Abstract
Palatable food is known for its ability to enhance reinforcing responses. Studies have suggested a circadian variation in both drug and natural reinforcement, with each following its own time course. The goal of this study was to determine the role of the MT1 and MT2 melatonin receptors in palatable snack food-induced reinforcement, as measured by the conditioned place preference (CPP) paradigm during the light and dark phases. C3H/HeN wild-type mice were trained for snack food-induced CPP at either ZT 6 – 8 (ZT: Zeitgeber time; ZT 0 = lights on), when endogenous melatonin levels are low, or ZT 19 – 21, when melatonin levels are high. These time points also correspond to the high and low points for expression of the circadian gene Period1, respectively. The amount of snack food (chow, Cheetos®, Froot Loops® and Oreos®) consumed was of similar magnitude at both times, however only C3H/HeN mice conditioned to snack food at ZT 6–8 developed a place preference. C3H/HeN mice with a genetic deletion of either the MT1 (MT1KO) or MT2 (MT2KO) receptor tested at ZT 6 – 8 did not develop a place preference for snack food. Although the MT2KO mice showed a similar amount of snack food consumed when compared to wild-type mice, the MT1KO mice consumed significantly less than either genotype. We conclude that in our mouse model snack food-induced conditioned place preference is dependent on time of day and the presence of the MT1 or MT2 receptors, suggesting a role for melatonin and its receptors in snack food-induced reinforcement.
Keywords: melatonin, melatonin receptors, C3H/HeN mouse, conditioned place preference, MT1 or MT2 knockout mice
1. Introduction
Obesity is a growing health problem within the United States, with over 30% of adults displaying an obese phenotype [1] characterized by a body mass index greater than 30 [2]. Studies have suggested a potential role for “food addiction” as a component of obesity [3, 4]. This push toward classifying certain types of excessive food intake as “food addiction” is supported by studies demonstrating food, particularly palatable foods, interact with the brain in a similar manner to drugs of abuse [2, 5–7].
A reinforcer is a stimulus, which increases the probability of an associated behavior occurring again. Food is considered to be a rewarding/reinforcing substance [8, 9], as evidenced by the fact that mice and rats will work to obtain food in an operant conditioning paradigm and food-paired with a distinct contextual cues will result in the formation of a place preference. The rewarding/reinforcing properties of food can be altered by taste, texture, and/or palatability [10]. For humans one type of food considered to have elevated reinforcing properties are “snack foods,” which refers to a subset of commercially produced highly palatable foods [11]. Using the definition of “snack foods” proposed by Hess et al., 2016, these foods are “energy-dense, nutrient-poor foods high in sodium, sugar, and/or fat” [12]. Ingestion of these foods has markedly increased [13, 14] and is suggested to be a contributing factor in the elevated incidence of obesity [11, 15]. The brain reward pathways play an important role in food intake behavior, particularly the dopamine system and μ opioid system [2]. Within the reward pathway food and food-related cues activate numerous areas including the striatum and ventral tegmental area, as well as other structures [16–18]. In contrast food and food-related cues decrease activation of the lateral and medial habenula [18].
Recent studies have demonstrated that while both natural reinforcement and drug reinforcement display a diurnal variation, they exhibit different times of maximum and minimum sensitivity [19]. This suggests the reward pathway may be preferentially primed for natural vs. drug reinforcement at various times, which in turn suggests susceptibility to regulation by a circadian hormone and/or molecule. The circadian molecule, melatonin, is released from the pineal gland with elevated levels at night and the low levels during the day [20]. Melatonin exerts its effects through action at two G protein-coupled receptors, termed MT1 and MT2 [21]. Studies in our laboratory have linked deletion of either the MT1 (MT1KO) or MT2 (MT2KO) melatonin receptor to a complete abrogation of methamphetamine-induced reinforcement, as quantified by the conditioned place preference paradigm (CPP) [22]. Accordingly, the melatonin receptors are located throughout the brain including areas of the reward pathway such as the nucleus accumbens [23], ventral tegmental area [23], substantia nigra reticulate [24], hippocampus [25–27], and habenula [28]. Furthermore, suppression of melatonin has been linked to obesity and metabolic syndrome [29], suggesting melatonin may be prime candidate for modulating food intake and reinforcement.
The goal of this study was first to assess the potential diurnal variation in snack food-induced CPP and the role of the melatonin receptors (MT1 and MT2) in this behavior. The CPP paradigm works as a model of classical conditioning by pairing a reinforcing stimulus with a previously neutral set of environmental cues, in the form of wall color and floor texture. Following multiple pairings, the reinforcing properties of the stimulus are transferred to the previously neutral environmental cues resulting in increased time spent in the compartment where the reinforcer was provided [30]. This paradigm has frequently been used to assess the reinforcing properties of drugs of abuse [19, 22, 30] as well as natural reinforcement such as food [11, 31, 32].
