Abstract
Objective
Find the optimal continuous electroencephalographic (CEEG) monitoring duration for seizure detection in critically ill patients.
Methods
We analyzed prospective data from 665 consecutive CEEGs, including clinical factors and time-to-event emergence of electroencephalographic (EEG) findings over 72 hours. Clinical factors were selected using logistic regression. EEG risk factors were selected a priori. Clinical factors were used for baseline (pre-EEG) risk. EEG findings were used for the creation of a multistate survival model with 3 states (entry, EEG risk, and seizure). EEG risk state is defined by emergence of epileptiform patterns.
Results
The clinical variables of greatest predictive value were coma (31% had seizures; odds ratio [OR] = 1.8, p<0.01) and history of seizures, either remotely or related to acute illness (34% had seizures; OR = 3.0, p<0.001). If there were no epileptiform findings on EEG, the risk of seizures within 72 hours was between 9% (no clinical risk factors) and 36% (coma and history of seizures). If epileptiform findings developed, the seizure incidence was between 18% (no clinical risk factors) and 64% (coma and history of seizures). In the absence of epileptiform EEG abnormalities, the duration of monitoring needed for seizure risk of <5% was between 0.4 hours (for patients who are not comatose and had no prior seizure) and 16.4 hours (comatose and prior seizure).
Interpretation
The initial risk of seizures on CEEG is dependent on history of prior seizures and presence of coma. The risk of developing seizures on CEEG decays to <5% by 24 hours if no epileptiform EEG abnormalities emerge, independent of initial clinical risk factors.
Electrographic seizures occur in approximately 20% of critically ill patients undergoing continuous electroencephalogram (CEEG). Most occur without overt clinical signs and can only be reliably identified with CEEG monitoring.1 An increasing list of studies indicates that these seizures exert adverse hemodynamic and metabolic effects on the brain and are associated with unfavorable outcome.2–6 Accordingly, current guidelines from various professional societies7–12 recommend that CEEG be performed in critically ill patients with acute brain injuries and impaired mental status, or with unexplained or fluctuating encephalopathies. The first seizure recorded during CEEG can be delayed by several hours or days, so recommendations are to monitor patients for at least 24 hours if they are not comatose and 48 hours if they are comatose.
Systematic detection of electrographic seizures with CEEG requires time from electroencephalographic (EEG) technologists and clinical neurophysiologists as well as financial support from payers.13,14 This constitutes a barrier to the development of CEEG monitoring programs, especially in resource-limited settings.
Prior studies sought to identify means to reduce the burden of CEEG. A first line of studies investigated quantitative EEG analysis and time-compressed EEG displays to decrease reviewing time.15,16 Although successful and already implemented in some institutions, such approaches do not reduce the need for personnel and technical supply. A second group of studies aimed to identify a subgroup of high-risk patients on whom to focus CEEG efforts. These studies relied on clinical factors, such as a history of epilepsy, coma, and clinical seizures prior to monitoring,1,17 early EEG findings that are detected prior to electrographic seizures, or a combination of both.7,17–19 A first issue with these studies is that they did not consider all the known EEG risk factors for electrographic seizures.1,17,18,20,21 Some were performed in highly selected populations.19 Another limitation in most prior studies is the failure to account for subject dropout, which might underestimate the risk of further seizures, especially in those considered to be at low risk clinically (because they receive shorter monitoring). In practice, if epileptiform discharges are noted during CEEG, many interpreting physicians tend to continue monitoring longer. A longer duration of monitoring increases the chance of capturing a seizure. This is true even if the epileptiform discharges do not modify the risk of seizures at all—leading to false correlations and self-fulfilling prophecies. A somewhat different but related error is the overestimation of incidence of seizures at later time points. Patients with a high clinical suspicion of seizures are on CEEG longer than those with a lower risk, independent of EEG findings. This bias will increase the proportion of high-risk patients at later time points and result in an overestimation of the risk of seizures at later time points.
A principled approach to address the problem of subject dropout as described above is survival analysis. Survival analysis has been applied to this problem on 2 previous occasions.17,18 In both studies, it was shown that the risk for seizures decays more rapidly than previously suspected, giving credence to the hypothesis of overestimation of seizure risk with long monitoring. However, their analyses did not account for the emergence of EEG risk factors during monitoring and how this affected the subsequent risk of seizures.
The purpose of this study was to develop models for the time-dependent risk of electrographic seizures in critically ill patients as a function of baseline clinical risk factors and of abnormalities that may emerge during EEG monitoring. Such models provide a way to personalize the duration of EEG monitoring based on a patient’s specific baseline risk factors and EEG findings. Multistate survival analysis provides a principled framework for our analysis.22
Subjects and Methods
Subjects
Following institutional review board approval, the enrolling institutions (Yale New Haven Hospital and Erasme Hospital, Free University of Brussels) prospectively entered consecutive adult (age≥16 years) subject data into an anonymized database between October 1, 2014 and September 30, 2015. To ensure adequate seizure detection and to mitigate bias, CEEG recordings of <24-hour duration and those that were interrupted for >2 hours (consecutive or not) were excluded. Patients with postanoxic coma were excluded as well, as the clinical significance of seizures is often ambiguous in this setting and may differ from the broader intensive care unit population.23 An inclusion/exclusion flowchart is provided in Figure 1.
