Abstract
The objective was to assess dynamic functional connectivity (FC) and local/global connectivity in Parkinson's disease (PD) patients with mild cognitive impairment (PD-MCI) and with normal cognition (PD-NC).
The sample included 35 PD patients and 26 healthy controls (HC). Cognitive assessment followed an extensive neuropsychological battery. For resting-state functional MRI (rs-fMRI) analysis, independent component analysis (ICA) was performed and components were located in 7 networks: Subcortical (SC), Auditory (AUD), Somatomotor (SM), visual (VI), cognitive-control (CC), default-mode (DMN), and cerebellar (CB). Dynamic FC analysis was performed using the GIFT toolbox. FC differences between groups in each FC state were analysed with the network-based statistic (NBS) approach. Finally, a graph-theoretical analysis for local/global parameters was performed.
The whole sample showed 2 dynamic FC states during the rs-fMRI. PD-MCI patients showed decreased mean dwell time in the hypo-connectivity state (p = 0.030) and showed increased number of state transitions (p = 0.007) compared with the HC. In addition, in the hypo-connectivity state, PD-MCI patients showed reduced inter-network FC between the SM-CC, SM-VI, SM-AUD, CC-VI and SC-DMN compared with the HC (p < 0.05-FDR). These FC alterations in PD-MCI were accompanied by graph-topological alterations in nodes located in the SM network (p < 0.001). In contrast, no differences were found between the PD-NC and HC.
Findings suggest the presence of dynamic functional brain deteriorations in PD-MCI that are not present in PD-NC, showing the PD-MCI group dynamic FC dysfunctions, reduced FC mostly between SM-CC networks and graph-topological deteriorations in the SM network. A dynamic FC approach could be helpful to understand cognitive deterioration in PD.
Keywords: Dynamic functional connectivity, Graph theory, Parkinson's disease, Mild cognitive impairment, PD-MCI, Networks
Highlights
-
•
Dynamic FC alterations were found in PD-MCI patients but not in PD-NC.
-
•
PD-MCI patients showed reduced mean dwell time in the hypo-connected state.
-
•
PD-MCI patients showed increased state transitions compared to HC.
-
•
PD-MCI patients showed inter-network FC reductions and graph parameter alterations.
-
•
Dynamic FC could be a useful approach in the study of cognitive impairment in PD.
1. Introduction
Cognitive deficits in Parkinson's disease (PD) patients are common from early to moderate stages (Aarsland et al., 2009, Elgh et al., 2009) and up to 80% of the patients may develop dementia in the course of the disease (Hely et al., 2008). Moreover, the presence of cognitive impairment is related to a reduction in quality of life and functional disability in PD (Leroi et al., 2012, Rosenthal et al., 2010).
Magnetic resonance imaging (MRI) studies have shown that cognitive deficits in PD patients are correlated with structural (Duncan et al., 2016) and functional brain dysfunctions (Christopher and Strafella, 2013, Gao and Wu, 2016). More specifically, resting-state functional MRI (rs-fMRI) is a non-invasive method that shows reliability and high reproducibility to easily explore the functional activity of the different brain networks (Biswal et al., 2010, Van Den Heuvel et al., 2010). To date, most rs-fMRI studies in PD have investigated functional connectivity (FC) patterns as a static phenomenon. While these studies have shown a general impairment in PD patients compared with healthy controls (HC), [for a review see: (Gao and Wu, 2016)], others have focused on assessing differences between PD patients with mild cognitive impairment (PD-MCI) and with normal cognition (PD-NC). PD-MCI patients seemed to present FC alterations within (Amboni et al., 2015, Baggio et al., 2015, Gorges et al., 2015) and between networks (Baggio et al., 2015) compared with HC, and also compared with PD-NC (Baggio et al., 2015, Gorges et al., 2015). Less pronounced FC differences between PD-NC and HC have also been reported (Amboni et al., 2015, Baggio et al., 2015, Gorges et al., 2015).