Here, we demonstrate snack food-induced place preference in C3H/HeN mice was dependent on time of day and on the presence of the MT1 and MT2 melatonin receptors. Snack food induced a statistically significant place preference in wild-type mice when provided during the light period (ZT 6 – 8), however this preference was abrogated during the dark period (ZT 19 – 21), when melatonin levels are elevated. Furthermore, deletion of either melatonin receptor (MT1 or MT2) resulted in a complete abrogation of place preference during the light phase.
2. Materials and Methods
2.1 Animals and Husbandry
C3H/HeN (104 males) mice were bred and maintained in the Laboratory Animal Facility at the University at Buffalo. Wild-type C3H/HeN mice and C3H/HeN mice homozygous for receptor deletions of the MT1 melatonin receptor (MT1KO) or MT2 melatonin receptor (MT2KO) were generated in our former laboratory at Northwestern University by backcrossing C57Bl/6J MT1KO mice (donated by Dr. Steven Reppert; Massachusetts General Hospital, Boston, MA, USA) and C3H/HeN MT2KO mice (donated by Dr. Steven Reppert; Massachusetts General Hospital, Boston, MA, USA) [33–35] with C3H/HeN mice (Harlan/Envigo, Indianapolis, IN, USA) as previously described [22, 36, 37].
Mice were maintained in humidity and temperature-controlled (22±1°C) rooms with food (Harlan Teklad 2018sx) and water provided ad libitum prior to the onset of the experimental protocol. All animal procedures were approved by the University at Buffalo Institutional Animal Care and Use Committee and adhered to the National Institutes of Health guidelines.
Male mice were group-housed (3 – 5 per cage) at weaning and maintained in a 14h: 10h light/dark cycle, with 150 to 200 lux light illumination at the level of the cage. Animals were housed in standard polycarbonate cages (30 x 19 cm) with corncob bedding. To maintain consistency with previous studies in our laboratory examining methamphetamine-induced place preference mice were switched to a 12h: 12h light dark cycle within ventilated, light-tight cabinets at 4 – 7 weeks of age and 10 – 14 days prior to experiment onset.
2.2 Food Preparation
Food was chosen based on previous papers detailing the reinforcing effects through the CPP paradigm [11]. Wild-type mice were randomly assigned to three groups: “chow,” “snack,” or “no food.” The “chow” group received chow only (Harlan Teklad 2018sx); the “snack” group received a mixture of equal parts chow, Oreos ®, Cheetos ® and Froot Loops ®, and the “no food” group did not receive any food during the conditioning session. Foods for the snack food group were chosen based on their ability to induce place preference in the literature [11, 32, 38]. The nutritional characteristics of the foods used are described in Table 1. Based on the results obtained from the wild-type mice at ZT 6 – 8, all subsequent testing (wild-type mice at ZT 19 – 21, MT1KO mice at ZT 6 – 8, and MT2KO mice at ZT 6 – 8) were only divided into the “snack” and “no food” groups.
Table 1.
Nutritional comparison of food-types used
| Food Type | kcal/g | Carbohydrate | Fat | Protein | Added Sugar | Sodium |
|---|---|---|---|---|---|---|
| Chow (2018sx) | 3.1 | 44.2% | 13.2% | 18.6% | - | 0.2% |
| Froot Loops | 3.8 | 85.7% | 4.8% | 4.8% | 33.3% | 0.5% |
| Cheetos | 5.7 | 53.6% | 35.7% | 7.1% | - | 0.9% |
| Oreos | 4.7 | 73.5% | 20.6% | 2.9% | 41.2% | 0.5% |
2.3 Video Tracking System and Apparatus
Mouse location and distance traveled in the CPP apparatus were monitored by TopScan (CleverSys Inc, Reston, VA, USA) video tracking software as previously described [22]. Briefly 8 testing chambers were monitored via top-view imaging provided by four Sony video cameras. Chambers were placed upon a light panel with the ability to emit white light (daytime studies: ZT 6 – 8) or infrared light (nighttime studies: ZT 19 – 21). Each chamber consisted of two choice compartments measuring 15 x 15 x 25 cm with distinct contextual cues (wall color and floor texture) and one neutral central compartment measuring 10 x 15 x 25 cm. Compartments were separated via guillotine door.
2.4 Experimental Design
Experiments were conducted at two time points representing the peak and trough of melatonin production in wild-type C3H/HeN mice (Figure 1) [39]. In MT1KO and MT2KO experiments were only conducted during the light phase, as no diurnal variation in preference was observed in previous studies examining methamphetamine CPP in these mice. Daytime experiments, representing the trough of melatonin production, were performed from Zeitgeber time (ZT) 6 – 8 (ZT 0 = lights on). Nighttime experiments, representing the peak of melatonin production, were conducted from ZT 19 – 21 (Figure 1). For all experiments mice were moved into the testing room one hour prior to test onset. A new cohort of mice was used for each experiment and treatment group.
Figure 1. Experimental Design.
A: Experiments for wild-type mice were conducted at two time points during the light/dark cycle, ZT 6 – 8 during the light phase and ZT 19 – 21 during the dark phase. These two time points correspond to the lowest and highest levels of melatonin, respectively [39]. Experiments for MT1KO and MT2KO mice were conducted only at ZT 6 – 8.
