Abstract
Introduction
Pre-clinical testing of hemiarthroplasty devices requires that the tribological conditions present in vivo with live cartilage be closely duplicated. A current limitation in the tribological testing of live cartilage involves the use of cell-culture media as lubricant.
Study Aim
to develop and test a new hyaluronan-phospholipid based medium (HA–phospholipid medium) that combines the rheological and frictional properties of synovial fluid with the nourishing properties of culture media to keep cells alive.
Materials and Methods
The HA–phospholipid medium consisted of culture medium with added phospholipid dipalmitoylphosphatidylcholine (0.3 mg/mL), and hyaluronic acid (2.42 mg/mL). A standard cell culture medium was used as the control. The rheology of each medium was determined using a flat plate configuration. Bovine calf cartilage was used to assess cell viability and friction in each medium. For friction measurements, a cobalt-chrome alloy ball was articulated against cartilage disks immersed in medium.
Results
Lipid vesicles 0.1 to 50 μm in diameter were identified in the HA–phospholipid medium. Cartilage cell viability was significantly higher in the HA–phospholipid medium (62% ± 8%, 95% CI) than in control medium (49.5% ± 5%) (p = 0.009). The HA–phospholipid medium exhibited strong shear-thinning behavior, similar to synovial fluid, with viscosities ~100-fold higher at 10 s−1 and 5-fold higher at 20,000 s−1 than the approximately Newtonian control medium. The HA–phospholipid medium also yielded 20% lower friction values than the control medium after one hour of testing.
Conclusions
The rheological and friction results indicate that the HA–phospholipid medium is superior to the control cell culture medium in emulating the shear thinning and lubricative properties of natural synovial fluid, making it more clinically relevant for in vitro wear and friction testing with live cartilage.
Keywords: cartilage, synovial fluid, lubrication, phospholipids, hyaluronic acid
1. Introduction
The prevalence of arthritis increases with age, reaching an estimated 55.8% in men and 68.7% in women ages 65 and older in the USA. [1]. A dramatic increase in the demand for orthopedic care is projected as the baby-boomer generation approaches retirement age [2]. Severe cartilage deterioration, as seen in the later stages of osteoarthritis, can require surgical interventions such as hemiarthroplasty or total joint replacement. Hemiarthroplasty is attractive because it preserves the healthy half of the joint, with only the diseased half being replaced by an implant. As such, it has been applied to hip, knee, shoulder, elbow, and finger joints [3–7]. Hemiarthroplasty results in the articulation of an implant material, in most cases cobalt–chromium–molybdenum (CoCrMo) alloy, against healthy cartilage. For proper pre-clinical testing of hemiarthroplasty devices, it is therefore necessary to replicate the precise tribological and biological conditions that are present when articulating against live cartilage.
A current limitation in the tribological testing of live cartilage involves the use of cell-culture medium as lubricant [7,8]. The composition of this medium is standardized and therefore wear markers can be easily quantified using a wide range of assays and analysis methods. In addition, cell-culture medium is able to nourish the tissue and keep it alive throughout testing [10]. Despite these positive characteristics, the rheological properties of cell-culture medium make it a poor substitute for the joints natural lubricant, synovial fluid (SF). SF provides a nearly frictionless interface for diathrodial joint motion due to its viscosity and lubricating constituents [11,12]. The main lubricating components of SF are high molecular weight hyaluronic acid (HA), superficial zone protein (SZP), and surface-active phospholipids [13]. These components have been shown to accumulate within the joint interface, providing surface boundary lubrication that helps support load throughout start-up and steady joint motion [14,15]. Previous work in the Tribology Laboratory at Rush University Medical Center has looked to SF as a potential lubricant for in vitro studies. Compared with control cell culture medium, cell viability dropped by 50%, and it was concluded that the lack of nutrition present in SF causes cell death [16]. In contrast, SF outperformed cell culture medium tribologically: when articulating against a cobalt–chromium hip ball, the arithmetic mean surface roughness (Ra) of the cartilage surface increased by 7% in SF, but by 25% in the control medium. Clearly, the viscous and lubricious properties of SF are vital for the tribological function of diathrodial joints; however, its lack of cellular nutrition makes it a poor candidate for use in in vitro live tissue studies.
It was therefore our goal to identify a more suitable lubricant for our studies with live cartilage tissue in the bioreactor. Conducting a literature review, we found that researchers at the Institut National des Sciences Appliquées de Lyon (INSA-Lyon) have developed a hyaluronan–phospholipid solution, which contained the phospholipids in form of large spherical vesicles (liposomes) [17,18], similar to those identified in synovial fluid [19]. These vesicles work in concert [20] with the previously described lipid multilayer on opposing sides of the articular cartilage [21]. Phospholipid vesicles can be several micrometers in diameter and are distributed across the articulating surface. Using surface force apparatus, it has been shown that phospholipid vesicles are more resilient against normal and shear loads and more effective in reducing friction compared with lipid bilayers [22]. In the aforementioned study, which was performed within the group of Klein et al., friction coefficients as small as 0.0001 were observed at pressures that reached as high as 18 MPa. We therefore asked the question, whether a phospholipid vesicle containing cell culture medium would improve the tribological properties over pure cell culture medium, while keeping the cells alive.
In synovial fluid, hyaluronic acid (HA) and phospholipids work in concert for ideal lubrication properties. It has been proposed that together with HA this forms a gel-like structure with proteins in the synovial fluid, and the lipid multilayer provides the fluid with better lubricating properties, helping to reduce friction within the joint [23]. Recently, using atomic force microscopy (AFM), Raj et al. [24] determined that hyaluronic acid and phospholipid vesicles adsorbed onto silica act synergistically to produce multilayer structures that yield a very low friction coefficient (<0.01). We also observed in our laboratory that the addition of HA can mitigate the activity of reactive oxygen species (ROS). ROS can cause death of chondrocytes when present in concentrations above 0.9 mM [25]. The palliation by HA is attributable to the redox capability of the molecule, which can give up and/or grab two electrons per base unit [26]. We hypothesized that a cell culture medium with added phospholipid vesicles and HA would lead to improved cell viability and lower friction than the culture medium alone for tribological testing of cartilage.
2. Materials and Methods
2.1. Study Overview
The HA–phospholipid medium, whose preparation we describe in detail, was characterized with a view to using it for tribological testing of live articular cartilage and to test our hypothesis that such a medium would lead to improved cell viability and lower friction than using culture medium alone. As such, the HA–phospholipid medium was characterized with respect to (1) its ability to maintain cell viability in live cartilage; (2) phospholipid liposome formation, determined by fluorescence microscopy; (3) its rheological properties for shear strain rates in the range of 10–20,000 s−1 using a plate rheometer; and (4) the friction that results when it is used as a lubricant for metal sliding against cartilage in a ball-on-flat configuration. A standard cell culture medium that we have used to perform tribological testing of live cartilage was used as a control and point of comparison throughout and is referred to as the “control medium”.
