± 0.23 | 9.50 ± 0.34 | 9.58 ± 0.34 | 9.73 ± 0.30 | 0.681 |
| Bayley motor | 18.52 ± 0.79 | 19.17 ± 1.14 | 18.83 ± 1.14 | 18.13 ± 1.02 | 0.742 |
| Fine | 9.76 ± 0.42 | 10.08 ± 0.60 | 10.50 ± 0.60 | 9.60 ± 0.54 | 0.970 |
| Gross | 8.76 ± 0.52 | 9.08 ± 0.76 | 8.33 ± 0.76 | 8.53 ± 0.68 | 0.601 |
| Median estimated total drinks (number of standard drinks) | – | – | 143 ± 278 | 39 ± 141 | 0.39b |
| Q1 = 48 | Q1 = 22 | ||||
| Q3 = 760 | Q3 = 68 | ||||
| Number of MEG rest epochs | 15.1 ± 1.4 | 20.4 ± 2.0 | 17.2 ± 2.2 | 14.7 ± 1.9 | 0.283 |
p-Values reported for main effect PAE with the exception of estimated total drinks.
Barratt simplified measure of social status.
Log(estimated number of drinks) one-way ANOVA between PAE and PAE + OMT groups.
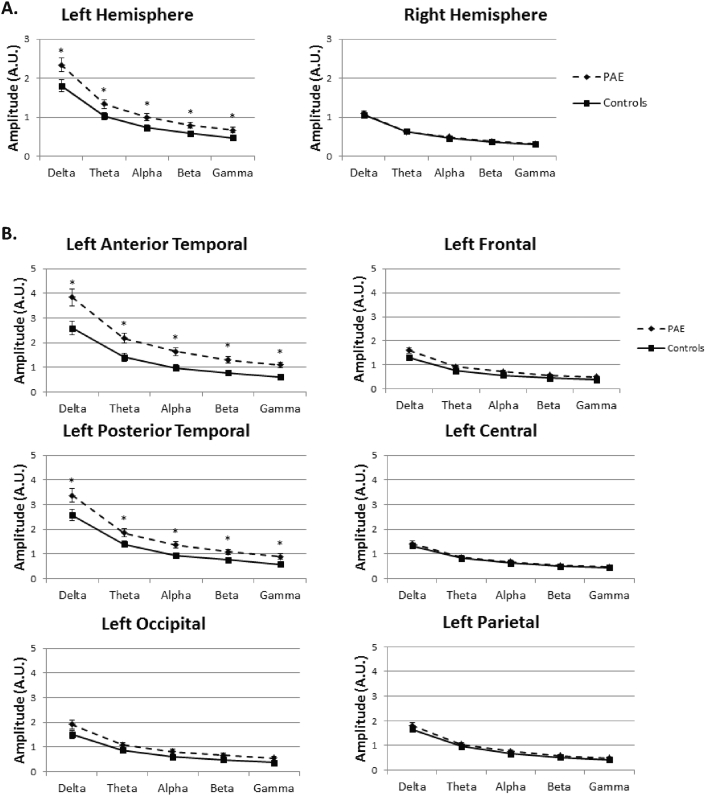
Hypersynchrony was tested by analyzing the spectral amplitude in all regions across the 5 frequency bands. The ANOVA included hemisphere, region and band as within subjects factors, PAE and OMT groups as between subjects factors and age at scan and SES as covariates. In the omnibus test there was a significant interaction between PAE, region, band, and hemisphere (F(1.66,92.8) = 7.56, p = 0.002). Follow-up tests revealed that this result was driven by left hemisphere effects revealing a main effect of group (F(1,56) = 10.3, p = 0.002) with PAE (2.029 sem 0.18) showing larger overall spectral amplitude than controls (1.25 sem 0.16) as shown in Fig. 4. There were no significant main effects or interactions in right hemisphere (p's > 0.49). Examining right hemisphere regions in separate analyses also showed no group effect of PAE on spectral amplitude (p's > 0.52). Left hemisphere also demonstrated a significant interaction between PAE, region and band (F(2.18,126.2) = 4.63, p = 0.01). There was no significant interaction with OMT exposure and PAE in the omnibus test so this was not examined for the follow-up within hemisphere analysis. Two regions drove the PAE, region and band interaction: left anterior temporal and left posterior temporal. The remaining regions did not reveal a significant group difference or a group by band interaction. Despite a band by group interaction, each band individually revealed a significant main effect of group (p < = 0.005) for left anterior temporal region and (p < 0.05) for left posterior temporal region demonstrating hypersynchrony across the frequency spectrum. The interaction with band revealed greater differences in high frequency (alpha, beta, gamma) than in low frequency (delta, theta) bands. In a follow-up analysis both gestational age at birth and maternal age were also included as covariates in the model based on the group differences observed in these variables. Neither of these contributed significantly to the model (p's > 0.1) and were subsequently removed.