B – D: For each experiment a new cohort of mice was used. All mice were subjected to a general experimental time line consisting of three days of handling prior to the onset of the experiment. Day 1 (Habituation) and Day 2 (Pre-CPP) consisted of free access to the entire test chamber for 20 min in order to eliminate novelty. Time spent in each choice compartment during the Pre-CPP was used to determine the initial compartment preference. Days 3 – 8 consisted of 60 min daily conditioning sessions during which mice were confined to one choice compartment. On Days 3, 5, and 7 mice were confined to their initially least-preferred compartment and received either no food, chow, or snack based on their group assignment. On Days 4, 6, and 8 mice were confined to their initially most-preferred compartment and received no food. Day 9 (Post-CPP) consisted of free access to the entire test chamber for a 20 min test session to determine final compartment preference.
One week prior to experiment onset mice were individually housed, with food and water provided ad libitum. Mice were weighed daily, for 7 days; this was averaged together to establish the initial baseline weight. Mice were handled and received enough food (chow, Oreos®, Cheetos ® and Froot Loops ®) to maintain 90% free feeding weight, during the three days prior to the initiation of the experimental protocol (Figure 1). This was done in order to acclimate mice to the experimental food.
The CPP paradigm consisted of nine days of testing (Figure 1). Day 1 and Day 2 consisted of a 20-min habituation and Pre-CPP test respectively. Habituation was used to eliminate chamber novelty, while Pre-CPP was used to establish initial compartment bias. The conditioning period took place from Day 3 to Day 8. Mice were confined to one compartment for each 60-min daily conditioning session. Mice were confined to their initially least preferred compartment on Days 3, 5, and 7, as determined by the Pre-CPP (Day 2) and received chow, snack (chow, Oreos®, Cheetos®, and Froot Loops®), or no food. Mice were confined to their initially most preferred compartment on Days 4, 6, and 8 and received no food. The amount of food consumed during the test was measured on Days 3, 5, and 7. Mice were provided with an appropriate amount of chow to maintain 90% free-feeding weight in their home cages after lights-off.
Day 9 consisted of the 20 min Post-CPP. From this session the place preference score was calculated by subtracting the Post-CPP time spent in the initially preferred compartment from the Post-CPP time spent in the opposite compartment, where food was presented in the chow and snack groups.
Mice were excluded for spending greater than 80% (960s) of the test in any given compartment during the Pre-CPP (Day 2) or for excessive jumping out of test chambers during conditioning. Of the total 64 C3H/HeN wild-type mice tested at both ZT 6 – 8 and ZT 19 – 21, 4 were excluded based on these criteria. Similarly, 4 of 16 MT1KO mice and 8 of 24 MT2KO were also excluded.
2.5 Statistical Analysis
2.5.1 CPP
Post-CPP data for the whole 20-min post-test were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests for the wild-type mice at ZT 6 – 8. Student t-test was used to compare knockout mice data at ZT 6 – 8 and wild-type data at ZT 19 – 21. Analyses were conducted using GraphPad Prism v. 6.01 (GraphPad Software Inc., LaJolla, CA). For all analyses p <0.05 was considered statistically significant.
2.5.2 Food Consumed
Food consumed (g) during the conditioning phase was measured for Days 3, 5, and 7. Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests for wild-type mice at ZT 19 – 21 as well as MT1KO and MT2KO mice at ZT 6 – 8. For the wild-type mice at ZT 6 – 8 data were analyzed by two-way repeated measures ANOVA, with the within subjects factors being day and the between subjects factor being treatment. This was followed by Bonferroni post-hoc tests.
3. Results
Assessments of place preference were systematically conducted between ZT 6 – 8 and ZT 19 – 21, corresponding to the peak and trough of melatonin respectively [39]. Additionally these time points correspond to trough (ZT 6 – 8) and peak (ZT 19 – 21) levels of food intake in the C3H/HeN mice [40].