However, more recently, rs-fMRI studies have shown that FC may actually vary during the acquisition time (i.e. dynamic FC) (Allen et al., 2014, Calhoun et al., 2014, Hutchison et al., 2013). A widely applied method for temporal dynamic FC analysis is the sliding time window method (Allen et al., 2014, Damaraju et al., 2014, Du et al., 2016, Hutchison et al., 2013). This method divides acquired rs-fMRI into windows and calculates the variation of FC across those windows. The results represent the dynamic characteristic of FC. Given that static FC has helped to understand the cerebral correlates of cognitive impairment in PD, a dynamic FC approach may add relevant information as it represents more accurately the dynamic nature of the brain (Calhoun et al., 2014, Hutchison et al., 2013).Therefore, a dynamic approach to study FC may help clarify the neurobiological substrates of presence of MCI in PD. To date, only one dynamic FC study has been recently published in PD, and showed dynamic FC alterations in PD patients compared to HC (Kim et al., 2017).
Furthermore, the characteristics of the brain networks can be investigated with graph-theory, which divides the networks in nodes (the brain regions) and in edges, representing the connections between the nodes (Bullmore and Sporns, 2009, Van Den Heuvel et al., 2010). Most graph-theoretical studies in PD showed significant reductions in both global and local parameters compared with HC (Göttlich et al., 2013, Koshimori et al., 2016, Luo et al., 2015, Skidmore et al., 2011, Tinaz et al., 2016) but increased graph parameters have also been found in PD compared with HC (Göttlich et al., 2013, Zhang et al., 2015). A graph theory approach may contribute to understanding of cognitive impairment in PD. To date, only one study has assessed the differences in graph parameters between PD-MCI and PD-NC, and found that PD-MCI patients showed decreased graph characteristics compared with PD-NC and HC (Baggio et al., 2014), but also increased graph parameters that correlated negatively with cognitive performance, suggesting the presence of compensatory mechanisms (Baggio et al., 2014).
Despite the fact that some steps have been taken towards understanding the FC differences between PD-MCI and PD-NC, the literature is still scarce and more specific studies are needed in this field. Dynamic FC is a novel FC approach that could add relevant information about the presence of MCI in PD, and the combination with other neuroimaging methods of analysis could help to better understand the neurodegenerative process that underlies cognitive impairment in PD. Therefore, the objective of this study was to assess the dynamic FC and local/global connectivity in PD-MCI and PD-NC using the combination of dynamic FC and graph-theoretical approaches during rs-fMRI.
2. Materials and methods
2.1. Subjects
The sample included 37 PD patients and 26 HC, matched with PD patients by age, gender and years of education. PD patients were recruited from the Department of Neurology at the Hospital of Galdakao and from the PD Biscay Association (ASPARBI). PD patients were enrolled in the study if they fulfilled the UK PD Society Brain Bank diagnostic criteria. Other inclusion criteria were: i) age between 45 and 75; ii) Hoehn and Yahr (H&Y) disease stage (Hoehn and Yahr, 1998) ≤ 3; iii) Unified PD Rating Scale (UPDRS) (Martinez-Martin et al., 1994) evaluated by the neurologist. Exclusion criteria were: i) presence of dementia as defined by the DSM-IV-TR and the Movement Disorders Society clinical criteria; ii) presence of other neurological illness/injury; iii) unstable psychiatric disorders; iv) visual hallucinations as assessed by the Neuropsychiatric Inventory Questionnaire (Kaufer et al., 2000); v) depression evaluated with the Geriatric Depression Scale (GDS) (score > 5) (Yesavage and Sheikh, 1986).
All patients were symptomatically stable, and tested while on their medication. Their Levodopa equivalent daily dose (LEDD) was recorded (Tomlinson et al., 2010).
