Abstract
Purpose
Pancreatic ductal and lung adenocarcinomas are the most common and prevalent types of human neoplasms with a greater than 80% mortality rate. The poor prognosis of both these cancers are likely due to the absence of valid approaches for early detection, the frequency of its metastases at the time of diagnosis, frequent recurrence after surgery, and poor responsiveness to chemotherapy. Most notably, the early development of pancreatic intraepithelial neoplasia and lung lesions is suggested to be the result of a mutation in the K-ras (G12D) oncogene. Tumor necrosis factor-related-apoptosis-inducing-ligand (TRAIL) has been shown to have great potential for the treatment of most human tumor cells, while leaving normal cells unharmed. However, some cancers show resistance to TRAIL treatment, leaving a gap in the understanding of its exact etiology.
Methods
TRAIL-induced resistance to cell death was investigated in pancreatic and lung cancer cell lines. Cell survival was determined by SRB and apoptosis by ELISA-based cell death assay. Activation of bid and caspases were evaluated by Western blotting.
Results
Our study demonstrated that TRAIL significantly suppressed cell survival, by inducing apoptosis in a dose-dependent manner, in the pancreatic cancer BxPC-3 (wild type G12) and lung cancer A549 (G12S) cell lines. In contrast, Panc-1 pancreatic and SK-LU-1 lung cancer cell lines, which have a mutated (G12D) K-ras genotype, were resistant to the actions of TRAIL.
Conclusions
This study demonstrates an association between TRAIL resistance to apoptosis in human pancreatic and lung cancer cell lines and G12D K-ras12 mutation.
Keywords: Apoptosis, TRAIL resistance, K-RAS, Pancreatic cancer, Lung cancer, Chemotherapeutic resistance
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal human malignancies with an overall 5-year survival rate of <5% and fourth leading cause of cancer-related deaths in the United States [1]. PDAC develops from a progressive cascade of cellular, morphological, and architectural changes from normal ductal epithelium through proneoplastic lesions named as pancreatic intraepithelial neoplasia (PanIN). Specific PanIN stages have been found to be associated with different mutations including the loss of tumor suppressor proteins CDKN2A/p16INK4A, p53, DPC4/SMAD4, and BRACA2 as well as the activation of HER-2/NEU and K-RAS2 genes [2].
Approximately 40% of the early PanIN lesions have been shown to be associated with the activating mutations in the KRAS2 locus. However, in advanced PDAC, KRAS2 mutations are detected in nearly 100% of the cases [3]. Among different mutations in the K-ras oncogene, the glycine to aspartic acid mutation within codon 12 (G12D) is the most common and is found in greater than 90% of PDACs. Hingorani et al. have demonstrated that mice constitutively expressing KrasG12D mutation, via the Pdx1-Cre transgene, in the pancreatic ductal epithelium showed the full spectrum of preinvasive PanIN-like lesions and supporting the fact that constitutive Kras activation is a critical and initiating step in the progression of PDAC [4].
Lung cancer is the most commonly diagnosed cancer worldwide, with nonsmall cell lung cancer (NSCLC) accounting for about 80% of all cases diagnosed [1]. Adenocarcinoma of the lung is more prevalent and currently the most common type of lung cancer in the United States. Activating mutations of the K-ras oncogene within codon 12 (G12D) have been reported in 25–50% of human lung adenocarcinomas [5]. Johnson et al. reported that mice harboring a latent allele of K-ras G12D mutation is capable of spontaneously activating the K-ras oncogene that results in the development of multiple, early-onset lung tumors [6].
The extremely poor prognosis of both of these fatal human cancers is due to the difficulties in its early detection, advance stage of the disease at the time of clinical diagnoses, frequent recurrence after surgery, and poor responsiveness to chemotherapy or radiotherapy regimens [1]. In spite of the various modern treatment modalities, the overall mortality rate of both of these cancers has not been significantly reduced in decades [1]. In addition to chemotherapeutic drugs use to treat pancreatic and lung cancers, tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)/APO-2L has been reported to be a novel and potential candidate for the treatment of human cancers [7, 8].