3.1 Conditioned Place Preference
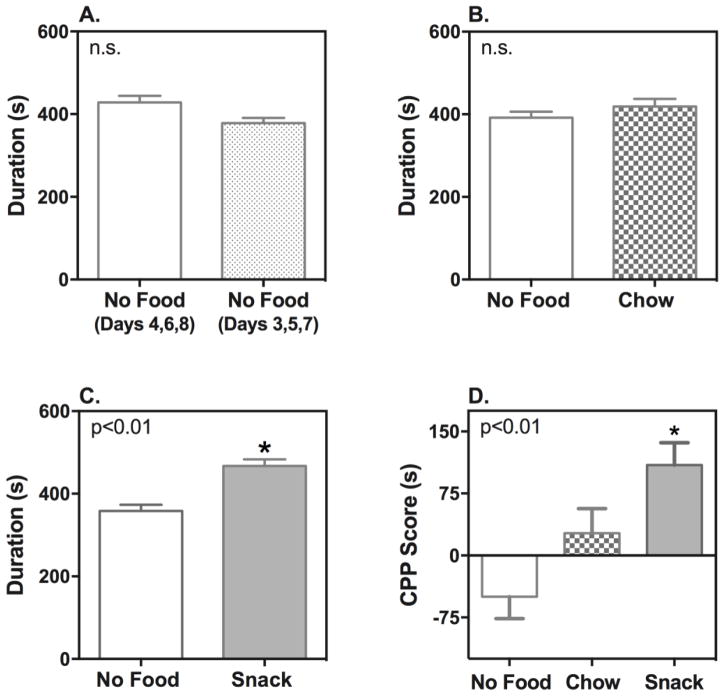
Food-induced CPP was first examined during the light phase (ZT 6 – 8) when melatonin levels [39] and food consumption for the C3H mice are at their lowest point [40]. During the light phase wild-type mice receiving no food during the conditioning phase did not display any difference in time spent in the two choice compartments during the 20-min Post-CPP test session (Figure 2A; n = 14). Similarly wild-type mice receiving only chow during the conditioning phase spent approximately equal time in both choice compartments during the Post-CPP test session (Figure 2B; n = 14). Conversely, wild-type mice receiving snack food during the conditioning phase displayed a significant increase in time spent in the food-paired compartment compared to the compartment paired with no food (Figure 2C; n = 13, p<0.01). Place preference scores were calculated by subtracting time spent in the initially most preferred compartment (no food presented) from time spent in the initially least preferred compartment (food presented in the chow and snack groups) during the Post-CPP test. Mice provided the snack food mixture displayed a strong preference score compared to the no food group (Figure 2D; F [2, 38] = 8.066, p<0.01).
Figure 2. Effect of Chow or “Snack” on Food-Induced Place Preference During the Light (ZT 6 – 8) Phase.
Time spent in each compartment during Post-CPP was measured across the whole 20-minute test session for the wild-type mice following treatment with no food (A: n = 14), chow (B: n = 14) or snack food (C: n = 13). CPP scores were calculated by subtracting time spent in the initially preferred compartment (no food presented) from time spent in the initially non-preferred compartment (food presented in chow and snack groups) (D). Data represent mean ± S.E.M. of time (s) spent in each compartment or as mean ± S.E.M of CPP score (s). Compartment duration was compared using Student t-test. *p<0.05 when compared to other compartment. CPP scores were compared using one-way ANOVA, with a main effect of treatment indicated by the p value in the upper left corner. *p<0.05 when compared to no food group (Bonferoni post-test). s: second; n.s.: non-significant.
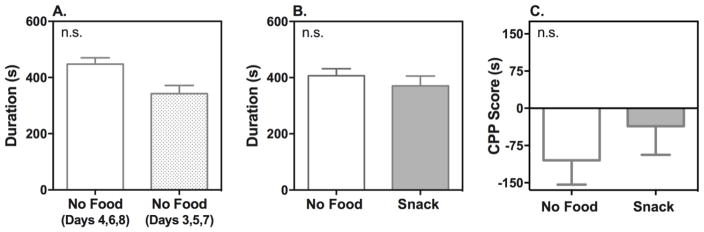
Snack food-induced CPP during the dark phase (ZT 19 – 21), when melatonin levels [39] and food consumption for the C3H mice are at their peak [40], was subsequently examined. Wild-type mice receiving no food during the conditioning phase displayed no significant differences in time spent in either compartment (Figure 3A; n = 10). Similarly, mice receiving snack food during the conditioning phase spent approximately the same amount of time in the compartment paired with snack food as the compartment paired with no food (Figure 3B; n = 9). Preference scores for both groups were not significantly different from each other, suggesting a preference for the snack food was not formed (Figure 3C).
Figure 3. Effect of Snack Food-Induced Place Preference During the Dark (ZT 19 – 21) Phase.
Time spent in each compartment during Post-CPP was measured across the whole 20-minute test session for the wild-type mice following treatment with no food (A: n = 10), or snack food (B: n = 9). CPP scores were calculated by subtracting time spent in the initially preferred compartment (no food presented) from time spent in the initially non-preferred compartment (food presented in snack group) (C). Data represent mean ± S.E.M. of time (s) spent in each compartment or as mean ± S.E.M of CPP score (s). Compartment duration and CPP scores were compared using Student t-test. s: second; n.s.: non-significant.
Next the role of the MT1 and MT2 melatonin receptors in food-induced place preference was assessed. Place preference for MT1KO and MT2KO mice was assessed at ZT 6 – 8, as no diurnal variation in preference was determined in previous studies involving these mice [22]. No differences in the time spent in either compartment were observed for the MT1KO mice provided no food (Figure 4A; n = 5) or snack food (Figure 4B; n = 7) when examined over the whole 20-min test session. This pattern was similar to that observed in MT2KO mice, as mice provided with no food (Figure 4D; n = 8) or snack food (Figure 4E; n = 8) did not show any statistically significant differences in time spent in either compartment. Preference scores for MT1KO and MT2KO mice (Figures 4C and F) showed no differences between the no food and snack food groups.
Figure 4. Effect of MT1 or MT2 Receptor Deletion on Snack Food-Induced Place Preference During the Light (ZT 6 – 8) Phase.