Fig. 4.
Spectral amplitude for left versus right hemisphere. Clear group differences are seen in left hemisphere that are not present in right hemisphere sensors for children with prenatal alcohol exposure (PAE) versus controls (A). By examining the left hemisphere group by region interaction, post hoc tests reveal that group differences are driven by hypersynchrony measured in anterior and posterior temporal channels (B).
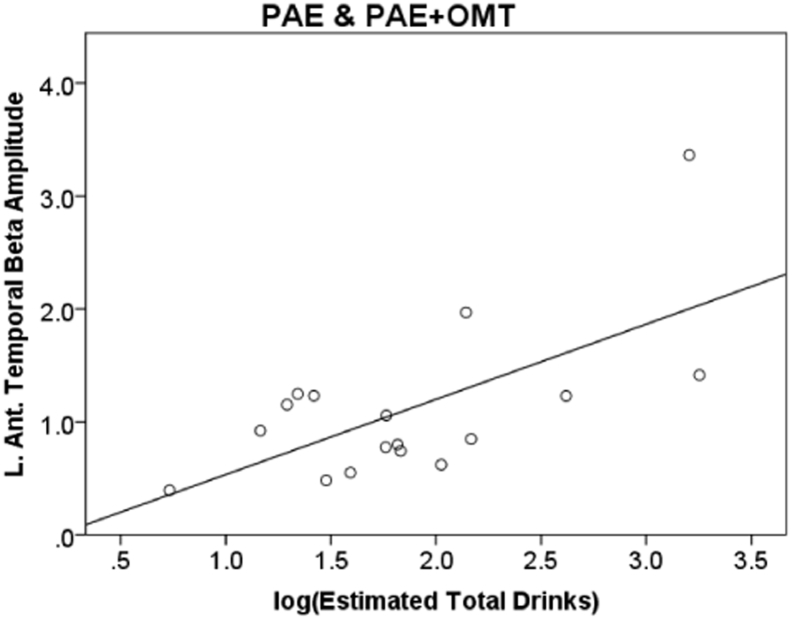
To examine if quantity of alcohol consumption in the PAE groups was related to hypersynchrony, we correlated left hemisphere anterior and posterior temporal regions based on the group differences revealed above relative to the log of the estimated total number of drinks consumed during pregnancy. The number of drinks was transformed due to the skewed distribution within the alcohol groups. There were significant positive correlations across all frequency bands in these two regions indicating greater alcohol consumption was associated with greater spectral amplitude or hypersynchrony in PAE infants (see Fig. 5; r = 0.55 to 0.68, N = 14). Correlations were corrected for multiple comparisons using FDR correction with 10 comparisons. There were no significant correlations between left anterior or posterior temporal amplitude and Bayley-III scores after correcting for multiple comparisons. Examining left anterior or posterior temporal amplitude and Bayley-III scores in the PAE group only showed an uncorrected significant association between left posterior temporal amplitude and gross motor function for both beta (r = − 0.398, p = 0.044) and gamma (r = − 0.401, p = 0.042) bands.
Fig. 5.
Correlation of beta band amplitude summed across left anterior temporal sensors and estimated total number of drinks across pregnancy in PAE infants. r = 0.68, p = 0.004.