TRAIL is a member of the TNF family that induces apoptosis by binding to its cognate transmembrane death receptors, TRAIL-R1/DR4, and TRAIL-R2/DR5 [9]. These death receptors are in high abundance on the surface of the transformed cancer cells and are not present on the cell surface of almost all normal cells. TRAIL induces its cytotoxic effects via binding to its death receptors on human tumor cells without affecting normal cells [10]. TRAIL has been shown to induce apoptotic cell death in human pancreatic and lung cancer cells in vitro and in vivo [8, 9]. Notably, some human pancreatic and lung cancer cells develop resistance to TRAIL-induced apoptosis [11]. However, evidence of the K-ras G12D mutation associated with resistance to TRAIL-induced apoptosis in pancreatic and lung cancer cells has yet to be reported.
In the present study, we investigated the possible explanations for the resistance of pancreatic and lung cancer cells to TRAIL-induced apoptosis. Based on the mutation status in codon 12 of the K-ras gene, we selected pancreatic BxPC-3 (G12, Wild Type) and Panc-1 (G12D) cells as well as lung cancer cell lines A549 (G12S) and SK-LU-1 (G12D) [12–17]. Our data indicated that TRAIL significantly inhibited the growth of wild-type (G12) pancreatic BxPC-3 and (G12S) lung A549 cells by inducing apoptosis in a dose-dependent manner and was associated with the cleavage of caspase-8, BID, caspase-3, and PARP. In contrast, K-ras-mutated (G12D) Panc-1 pancreatic and SK-LU-1 lung cancer cell lines were resistant to TRAIL-induced apoptosis and were associated with the inability to activate the downstream apoptotic pathways.
Materials and methods
Reagents
The well-differentiated epithelial human pancreatic adenocarcinoma BxPC-3, Panc-1, and lung carcinoma SK-LU-1 cell lines were obtained from American Type Cell Culture (ATCC, Manassas, VA). A549 lung cancer cells were obtained from Dr. Jayarama Gunaje, Texas Tech University Health Sciences Center. Heat-inactivated fetal bovine serum and RPMI-1640, DMEM and F-12K medium were purchased from Mediatech Inc. Human recombinant TRAIL was purchased from R&D Systems. Calcium ionophore A23187 and antibody against β-actin were purchased from Sigma–Aldrich. The cell death detection apoptosis ELISA kit was purchased from Roche Applied Science. Western blotting enhanced chemiluminescence (ECL) reagent was purchased from Perkin Elmer. Antibodies against full-length BID (cat#2002), cleaved fragment of caspase-8 (cat#9496), full-length caspase 3 (cat#9664), and poly (ADP-ribose) polymerase (PARP) (cat#9541), were from Cell Signaling Technology Inc.
Cell culture
Monolayer culture of BxPC3 cells were maintained in RPMI-1640 medium (Sigma–Aldrich, St. Louis, MO) adjusted to contain 10% heat-inactivated fetal bovine serum (FBS) supplemented with 2 mM l-glutamine 1.5 g/L sodium bicarbonate 4.5 g/L glucose, 10 mM HEPES, 1.0 mM sodium pyruvate, and 1% (v/v) penicillin/streptomycin. Panc-1 cells were cultured and maintained in DMEM supplemented with 4 mM l-glutamine and adjusted to contain 10% FBS and 1% (v/v) penicillin/streptomycin. Monolayer cultures of SK-LU-1 and A549 were maintained in F-12 K medium adjusted to contain 10% FBS and 1% (v/v) penicillin/streptomycin.
SRB Cell survival assay
The effect of TRAIL and A23187 on cell survival in BxPC-3, Panc-1, SK-LU-1, and A549 cells was determined by Sulforhodamine B assay (SRB, Sigma–Aldrich) as described previously [18–20]. Cells were treated with hrTRAIL for 6 h or A23187 for 24 h. The plates were read at 590 nm using the Bio Kinetics plate reader EL-800 (BioTek Instrument Inc). IC50 values were obtained from GraphPad Prism.
Western blot analysis
BxPC-3, Panc-1, SK-LU-1, and A549 cells (1×106) were plated and allowed to attached overnight, followed by treatment with varying concentrations of hrTRAIL (0, 5, 10 and 20 ng/ml) for 6 h. Whole cell extracts were prepared and 60 µg of protein were analyzed for caspase-8, BID, caspase-3, and PARP by immunoblotting, as previously described [18–22].