FIGURE 1.
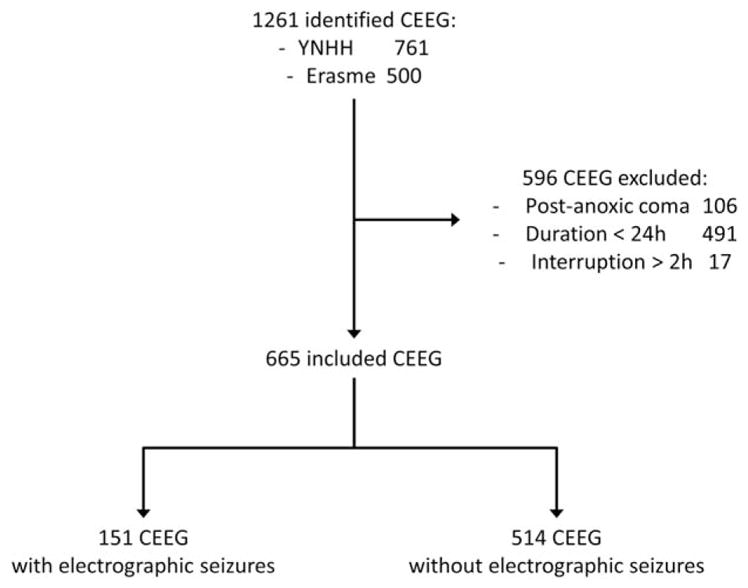
Inclusion/exclusion flow chart. CEEG = continuous electroencephalogram; YNHH = Yale New Haven Hospital.
Clinical Variables
The following binary variables were prospectively collected from medical charts and discussion with the clinical team at CEEG onset, as part of routine clinical practice: gender, presence of acute brain injury, history of remote brain injury, history of epilepsy (defined as diagnosis of epilepsy prior to the current admission), witnessed acute clinical seizures prior to CEEG (either during or immediately prior to the current admission, as reported by the treating physicians), coma (defined as lack of purposeful responses, including to noxious stimulation) at time of CEEG, and focal neurological deficit at time of CEEG. Ordinal discrete variables included age (in years) and duration of monitoring (in hours).
EEG Variables
CEEG was performed as clinically indicated using 21 electrodes placed per the 10–20 international electrode system. At both institutions, CEEGs are performed to rule out electrographic seizures in patients with partly unexplained alteration of consciousness. EEGs were reviewed and scored for this study by 1 of the authors (G.O., N.R., or N.G.) according to the American Clinical Neurophysiology Society Critical Care EEG Terminology, 24 which shows an almost perfect inter-rater reliability for the patterns used in this study.25 Prior to the study, all 3 EEG scorers first passed a standardized certification test provided by the Critical Care EEG Monitoring Research Consortium to ascertain their ability to use the terminology and to ensure adequate levels of inter-rater agreement in applying the terminology. We reported the presence and time of emergence (in minutes from onset of EEG recording) of periodic and rhythmic patterns, sporadic epileptiform discharges, brief potentially ictal rhythmic discharges (BIRDs),20 and seizures.24
Statistical Analysis
Variable selection for use in the multistate survival analysis was performed separately for EEG (time-dependent) and clinical factors (time-independent). Clinical features associated with seizures were first identified using chi-square or Fisher exact test for categorical variables and Mann–Whitney U test for ordinal variables, with an unadjusted alpha of 0.05. Multivariate logistic regression was then employed to determine the clinical risk factors independently associated with seizures that were further used for the survival analysis. The EEG factors used to define the “EEG risk state” were selected a priori based on large cohort studies showing an association of these factors with seizures. 1,20,21,26 Their association was confirmed in the study dataset using chi-square or Fisher exact test and time-dependent survival analysis to ensure time dependency did not affect the results. The EEG factors were combined into a single factor to simplify the prediction model and increase the effect size. Specifically, EEG patterns predictive of seizures were combined to create a single risk state with multiple different EEG findings qualifying as single-entry criteria.