The second hypothesis proposing that infants with PAE would have a different distribution of low and high frequency spectral amplitude relative to controls was tested by comparing the ratio of low frequency (summed delta and theta) versus the high frequency amplitude (summed beta and gamma) described as the low frequency/high frequency ratio (LFHFR). We used a mixed measures ANOVA with PAE and OMT exposure as between subjects factors and region (6 regions), band (low vs. high frequency amplitude) and hemisphere (left/right) as within subjects factors. The omnibus ANOVA did not reveal a significant main effect or interaction based on PAE or OMT exposure (p's > 0.05).
Finally, the LFHFR was correlated with Bayley-III scores to determine if there was a relationship between behavioral measures and oscillatory amplitude ratio. No significant correlations were identified when all groups were included in the analysis. However, significant correlations between Bayley-III score and MEG LFHFR were found when correlations were performed separately by group. Specifically, right parietal LFHFR was negatively correlated with Bayley-III cognitive score (r = − 0.81, p = 0.003) in the PAE only group such that higher ratio was related to poorer cognitive performance. Furthermore, the left central LFHFR was significantly correlated with Bayley-III fine motor score (r = 0.72, p = 0.004) in the PAE + OMT exposure group such that higher LFHFR was associated with better performance. Correlations were corrected for multiple comparisons across Bayley-III scores using FDR. Other correlations that did not meet this adjusted significance value are not reported.
4. Discussion
The results revealed persistent differences in neural spectral amplitude in PAE children relative to controls with hypersynchrony observed in PAE infants at 6 months of age. However, there were no differences in the pattern of low versus high frequency amplitude in infants exposed to alcohol versus controls. Furthermore, in the current study, OMT exposure did not significantly interact with PAE.
Our results are consistent with prior studies indicating hypersynchrony in neonates born to mothers with alcohol use disorders. Havlicek et al. (1977) reported 150–200% increased spectral amplitude in sleeping neonates with the largest effects occurring during REM sleep. This is similar to our range of 130–178% in the left temporal regions. Later studies determined that nicotine did not alter this hypersynchrony (Chernick et al., 1983) and hypersynchrony extended beyond the initial withdrawal period (Ioffe et al., 1984). However, there are at least three important differences between the current study and the prior studies reporting hypersynchrony. First, the current study examined infant neurophysiological measures at 6 months of age, whereas the initial reports of hypersynchrony were measured in neonates in the 0–1.5 month age range. Second, the current study primarily recruited women with moderate drinking during pregnancy rather than women with heavy alcohol consumption. Third, the prior EEG studies were collected during sleep, whereas the current data was collected while infants were awake. Therefore, the current results extend the prior findings by providing evidence that hypersynchrony persists at least until 6 months of age, it is present in the awake state, and lower levels of alcohol consumption than those reported previously also lead to the altered neural architecture that enables hypersynchrony in infants with PAE. However, unlike the results reported by Ioffe and Chernick (1990), we did not find a significant association between hypersynchrony and Bayley-III score when accounting for multiple comparisons. However, there was an (uncorrected) negative association between the Bayley-III gross motor and hypersynchrony showing a similar pattern indicating that hypersynchrony was associated with poorer performance in the PAE infants. This uncorrected association may indicate a reduced sensitivity of the Bayley-III to developmental effects in infants at 6 months of age, in contrast to the assessment at 4–7 years of age employed by Ioffe and Chernick (1990).
While the current neuroimaging literature cannot directly determine the underlying mechanism that leads to hypersynchrony, there is considerable evidence from animal studies indicating an altered inhibitory/excitatory ratio due to PAE (Larsen et al., 2016). For example, Skorput et al. (2015) determined that binge drinking led to an increase in inhibitory parvalbumin-positive interneurons in prefrontal cortex that, in turn, decreases excitatory transmission. However, third trimester equivalent binge episodes lead to decreased parvalbumin-positive interneurons (Smiley et al., 2015), which may be consistent with hypersynchrony measured here. Therefore, a possible straightforward explanation for the current results may be that these PAE infants experience impaired development of their inhibitory interneuron networks which leads to over-activation of neuronal populations leading to a general increase in synchronized neural oscillations. To the best of our knowledge, this hypersynchrony does not persist in older children with FASD based on the reported reductions in alpha amplitude in adolescents with FAS (Kaneko et al., 1996); however, the literature is sparse - providing strong motivation to further examine this across development. Overall, the result is consistent with our prior studies indicating altered gamma oscillations in children with FASD (Stephen et al., 2013), where inhibitory interneurons are thought to contribute directly to the generation of gamma oscillations.