Time spent in each compartment during Post-CPP was measured across the whole 20-minute test session for the MT1KO mice following treatment with no food (A: n = 5) or snack food (B: n = 7). CPP scores were calculated by subtracting time spent in the initially preferred compartment from time spent in the initially non-preferred compartment (C). The same comparisons were performed for the MT2KO mice including compartment duration for the no food group (D: n = 8) and snack food group (E: n = 8). CPP scores were also calculated for MT2KO mice (F). Data represent mean ± S.E.M. of time (s) spent in each compartment or as mean ± S.E.M of CPP score (s). Compartment duration and CPP scores were compared using Student t-test. *p<0.05, when compared to other compartment or other treatment group. s: second; n.s.: non-significant.
3.2 Food Consumed
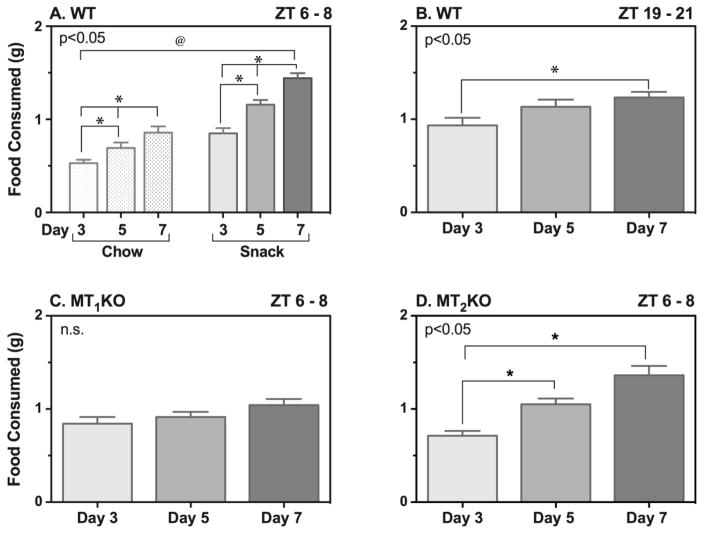
The food consumed throughout conditioning was assessed by within-group comparisons between subsequent days of food presentation. Wild-type mice provided with chow or snack food during the light phase, significantly increased the amount of food consumed during testing from Day 3 to Day 5 and Day 5 to Day 7 (Figure 5A: F [2, 52] = 75.06, n = 14, p<0.05). Interestingly mice provided with snack food consumed more food on each day of conditioning than mice only provided with chow (Figure 5A: F [1, 26] = 51.25, n = 14, p<0.05). During the dark phase wild-type mice increased snack food intake on Day 7 vs. Day 3 (Figure 5B: p<0.05). In contrast to wild-type mice, MT1KO mice failed to display any change in food intake from Day 3 to Day 7 (Figure 5C). MT2KO mice however displayed a similar patter of escalation in food intake as observed in the wild-type during the light phase, with a significant increase occurring on Day 5 vs. Day 3 and Day 7 vs. Day 5 (Figure 5D, p<0.05).
Figure 5. Food Consumed During Conditioning Days During the Light (ZT 6–8) and Dark (19–21) Phases.
Food consumed was measured for the chow and snack food treated groups during each conditioning day on which food was presented (Days 3, 5, and 7). Wild-type mice tested at ZT 6 – 8 were divided into a chow group receiving chow only (Harlan Teklad 2018sx) and a snack group receiving a mixture of chow, Oreos, Cheetos, and Froot Loops. Wild-type mice tested at ZT 19 – 21 as well as MT1KO and MT2KO mice tested at ZT 6 – 8 received the snack mixture. Effect of treatment was compared using two-way ANOVA for the wild-type mice at ZT 6 – 8 (A). One-way ANOVA was used to compare effect of treatment for wild-type mice at ZT 19 – 21 (B) as well as the MT1KO (C) and MT2KO (D) mice at ZT 6 – 8. Bars represent the mean ± S.E.M. of food consumed in grams. *p<0.05 when comparing food consumed between treatment days; @p<0.05 when comparing between treatment groups
4. Discussion
Melatonin receptors and time of day play an important role in modulating the reinforcing properties of food, specifically palatable snack food. Here we demonstrated that the maximal effect of food-induced CPP was observed during the light phase (ZT 6 – 8), but not during the dark phase (ZT 19 – 21), potentially through an MT1 or MT2 dependent effect. These findings suggest a role for a circadian component, likely an oscillator or signaling molecule, in food-induced reinforcement. Prospective candidates for such a role could include melatonin, its receptors, and/or clock genes.
This study represents the first demonstration of snack food-induced place preference in C3H/HeN mice, though this strain has previously been used to examine the reinforcing properties of several drugs of abuse [22, 41, 42]. Wild-type mice developed a significant place preference for snack food during the light phase (ZT 6 – 8), but not the dark phase (ZT 19 – 21). Interestingly this corresponds to the time periods associated with the trough and peak of melatonin [39]. Furthermore, this is similar to what has been observed in studies examining place preference for cocaine [41, 43] and methamphetamine [22], suggesting a similar mechanism may be governing the diurnal variation in drug and natural reinforcement.