Multistate survival analysis is an extension of standard survival analysis. The primary difference is the presence of more states. In standard survival analysis, the primary goal is to estimate the hazard rate. The hazard rate is the instantaneous chance that a subject would transition from one state to another, that is, alive to dead or healthy to ill. Where in standard survival analysis this can be defined using a single rate, in multistate survival analysis, a rate needs to be estimated for each potential transition. Typically, this is represented as a matrix, commonly called the transition matrix. The simplest of these, and the one used in this model, allows for only 3 states—in our case, entry, EEG risk, and seizure. The transitions allowed are entry to EEG risk, entry to seizure, and EEG risk to seizure. This is often referred to as an illness death model. Importantly, transitions back from EEG risk to entry or from seizure to the either of the other 2 states are not allowed, hence the death in the illness death model. This simplifies the model, because only 3 rates in the transition matrix need to be estimated (still much greater than the one). Another simplifying assumption used in this analysis is the Markov assumption. This means that the transition rate is not dependent on the prior states or on the time within any given state. Similar to standard survival analysis, factors such as gender and treatment that could modify the hazard rate can be considered. Whereas in standard survival analysis only 1 coefficient needs to be estimated per a binary factor, in multistate analysis a coefficient is needed for every transition possibility. Multistate survival analysis is attractive because it allows for excellent visualization and estimation of how an intervening intermediate state can affect the transition between 2 other states. In contrast, the primary limitation is that the number of parameters needed to be estimated rapidly increases as the complexity of the model increases. Therefore, the number of subjects needed to reliably estimate those parameters also increases nonlinearly; this is the so-called curse of dimensionality. Further reading on multistate survival analysis can be found in Putter et al 2007.22
We used 3 states for the multistate survival analysis over a time window of 72 hours. The multistate model was used to predict the overall risk of seizures, the risk of seizures after the development of electrographic seizure risk factors, and the risk of seizures as a function of EEG monitoring duration. The 3 states were entry, risk state, and seizure. Transitioning into the risk state required development of at least 1 of the findings identified as conferring increased risk of seizures. These include BIRDs, lateralized periodic discharges (LPDs), lateralized rhythmic delta activity (LRDA), bilateral independent periodic discharges (BIPDs), lateralized rhythmic spike-and-wave (LSW), and sporadic epileptiform discharges. Transitions were allowed only in one direction in the so-called illness death model.27 In this case, “illness” represents development of EEG risk factors and seizure represents the absorbing “death” state. A 72-hour time window from the start of the recording was utilized to minimize subject dropout and informative censoring.
The survival analysis was performed using 2 clinical covariates to define 4 risk categories. These covariates were history of seizure (including both remote and acute) and coma, all found to be independent clinical risk factors (see Results). These covariates were used for calculation of unique hazard function for each state transition using stratified Markov methodology, meaning there was no assumption of proportionality of hazard functions for the different clinical covariates. Multistate survival analysis was performed with bootstrap resampling over 500 trials to calculate 95% confidence bands. Multistate survival analysis was performed with the mstate package in R (R Foundation).22,27 Other statistical analysis and modeling was performed with MATLAB 2016a (MathWorks, Natick, MA).
Results
Demographics and Clinical Risk Factors
A total of 1,261 subjects were identified, and 665 were included. Reasons for exclusion are detailed in Figure 1. Electrographic seizures ultimately occurred in 151 of 665 (23%) subjects. Demographics and clinical risk factors of electrographic seizures are presented in Table 1. In brief, prior clinical seizures (history of epilepsy or acute clinical seizures) and coma were both found to be independent clinical risk factors for seizures. Both history of epilepsy and acute clinical seizures were significant predictors of seizures and were combined into a single risk factor group to maintain statistical power for survival analysis. The use of antiepileptic drugs at CEEG onset was also associated with electrographic seizures. It was, however, strongly associated, and considered to be a surrogate of, a history of epilepsy (53 of 60 [88%] vs 278 of 605 [46%]; odds ratio [OR] = 8.9, 95% confidence interval [CI] = 4.0–20, p<0.001) and the presence of acute clinical seizures (200 of 246 [81%] vs 131 of 419 [31%]; OR = 9.6, 95% CI = 6.5–14.0; p<0.001), and was not included as a risk factor per se. Four subgroups of patients were defined based on 2 clinical risk factors and showed an increasing risk of electrographic seizures: no prior seizure/no coma (40 of 313 [13%]), coma/no prior seizure (19 of 85 [22%]), prior seizure/no coma (71 of 223 [30%]), and prior seizure and coma (21 of 44 [48%]).
TABLE 1.