Interestingly, the current results implicate left hemisphere hypersynchrony with no evidence of hypersynchrony in right hemisphere sensors. This is a novel result relative to prior EEG studies. This discrepancy may be explained by the smearing of the EEG signal due to skull conduction that often limits the ability of EEG studies to identify lateralized effects (Leahy et al., 1998, Mosher et al., 1999) that does not impact MEG signals. MEG signal is also less affected relative to EEG by the presence of fontanels in infants at 6 months of age (Okada et al., 1999) which is expected to lead to more between subject variability in EEG. However, it is also important to note that the default head position for the 6-month-old infants that best matched the average head position across infants was slightly biased to the left side of the helmet. This can be seen by the overall larger amplitude in left vs. right hemisphere across the frequency spectrum. However, despite the weaker signal in right hemisphere, the variance is also reduced and the non-significant pattern (p's > 0.5) is for controls to have greater amplitude in right hemisphere than alcohol-exposed infants, suggesting that signal strength and head position do not account for the hemispheric difference. While some hemispheric effects have been reported in children with FASD, the results are mixed in terms of which hemisphere is affected. For example, our prior study revealed differences in gamma oscillations that were primarily in right hemisphere, but this was measured during performance of a prosaccade task rather than during rest. Interestingly, the adolescent EEG study performed by Kaneko et al. (1996) corroborates our results indicating greater alterations of alpha amplitude in children with FAS in left hemisphere. However, structural studies which have examined whole brain effects indicate that PAE impacts both hemispheres (Gautam et al., 2015, Sowell et al., 2002).
Although examining PAE was the primary aim of this study, we included two additional groups who were exposed to OMT (with or without concurrent PAE). As described previously (Bakhireva et al., 2015), our primary motivation for including these groups was to help control for other socio-economic and home-rearing environmental factors. Despite these other confounding factors, the primary result that emerged from the current study was that PAE led to hypersynchrony and OMT exposure did not significantly alter that result. Despite the lack of significance, OMT exposure did reveal some strong trends in the high frequency/low frequency analysis and may suggest that the current study is underpowered to measure these effects. Prior studies have revealed delays in visual latencies measured with EEG in infants exposed to OMT during pregnancy providing some evidence that persistent effects on neurodevelopment may also be present due to opioid use. However, total SES was also included as a covariate in the model to account for variance in SES factors across groups. SES emerged as a significant main effect in both models (hypersynchrony and low/high frequency amplitude) confirming the persistent effects of SES on brain development as reported previously (Hurt et al., 2009, Otero, 1994, Tomalski et al., 2013). It is important to note that although there were no significant effects in LFHFR by PAE, LFHFR did differ based on SES such that LFHFR was greater in high risk infants (low SES) relative to low risk infants (high SES). This is consistent with prior studies determining that infants with a disadvantaged upbringing reveal a delay in brain development – represented by a delayed shift to high frequency amplitude (Sanjuan et al., 2016). This underlines the importance of including samples matched on SES when studying the effects of alcohol on brain development.
While the infant literature examining the effects of PAE are still quite sparse, the emergence of functional connectivity analysis using fMRI reveals future directions for this work. A clear path forward will be to extend the current results to examine brain connectivity at the source level using both MEG and fMRI. The current study did not obtain individual MRIs from each infant due to the additional challenge of obtaining infant MRIs, limiting our ability to perform connectivity at the source level. Despite some evidence for altered connectivity in older children with FASD (Wozniak et al., 2016), little evidence indicates effects in infancy. For example, recent rsfMRI functional connectivity studies have assessed the effects of alcohol in conjunction with prenatal cocaine exposure and did not find a significant effect of PAE on infant FNC (Salzwedel et al., 2015). However, the primary aim of that study was on cocaine exposure and the lack of results for alcohol effects may represent limited statistical power rather than no effect. It remains to be seen whether EEG/MEG provide unique sensitivity to this hypersynchrony effect or if normal connectivity patterns are observed in these same infants using rsfMRI.