2.2. The Media
2.2.1. The Control Medium
This is the standard medium used in the Tribology Laboratory at Rush University because we have found that it provides nourishment for the articular cartilage tissue throughout culture and mechanical testing [7].
The materials for the preparation of the control medium are listed in Table 1. The medium is composed of Ham’s F-12, a nutrient mixture, and Dulbecco’s Modified Eagle’s Medium (DMEM); a solution containing glucose, amino acids, salts, and vitamins. Both of these components serve to promote cell viability throughout culture. Mini-ITS+, a solution containing insulin, was added to maintain the cartilage metabolic activity without introducing an unknown concentration of growth factors, as is the case when using bovine serum in the culture medium. In addition, a combination of antibiotics and antimycotics were used in order to prevent bacterial and fungal contamination. These included gentamicin, and an antibiotic–antimycotic mixture containing penicillin, streptomycin, and amphotericin B. To form the medium, all components were combined and run through a 0.22 μm sterile vacuum filter, then stored at ~1.6°C prior to use.
Table 1.
Materials for the preparation of the control medium.
|
|
|
|
|
|
|
|
|
|
2.2.2. The HA–Phospholipid Medium
The preparation and characterization of phospholipids for the use in media are detailed in the doctoral thesis of A.M Trunfio-Sfargiu [16] and related publications [28–30]. This work provided the basis for the HA–phospholipid medium used herein, which contains three major components: (1) the standard culture (control) medium described above, which provides nourishment for the tissue; (2) dipalmitoylphosphatidylcholine (DPPC); and (3) HA. The three components that make up this medium work in conjunction to not only keep the cartilage tissue alive and healthy, but to reduce friction and wear upon articulation. The DPPC should allow us to create liposomes within the cell culture medium, a characteristic that needed to be investigated since the formation of lipid vesicles is specific to the medium properties [31]. Hyaluronic acid enhances the viscosity and boundary-lubricating properties of cartilage surfaces either individually or in combination with other SF constituents [32,33]. Recently, a synergistic action between HA and phosphocholine liposomes has been discovered [24,34]. The materials used to prepare the HA–phospholipid medium are listed in Table 2.
Table 2.
Materials for the preparation of Phospholipid Medium.
| Hyaluronic acid sodium salt from Streptococcus equi |
|
| 16:0 PC (DPPC) 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
|
| Ethyl Alcohol 200 Proof |
|
| Chloroform |
|
| 14:0-06:0 NBD PC Fluorescent Marker |
|
Thirty mg (0.3 mg/mL) of DPPC were added to 100 mL of a solvent consisting of 90% chloroform and 10% ethanol, followed immediately with the addition of 15 μL of the fluorescent marker 1-myristoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (14:0-06:0 NBD PC, Cat. No. 810122, Avanti Polar Lipids, Inc., Alabaster, AL, USA). The concentration of DPPC was based on previous work in the laboratories of Hills [21] and LaBerge [35]. The solvent was slowly removed at 47°C by rotary evaporation under a gentle nitrogen flow. This slow process allowed an even, thin lipid film to gradually form on the walls of the glassware. Any residual chloroform was removed under a vacuum provided by a water jet pump. The procedure was repeated for five rounds (adding chloroform, then evaporating) until all DPPC was affixed to the sides of the glassware [36]. We then proceeded to the hydration step. A total of 100 mL of control medium was heated to 47°C and added to the flask that contained the lipids. This solution was agitated for 10 min until all the DPPC (0.3 mg/mL) had dissolved. Powdered hyaluronic acid (2.42 mg/mL, Sigma-Aldrich (St. Louis, MO, USA), ≤1% protein, molecular weight ~1.5–1.8 × 106 Da) was then added. The HA–phospholipid medium was rotated at this temperature until the HA dissolved. The final solution had a pearlescent appearance and was noticeably more viscous than the control medium.
The resulting HA–phospholipid medium was imaged using a fluorescence-light microscope set-up consisting of an Eclipse TE2000-Sinverted microscope (Nikon Instruments Inc., Melville, NY, USA), a charge-coupled device (CCD) camera (SPOT RT-KE Color 3-Shot, Model 7.3×l Diagnostic Instruments, Sterling Heights, MI, USA), and a 5× objective. The size range of vesicles was determined by analyzing the acquired images with the ImageJ software (version 1.51j, National Institutes of Health, Bethesda, MA, USA) [36].
2.3. Cartilage Procurement and Cell Viability
Cartilage from the stifle joints of 6–8-month-old steers was obtained from a local abattoir (Chiappetti’s Meats, Chicago, IL, USA). The animals were slaughtered approximately 48 h prior to acquisition, and joints were kept intact and refrigerated prior to arrival at the lab. Joints were dissected in order to expose the femoral trochlear groove. Integrity of the joint tissues (i.e., lack of bruising and blood) and cartilage cell viability better than 80% was confirmed prior to use [7].
For cell viability studies, explants were harvested from the femoral trochlear groove using a 4 mm in diameter biopsy punch (n = 4). Biopsies were then randomized into both lubricant groups. Biopsies were cultured in the phospholipid medium or control medium for seven days. Viability tests were performed to ensure the constituents of the phospholipid medium would not harm the cells.
All samples were kept in an incubator to control the temperature (37°C), humidity (95%) and CO2 concentration (5%). After the seven-day culture, samples were incubated in a solution of calcein AM and ethidium homodimer in 1× PBS at 37°C for 45 min (Invitrogen, Eugene, OR, USA). Calcein AM passes through the membranes of live cells and is converted to fluorescent calcein. This only occurs in live cells, due to the fact that dead cells do not possess the necessary active esterases [38]. Ethidium homodimer acts by binding to the nucleic acids (DNA and RNA) of dead cells, while its positive charge and large size prevent it from penetrating live cells. After incubation, cartilage samples were imaged with a confocal laser scanning microscope (Nikon E200, Melville, NY, USA) equipped with a 5× objective and a charge-coupled device camera (SPOT RT-KE Color 3-sho, Model 7.3×; Diagnostic Instruments, Sterling Heights, MI, USA). Images were taken and live and dead cell counts were obtained through analysis with Image J [36].
For frictional testing, full thickness (~3 mm) oval articular cartilage explants were procured and press-fitted into a porous polyethylene scaffold (Figure 1a). Both cartilage groups (phospholipid medium versus control medium) were pre-cultured for three days in culture medium prior to tribological testing. During the pre-culture period, explants were stored in separate baths of control medium (3 mL), which was replaced every 24 h.