Apoptosis detection by cell death ELISA
Apoptotic cell death was measured by Cell Death ELISA assay, as previously described [14–16]. Briefly, 1×104 BxPC-3, Panc-1, SK-LU-1, and A549 cells were seeded in 96 well plates and treated with hrTRAIL (0, 5, 10 or 20 ng/ml) for 6 h. Each sample was analyzed in triplicate. The plates were read at 405 nm and at 490 nm on EL800 ELISA plate reader (BioTek Instruments).
Statistical analysis
All experiments were repeated independently, a minimum of three times and expressed as mean values with 95% confidence. All statistical calculations were performed using InStat software and GraphPad Prizm 4.0. Nonparametric analysis of variance (ANOVA) followed by Bonferroni or Newman–Keuls post hoc multiple comparison tests was used to test the statistical significance between multiple control and treated groups. Differences were considered significant when P ≤ 0.05.
Results
TRAIL inhibits the growth of human pancreatic and lung cancer cells
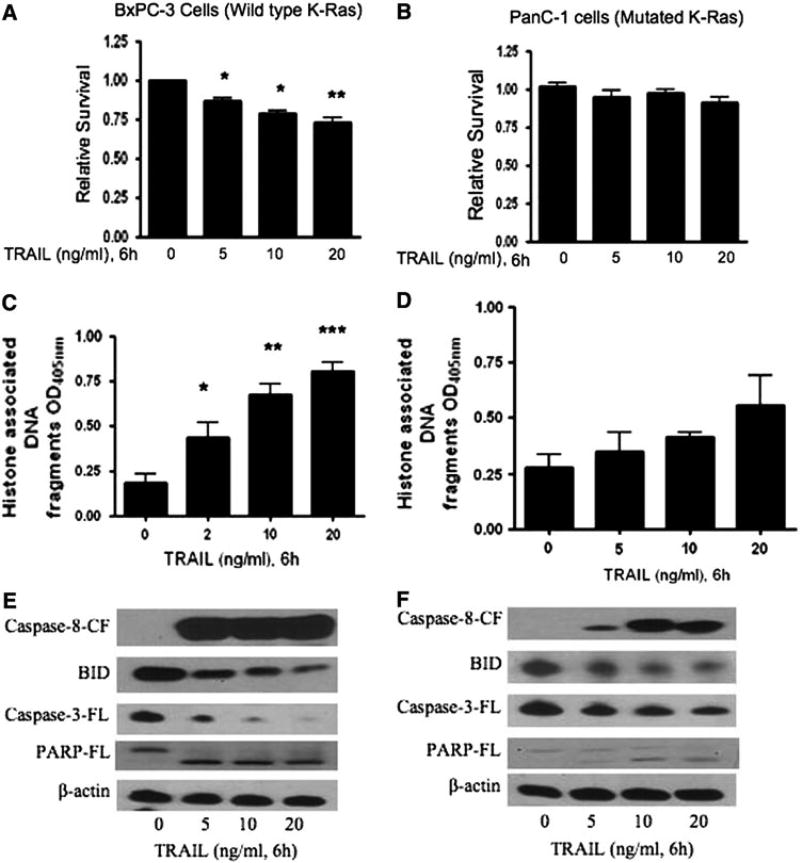
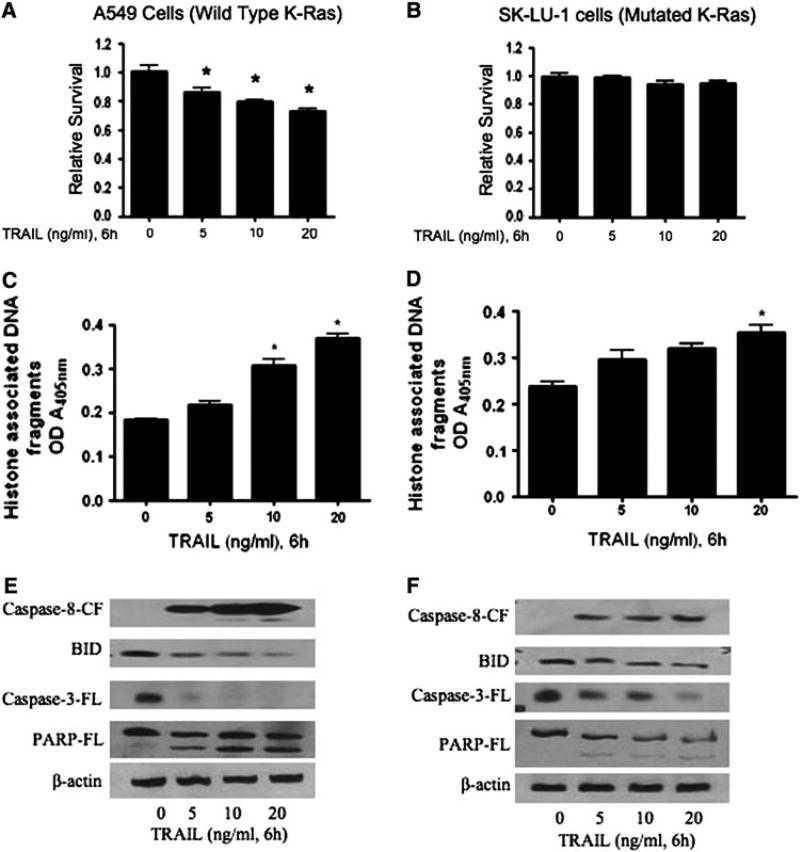
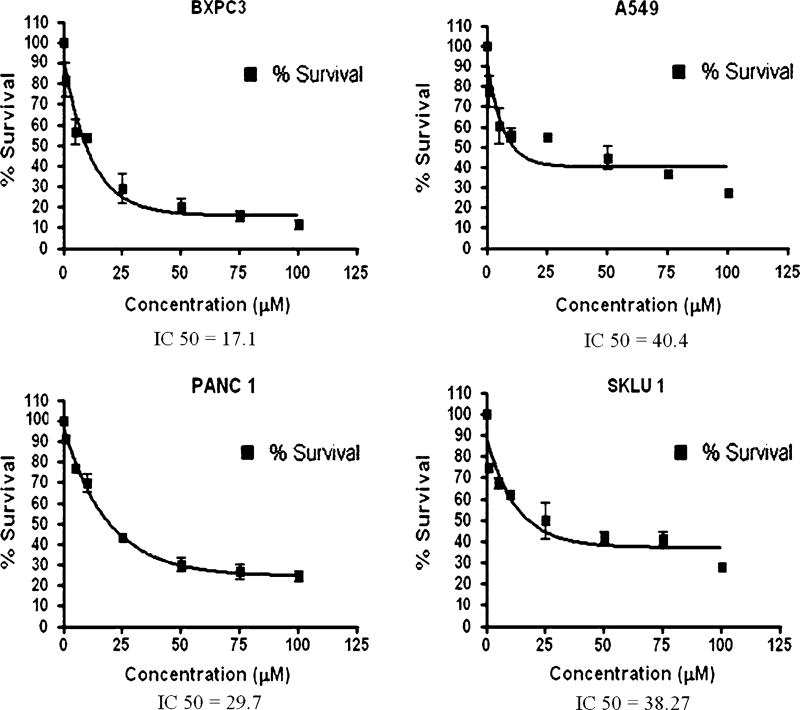
As evidenced from previous studies, TRAIL can induce cytotoxic effects on human cancer cells and tumors by inhibiting cell proliferation through various signaling pathways [19, 20, 22]. To determine the optimal concentration of TRAIL to use that might