The primary limitations to this study are related to the challenge of obtaining reliable MEG data from awake infants. This limitation was minimized by taking a number of deliberate measures to minimize variability in data quality across infants. First, some infant data was deemed unusable due to excessive motion and the subsequent movement artifacts that result. Second, the MEG data collection procedure was video-taped and coded to identify reliable periods of rest (child is resting quietly and is not actively moving their arms). The rest epochs identified via the video and the synchronously collected EMG/pressure transducer signals were extracted from the MEG continuous recording. Third, the MEG data from each infant were transformed to a default head position across the continuous recording using the movement compensation feature of the Maxfilter software, thereby minimizing both the effect of head motion during the scan and differences in head placement within the helmet across infants. A few subjects were eliminated at this stage due to noise amplification in the cases where head movement was too great such that noise amplification occurred (Wehner et al., 2008). These factors led to the limitation of a relatively small sample size that may have hampered our ability to detect effects of opioid exposure in these infants. An additional limitation was the asymmetric head position in the sensor array chosen to optimize sensitivity to the left hemisphere in these infants. The primary results were obtained in left hemisphere and while the right hemisphere shows no pattern consistent with group effects suggesting a lateralized effect, this finding will be better confirmed with source analysis that accounts for head position in amplitude calculations. Establishing an efficient pipeline for source analysis of infant data sets is an important future direction to address this limitation.
5. Conclusions
The current results reveal a persistent finding of hypersynchrony in infants prenatally exposed to alcohol relative to healthy controls regardless of prenatal opioid exposure in these infants. However, our results do not support an altered developmental pattern based on low frequency to high frequency ratio. Yet, SES emerged as a persistent factor that also plays a role in infant development and underscores the importance of matching on SES factors when examining the effects of prenatal exposures on infant brain development. The current results in combination with the behavioral results indicate that MEG may provide an early indicator of atypical brain development in the absence of measurable developmental effects as assessed with the Bayley-III. Future longitudinal studies will need to determine if these early alterations in brain development are predictive of long-term deficits in children with PAE. Furthermore, an increasing number of studies, including our own (Lowe et al., 2017), have demonstrated the effect of infant-maternal interaction on development and may provide a more sensitive marker to altered brain development at this early age than emerging cognitive measures. Finally, the results indicate lateralization of the hypersynchrony effect relative to prior EEG studies. With future localization of MEG signals, we may identify the source of this hypersynchrony to better guide interventions at this young age.
Acknowledgements
We would like to thank the parents and their infants for participating in this study. We also thank Shikhar Shrestha, Sonnie Williams, Joy VanMeter, and Laura Garrison for their help with data collection and data organization. This work was supported by the National Institutes of Health [grant numbers R01AA021771 and P50AA022534] and the National Science Foundation [grant number 1539067].