Figure 1.
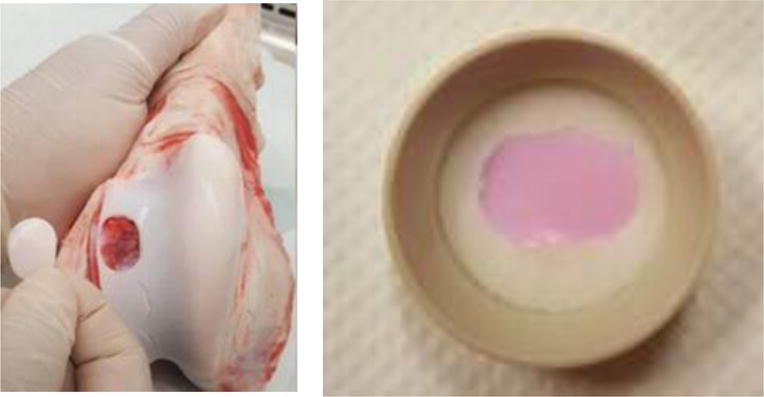
Cartilage procurement for friction testing. (a) Cartilage explant from the trochlear groove 20 mm in length and 14 mm in width (b) cartilage explant contained in porous polyethylene scaffold and stained pinkish after three days of pre-culture in culture medium.
2.4. Rheological Measurements
Viscosity versus shear strain rate curves for the phospholipid and control media were generated using an MCR 302 Rheometer (Anton Paar, GmbH, Graz, Austria) with a plate-on-plate configuration. The rheological measurements were performed at 37°C using a 50 mm flat plate up to a shear strain rate of 20,000 s−1. Each test required less than 0.5 mL of fluid. For each medium, three distinct samples (n = 3) were tested and the resulting three viscosity curves were averaged to produce the final viscosity curve.
2.5. Friction Test
Friction was measured using a ball-on-flat configuration in which a unidirectionally rotating wrought CoCrMo ball (Zimmer Inc., Warsaw, IN, USA) made equatorial contact against a flat bovine articular cartilage disc immersed in medium (Figure 2). The set-up was part of a bioreactor described previously [39,40]. The ball axis of rotation was thus parallel to the cartilage surface and was stationary. The ball was 32 mm in diameter with an arithmetic mean roughness of Ra = 12.8 ± 2.8 nm. The set-up was housed in an incubator maintaining 95% humidity and 5% atmospheric CO2 at 37°C. During the one-hour test, the cartilage explants were loaded to 40 N (approx. 2 MPa [40]) against the CoCrMo ball rotating with 10 revolutions per minute (linear velocity of 0.02 m/s). The frictional force was measured six times per minute. The coefficient of friction (COF) was then computed by dividing this force by the normal force. Three independent tests (n = 3) were conducted for each medium. For each test, fresh cartilage and fresh medium were used. To increase the statistical power, the data from the three tests were combined and the average coefficient of friction computed at 5 min intervals. Thus, the reported COF at the 5-min time point is the average of all the COF values (typically 90) during the first five minutes of testing, and so forth. The last time point, at 60 min, is therefore the average of all the COF values the last five minutes of testing.
Figure 2.
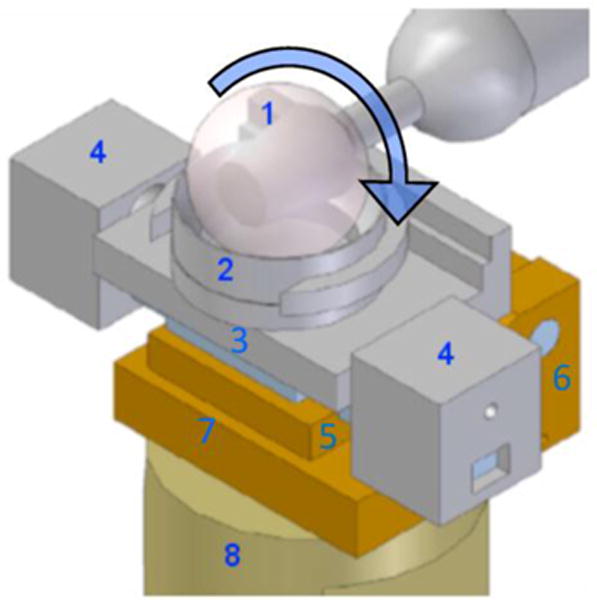
Sketch of the set-up for the friction measurements. A 32 mm diameter CoCrMo ball (1) rotates equatorially against a bovine articular cartilage plug held in the explant holder (2) mounted onto a sliding stage (3) that is free to slide laterally on a linear bearing. Movements of the sliding stage are prevented by lateral constraints (4) that house the load cells measuring tangential force. The jack plate (5), moving around hinge joint (6), rests on a load cell (embedded in bottom plate (7)) that measures normal force. The whole friction device is mounted onto a single stage (8) of a four-station bioreactor [39].
2.6. Statistics
Comparisons between the phospholipid and control media groups with respect to cell viability, viscosity at various shear rates, and friction coefficients at the various time points were performed using pairwise two-tailed Student’s t-tests. The statistical calculations were performed using Excel (Microsoft Corp, Redmond, CA, USA) and Design Expert 9 (Stat-Ease, Inc., Minneapolis, MN, USA). The reference significance level was p = 0.05. Unless otherwise stated, the plus/minus values are standard deviations.
3. Results
3.1. Cell Viability
Prior to performing tribological testing, we checked that the HA–phospholipid medium would not harm cartilage cells. After the seven-day culture we found a significant difference in cell viability between the HA–phospholipid medium (62% ± 8%, 95% CI) and the control medium (49.5% ± 5%) (p = 0.009). Representative histological section micrographs are shown in Figure 3.
Figure 3.
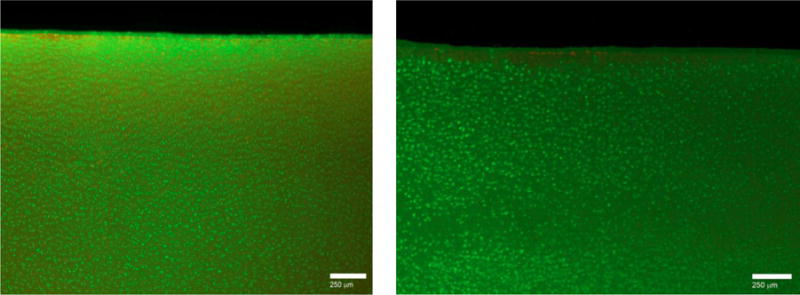
Histological sections of a tangential layer of a 3 mm × 2 mm cartilage biopsy punch after seven days of culture in control medium (left) and in HA–phospholipid medium (right). Calcein AM and ethidium homodimer-1 were used to stain live cells green and dead cells red-brown.