alter the survival of human pancreatic BxPC-3 and lung A549 cancer cells, we tested the effect of different doses (0, 5, 10 and 20 ng/ml) of TRAIL and different time points of analysis (0, 3, 6, 12 and 24 h). We found that TRAIL decreased the survival of both BxPC-3 and A549 cells and this was maximal at 6 h, when compared to 3, 12, and 24 h of treatment (data not shown). Therefore, in all subsequent experiments, cells were treated with TRAIL for 6 h to observe the maximum effect. As shown in Fig. 1, treatment for 6 h with increasing concentrations of TRAIL significantly inhibited the survival of human pancreatic BxPC-3 cancer cells in a dose-responsive manner, but had no effect on the survival of Kras12-mutated PanC-1 pancreatic cancer cells (Fig. 1a, b). Similarly, increasing concentrations of TRAIL significantly decreased the survival of human lung cancer A549 cells dose responsively, but had no effect on the survival of Kras12-mutated SK-LU-1 lung cancer cells (Fig. 2a, b). The calcium ionophore, A23187, was used as a nonspecific inducer of cell death (Fig. 3) [27]. In BxPC3 and PanC-1 pancreatic cancer cell lines, 24-h treatment of A23187 induced cell death with an IC50 of 17.1 and 29.7 µM, respectively. In A549 and SK-LU-1 lung cancer cell lines, treatment of A23187 induced cell death with an IC50 of 40.4 and 38.27 µM, respectively.