One potential mechanism underlying the observance of a place preference at ZT 6 – 8 and not at ZT 19 – 21, would be the difference in the presence of a reward prediction error. Reward prediction errors are thought to support new learning by strengthening the association between a reinforcer and the cues preceding its presentation [44]. Unexpected or unpredicted reinforcers generate a positive prediction error, while an expected or predicted reinforcer does not generate a prediction error. As our mice only display a place preference during their inactive phase, when food would normally not be consumed, it is possible the presentation of food, particularly snack food, at this time results in the generation of a positive prediction error thus strengthening the association between the presence of the reinforcer and the compartment in which it was presented. In contrast, presentation of snack food during the active phase of the mouse, when food would normally be consumed, would likely either not result in a reward prediction error or would result in one to a lesser degree, thus not supporting the formation of a place preference.
The diurnal variation observed in the wild-type mice in our studies is likely not caused by an alteration in the amount of food consumed at the two time points examined. Although mice at ZT 6 – 8 provided with chow consumed significantly less food than mice receiving snack food at either time of day, there were no differences in snack food consumption between the two times of day. Indeed mice displayed similar consummatory behavior during both time periods tested.
The melatonin receptors play an important role in snack food-induced CPP, with deletion of either receptor at ZT 6 – 8 resulting in an abrogation of place preference. For the MT1KO mice this lack of preference may be partially accounted for by the difference in the amount of food consumed during the conditioning. MT1KO mice consumed significantly less snack food throughout conditioning than the wild-type mice, suggesting this food may not have had the same positive reinforcement for this genotype. In contrast, there were no differences observed for the amount of snack food consumed between the wild-type and MT2KO mice. During conditioning the increase in snack food intake with each subsequent exposure is similar to that observed in a sensitized response. Wild-type mice and MT2KO mice show such an elevation, with significantly more food consumed on Day 7 compared to Day 3. Interestingly, previous studies have demonstrated an abrogation of methamphetamine sensitization in mice lacking the MT1 receptor [45]. This is in accordance with the MT1KO mice being the only genotype to not display an increase in food intake throughout the conditioning phase. Furthermore, deletion of the MT1 receptor has been associated with anhedonia, as evidenced by decreased performance in a sucrose consumption test [46] and depressive-like behavior, as evidenced by a significant increase in immobility in the forced swim test [47]. Overall these data suggest a potentially altered reward pathway associated with deletion of the MT1 receptor.
Recent studies in vivo have demonstrated the formation of MT1/MT2 heterodimers, which signal through a different pathway than the MT1 or MT2 homodimers [48]. If these heterodimers represent the necessary component behind place preference expression in the wild-type mice, deletion of either receptor would disrupt heterodimer formation and thus could account for the lack of CPP observed in the MT1KO and MT2KO. Although the exact contributions of melatonin receptor heterodimers vs. homodimers in the modulation of reinforcing behaviors have yet to be elucidated, this represents an important area of future study.
Several studies have tied CPP for food, specifically snack foods, to the opioid system with the opioid receptor antagonist naltrexone dose-dependently inhibiting place preference [11, 49, 50]. Melatonin has been tied to the opioid system through studies examining morphine CPP. In particular one study showed systemic or intracerebral administration of melatonin in mice abrogates the expression of morphine-induced CPP in a dose dependent manner [51]. Therefore, it is possible the abrogation of place preference for food observed at night could be attributed to melatonin’s action within the opioid system. Furthermore, this study showed that melatonin’s ability to abrogate morphine-induced CPP was reversed through treatment with luzindole or the selective MT2 antagonist K185 [51]. Together these results suggest that melatonin abrogates the formation of a place preference for morphine through the MT2 receptor [51]. The importance of the MT2 receptor in the formation of place preference in this model could potentially implicate this receptor as an important contributor for our food-induced place preference as well.
The suppression of CPP observed during the dark phase could be attributed to the functional effects of Period 1 (PER1), a circadian protein, which is elevated during the light phase peaking around ZT 5 and reaches its lowest levels during the dark phase between ZT 17 – 21 [52]. Studies have shown palatable foods, such as chocolate, are able to induce expression of PER1 in corticolimbic structures [53]. This is similar to what is observed following acute methamphetamine treatment [54]. This is of interest as PER1 expression has been shown to be essential for the expression of CPP to addicting substances such as cocaine [43]. Furthermore, altered PER1 phosphorylation results in an altered pattern of food intake and an obese phenotype in mice [55]. The lack of preference observed in our studies corresponds to the trough in PER1 rhythmicity. Interestingly melatonin has been shown to down-regulate PER1 through the MT1 receptor [56]. Therefore, it is possible our MT1KO mice have an altered pattern of PER1 expression compared to the wild-type mice. In fact Per1 rhythmicity is completely abolished in the suprachaismatic nucleus of these mice, leading to a continuous low level of PER1 that in turn likely abrogates place preference [57].