Demographics and Clinical Risk Factors of Electrographic Sz
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Whole Cohort, n = 665 | EEG Sz, n = 151 | No EEG Sz, n = 514 | p | OR [95% CI] | p | OR [95% CI] | |
| Center, YNHH | 290 (44%) | 72 (48%) | 218 (42%) | 0.264 | 1.2 [0.9–1.8] | ||
|
| |||||||
| Duration, h | 44 [27–63] | 67 [48–102] | 39 [25–48] | <0.001 | N/A | ||
|
| |||||||
| Age, yr | 63 [52–76] | 65 [52–76] | 63 [52–77] | 0.887 | N/A | ||
|
| |||||||
| Gender, female | 336 (51%) | 81 (54%) | 255 (50%) | 0.406 | 1.2 [0.8–1.7] | ||
|
| |||||||
| Acute brain injury | 399 (60%) | 87 (58%) | 312 (61%) | 0.509 | 0.9 [0.6–1.3] | ||
|
| |||||||
| Remote brain injury | 93 (14%) | 32 (21%) | 61 (12%) | 0.005 | 2.0 [1.2–3.2] | 0.294 | 1.3 [0.8–2.2] |
|
| |||||||
| History of Sz | 267 (40%) | 92 (61%) | 175 (34%) | <0.001 | 3.0 [1.7–5.1] | <0.001 | 3.1 [2.1–4.6] |
|
| |||||||
| History of epilepsy | 60 (9%) | 26 (17%) | 34 (7%) | ||||
|
| |||||||
| Acute clinical Sz | 246 (37%) | 83 (55%) | 163 (32%) | ||||
|
| |||||||
| Coma | 129 (19%) | 40 (26%) | 89 (17%) | 0.014 | 1.7 [1.1–2.6] | 0.002 | 2.1 [1.3–3.2] |
|
| |||||||
| Focal neurological deficit | 265 (40%) | 72 (48%) | 193 (38%) | 0.03 | 1.5 [1.1–2.2] | 0.090 | 1.6 [0.9–2.3] |
|
| |||||||
| Admitted to an ICU | 569 (85%) | 125 (83%) | 444 (86%) | 0.29 | 0.8 [1.5–1.2] | ||
|
| |||||||
| AEDs at CEEG onset | 331 (50%) | 107 (71%) | 224 (44%) | <0.001 | 3.1 [2.1–4.7] | ||
Data are presented as No. (%) or median [interquartile range]. Multivariate logistic regression model included the following variables: remote brain injury, history of Sz, coma, and focal neurological deficit. Multivariate model statistics: −2 log likelihood = 661.7; goodness-of-fit chisquare = 50.7; df = 4; p<0.001. Acute brain injury included intracerebral hemorrhage (n = 78), subarachnoid hemorrhage (n = 70), ischemic stroke (n = 52), traumatic brain injury (n = 51), subdural hematoma (n = 33), CNS infection (n = 32), CNS inflammatory disorder (n = 10), recent neurosurgery (n = 13), and posterior reversible encephalopathy syndrome (n = 6); other etiologies included CNS neoplasm (n = 54), toxic–metabolic encephalopathy (n = 103), sepsis (n = 58), seizure disorder (n = 92), and others (n = 13).
AED = antiepileptic drug; CEEG = continuous electroencephalogram; CI = confidence interval; CNS = central nervous system; EEG = electroencephalographic; ICU = intensive care unit; N/A = not applicable; OR = odds ratio; Sz = seizures; YNHH = Yale New Haven Hospital.
EEG Risk Factors
LPDs, LRDA, and BIRDs were all associated with electrographic seizures (Table 2). Sporadic epileptiform discharges, BIPDs, and LSW showed a trend towards an association but were not statistically significant. In the case of BIPDs and LSW, the low prevalence of findings decreased the power of detecting a statistical difference. Of note, 61 of 151 (40%) electrographic seizures occurred in patients with no periodic, rhythmic, or epileptiform discharges. The presence of LPDs, LSW, LRDA, BIRDs, BIPDs, or sporadic epileptiform discharges define the EEG risk state for survival analysis based on the univariate analysis of this study and previous studies, demonstrating an association of these EEG factors and seizures (see Subjects and Methods). The combined EEG risk state featured an OR for seizures of 2.5 (95% CI = 1.8–3.7, p<0.001).
TABLE 2.
Electrographic Risk Factors of Electrographic Sz
| Univariate | |||||
|---|---|---|---|---|---|
| Factor | Whole cohort, n = 665 | EEG Sz, n = 151 | No EEG Sz, n = 514 | p | OR [95% CI] |
| LPDs | 90 (14%) | 41 (27%) | 49 (10%) | <0.001 | 3.6 [2.2–5.9] |
| BIPDs | 6 (1%) | 2 (1%) | 4 (1%) | 0.326 | 2.1 [0.4–11.2] |
| GPDs | 66 (10%) | 7 (5%) | 59 (11%) | 0.113 | 0.5 [0.2–1.2] |
| LRDA | 49 (7%) | 17 (11%) | 32 (6%) | 0.049 | 1.9 [1.03–3.55] |
| GRDA | 44 (7%) | 4 (3%) | 40 (8%) | 0.140 | 0.43 [0.2–1.2] |
| LSW | 2 (0%) | 1 (1%) | 1 (0%) | 0.348 | 4.3 [0.3–69.1] |
| SED | 133 (20%) | 35 (23%) | 98 (19%) | 0.100 | 1.5 [0.9–2.5] |
| BIRDs | 9 (1%) | 7 (5%) | 2 (0%) | <0.001 | 14.9 [3.0–73.6] |
| Combined LPD, BIPD, LRDA, LSW, SED, and/or BIRDs | 250 (38%) | 83 (55%) | 167 (32%) | <0.001 | 2.5 [1.8–3.7] |
| No discharge | 321 (48%) | 61 (40%) | 260 (51%) | Reference | |
Data are presented as No. (%).