Fig. 1.
TRAIL sensitivity and resistance in Pancreatic BxPC-3 and PanC-1 cancer cells. BxPC-3 (pancreatic), having wild-type K-Ras gene, and PanC-1 (pancreatic) cancer cells, having a mutation (G–D) at codon 12 in K-Ras gene, were treated with different concentrations of TRAIL (0, 5, 10 and 20 ng/ml) for 6 h. Effect of TRAIL treatment on the survival of a BxPC-3 and b PanC-1 cells was analyzed by Sulforhodamine B assay. Apoptosis by TRAIL was evaluated by cell death detection apoptosis ELISA in c BxPC-3 and d PanC-1 cells. The values are represented as mean ± SEM of three independent experiments (each conducted in triplicate). Statistically significant when compared with control, *P < 0.05, **P < 0.01, and ***P < 0.001. For Western blotting, total cell lysates were prepared as described in the “Materials and methods” section. Representative immunoblots show the effect of TRAIL treatment in e BxPC-3 and f PanC-1 cells, on the cleavage of caspase-8, caspase-3, and full-length Bid and PARP. Each blot was stripped and reprobed with anti-β-actin antibody to ensure equal protein loading
Fig. 2.
TRAIL sensitivity and resistance in Lung A549 and SK-LU-1 cancer cells. A549, having a (G–S) mutation at codon 12 in the K-Ras gene, and SK-LU-1 lung cancer cells, having a mutation (G–D) at codon 12 in the K-Ras gene, were treated with different concentrations of TRAIL (0, 5, 10 and 20 ng/ml) for 6 h. Effect of TRAIL treatment on the survival of a A549 and b SK-LU-1 cells was analyzed by Sulforhodamine B assay. Apoptosis by TRAIL was evaluated by cell death detection apoptosis ELISA in c A549 and d SK-LU-1 cells. The values are represented as mean ± SEM of three independent experiments (each conducted in triplicate). Statistically significant when compared with control, *P < 0.05, **P < 0.01, and ***P < 0.001. For Western blotting, total cell lysates were prepared as described in the “Materials and methods” section. Representative immunoblots show the effect of TRAIL treatment in e A549 and f SKLU-1 cells, on the cleavage of caspase-8, caspase-3, and full-length Bid and PARP. Each blot was stripped and reprobed with anti-β-actin antibody to ensure equal protein loading
Fig. 3.
A23187 uniformly decreases cell survival in pancreatic and lung cancer cell lines. BxPC-3 (pancreatic), having wild-type K-Ras gene; PanC-1 (pancreatic) cancer cells, having a mutation (G–D) at codon 12 in K-Ras gene; A549, having a (G–S) mutation at codon 12 in the K-Ras gene; and SK-LU-1 lung cancer cells, having a mutation (G–D) at codon 12 in the K-Ras gene were treated with varying concentrations of the calcium ionophore A23187 (0–100 µM) for 24 h to examine the effects on cell survival as analyzed by Sulforhodamine B assay. IC50 was determined by GraphPad Prism
TRAIL induces apoptosis in pancreatic and lung cancer cells
In order to determine how TRAIL might inhibit cell growth, pancreatic BxPC-3 and lung A549 cells were treated with increasing concentrations of TRAIL for 6 h, and the degree of apoptosis was measured by cell death detection ELISA assay as described in Methods. As shown, TRAIL significantly induced increasing levels of apoptosis in both BxPC-3 (Fig. 1c) and A549 (Fig. 2c) cells in a dose-responsive manner. In contrast, increasing TRAIL concentrations did not induce apoptosis in pancreatic PanC-1 cells, even at the highest doses (Fig. 1d). In lung SK-LU-1 cells, TRAIL does not significantly induce apoptosis, except at the very highest concentration tested, 20 ng/ml (Fig. 2d).