3.2. Liposome Formation
By adding 14:0-06:0 NBD-PC fluorescent marker, we were able to visualize the successful formation of lipid vesicles. Lipids that are labeled with NBD (7-nitrobenz-2-oxa-1,3-diazol-4-yl) are commonly used to monitor the organization and dynamics which occur in lipid membranes [41]. The vesicles formed ranged from 0.1 to 50 μm in diameter (Figure 4).
Figure 4.
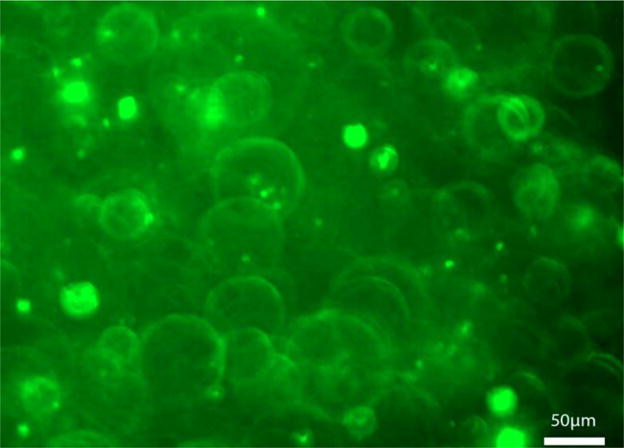
Fluorescent image of lipid vesicles in HA–phospholipid medium (20×).
3.3. Rheological Measurements
The viscosity of the HA–phospholipid medium was distinctly shear-thinning, whereas that of the control medium was approximately Newtonian with an average viscosity of 0.71 ± 0.03 mPa·s (±standard deviation) (Figure 5). The viscosity of the HA–phospholipid medium was also much higher than that of the control medium for all shear strain rates, being almost 100-fold higher at 10 s−1 (60.6 ± 2.3 mPa·s, 0.63 ± 0.07 mPa·s, respectively, p <0.001) and still five-fold higher at 20,000 s−1 (3.92 ± 0.07, 0.73 ± 0.01, respectively, p < 0.001). Within the range of strain rates of 10 to 20,000 s−1 used here, the viscosity curve of the HA–phospholipid medium fits well the Carreau–Yasuda model [42] given by Equation (1):
| (1) |
where η is the viscosity at shear strain rate γ, η∞ is the viscosity at infinite shear rate, η0 is the zero-shear-rate viscosity, γ* is the normalizing shear rate for shear-thinning onset, a and n are dimensionless power parameters controlling the steepness of the shear-thinning curve. The fit values found for these parameters were η0 = 70.7 mPa·s, η∞ = 1.60 mPa·s, γ* = 53.8 s−1, a = 0.807, n = 0.433, yielding a curve that closely matches the data (Figure 5), with an R-squared value of 0.999. The value of the exponent n (0.433) is indicative of pronounced shear-thinning behavior for shear rates above γ*, noting that n = 0 maximizes shear-thinning and n = 1 implies no shear-thinning.
Figure 5.
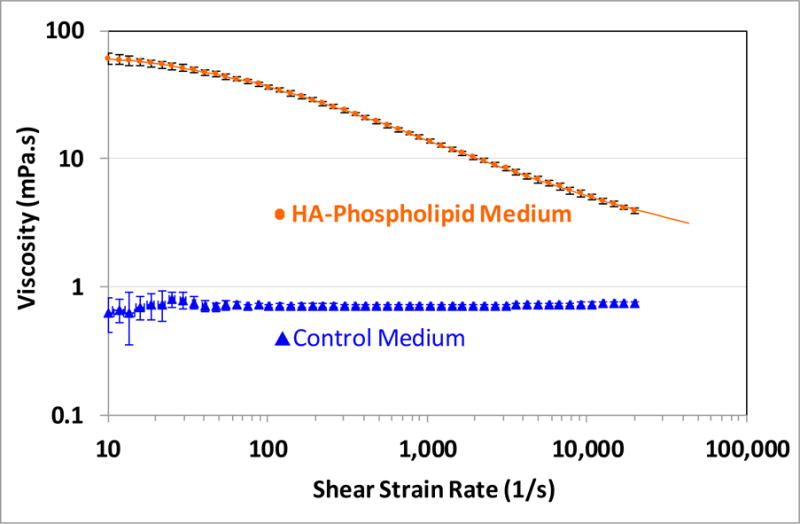
Viscosity curves for the HA–phospholipid and control media. The error bars denote the 95% confidence intervals. The solid curve for the phospholipid plot is a fit to the Carreau–Yasuda model (Equation (1)); the curve has been extended beyond the measurement range for visibility.
3.4. Friction Measurements for a CoCrMo Ball Articulating against Bovine Cartilage
The average friction coefficient values were lower for the HA–phospholipid medium than for the control medium at all 12 rotation time points, although the difference did not achieve statistical significance consistently until the 40-min time point, where the friction values for two media clearly diverge (Figure 6). Starting at the 40-min time point, when the curves start to plateau to a steady state, the friction coefficient for the phospholipid medium was at least 19.6% lower than for the control medium (p ≤ 0.005). The average friction coefficient at the 60-min time point, marking the end of the test, was 0.287 ± 0.035 (95% confidence interval) for the HA–phospholipid medium and 0.358 ± 0.033 for the control medium.
Figure 6.
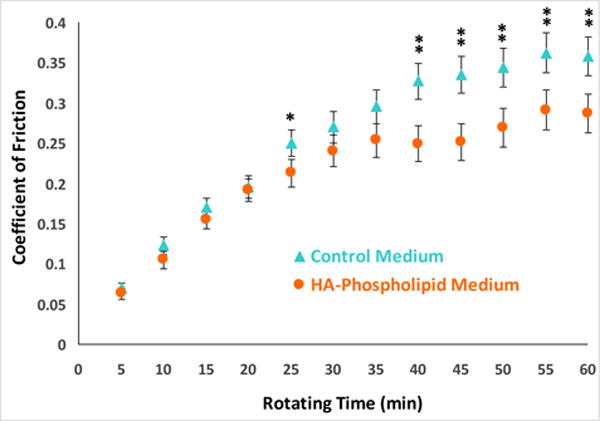
Evolution of the average friction coefficient of cartilage articulating against a CoCrMo ball in the HA–phospholipid medium (circles) and in the control medium (triangles) during one hour of ball rotating time. Each point corresponds to the average friction over the previous 5 min. The error bars denote the least significant difference intervals at the 95% confidence level. A statistically significant difference between the HA–phospholipid and control media averages is shown with one asterisk (*) for p ≤ 0.05 and two asterisks (**) for p ≤ 0.005.