BIPD = bilateral independent periodic discharge; BIRD = brief potentially ictal rhythmic discharge; CI = confidence interval; EEG = electroencephalographic; GPD = generalized periodic discharge; GRDA = generalized rhythmic delta activity; LPD = lateralized periodic discharge; LRDA = lateralized rhythmic delta activity; LSW = lateralized spike-and-wave discharge; OR = odds ratio; SED = sporadic epileptiform discharge; Sz = seizures.
Individual CEEG timelines are summarized in the swimmer plot in Figure 2. Median observed time of seizure emergence (time to first seizure) was 44 minutes (interquartile range = 1–332 minutes, range = 0–4,380 minutes), and median observed time of EEG risk pattern (time to first appearance of any epileptiform pattern) was 4 minutes (interquartile range = 0–103 minutes, range = 0–5,230 minutes).
FIGURE 2.
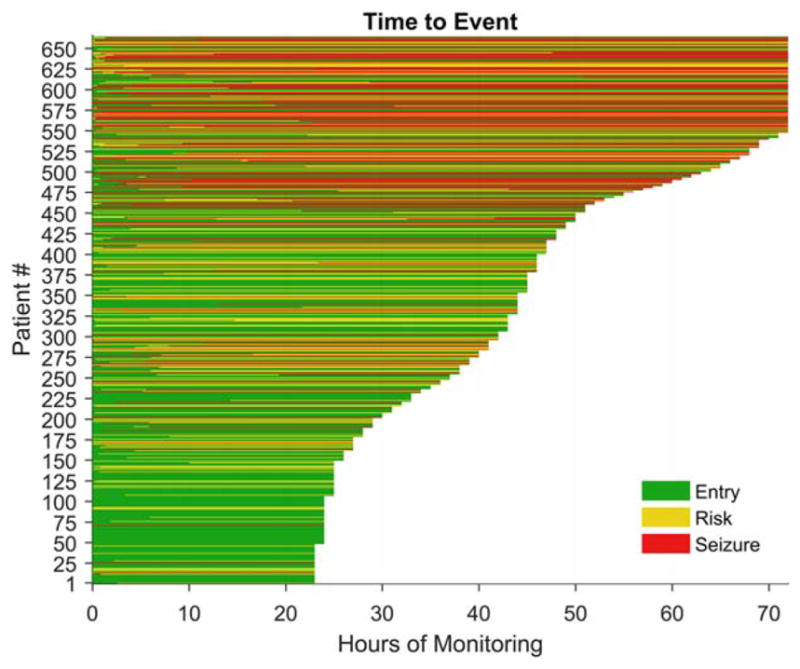
Swimmer plot showing the raw data with a line for each subject. The length of the line is the duration of monitoring, and the color reflects the state (entry, risk, or seizure). The choice of a 72-hour time window was chosen to minimize the number of censored subjects balanced against the clinical utility of the model. [Color figure can be viewed atwileyonlinelibrary.com]
Multistate Survival Analysis
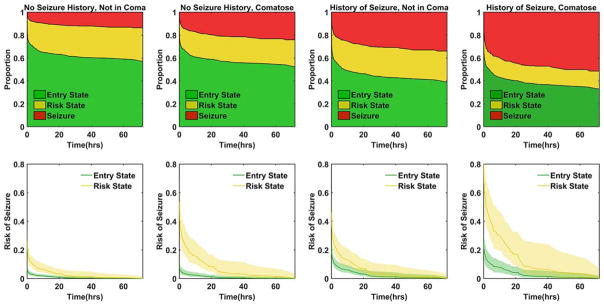
The calculated proportion of subjects in each state during the 72-hour time window from the survival analysis model is presented for each clinical group in Figure 3 (upper panels). The proportion of subjects in each state at selected time points is found in Table 3.
FIGURE 3.