References
- Bakhireva L.N., Lowe J.R., Gutierrez H.L., Stephen J.M. Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) prospective cohort: study design considerations. Adv. Pediatr. Res. 2015;2 doi: 10.12715/apr.2015.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Methodol. 1995;57:289–300. [Google Scholar]
- Berchicci M., Zhang T., Romero L., Peters A., Annett R., Teuscher U., Bertollo M., Okada Y., Stephen J., Comani S. Development of mu rhythm in infants and preschool children. Dev. Neurosci. 2011;33(2):130–143. doi: 10.1159/000329095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernick V., Childiaeva R., Ioffe S. Effects of maternal alcohol intake and smoking on neonatal electroencephalogram and anthropometric measurements. Am. J. Obstet. Gynecol. 1983;146:41–47. doi: 10.1016/0002-9378(83)90924-9. [DOI] [PubMed] [Google Scholar]
- Clarke A.R., Barry R.J., Mccarthy R., Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin. Neurophysiol. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Fan J., Jacobson S.W., Taylor P.A., Molteno C.D., Dodge N.C., Stanton M.E., Jacobson J.L., Meintjes E.M. White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Hum. Brain Mapp. 2016;37:2943–2958. doi: 10.1002/hbm.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Lin W., Grewen K., Gilmore J.H. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. 2016 doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P., Lebel C., Narr K.L., Mattson S.N., May P.A., Adnams C.M., Riley E.P., Jones K.L., Kan E.C., Sowell E.R. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum. Brain Mapp. 2015;36:2318–2329. doi: 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Lin W., Gao W., Fair D.A. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlicek V., Childiaeva R., Chernick V. EEG frequency spectrum characteristics of sleep states in infants of alcoholic mothers. Neuropadiatrie. 1977;8:360–373. doi: 10.1055/s-0028-1091532. [DOI] [PubMed] [Google Scholar]
- Hurt H., Betancourt L.M., Malmud E.K., Shera D.M., Giannetta J.M., Brodsky N.L., Farah M.J. Children with and without gestational cocaine exposure: a neurocognitive systems analysis. Neurotoxicol. Teratol. 2009;31:334–341. doi: 10.1016/j.ntt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C., Bittigau P., Ishimaru M.J., Wozniak D.F., Koch C., Genz K., Price M.T., Stefovska V., Horster F., Tenkova T., Dikranian K., Olney J.W. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ioffe S., Chernick V. Prediction of subsequent motor and mental retardation in newborn infants exposed to alcohol in utero by computerized EEG analysis. Neuropediatrics. 1990;21:11–17. doi: 10.1055/s-2008-1071450. [DOI] [PubMed] [Google Scholar]
- Ioffe S., Childiaeva R., Chernick V. Prolonged effects of maternal alcohol ingestion on the neonatal electroencephalogram. Pediatrics. 1984;74:330–335. [PubMed] [Google Scholar]
- Kaneko W.M., Ehlers C.L., Philips E.L., Riley E.P. Auditory event-related potentials in fetal alcohol syndrome and Down's syndrome children. Alcohol. Clin. Exp. Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P.W. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev. Disabil. Res. Rev. 2009;15:218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku P., Segall J.M., Beatty G.K. Cognitive and behavioral effects of prenatal alcohol exposure. Future Neurol. 2011;6:237–259. [Google Scholar]
- Larsen Z.H., Chander P., Joyner J.A., Floruta C.M., Demeter T.L., Weick J.P. Effects of ethanol on cellular composition and network excitability of human pluripotent stem cell-derived neurons. Alcohol. Clin. Exp. Res. 2016;40:2339–2350. doi: 10.1111/acer.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy R.M., Mosher J.C., Spencer M.E., Huang M.X., Lewine J.D. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr. Clin. Neurophysiol. 1998;107:159–173. doi: 10.1016/s0013-4694(98)00057-1. [DOI] [PubMed] [Google Scholar]
- Li D., Karnath H.O., Xu X. Candidate biomarkers in children with autism spectrum disorder: a review of MRI studies. Neurosci. Bull. 2017;33:219–237. doi: 10.1007/s12264-017-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Qeadan F., Leeman L., Shrestha S., Stephen J.M., Bakhireva L.N. The effect of prenatal substance use and maternal contingent responsiveness on infant affect. Early Hum. Dev. 2017;115:51–59. doi: 10.1016/j.earlhumdev.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A., Nelson C.A., 3rd, Zeanah C.H. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Dev. Psychopathol. 2008;20:861–880. doi: 10.1017/S0954579408000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher J.C., Leahy R.M., Lewis P.S. EEG and MEG: forward solutions for inverse methods. IEEE Trans. Biomed. Eng. 1999;46:245–259. doi: 10.1109/10.748978. [DOI] [PubMed] [Google Scholar]