4. Discussion
In this study, we evaluated a new test medium that combines the cell-nourishing properties of culture medium with the viscosity-enhanced properties of synovial fluid to facilitate lubrication. During our tribological experiments, our hypothesis was therefore that the new test medium would lead to higher cell viability and to lower friction than the culture medium when articulating bovine articular cartilage against a metal counterface in our bioreactor tribometer. We elected to use cobalt–chromium (CoCr) alloy, as it is the most commonly used material in hemiarthroplasty and cartilage defect repair. In both procedures, the articular cartilage layer articulates against the metal bearing surface, replacing the natural cartilage‐on‐cartilage interface [43]. The goal of this study was to continue to improve upon our current in vitro model of hemiarthroplasty, by employing a lubricant that more closely models synovial fluid.
4.1. Cell Viability
We performed a viability test with cartilage biopsy punches in order to determine whether the phospholipid would harm the cells. After a seven-day culture period, we found that the HA–phospholipid medium did no harm to the cells relative to the control medium. In fact, the HA–phospholipid medium performed significantly better than the control. Due to the lack of proteinaceous components and active loading in either medium, cell viability was expectedly moderate for both media.
While the difference in viability between the HA–phospholipid medium and control groups may be attributed to randomization artifacts in cartilage quality, another explanation involves the anti-oxidative properties of HA. A study by Sato et al. [44] has shown that HA caused a marked reduction in reactive oxygen species (ROS) within synovial fluid. Their results suggest that HA acts as a scavenger to ROS, and will impact the concentration of ROS in a dose-dependent manner. The presence of HA within the HA–phospholipid medium may therefore cause a reduction in the ROS present in culture, thus providing protection to the cartilage cells and improving viability.
4.2. Liposome Formation
In the natural joint, plane lipid multilayers exist upon the surface of articular cartilage. Within these layers there are anywhere from three to seven lipid bilayers separated by saline layers. Lipids exist in the synovial fluid at a very high concentration—approximately 10 times greater than what is required to cover all of the articular surfaces with up to seven lipid bilayers. These lipids create an outer layer, which envelops hyaluronic acid, forming a tubular or vesicular structure [17,18]. Previous studies have shown that these vesicles can both merge and divide due to changes in permeability and or and exposure to stress. If a vesicle undergoes significant mechanical stress (traction or compression), the lipid membrane will stretch until it fractures and then divides into several smaller vesicles [45,46].
In order to model the tribological properties of synovial fluid, the 0.3 mg/mL concentration of DPPC used here is within the range of 0.26–0.98 mg/mL reported for osteoarthritic synovial fluid and only twice the concentration of 0.13–0.15 mg/mL reported for normal synovial fluid [15]. DPPC is also a significant component of the phosphatidylcholine lipids adsorbed onto the surface of articular cartilage [35] and is pervasive in joints [34]. As the DPPC is mixed into the control medium, the non-polar portions of the DPPC molecule turn inward, forming vesicles. As these hydrophobic interactions occur at different rates, a wide range of vesicle sizes is formed [47]. The size range of 0.1 to 50 μm in diameter (Figure 4) for the vesicles synthesized in this study overlaps with the range of 0.1 μm to a few hundred nanometers of the vesicles found naturally in synovial fluid [48], but it also indicates the presence of much larger vesicles. However, this is the range prior to tribological testing, and, as pointed out above, the larger vesicles are expected to divide into smaller vesicles under shear and compressive stresses. A comparison of the size distribution of vesicles before and after testing will be the subject of a future study.
4.3. Rheological measurements
The viscosity values for the HA–phospholipid medium are toward the bottom of the range of viscosities reported by Schurz and Ribitsch [49] for degenerative synovial fluids in the 10 to 1000 s‐1 interval where the studies overlap (Figure 7). The HA-P viscosity values overlap with those of healthy synovial fluid from 70 to 500 s−1, but they are markedly lower below 50 s−1 (Figure 7). Thus, at 10 s−1 the HA–phospholipid medium viscosity of 60.6 mPa·s is much lower than the corresponding viscosity of approximately 540 mPa·s for healthy synovial fluid [49]. The HA concentration of 2.43 mg/mL in the HA–phospholipid medium is well within the range of 0.35–4.22 mg/mL that has been reported for normal synovial fluid [50] and therefore does not account for the much lower viscosities of the HA–phospholipid medium compared to normal synovial fluid at the lower shear rates. The lower viscosities may therefore arise from the lower molecular weight of the HA used for the HA–phospholipid medium (1.5–1.8 MDa) compared to the molecular weight in normal synovial fluid, which has been reported in the range of 6.3–7.6 MDa [51]. The viscosity versus shear rate values of the HA–phospholipid medium are in good alignment with those reported by Haward et al. [52] for a solution of 0.3 wt % HA (MW = 1.6 MDa) in a physiological phosphate buffered saline (0.01 M, pH 7.4). The viscosity values in the range of 10–10,000 s−1, where the two studies overlap, are a little higher for their fluid, as expected from its higher HA concentration (Table 3).
Figure 7.
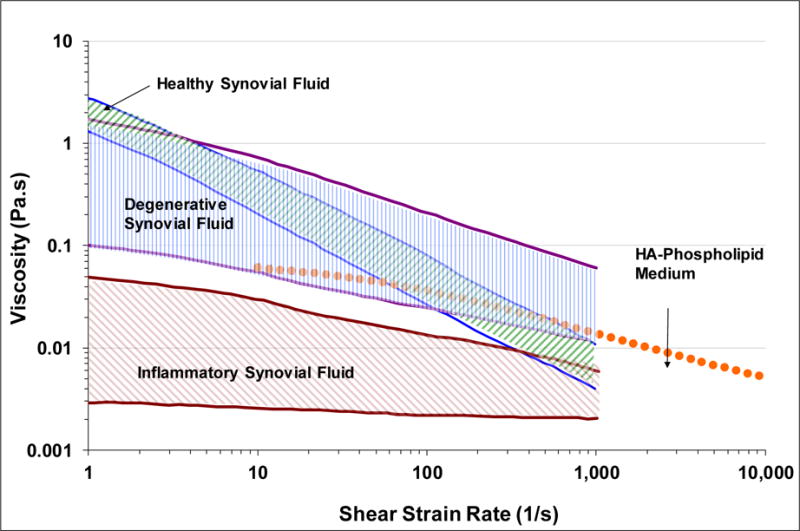
Viscosity ranges versus shear strain rate for healthy (green upward hatch), degenerative (blue vertical hatch), and inflammatory (plum downward hatch) synovial fluids [49] compared to viscosity curve for the HA–phospholipid medium.