2.2. Neuropsychological assessment
PD and HC underwent a neuropsychological battery that included the Mini-Mental State Examination (MMSE) as a screening measure (Lobo et al., 2001). Five cognitive domains were assessed: 1) Attention and working memory measured with the Digit Span Backward (Pena-Casanova et al., 2009), the Brief Test of Attention (BTA) (Schretlen, 1989), the Trail Making Test (A) (Pena-Casanova et al., 2009) and the Stroop Test (Words and Color) (Golden, 1994); 2) Executive functions measured with the Trail Making Test (B) and the Clock Drawing Test (order) (Mainland and Shulman, 2013); 3) Language evaluated with the Boston Naming Test (abbreviated version) and the Verbal Fluency Test (semantic); 4) Memory assessed with the Hopkins Verbal Learning Test (HVLT) (Brandt, 1991) and the Brief Visual Memory Test (BVMT) (Benedict et al., 1996); 5) Visuospatial ability measured with the subtest Incomplete letters from the Visual Object and Space Perception (VOSP) (Rapport et al., 1998) and the subtest cube analysis from the VOSP.
Classification for PD-MCI followed Level II of Movement Disorders Society Task Force criteria corresponding to a comprehensive assessment (Litvan et al., 2012). PD patients that showed impairment in two tests within a single cognitive domain or impairment in at least two tests in different cognitive domains were classified as PD-MCI. PD scores were considered impaired when the score was 1.5 standard deviations (SD) below the mean of the matched HC group. PD patients who failed to meet these specific criteria were classified as PD-NC.
2.3. Neuroimaging acquisition
Imaging data were acquired in a 3 T MRI scanner (Philips Achieva TX) at OSATEK, Hospital of Galdakao. All sequences were acquired during a single session. T1-weighted images acquisition were obtained in a sagittal orientation (TR = 7.4 ms, TE = 3.4 ms, matrix size = 228 × 218; flip angle = 9°, FOV = 250x250mm, slice thickness = 1.1 mm, 300 slices, voxel size = 0.98 × 0.98 × 0.60 mm, acquisition time = 4′55″).
The rs-fMRI was obtained in an axial orientation in an anterior-posterior phase direction using sequence sensitive to blood oxygen level dependent (BOLD) contrast and multi-slice gradient echo EPI sequence (TR = 2100 ms, TE = 16 ms, matrix size = 80 × 78, flip angle = 80°, FOV = 240x240mm, slice thickness = 3 mm, slice gap = 0.25 mm, 214 volumes, 40 slices, voxel size = 3.00 × 3.00 × 3.00 mm, acquisition time = 7′40″).
Rs-fMRI data were acquired during a so-called resting-state block. Subjects were instructed to neither engage in any particular cognitive nor motor activity, to keep their eyes closed without thinking about anything in particular and they were told they could not fall asleep. Once the rs-fMRI acquisition terminated, the participant was asked whether they had fallen asleep or not. No patient reported having fallen asleep. Foam padding and headphones were used to limit head movement and reduce scanner noise for the subject.
2.4. Neuroimaging preprocessing
Preprocessing for rs-fMRI data was performed using the Conn Functional Connectivity Toolbox 14.p (Whitfield-Gabrieli and Nieto-Castanon, 2012). All preprocessing steps were conducted using the default preprocessing pipeline for volume-based analysis (to MNI-space). Three volumes were acquired previous to the 214 volumes of the rs-fMRI acquisition and were then discarded prior to the analysis. First, each subject's 214 functional images were realigned to the first volume and unwarped (which implements the removal of dynamic EPI distortions, movement-by-susceptibility interactions as described in http://www.fil.ion.ucl.ac.uk/spm/toolbox/unwarp/), slice-timing corrected (interleaved botton-up), co-registered with structural data, spatially normalized into the standard MNI space (Montreal Neurological Institute) and finally images were smoothed using a Gaussian kernel of 6 mm FWMH. Moreover, noise was reduced via the anatomical CompCor approach, which extracts principal components from white matter and cerebrospinal fluid time series. These components were added as confounds in the denoising step of the CONN toolbox. The six head motion parameters derived from spatial motion correction were also added as cofounds. As recommended band-pass filtering was performed with a frequency window of 0.008 to 0.09 Hz (Weissenbacher et al., 2009). Linear detrending was additionally performed.