- Mwansisya T.E., Hu A., Li Y., Chen X., Wu G., Huang X., Lv D., Li Z., Liu C., Xue Z., Feng J., Liu Z. Task and resting-state fMRI studies in first-episode schizophrenia: a systematic review. Schizophr. Res. 2017 doi: 10.1016/j.schres.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Newville J., Valenzuela C.F., Li L., Jantzie L.L., Cunningham L.A. Acute oligodendrocyte loss with persistent white matter injury in a third trimester equivalent mouse model of fetal alcohol spectrum disorder. Glia. 2017;65:1317–1332. doi: 10.1002/glia.23164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y.C., Lahteenmaki A., Xu C. Experimental analysis of distortion of magnetoencephalography signals by the skull. Clin. Neurophysiol. 1999;110:230–238. doi: 10.1016/s0013-4694(98)00099-6. [DOI] [PubMed] [Google Scholar]
- Otero G.A. EEG spectral analysis in children with sociocultural handicaps. Int. J. Neurosci. 1994;79:213–220. doi: 10.3109/00207459408986082. [DOI] [PubMed] [Google Scholar]
- Paolozza A., Treit S., Beaulieu C., Reynolds J.N. Diffusion tensor imaging of white matter and correlates to eye movement control and psychometric testing in children with prenatal alcohol exposure. Hum. Brain Mapp. 2017;38:444–456. doi: 10.1002/hbm.23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel A.P., Grewen K.M., Vachet C., Gerig G., Lin W., Gao W. Prenatal drug exposure affects neonatal brain functional connectivity. J. Neurosci. 2015;35:5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan P.M., Poremba C., Flynn L.R., Savich R., Annett R.D., Stephen J. Association between theta power in 6-month old infants at rest and maternal PTSD severity: a pilot study. Neurosci. Lett. 2016 doi: 10.1016/j.neulet.2016.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Skorput A.G., Gupta V.P., Yeh P.W., Yeh H.H. Persistent interneuronopathy in the prefrontal cortex of young adult offspring exposed to ethanol in utero. J. Neurosci. 2015;35:10977–10988. doi: 10.1523/JNEUROSCI.1462-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J.F., Saito M., Bleiwas C., Masiello K., Ardekani B., Guilfoyle D.N., Gerum S., Wilson D.A., Vadasz C. Selective reduction of cerebral cortex GABA neurons in a late gestation model of fetal alcohol spectrum disorder. Alcohol. 2015;49:571–580. doi: 10.1016/j.alcohol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen R.J., Van't Klooster B.J., Van Der Molen M.W., Van Leeuwen H.M., Licht R. Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biol. Psychol. 1997;44:187–209. doi: 10.1016/s0301-0511(96)05218-0. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Peterson B.S., Mattson S.N., Welcome S.E., Henkenius A.L., Riley E.P., Jernigan T.L., Toga A.W. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. NeuroImage. 2002;17:1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Stephen J.M., Coffman B.A., Stone D.B., Kodituwakku P. Differences in MEG gamma oscillatory power during performance of a prosaccade task in adolescents with FASD. Front. Hum. Neurosci. 2013;7:900. doi: 10.3389/fnhum.2013.00900. (eCollection 2013.</p >) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth A.P., Bookstein F.L., Barr H.M., Sampson P.D., O'malley K., Young J.K. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J. Dev. Behav. Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. (doi: 00004703-200408000-00002 [pii]) [DOI] [PubMed] [Google Scholar]
- Takamura T., Hanakawa T. Clinical utility of resting-state functional connectivity magnetic resonance imaging for mood and cognitive disorders. J. Neural Transm. (Vienna) 2017;124:821–839. doi: 10.1007/s00702-017-1710-2. [DOI] [PubMed] [Google Scholar]
- Tomalski P., Moore D.G., Ribeiro H., Axelsson E.L., Murphy E., Karmiloff-Smith A., Johnson M.H., Kushnerenko E. Socioeconomic status and functional brain development - associations in early infancy. Dev. Sci. 2013;16:676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Uusitalo M.A., Ilmoniemi R.J. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Wehner D.T., Hamalainen M.S., Mody M., Ahlfors S.P. Head movements of children in MEG: quantification, effects on source estimation, and compensation. NeuroImage. 2008;40:541–550. doi: 10.1016/j.neuroimage.2007.12.026. (doi: S1053-8119(07)01148-2 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Mattson S.N., Coles C.D., Kable J.A., Jones K.L., Boys C.J., Lim K.O., Riley E.P., Sowell E.R., Cifasd Functional connectivity abnormalities and associated cognitive deficits in fetal alcohol spectrum disorders (FASD) Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]