Table 3.
Comparison of the viscosity at various shear rates for the HA–phospholipid medium and an HA solution in phosphate buffered saline.
| Viscosity (mPa·s) at Various Shear Rates | |||||
|---|---|---|---|---|---|
|
| |||||
| HA Concentration (wt %) | 10 s−1 | 100 s−1 | 1000 s−1 | 10,000 s−1 | |
| Phosphate Buffered Solution [52] | 0.3 | 68.0 | 40.8 | 16.3 | 6.3 |
| HA–Phospholipid Medium | 0.243 | 60.4 | 36.4 | 13.9 | 5.1 |
4.4. Friction Measurements
Our test demonstrated a 20% lowering of the coefficient of friction with the HA–phospholipid medium compared to the control medium when friction levels had started to plateau toward a steady state wherein fluid exudation from the cartilage surface had presumably abated and boundary lubrication was dominant. At that point, boundary lubrication by the HA and phospholipid through chemical interaction with the cartilage and metal surfaces can have more effect. The evolution of the friction coefficient over time is in agreement with experiments conducted by Caligaris and Ateshian [53], who investigated the effects of loss of interstitial fluid pressurization on cartilage friction. Using a set-up of reciprocating osteochondral plugs on a glass plate they found 1.8-times better lubricating properties of SF compared with PBS (phosphate-buffered physiological salt solution) during steady state. In our testing series, the friction improvement of HA–phospholipid medium over cell culture medium, which should be tribologically comparable to PBS, was less than 1.3. Hence, this suggests that we are still missing other important lubricating constituents of SF, like lubricin (proteoglycan 4 or PRG4) [32].
With a similar testing setup, Li, et al. [54] demonstrated only an 8% decrease in the friction coefficient between cartilage articulated in a bath of Ringer’s solution versus an HA solution, suggesting that the unique lipid structure within the HA–phospholipid medium does indeed reduce friction beyond the effects of HA; although, some of the differences may stem from the use of different counterfaces during testing. A study by Forsey et al. [55] utilized a similar medium which contained DPPC and HA, and came to a similar conclusion regarding the unique role of lipid vesicles in reducing friction. They found that the lubricative properties of their solution were not concentration dependent for HA; however, the concentration of DPPC was directly related to the coefficient of friction during testing.
4.5. Limitations
This study had several limitations. First, immature bovine tissue is commonly used in tribological research due to the ease of use (tissue is thicker and more abundant than human tissue, as well as more readily available). Although bovine tissue is considered an acceptable alternative in many in-vitro studies, the translatability to mature human cartilage can be limited due to the differences in biological and mechanical properties [56,57]. Currently the way the workflow is set up would pose challenges for the use of human tissue. However, it is possible that various adjustments could be made to better allow for the tribological testing of human tissue. Second, in addition to the tissue source, variability in tissue quality and the manual procurement process have an impact on the size/thickness of explants procured. The tissue was minimally handled; however, it is possible that damage incurred during procurement could have contributed to decreased cell viability. Also, there may have been an increase in cartilage surface roughness that was not assessed. Third, the rheology of the media at shear rates below 10 s−1 was not determined. However, the viscosity associated with the HA is expected to plateau as the shear rate approaches zero, in line with the Carreau–Yasuda model (Equation (1)) and observed experimentally [52]. The effect, if any, of the phospholipids on the viscosity of the HA–phospholipid medium at low shear rates remains to be determined. Fourth, as discussed above, the viscosity values for the HA–phospholipid medium were markedly lower than those for normal synovial fluid, particularly at shear rates below 1000 s−1. However, for preclinical testing of materials against cartilage, it is probably more conservative and representative to have a fluid that simulates osteoarthritic and inflammatory joint fluid, while still emulating the shear-thinning behavior of these fluids [58]. Further mechanistic studies are necessary to elucidate the lubricating contribution of each of the constituents in the HA–phospholipid medium.
5. Conclusions
The overall purpose of this study was to investigate the biological and mechanical properties of a new HA–phospholipid medium, and compare it to a standard culture medium for use in tribological studies. The rheological and friction results indicate that the HA–phospholipid medium is superior to the control cell culture medium in emulating the shear thinning and lubricative properties of natural synovial fluid. Furthermore, the HA–phospholipid medium is not only able to nourish the cells while in culture, but to potentially increase overall cell viability compared to the control medium, perhaps due to the anti-oxidative properties of HA. These results thus confirm our hypothesis that a cell culture medium with added phospholipid vesicles and HA leads to better cell viability and lower friction than culture medium alone. The reliance on cell culture medium as a lubricant for wear testing of live cartilage to maintain cell viability is therefore no longer necessary, a significant development given that the mechano-biological properties of cell culture media are vastly different from those of natural synovial fluid. An HA–phospholipid medium thus has the potential to make in-vitro wear and friction experiments with live cartilage and implant materials more clinically relevant. In particular, this medium could contribute to the more realistic evaluation of new artificial materials for improving the clinical performance of hemiarthroplasties.
Acknowledgments
This study was funded with partial support from grant R01 AR066635 from the National Institutes of Health (NIH).