TRAIL activates the extrinsic apoptotic pathway in Kras12 wild-type pancreatic and lung cancer cells
In order to further address the mechanism of TRAIL-induced apoptosis, we evaluated the effect of TRAIL on the apoptotic signaling molecules, such as caspase-8, Bid, caspase-3, and PARP, of the extrinsic or death receptor pathway. Treatment of pancreatic BxPC-3 and lung A549 cells with TRAIL resulted in the extensive cleavage of caspase-8, Bid, caspase-3, and PARP in a dose-dependent manner (Figs. 1e, 2e). The loss of full-length Bid and procaspase-3 is indicative of the cleavage of these proteins (Figs. 1e, 2e). These results suggest that both BxPC-3 and A549 cells are sensitive to TRAIL-induced apoptosis via cleavage of caspase-8, Bid, caspase-3, and PARP.
Resistance of TRAIL-induced apoptosis in human Pancreatic PanC-1 and SK-LU-1 Lung cancer cells
Resistance to TRAIL-induced apoptosis has previously been reported in pancreatic and lung cancer cells [22–25]. We selected PanC-1(pancreatic) and SK-LU-1(lung) cancer cells that have a mutation in the codon 12 (G12D) of K-ras gene, as it is the most common mutation in human pancreatic and lung tumors, as a means to determine a mechanism of resistance to TRAIL-induced apoptosis. PanC-1 and SK-LU-1 cells treated with TRAIL barely altered the cell survival of either of the cell lines (Figs. 1d, 2d). We failed to observe statistically significant apoptosis induced by TRAIL in PanC-1 cells (Fig. 1d). In SK-LU-1, TRAIL modestly increased apoptosis but only at the highest concentration (Fig. 2d). The effect of TRAIL on PanC-1 and SK-LU-1 were completely opposite to what was observed in wild-type KRas12 (BxPC-3) and G12S K-Ras12 (A549) cells. Consistent with apoptosis data by Cell Death ELISA, we observed substantially reduced cleavage of caspase-8, Bid, caspase-3, and PARP in both PanC-1 and SK-LU-1 cells (Figs. 1f, 2f), when compared to BxPC-3 and A549 cells. This suggests that the glycine (G) to aspartate (D) mutation in codon 12 of the K-ras gene confers resistance of these cells to TRAIL-induced apoptosis.
Discussion
In the present study, we attempted to determine an association between K-Ras12 mutation and resistance to TRAIL-induced apoptosis in pancreatic and lung cancer cells. For this purpose, we used BxPC-3 pancreatic that have a wild-type amino acid glycine at codon 12 of the K-ras gene, A549 lung cancer cells that have a glycine to serine mutation at codon 12, and PanC-1 pancreatic and SK-LU-1 lung cancer cells, both of which harbor a single point mutation in codon 12 that changes glycine to aspartic acid (G12D). We demonstrated that treatment with varying concentrations of TRAIL in BxPC-3 and A549 cells, significantly inhibited the growth and induced apoptosis as evidenced by cell survival, cell death detection apoptosis ELISA assay, and immunoblotting. Our data are consistent with a previously published report that showed that both the pancreatic BxPC-3 and PanC-1 cells express TRAIL receptor, whereas BxPC-3 cells were susceptible and PanC-1 cells were found to be resistance to TRAIL-mediated apoptosis [23].
TRAIL can induce apoptosis through an extrinsic apoptotic pathway via binding to its death receptors. TRAIL receptors are predominantly expressed in a majority of cancer cells, but not in noncancerous cells [7, 9]. Upon binding to its receptors, TRAIL mediates an apoptotic response through the activation and cleavage of caspase-8 that in turn cleaves caspase-3. Our results demonstrate that TRAIL induces apoptosis in BxPC-3 pancreatic and A549 lung cancer cells via enhanced cleavage of caspase-8. Previous studies also suggest that in some cells caspase-8 may additionally trigger the activation of the mitochondrial apoptotic pathway following cleavage of BID, thus resulting in the loss of mitochondrial membrane integrity. We found that treatment of BxPC-3 and A549 cells with TRAIL leads to the cleavage of Bid suggesting the activation of the mitochondrial pathway, in addition to the extrinsic pathway, in TRAIL-induced apoptosis. Both the extrinsic and intrinsic cell death pathways culminate in the cleavage of the final executioner of apoptotic protein caspase-3 and its direct downstream substrate, PARP. Our results suggest that TRAIL inhibits cell growth by inducing apoptosis in those cells harboring a wild-type allele at codon 12 in the K-ras gene. This is in agreement with a previously published study, in which the authors showed that TRAIL-induced apoptosis in A549 lung cancer cells by stimulating the cleavage of BID [26].