Results of the multistate survival analysis. The upper panels show the estimated proportion of subjects in each state (entry, risk, and seizure) based on the respective clinical risk factors of coma and history of seizures. The lower panels show the remaining risk of developing seizures in the 72-hour period for the entry and risk states respective to their clinical risk factors. The shaded area represents the bootstrapped 95% confidence interval. [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Multistate Survival Analysis: Estimated Incidence of Sz and Remaining Chance of Sz
| Remaining Probability of Sz | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Clinical Factors | State | 1 Hour | 6 Hours | 12 Hours | 24 Hours | Hours to < 5% Sz | Overall Probability of Sz | |
| No coma & No prior Sz, | Entry | 0.04 (0.03–0.06) | 0.02 (0.01–0.03) | 0.02 (0.01–0.03) | 0.01 (0.00–0.02) | 0.4 | Sz from entry state | 0.09 (0.06–0.12) |
| n = 312 [101]a | EEG risk | 0.15 (0.10–0.20) | 0.08 (0.05–0.12) | 0.06 (0.04–0.09) | 0.02 (0.01–0.06) | 14.4 | Sz from risk state | 0.18 (0.12–0.24) |
| Enter risk state | 0.33 (0.29–0.40) | |||||||
|
| ||||||||
| No coma & prior Sz, | Entry | 0.12 (0.10–0.17) | 0.07 (0.05–0.11) | 0.05 (0.04–0.09) | 0.02 (0.01–0.05) | 12.7 | Sz from entry state | 0.30 (0.23–0.37) |
| n = 223 [91]a | EEG risk | 0.28 (0.21–0.37) | 0.15 (0.10–0.24) | 0.12 (0.08–0.19) | 0.04 (0.02–0.12) | 21.7 | Sz from risk state | 0.35 (0.25–0.48) |
| Enter risk state | 0.40 (0.21–0.50) | |||||||
|
| ||||||||
| Comatose & no prior Sz, | Entry | 0.05 (0.03–0.08) | 0.03 (0.01–0.05) | 0.02 (0.01–0.04) | 0.01 (0.00–0.02) | 1.2 | Sz from entry state | 0.13 (0.08–0.17) |
| n = 85 [34]a | EEG risk | 0.33 (0.23–0.47) | 0.18 (0.11–0.29) | 0.14 (0.09–0.24) | 0.05 (0.02–0.14) | 16.7 | Sz from risk state | 0.39 (0.28–0.52) |
| Enter risk state | 0.36 (0.26–0.48) | |||||||
|
| ||||||||
| Comatose & prior Sz, | Entry | 0.16 (0.10–0.24) | 0.09 (0.05–0.14) | 0.07 (0.04–0.12) | 0.02 (0.01–0.07) | 16.4 | Sz from entry state | 0.36 (0.24–0.50) |
| n = 44 [19]a | EEG risk | 0.57 (0.41–0.74) | 0.34 (0.22–0.52) | 0.26 (0.17–0.43) | 0.10 (0.04–0.28) | 44.2 | Sz from risk state | 0.64 (0.48–0.86) |
| Enter risk state | 0.42 (0.29–0.58) | |||||||
Data are presented numbers in parentheses representing 95% confidence intervals from bootstrap resampling. EEG risk state in this context refers to subjects who develop the EEG findings of lateralized periodic discharges, bilateral periodic discharges, lateralized rhythmic discharges, lateralized spike wave, sporadic epileptiform discharges, and/or brief ictal rhythmic discharges.
Number of subjects in each category in brackets is the number who developed an EEG risk factor.
EEG = electroencephalographic; Sz = seizures.
In further analysis, we examined the decaying risk of seizure versus duration of monitoring and how this risk is affected by the development of EEG findings, as depicted in Figure 3 (lower panels). The risk of seizures decays quickly if EEG risk factors do not develop and if there is an absence of clinical risk factors. Numeric data for select time points are found in Table 3, including the duration of monitoring needed for the risk of seizures in the subsequent 72 hours to fall below 5% for the clinical risk states and whether EEG risk factors develop. This table can be used as a quick clinical reference. If the patient is not comatose and has no history of seizures, a routine EEG of at least 30 minutes is sufficient to place them in <5% seizure risk for the next 72 hours. Patients either with a history of seizure or in coma need longer monitoring than a routine EEG regardless of whether an EEG abnormality is present. If any EEG risk factor develops, the patient needs at least 15 to 44 hours, dependent on clinical risk factors.
EEG risk factors are more influential in comatose patients regardless of whether they have a history of seizures, as evidenced by the greater width between the seizure decay plots (see Fig 3, lower panels) in the comatose versus noncomatose subjects.
Discussion
We investigated the combined value of clinical and time-dependent EEG risk factors for the stratification of risk of electrographic seizures in critically ill patients. We used multistate survival analysis to adjust for differing durations of EEG monitoring on a prospective cohort of 665 patients undergoing CEEG. Univariate and multivariate analysis of non-EEG factors found history of seizure and coma were the most relevant predictors. In terms of EEG factors, we used results from prior investigations, in part confirmed in this study, which found that sporadic epileptiform discharges, LPDs, LSW, LRDA, BIRDs, and BIPDs are predictive of seizures. These EEG factors were combined into a single risk state for the purpose of survival analysis. We found that the yield of CEEG for detecting new development of electrographic seizures declines quickly if clinical and EEG risk factors are not present; even with these factors, the yield decays exponentially if the EEG remains negative for these epileptiform patterns.