Footnotes
Author Contributions: Markus A. Wimmer and Ana-Maria Trunfio-Sfarghiu conceived and designed the experiments; Gregoire Aldebert, Teresa Veselak, and Michel P. Laurent performed the experiments; Thomas M. Schmid provided experimental assistance; Michel P. Laurent analyzed the data; Teresa Veselak, Michel P. Laurent, and Markus A. Wimmer wrote the paper.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Jafarzadeh SR, Felson DT. Updated estimates suggest a much higher prevalence of arthritis in US adults than previous ones. Arthritis Rheumatol. 2017 doi: 10.1002/art.40355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martini EM, Garrett N, Lindquist T, Isham GJ. The boomers are coming: A total cost of care model of the impact of population aging on health care costs in the United States by Major Practice Category. Health Serv Res. 2007;42:201–218. doi: 10.1111/j.1475-6773.2006.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pala E, Trono M, Bitonti A, Lucidi G. Hip hemiarthroplasty for femur neck fractures: Minimally invasive direct anterior approach versus postero-lateral approach. Eur J Orthop Surg Traumatol. 2016;26:423–427. doi: 10.1007/s00590-016-1767-x. [DOI] [PubMed] [Google Scholar]
- 4.Springer BD, Scott RD, Sah AP, Carrington R. McKeever hemiarthroplasty of the knee in patients less than sixty years old. J Bone Jt Surg Am. 2006;88:366–371. doi: 10.2106/JBJS.E.00123. [DOI] [PubMed] [Google Scholar]
- 5.Ferrel JR, Trinh TQ, Fischer RA. Reverse total shoulder arthroplasty versus hemiarthroplasty for proximal humeral fractures: A systematic review. J Orthop Trauma. 2015;29:60–68. doi: 10.1097/BOT.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 6.Phadnis J, Watts AC, Bain GI. Elbow hemiarthroplasty for the management of distal humeral fractures: Current technique, indications and results. Shoulder Elb. 2016;8:171–183. doi: 10.1177/1758573216640210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettersson K, Amilon A, Rizzo M. Pyrolytic carbon hemiarthroplasty in the management of proximal interphalangeal joint arthritis. J Hand Surg Am. 2015;40:462–468. doi: 10.1016/j.jhsa.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Trevino RL. Thesis for the Degree of Doctor of Philosophy. Rush University; Chicago, IL, USA: 2016. Mechanobiological Response of Cartilage to Self-Mating and Prosthetic Contact through In Vitro Modeling of Joint Articulation. [Google Scholar]
- 9.Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40–49. doi: 10.1016/j.tvjl.2015.07.011. ISSN 1090-0233. [DOI] [PubMed] [Google Scholar]
- 10.Yao T, Asayama Y. Animal-cell culture media: History, characteristics, and current issues. Reprod Med Biol. 2017;16:99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mow VC, Huiskes R. Basic Orthopaedic Biomechanics & Mechano-Biology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2005. [Google Scholar]
- 12.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt T, Sah R. Effect of Synovial Fluid on Boundary Lubrication of Articular Cartilage. Osteoarthr Cartil. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Chan SMT, Neu CP, Komvopoulos K, Reddi AH. The Role of Lubricant Entrapment at Biological Interfaces: Reduction of Friction and Adhesion in Articular Cartilage. J Biomech. 2011;44:2015–2020. doi: 10.1016/j.jbiomech.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Fam H, Bryant JT, Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007;44:59–74. [PubMed] [Google Scholar]
- 16.Stoia JL. Master’s Thesis. University of Illinois at Chicago; Chicago, IL, USA: 2011. Cartilage-on-Cartilage Articulating System as a Wear Benchmark for Artificial Replacement Materials. [Google Scholar]
- 17.Trunfio-Sfarghiu AM. Thesis for the Degree of Doctor in Biomechanics. INSA de Lyon; Villeurbanne, France: 2006. Modèle bio-tribologique des articulations Rôle mécanique et physicochimique des assemblages moléculaires du fluide synovial. [Google Scholar]
- 18.Corneci MC, Dekkiche F, Trunfio-Sfarghiu AM, Meurisse MH, Berthier Y, Rieu JP. Tribological properties of fluid phase phospholipid bilayers. Tribol Int. 2011;44:1959–1968. doi: 10.1016/j.triboint.2011.08.015. [DOI] [Google Scholar]
- 19.Watanabe M, Leng CG, Toriumi H, Hamada Y, Akamatsu N, Ohno S. Ultrastructural study of upper surface layer in rat articular cartilage by “in vivo cryotechnique” combined with various treatments. Med Electron Microsc. 2000;33:16–24. doi: 10.1007/s007950000003. [DOI] [PubMed] [Google Scholar]
- 20.Mirea DA, Trunfio-Sfarghiu AM, Matei CI, Munteanu B, Piednoir A, Rieu JP, Blanchin MG, Berthier Y. Role of the biomolecular interactions in the structure and tribological properties of synovial fluid. Tribol Int. 2013;59:302–311. doi.org/10.1016/j.triboint.2012.06.015. [Google Scholar]
- 21.Hills BA. Oligolamellar lubrication of joints by surface active phospholipid. J Rheumatol. 1989;16:82–91. [PubMed] [Google Scholar]
- 22.Sorkin R, Dror Y, Kampf N, Klein J. Mechanical stability and lubrication by phosphatidylcholine boundary layers in the vesicular and in the extended lamellar phases. Langmuir. 2014;30:5005–5014. doi: 10.1021/la500420u. [DOI] [PubMed] [Google Scholar]
- 23.Sivan S, Schroeder A, Verberne G, Merkher Y, Diminsky D, Priev A, Maroudas A, Halperin G, Nitzan D, Etsion I, et al. Liposomes act as effective biolubricants for friction reduction in human synovial joints. Langmuir. 2010;26:1107–1116. doi: 10.1021/la9024712. [DOI] [PubMed] [Google Scholar]
- 24.Raj A, Wang M, Zander T, Wieland DCF, Liu X, An J, Garamus VM, Willumeit-Römer R, Fielden M, Claesson PM, et al. Lubrication synergy: Mixture of hyaluronan and dipalmitoylphosphatidylcholine (DPPC) vesicles. J Colloid Interface Sci. 2017;488:225–233. doi: 10.1016/j.jcis.2016.10.091. Epub 2016 Nov 1. [DOI] [PubMed] [Google Scholar]
- 25.Yu CJ, Ko CJ, Hsieh CH, Chien CT, Huang LH, Lee CW, Jiang CC. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid-regulated proteins involved in chondroprotective effect under oxidative stress. J Proteom. 2014;99:40–53. doi: 10.1016/j.jprot.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Jahn M, Baynes JW, Spiteller G. The reaction of hyaluronic acid and its monomers, glucuronic acid and N-acetylglucosamine, with reactive oxygen species. Carbohydr Res. 1999;321:228–234. doi: 10.1016/s0008-6215(99)00186-x. [DOI] [PubMed] [Google Scholar]
- 27.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Mirea DA, Trunfio-Sfarghiu AM, Piednoir A, Rieu JP, Blanchin MG, Berthier Y. AFM Study of the Interactions Between Synovial Liquid’S Molecular Components in Boundary Lubrication. J Balk Tribol Assoc. 2011;17:642–651. [Google Scholar]