Our results further show that TRAIL administration failed to inhibit the growth or induce apoptosis in PanC-1 and SK-LU-1 cells, which harbor a G12D mutation. We found that the cleavage of caspase-8 and BID were modest when compared to BxPC-3 and A549 cells, whereas the cleavage of caspase 3 and PARP were barely detected. This suggests an important role of G12D mutation in conferring resistance to TRAIL-induced apoptosis in PanC-1 and SK-LU-1 cells, which also express constitutive levels of anti-apoptotic proteins. Further support for the specificity of K-ras mutation involvement in TRAIL resistance is provided by the analysis of A23187-induced cell death. A23187 nonspecifically induces cell death [27]. If K-Ras12 G12D mutations provided a general chemoresistance in cancer cells, treatment of G12D pancreatic and lung cancer cells should show a pronounced shift in IC50s; however, this was not observed (Fig. 3). This data suggests that the K-Ras12 G12D mutation confers a preferential chemoresistance to TRAIL-induced apoptosis in pancreatic and lung cancer cells. Other codon 12 K-Ras mutations have been identified and the data suggest that some mutations may confer less resistance, suggesting that even a particular single nucleotide point mutation may determine the overall strength of K-Ras activity and level of resistance to TRAIL-induced apoptosis. Future studies specifically evaluating K-Ras12 by gene knockdown and/or overexpression studies will help to determine the influence of other gene mutations (p53, p16, DPC4) on overall TRAIL resistance.
According to recent data, TRAIL is being used with several conventional chemotherapeutic drugs to induce tumor cell apoptosis because of its strong synergistic effects and potent cell killing properties and thus places it in a category of a promising candidate for cancer therapy. In our most recent study, we found that resistance to TRAIL-induced apoptosis in G12D K-Ras pancreatic cancer cells was overcome by sensitizing the cells with a low dose of benzyl isothiocyanate, a phytochemical present in cruciferous vegetables [22].
In conclusion, our studies indicate that a mutation within codon 12 of the K-Ras gene is an important event in conferring resistance to TRAIL-induced apoptosis in pancreatic and lung cancer cells. Our data suggest a clinical relevance in screening for G12D mutations in pancreatic and lung adenocarcinomas as a biomarker for resistance to TRAIL. This would provide a rationale for new therapeutic approaches of TRAIL sensitization, in combination with current chemotherapies.
Acknowledgments
This investigation was supported in part by R01 grants CA106953 and CA129038 (to S.K.S.) awarded by the National Cancer Institute. The funds from Texas Tech University Health Sciences Center, School of Pharmacy (to S.K.S.) are also acknowledged. The authors wish to thank Dr. Jayarama Gunaje, Texas Tech University Health Sciences Center for providing A549 cells.
Contributor Information
Ravi P. Sahu, Department of Biomedical Sciences and Cancer Biology Center, School of Pharmacy, Texas Tech University Health Sciences Center, Suite 1103, 1406 Coulter Drive, Amarillo, TX 79106, USA
Sanjay Batra, Department of Biomedical Sciences and Cancer Biology Center, School of Pharmacy, Texas Tech University Health Sciences Center, Suite 1103, 1406 Coulter Drive, Amarillo, TX 79106, USA.