Another circadian gene, which could be contributing to the abrogation in preference, is Clock. Deletion of this gene has been associated with an increase in place preference for cocaine [58]. Interestingly deletion of the MT2 melatonin receptor results in altered Clock gene expression in the suprachiasmatic nucleus [57]. This altered expression in the MT2 KO mice could change the period of sensitivity for reinforcing substances in these mice, thus resulting in the abrogation of preference observed at the time points we examined. Further studies should determine the alterations of rhythmic Per1 and Clock expression within areas of the reward pathway.
5. Conclusions
This study demonstrated a diurnal variation in place preference for snack food in C3H/HeN mice, with snack food reinforcement observed during the light phase but not the dark phase. The lack of food preference at night in the wild-type mice could potentially result from the action of melatonin within the opioid system or through regulation of clock gene expression, particularly PER1. Furthermore, this study demonstrated for the first time that the MT1 and MT2 melatonin receptors are necessary for the expression of snack food-induced CPP in the C3H/HeN mice during the light phase. Together these results suggest a link between melatonin and its receptors as regulators of reinforcing properties of natural stimuli. Therefore, further examination of this system may help elucidate mechanisms by which food, particularly snack food, induces reinforcement in humans.
Highlights.
Palatable snack food but not chow induced CPP in C3H/HeN wild-type mice during the light period.
CPP for palatable snack food was abrogated during the dark phase in wild-type mice, indicating a diurnal variation in wild-type mice.
MT1 and MT2 melatonin receptors are necessary for palatable snack food to induce CPP, as deletion of these receptors blocked CPP formation.
Acknowledgments
We want to acknowledge the technical assistance of Iwona Stepien and Michele Sveinsson.
Funding Sources
This work was supported by USPHS [grant number DA021870] to MLD and funds from the University at Buffalo.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Author’s contributions
All authors contributed to the conception and design, acquisition, and/or analysis and/or interpretation of data (SJC, RLH, MLD). SJC conducted all the in vivo experiments, analyzed and interpreted the data in consultation with MLD and all authors. RLH built the chambers and tested the equipment as necessary. SJC drafted the manuscript, which was edited by MLD, and subsequently by all authors. The final version of the manuscript was approved before submission by all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69(4):664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potenza MN. Obesity, food, and addiction: emerging neuroscience and clinical and public health implications. Neuropsychopharmacology. 2014;39(1):249–50. doi: 10.1038/npp.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gearhardt AN, Boswell RG, White MA. The association of “food addiction” with disordered eating and body mass index. Eat Behav. 2014;15(3):427–33. doi: 10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23(3):39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12(11):638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 8.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97(5):551–60. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 10.Berridge KC. 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97(5):537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarosz PA, Sekhon P, Coscina DV. Effect of opioid antagonism on conditioned place preferences to snack foods. Pharmacol Biochem Behav. 2006;83(2):257–64. doi: 10.1016/j.pbb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Hess JM, Jonnalagadda SS, Slavin JL. What Is a Snack, Why Do We Snack, and How Can We Choose Better Snacks? A Review of the Definitions of Snacking, Motivations to Snack, Contributions to Dietary Intake, and Recommendations for Improvement. Adv Nutr. 2016;7(3):466–75. doi: 10.3945/an.115.009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zizza C, Siega-Riz AM, Popkin BM. Significant increase in young adults' snacking between 1977–1978 and 1994–1996 represents a cause for concern! Prev Med. 2001;32(4):303–10. doi: 10.1006/pmed.2000.0817. [DOI] [PubMed] [Google Scholar]
- 14.Jahns L, Siega-Riz AM, Popkin BM. The increasing prevalence of snacking among US children from 1977 to 1996. J Pediatr. 2001;138(4):493–8. doi: 10.1067/mpd.2001.112162. [DOI] [PubMed] [Google Scholar]
- 15.Juul F, Hemmingsson E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr FirstView. 2015:1–12. doi: 10.1017/S1368980015000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144(1):344–55. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 17.Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav. 2008;93(3):637–50. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 1998;805(1–2):169–80. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- 19.Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009;24(6):465–76. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- 20.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79(1–3):C153–8. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 21.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–80. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clough SJ, Hutchinson AJ, Hudson RL, Dubocovich ML. Genetic deletion of the MT1 or MT2 melatonin receptors abrogates methamphetamine-induced reward in C3H/HeN mice. Physiol Behav. 2014;132:79–86. doi: 10.1016/j.physbeh.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, Pandey GN, Manev H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136(1–2):45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, Rivara S, Bedini A, Angeloni D, Fraschini F, Mor M, Tarzia G, Descarries L, Gobbi G. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31(50):18439–52. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Muller-Spahn F, Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J Pineal Res. 2005;38(1):10–6. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 26.Savaskan E, Olivieri G, Meier F, Brydon L, Jockers R, Ravid R, Wirz-Justice A, Muller-Spahn F. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer's disease patients. J Pineal Res. 2002;32(1):59–62. doi: 10.1034/j.1600-079x.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22(9):2231–7. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamah-Biassi EB, Zhang Y, Jung H, Vissapragada S, Miller RJ, Dubocovich ML. Distribution of MT1 melatonin receptor promoter-driven RFP expression in the brains of BAC C3H/HeN transgenic mice. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2014;62(1):70–84. doi: 10.1369/0022155413507453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiter RJ, Tan DX, Korkmaz A, Ma S. Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Annals of Medicine. 2011 doi: 10.3109/07853890.2011.586365. [DOI] [PubMed] [Google Scholar]