Compared to prior survival analysis, there was a similar incidence of seizures of 23% overall. This incidence is slightly higher than in large case series of CEEG in critically ill patients,1 likely because we excluded CEEGs that were stopped before 24 hours.
The projected risk of seizure without epileptiform discharges fell to <5% within 2 hours in a prior study,17 compared to between 4% and 16% at 1 hour in this study, depending on the presence of clinical risk factors. Compared to this study, ours included a more comprehensive set of EEG risk factors and excluded patients with a duration of monitoring <24 hours, thus mitigating the bias of self-fulfilling prophecy due to early dropout. Our study accounts for pre-EEG clinical risk factors to stratify the risk and importantly utilized the time dependence of the emergence of EEG factors to modify the risk during the course of the EEG. Our study also used a larger database. In terms of clinical and electrographic risk factors for seizures, the results of the current study are similar to a larger multi-institutional cohort of >4,000 subjects.26 The mixture of underlying etiologies was also similar to prior CEEG cohorts.1,18,26
Although multistate survival is an improvement over previous methodologies, it has limitations. First is the selection of subjects. This cohort of patients is from 2 tertiary care hospitals with wide use of CEEG. These results may not hold true in a hospital with a different mixture of pathologies and differing use of CEEG. Nonetheless, the demographics, including prevalence of coma and prior seizures, and the rate of electrographic seizure in this cohort are similar to many prior publications. 1,6,19,28–34 A second major limitation is inherent to survival analysis methods. Survival analysis accounts for the effects of subject dropout, but if the subjects who drop out are systematically different from the subjects who stay in, informative censorship can bias the resulting model. To limit the impact of this potential problem, we limited the monitoring sessions to 72 hours, similar to previous studies on this subject.17,18 Third, despite the size of our cohort, we were unable to stratify EEG risk beyond a single state. Different EEG patterns portend a variable risk of seizures, which is largest for BIRDs and LPDs and smallest for sporadic epileptiform discharges. 1,20,21,26 Further studies, necessarily of larger size, should attempt to refine this initial model by stratifying the EEG risk state to provide more precise prediction for each category of EEG pattern. Accounting for other features of abnormal EEG patterns may be important as well, such as prevalence (eg, occasional vs frequent vs abundant) or frequency. 26 Coma was defined as a lack of purposeful response to noxious stimulation. This differs from previous studies that have defined coma on the basis of the clinical scores, mainly the Glasgow Coma Scale (GCS). We believe that our definition, which is adapted from seminal texts in the field,35,36 is both simple and practical, and circumvents most of the known shortcomings of the GCS, in particular the limitations of the verbal assessment in intubated or aphasic patients.37 Finally, we combined history of epilepsy and acute clinical seizures into a single clinical variable. With more subjects, it should be feasible to incorporate their respective values independently, that is, by creating more clinical subgroups.
This study uses a state-of-the-art statistical method to approach a practical yet pernicious question at the core of CEEG in critically ill patients. The results of this study provide practitioners a simple quantitative rubric for the probability that a patient will have a seizure during the next 72 hours of CEEG monitoring with only 4 pieces of data: has the patient had seizures previously, is the patient comatose, how long has the patient been on CEEG monitoring, and are there any EEG risk factors during CEEG so far.
Despite the limitations acknowledged above, this approach can potentially translate into a substantial increase in cost-effectiveness of CEEG in critically patients, a procedure associated with substantial burden and cost. This study only addresses the use of CEEG for seizure detection; other indications of CEEG, such as postcardiac arrest prognosis and ischemia monitoring, require different monitoring durations.
Conclusions
The risk of seizures during CEEG is higher in patients with a history of seizures and coma. The risk of developing seizures decays rapidly if no epileptiform EEG patterns (LPDs, BIRDs, LRDA, BIPDs, and sporadic epileptiform discharges) or seizures emerge. The duration of CEEG monitoring could be tailored to each patient’s risk factors based on a combination of clinical and electrographic risk factors to match CEEG monitoring to that patient’s risk profile. Broadly speaking, to obtain a <5% risk of seizure, patients can be divided into 3 categories: (1) patient is not comatose, no history of seizure, and no EEG risk factors: 24 minutes of EEG will suffice; (2) patient either has a history of seizure, is comatose, or has an EEG risk factor: 24 hours of monitoring is indicated; and (3) patient has both a history of seizure and is comatose with an EEG risk factor: at least 48 hours of monitoring is reasonable.
The ability to do so moves the field of CEEG monitoring closer to the ideal of “precision medicine,” and can contribute to efforts to optimize the use of CEEG resources. These findings should be validated in prospective studies.