Prabodh K. Kandala, Department of Biomedical Sciences and Cancer Biology Center, School of Pharmacy, Texas Tech University Health Sciences Center, Suite 1103, 1406 Coulter Drive, Amarillo, TX 79106, USA
Thomas L. Brown, Department of Neuroscience, Cell Biology, and Physiology, Wright State University Boonshoft School of Medicine, 3640 Colonel Glenn Highway, Dayton, OH 45435, USA
Sanjay K. Srivastava, Department of Biomedical Sciences and Cancer Biology Center, School of Pharmacy, Texas Tech University Health Sciences Center, Suite 1103, 1406 Coulter Drive, Amarillo, TX 79106, USA
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 5.Rodenhuis S, Slebos RJ, Boot AJ, Evers SG, Mooi WJ, Wagenaar SS, van Bodegom PC, Bos JL. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48(20):5738–5741. [PubMed] [Google Scholar]
- 6.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava RK. TRAIL/Apo-2L: mechanisms, clinical applications in cancer. Neoplasia. 2001;3(6):535–546. doi: 10.1038/sj.neo.7900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 10.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Update. 2004;7(2):139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Song JJ, An JY, Kwon YT, Lee YJ. Evidence for two modes of development of acquired tumor necrosis factor-related apoptosis-inducing ligand resistance. Involvement of Bcl-xL. J Biol Chem. 2007;282(1):319–328. doi: 10.1074/jbc.M608065200. [DOI] [PubMed] [Google Scholar]
- 12.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR. Curr Protoc Hum Genet. 2008;Chapter 10 doi: 10.1002/0471142905.hg1011s57. http://wwwsangeracuk/perl/genetics/CGP/cosmic?action=study&study_id=3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori S, Sugahara K, Uemura A, Akamatsu N, Tutsumi R, Kuroki T, Hirakata Y, Atogami S, Hasegawa H, Yamada Y, Kamihira S. Rapid, simple, and accurate detection of K-ras mutations from body fluids using real-time PCR and DNA melting curve analysis. Lab Medicine. 2006;37:286–289. [Google Scholar]
- 14.Moore PS, Sipos B, Orlandini S, Sorio C, Real FX, Lemoine NR, Gress T, Bassi C, Klöppel G, Kalthoff H, Ungefroren H, Löhr M, Scarpa A. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- 15.Emonds E, Fitzner B, Jaster R. The mutation status in BxPC-3 cells (Wild type Kras). Molecular determinants of the antitumor effects of trichostatin A in pancreatic cancer cells. World J Gastroenterol. 2010;16(16):1970–1978. doi: 10.3748/wjg.v16.i16.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Wang C, Dong J, Zhao G, Chen X, Zhang M. Detection of K-ras gene point mutation’s style in human pancreatic cancer cell line PANC-1 by PCR-SSP. The Chinese-German Journal of Clinical Oncology. 2006;5:46–48. [Google Scholar]
- 17.Mitchell CE, Belinsky SA, Lechner JF. Detection and quantitation of mutant K-ras codon 12 restriction fragments by capillary electrophoresis. Anal Biochem. 1995;224(1):148–153. doi: 10.1006/abio.1995.1020. [DOI] [PubMed] [Google Scholar]
- 18.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101(3):176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100(9):1425–1433. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahu RP, Zhang R, Batra S, Shi Y, Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–1753. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni K, Selesniemi K, Brown TL. Interferon-gamma sensitizes the human salivary gland cell line, HSG, to tumor necrosis factor-alpha induced activation of dual apoptotic pathways. Apoptosis. 2006;11:2205–2215. doi: 10.1007/s10495-006-0281-8. [DOI] [PubMed] [Google Scholar]
- 22.Wicker CA, Sahu RP, Kulkarni-Datar K, Srivistava SK, Brown TL. BITC sensitizes pancreatic adenocarcinomas to TRAIL-induced apoptosis. Cancer Growth Metastasis. 2009;2:45–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim SM, Ringel J, Schmidt C, Ringel B, Müller P, Koczan D, Thiesen HJ, Löhr M. Pancreatic adenocarcinoma cell lines show variable susceptibility to TRAIL-mediated cell death. Pancreas. 2001;23(1):72–79. doi: 10.1097/00006676-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 25.Corazza N, Kassahn D, Jakob S, Badmann A, Brunner T. TRAIL-induced apoptosis: between tumor therapy and immunopathology. Ann N Y Acad Sci. 2009;1171:50–58. doi: 10.1111/j.1749-6632.2009.04905.x. [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Shin JN, Woo HN, Piya S, Moon AR, Seo YW, Seol DW, Kim TH. DOBI is cleaved by caspases during TRAIL-induced apoptotic cell death. BMC Rep. 2009;42(8):511–515. doi: 10.5483/bmbrep.2009.42.8.511. [DOI] [PubMed] [Google Scholar]
- 27.Kozian D, Proulle V, Nitsche A, et al. Identification of genes involved in Ca2 + ionophore A23187-mediated apoptosis and demonstration of a high susceptibility for transcriptional repression of cell cycle genes in B lymphoblasts from a patient with Scott syndrome. BMC Genom. 2005;6:146. doi: 10.1186/1471-2164-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]