2.5. Group ICA
After preprocessing the data, Group ICA of fMRI Toolbox (GIFT) was used to decompose the data into functional networks using group spatial independent component analysis (ICA) (Calhoun et al., 2001). First, subject-specific data was reduced to 120 independent components (ICs) with the principal component reduction as previously done (Allen et al., 2014, Damaraju et al., 2014). In a second step group-data was reduced to 100 ICs with the expectation maximization algorithm (Roweis, 1998). To ensure stability and validity we repeated 20 times the Infomax ICA algorithm in ICASSO (Himberg et al., 2004). Aggregated spatial maps were estimated. The back reconstruction approach (GICA) was used to obtain subject-specific maps and time courses as implemented in GIFT software (Calhoun et al., 2001). Visual inspection and the spatial correlation values between ICs and the template were used for ICs selection (Shirer et al., 2012), based on the FC atlas networks of mialab (http://mialab.mrn.org/data/index.html), according to these 7 categories: Subcortical (SC), Auditory (AUD), Somatomotor (SM), visual (VI), cognitive-control (CC), default-mode (DMN), and cerebellar (CB) networks (Allen et al., 2014). Components were classified as intrinsic connectivity networks (ICNs) if they exhibited peak activations in grey matter, high correlation values with resting-state networks, and had time courses dominated by low-frequency fluctuations (Cordes et al., 2000). This process resulted in 29 ICs out of the 100 ICs obtained, divided in: 2 ICs in the SC network, 2 ICs in the AUD network, 5 ICs in the SM network, 5 ICs in the VI network, 6 ICs in the CC (which included the salience network and language network), 7 ICs in the DMN and 2 ICs in the CB network (see Fig. 1; Supplementary Table 1).
Fig. 1.
Spatial Maps from the ICs (A) and the static FC between them in the whole sample (B)
A) Spatial Maps from the 29 Independent Components (ICs) and B) the static FC between them in the whole sample. 29 ICs are divided in 7 networks. Each IC has a specific color in A) which corresponds to the color in B). Each ICs in B) have a specific label, which represents bilateral activations unless it is specified Left (L) or Right (R). The colorbar represents the value of the correlations; Red color represents positive correlations, Blue color represents negative correlations. BA = Brodmann Area; TTG = transverse temporal lobe; STG = superior temporal gyrus; PreCG = precentral gyrus; SPL = superior parietal lobe; LingualG = lingual gyrus; IOL = inferior occipital lobe; MFG = middle frontal gyrus; IPL = inferior parietal lobe; MTG = middle temporal lobe; IFG = inferior frontal gyrus; MPFC = medial prefrontal Cortex; AngG = angular gyrus; InfTL = Inferior temporal lobe; CB = Cerebellum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
After ICs selection, subject-specific spatial maps and time courses were post-processed, following (Allen et al., 2014), and included a detrending, a filter cutoff of low frequency fluctuation set at 0.15, and despiking. Head movement effect was regressed out to obtain more accurate results.
2.6. Motion correction
To minimize the impact of head motion in the connectivity results, maximum displacement and mean frame displacement (FD) were calculated. Subjects were excluded if the maximum displacement (absolute value) in translation indexes x, y, or z was higher than 3.0 mm and in rotation indexes was higher than 3.0° (Chen et al., 2016). No subject was excluded due to this criterion. Moreover, mean frame displacement (FD) was calculated for each subject using the published formula (Power et al., 2012). Subjects with a mean FD of > 0.5 mm were excluded from the analysis. Two PD subjects were excluded from the analysis due to this criterion. Therefore, the analyses were carried out with 35 PD patients and 26 HC. Mean FD values for each group are included in Table 1.