- 30.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56(6):613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 31.Imaizumi M, Takeda M, Suzuki A, Sawano S, Fushiki T. Preference for high-fat food in mice: fried potatoes compared with boiled potatoes. Appetite. 2001;36(3):237–8. doi: 10.1006/appe.2001.0399. [DOI] [PubMed] [Google Scholar]
- 32.Levy A, Salamon A, Tucci M, Limebeer CL, Parker LA, Leri F. Co-sensitivity to the incentive properties of palatable food and cocaine in rats; implications for co-morbid addictions. Addiction Biology. 2013;18(5):763–73. doi: 10.1111/j.1369-1600.2011.00433.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19(1):91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 34.Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39(2):113–20. doi: 10.1111/j.1600-079X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 35.Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR. Targeted Disruption of the Mouse Mel1b Melatonin Receptor. Molecular and Cellular Biology. 2003;23(3):1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumaya IC, Masana MI, Dubocovich ML. The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res. 2005;39(2):170–7. doi: 10.1111/j.1600-079X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson AJ, Hudson RL, Dubocovich ML. Genetic deletion of MT(1) and MT(2) melatonin receptors differentially abrogates the development and expression of methamphetamine-induced locomotor sensitization during the day and the night in C3H/HeN mice. J Pineal Res. 2012;53(4):399–409. doi: 10.1111/j.1600-079X.2012.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarosz PA, Kessler JT, Sekhon P, Coscina DV. Conditioned place preferences (CPPs) to high-caloric, snack foods in rat strains genetically prone vs. resistant to diet-induced obesity: Resistance to naltrexone blockade. Pharmacology Biochemistry and Behavior. 2007;86(4):699–704. doi: 10.1016/j.pbb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Masana MI, Benloucif S, Dubocovich ML. Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse. J Pineal Res. 2000;28(3):185–92. doi: 10.1034/j.1600-079x.2001.280309.x. [DOI] [PubMed] [Google Scholar]
- 40.Adamah-Biassi EB, Stepien I, Hudson RL, Dubocovich ML. Automated video analysis system reveals distinct diurnal behaviors in C57BL/6 and C3H/HeN mice. Behav Brain Res. 2013;243:306–12. doi: 10.1016/j.bbr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489(3):203–5. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM. Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behavioral and brain functions : BBF. 2011;7(1):29. doi: 10.1186/1744-9081-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99(13):9026–30. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nature Neuroscience. 2013;16:966. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchinson AJ, Ma J, Liu J, Hudson RL, Dubocovich ML. Role of MT1 melatonin receptors in methamphetamine-induced locomotor sensitization in C57BL/6 mice. Psychopharmacology (Berl) 2014;231(1):257–67. doi: 10.1007/s00213-013-3228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, Gobbi G. Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int J Neuropsychopharmacol. 2015;18(3) doi: 10.1093/ijnp/pyu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68(6):425–9. doi: 10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Baba K, Benleulmi-Chaachoua A, Journe AS, Kamal M, Guillaume JL, Dussaud S, Gbahou F, Yettou K, Liu C, Contreras-Alcantara S, Jockers R, Tosini G. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013;6(296):ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delamater AR, Sclafani A, Bodnar RJ. Pharmacology of sucrose-reinforced place-preference conditioning: effects of naltrexone. Pharmacol Biochem Behav. 2000;65(4):697–704. doi: 10.1016/s0091-3057(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 50.Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. International Journal of Obesity (Lond) 2009;33(Suppl 2):S54–8. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- 51.Han J, Xu Y, Yu CX, Shen J, Wei YM. Melatonin reverses the expression of morphine-induced conditioned place preference through its receptors within central nervous system in mice. Eur J Pharmacol. 2008;594(1–3):125–31. doi: 10.1016/j.ejphar.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 52.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28(12):2117–23. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 53.Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C. Expectancy for food or expectancy for chocolate reveals timing systems for metabolism and reward. Neuroscience. 2008;155(1):297–307. doi: 10.1016/j.neuroscience.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59(4):894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Huang M, Wu X, Shi G, Xing L, Dong Z, Qu Z, Yan J, Yang L, Panda S, Xu Y. PER1 phosphorylation specifies feeding rhythm in mice. Cell Rep. 2014;7(5):1509–20. doi: 10.1016/j.celrep.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 56.Imbesi M, Arslan AD, Yildiz S, Sharma R, Gavin D, Tun N, Manev H, Uz T. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. J Pineal Res. 2009;46(1):87–94. doi: 10.1111/j.1600-079X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 57.von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann N Y Acad Sci. 2005;1040(1):508–11. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- 58.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102(26):9377–81. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]