Footnotes
Author Contributions
A.F.S. and G.O. share first authorship. The following authors were involved in study concept and design: A.F.S., G.O., S.B., M.B.W., L.H., N.G.; data acquisition and analysis: A.F.S., G.O., N.R., B.L., M.B.W., N.G.; drafting the manuscript and figures: all authors.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 2.Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579–590. doi: 10.1002/ana.24606. [DOI] [PubMed] [Google Scholar]
- 3.Struck AF, Westover MB, Hall LT, et al. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit Care. 2016;24:324–331. doi: 10.1007/s12028-016-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137(pt 5):1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–260. doi: 10.1212/WNL.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, chnical specifications, and clinical practice. J Clin Neurophysiol. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Clinical Neurophysiology Society. Guideline twelve: guidelines for long-term monitoring for epilepsy. J Clin Neurophysiol. 2008;25:170–180. doi: 10.1097/WNP.0b013e318175d472. [DOI] [PubMed] [Google Scholar]
- 10.Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–1351. doi: 10.1007/s00134-013-2938-4. [DOI] [PubMed] [Google Scholar]
- 11.Andre-Obadia N, Sauleau P, Cheliout-Heraut F, et al. French guidelines on electroencephalogram [in French] Neurophysiol Clin. 2014;44:515–612. doi: 10.1016/j.neucli.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(suppl 2):S1–S26. doi: 10.1007/s12028-014-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ney JP, van der Goes DN, Nuwer MR, et al. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology. 2013;81:2002–2008. doi: 10.1212/01.wnl.0000436948.93399.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abend NS, Topjian AA, Gutierrez-Colina AM, et al. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care. 2011;15:70–75. doi: 10.1007/s12028-010-9380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moura LM, Shafi MM, Ng M, et al. Spectrogram screening of adult EEGs is sensitive and efficient. Neurology. 2014;83:56–64. doi: 10.1212/WNL.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haider HA, Esteller R, Hahn CD, et al. Sensitivity of quantitative EEG for seizure identification in the intensive care unit. Neurology. 2016;87:935–944. doi: 10.1212/WNL.0000000000003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015;126:463–471. doi: 10.1016/j.clinph.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafi MM, Westover MB, Cole AJ, et al. Absence of early epileptiform abnormalities predicts lack of seizures on continuous EEG. Neurology. 2012;79:1796–1801. doi: 10.1212/WNL.0b013e3182703fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo JY, Rampal N, Petroff OA, et al. Brief potentially ictal rhythmic discharges in critically ill adults. JAMA Neurol. 2014;71:454–462. doi: 10.1001/jamaneurol.2013.6238. [DOI] [PubMed] [Google Scholar]
- 21.Gaspard N, Manganas L, Rampal N, et al. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:1288–1295. doi: 10.1001/jamaneurol.2013.3475. [DOI] [PubMed] [Google Scholar]
- 22.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 23.Hofmeijer J, Tjepkema-Cloostermans MC, Blans MJ, et al. Unstandardized treatment of electroencephalographic status epilepticus does not improve outcome of comatose patients after cardiac arrest. Front Neurol. 2014;5:39. doi: 10.3389/fneur.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 25.Gaspard N, Hirsch LJ, LaRoche SM, et al. Interrater agreement for critical care EEG terminology. Epilepsia. 2014;55:1366–1373. doi: 10.1111/epi.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181–188. doi: 10.1001/jamaneurol.2016.4990. [DOI] [PubMed] [Google Scholar]
- 27.Putter H. [Accessed February 28, 2016];Tutorial in biostatistics: competing risks and multi-state models: analyses using the mstate package. R. 2016 Available at: https://cran.r-project.org/web/packages/mstate/vignettes/Tutorial.pdf.
- 28.Jordan KG. Nonconvulsive status epilepticus in acute brain injury. J Clin Neurophysiol. 1999;16:332–340. doi: 10.1097/00004691-199907000-00005. discussion 353. [DOI] [PubMed] [Google Scholar]
- 29.Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155–166. doi: 10.1016/0920-1211(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 30.DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–840. doi: 10.1111/j.1528-1157.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 31.Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 32.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 33.Carrera E, Claassen J, Oddo M, et al. Continuous electroencephalographic monitoring in critically ill patients with central nervous system infections. Arch Neurol. 2008;65:1612–1618. doi: 10.1001/archneur.65.12.1612. [DOI] [PubMed] [Google Scholar]
- 34.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 35.Fisher CM. The neruological examination of the comatose patient. Acta Neurol Scand. 1969;45(suppl 36):1–56. doi: 10.1111/j.1600-0404.1969.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 36.Posner JB, Plum F. Plum and Posner’s diagnosis of stupor and coma. 4. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 37.Kornbluth J, Bhardwaj A. Evaluation of coma: a critical appraisal of popular scoring systems. Neurocrit Care. 2011;14:134–143. doi: 10.1007/s12028-010-9409-3. [DOI] [PubMed] [Google Scholar]