- 29.Sava MM, Boulocher C, Matei CI, Munteanu B, Schramme M, Viguier E, Roger T, Berthier Y, Blanchin MG, Trunfio-Sfarghiu AM. Structural and tribological study of healthy and biomimetic, SF. Comput Methods Biomech Biomed Eng. 2013;16(Suppl. 1):216–218. doi: 10.1080/10255842.2013.815866. [DOI] [PubMed] [Google Scholar]
- 30.Sava MM, Munteanua B, Renault E, Berthier Y, Trunfio-Sfarghiu AM. Tribological Analysis of UHMWPE Tibial Implants in Unicompartmental Knee Replacements: From Retrieved to In Vitro Studies. Biotribology. 2018;13:1–15. doi.org/10.1016/j.biotri.2017.11.001. [Google Scholar]
- 31.Dekkiche F, Corneci MC, Trunfio-Sfarghiu AM, Munteanu B, Berthier Y, Kaabar W, Rieu JP. Stability and tribological performances of fluid phospholipid bilayers: effect of buffer and ions. Colloids Surf B Biointerfaces. 2010;80:232–239. doi: 10.1016/j.colsurfb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 33.Corvelli M, Che B, Saeui C, Singh A, Elisseeff J. Biodynamic performance of hyaluronic acid versus synovial fluid of the knee in osteoarthritis. Methods. 2015;84:90–98. doi: 10.1016/j.ymeth.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Seror J, Day AJ, Kampf N, Klein J. Ultra-low friction between boundary layers of hyaluronan-phosphatidylcholine complexes. Acta Biomater. 2017;59:283–292. doi: 10.1016/j.actbio.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Sarma AV, Powell GL, LaBerge M. Phospholipid composition of articular cartilage boundary lubricant. J Orthop Res. 2001;19:671–676. doi: 10.1016/S0736-0266(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Franses EI. New protocols for preparing dipalmitoylphosphatidylcholine dispersions and controlling surface tension and competitive adsorption with albumin at the air/aqueous interface. Colloids Surf B Biointerfaces. 2005;43:256–266. doi: 10.1016/j.colsurfb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Pérez JMM, Pascau J. Image Processing with ImageJ. Vol. 11. Packt Publishing Ltd.; Birmingham, UK: 2003. pp. 36–42. [Google Scholar]
- 38.Pascual Garrido C, Hakimiyan AA, Rappoport L, Oegema TR, Wimmer MA, Chubinskaya S. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthr Cartil. 2009;17:1244–1251. doi: 10.1016/j.joca.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wimmer MA, Grad S, Kaup T, Hänni M, Schneider E, Gogolewski S, Alini M. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004;10:1436–1445. doi: 10.1089/ten.2004.10.1436. [DOI] [PubMed] [Google Scholar]
- 40.Shekhawat VK. PhD Thesis. University of Illinois at Chicago; Chicago, IL, USA: 2009. Influence of Kinematics on Mechano-Biological Response of Articular Cartilage—An In Vitro Investigation. [Google Scholar]
- 41.Raghuraman H, Shrivastava S, Chattopadhyay A. Monitoring the looping up of acyl chain labeled NBD lipids in membranes as a function of membrane phase state. Biochim Biophys Acta (BBA)-Biomembr. 2007;1768:1258–1267. doi: 10.1016/j.bbamem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Bird RB, Armstrong RC, Hassager O. Dynamics of Polymeric Liquids, Fluid Dynamics. Vol. 1. Wiley; New York, NY, USA: 1987. [Google Scholar]
- 43.Oungoulian SR, Durney KM, Jones BK, Ahmad CS, Hung CT, Ateshian GA. Wear and damage of articular cartilage with friction against orthopedic implant materials. J Biomech. 2015;48:1957–1964. doi: 10.1016/j.jbiomech.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato H, Takahashi T, Ide H, Fukushima T, Tabata M, Sekine F, Kobayashi K, Negishi M, Niwa Y. Antioxidant activity of synovial fluid, hyaluronic acid, and two subcomponents of hyaluronic acid. Synovial fluid scavenging effect is enhanced in rheumatoid arthritis patients. Arthritis Rheum. 1988;31:63–71. doi: 10.1002/art.1780310110. [DOI] [PubMed] [Google Scholar]
- 45.Sorkin R, Kampf N, Dror Y, Shimoni E, Klein J. Origins of extreme boundary lubrication by phosphatidylcholine liposomes. Biomaterials. 2013;34:5465–5475. doi: 10.1016/j.biomaterials.2013.03.098. [DOI] [PubMed] [Google Scholar]
- 46.Pawlak Z, Yusuf KQ, Pai R, Urbaniak W. Repulsive surfaces and lamellar lubrication of synovial joints. Arch Biochem Biophys. 2017;623–624:42–48. doi: 10.1016/j.abb.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Leckband D, Israelachvili J. Intermolecular Forces in Biology. Vol. 34. Cambridge University Press Quarterly Reviews of Biophysics; Cambridge, UK: 2001. pp. 105–267. [DOI] [PubMed] [Google Scholar]
- 48.Matei CI, Boulocher C, Boulé C, Schramme M, Viguier E, Roger T, Berthier Y, Trunfio-Sfarghiu AM, Blanchin MG. Ultrastructural analysis of healthy synovial fluids in three mammalian species. Microsc Microanal. 2014;20:903–911. doi: 10.1017/S1431927614000415. [DOI] [PubMed] [Google Scholar]
- 49.Schurz J, Ribitsch V. Rheology of synovial fluid. Biorheology. 1987;24:385–399. doi: 10.3233/bir-1987-24404. [DOI] [PubMed] [Google Scholar]
- 50.Bollet AJ. The intrinsic viscosity of synovial fluid hyaluronic acid. J Lab Clin Med. 1956;48:721–728. [PubMed] [Google Scholar]
- 51.Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haward SJ, Jaishankar A, Oliveira MS, Alves MA, McKinley GH. Extensional flow of hyaluronic acid solutions in an optimized microfluidic cross-slot device. Biomicrofluidics. 2013;7:044108. doi: 10.1063/1.4816708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caligaris M, Ateshian GA. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthr Cartil. 2008;16:1220–1227. doi: 10.1016/j.joca.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li F, Wang A, Wang C. Analysis of friction between articular cartilage and polyvinyl alcohol hydrogel artificial cartilage. J Mater Sci Mater Med. 2016;27:87. doi: 10.1007/s10856-016-5700-y. [DOI] [PubMed] [Google Scholar]
- 55.Forsey RW, Fisher J, Thompson J, Stone MH, Bell C, Ingham E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials. 2006;27:4581–4590. doi: 10.1016/j.biomaterials.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 56.Olivier P, Loeuille D, Watrin A, Walter F, Etienne S, Netter P, Gillet P, Blum A. Structural evaluation of articular cartilage: Potential contribution of magnetic resonance techniques used in clinical practice. Arthritis Rheum. 2001;44:2285–2295. doi: 10.1002/1529-0131(200110)44:10<2285::aid-art391>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 57.Torzilli PA, Deng XH, Ramcharan M. Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech Model Mechanobiol. 2006;5:123–132. doi: 10.1007/s10237-006-0030-5. [DOI] [PubMed] [Google Scholar]
- 58.Cooke AF, Dowson D, Wright V. The Rheology of Synovial Fluid and Some Potential Synthetic Lubricants for Degenerate Synovial Joints. Eng Med. 1978;7:66–72. [Google Scholar]