Table 1.
Sociodemographic and clinical characteristics of the sample.
| HC n = 26 |
PD-NC N = 12 |
PD-MCI N = 23 |
Statistic | p | |
|---|---|---|---|---|---|
| Age | 68.31 (7.52) | 65.17 (8.31) | 69.17 (4.48) | F = 1.44 | 0.243 |
| Gender (male) | 69% | 50% | 56% | χ2 = 1.42 | 0.489 |
| Education (years) | 11.31 (4.73) | 12.50 (4.33) | 9.22 (4.64) | H = 3.68 | 0.159 |
| MMSE | 28.85 (1.31) | 28.67 (1.30) | 26.86 (2.10) | H = 18.87 | < 0.001 |
| FD | 0.18 (0.07) | 0.18 (0.06) | 0.19 (0.08) | H = 0.463 | 0.793 |
| UPDRS III | – | 18.45 (6.91) | 22.65 (11.08) | t = 1.31 | 0.259 |
| H&Y | – | 1.79 (0.39) | 1.93 (0.50) | χ2 = 1.79 | 0.782 |
| Disease duration (years) | – | 5.93 (5.88) | 7.11 (5.67) | U = 108.00 | 0.294 |
| LEDD | – | 548.85 (459.64) | 904.52 (518.54) | t = − 2.05 | 0.054 |
All values are expressed in mean (SD).
HC = healthy controls; PD-NC = PD patients with normal cognition; PD-MCI = PD patients with mild cognitive impairment; MMSE = Mini-Mental State Examination; FD = Framewise displacement; UPDRS-III = Unified Parkinson's Disease Rating scale, Motor Score; H&Y = Hoehn and Yahr stages; LEDD = Levodopa Equivalent Daily Dose.
2.7. Dynamic FC analysis
Dynamic FC analysis was performed with the GIFT toolbox. A sliding time window of 22 TR method for each subject was applied (Allen et al., 2014), with a Gaussian window alpha value of 3, and a step between windows of 1 TR, resulting in the analysis of 192 windows. Due to the short time segments that could have insufficient information, the regularized inverse covariance matrix was used (Varoquaux et al., 2010). All the dynamic functional networks connectivity windows across all subjects were used to estimate the FC states. To do so, k-means clustering analysis was repeated 100 times to obtain the unbiased initial cluster, and was used to cluster the dynamic FC windows. K-means clustering applies Euclidean distance to regroup similar FC matrices of the different windows. The number of clusters (k) can be calculated in several ways. In this study we used the elbow criterion following previous dynamic FC studies (Allen et al., 2014, Damaraju et al., 2014) and the cluster number was set to 2. We used the Pearson correlation coefficient for clustering analysis, which is also the most widely used FC measure in rs-fMRI studies (Chang and Glover, 2010, Damaraju et al., 2014, Handwerker et al., 2012, Hutchison et al., 2013, Sakoğlu et al., 2010).
Indexes from dynamic FC were used to test differences between groups: 1) Mean dwell time defined as the number of consecutive windows in a specific state, or time that the subjects remain in the one FC state (Allen et al., 2014); 2) Number of transitions between states or state transition was calculated counting the total number of changes between states for each subject, and the differences between groups were assessed with two-sample t-test in the Statistical Package for Social Science (SPSS) (IBM SPSS Statistics 22).
In addition, FC differences between groups in each FC state were analysed with the network-based statistic (NBS) approach (Zalesky et al., 2010). The nodes were specified with the peak coordinates of each IC and the edges were represented with the correlation values in Z-scores. A nonparametric permutation approach (15,000 permutations) is applied. In each permutation, the group to which each subject belongs was randomly exchanged, and the statistical test is recalculated in each permutation in order to test the null-hypothesis. Then, p value was calculated FDR corrected for multiple comparisons.
2.8. Graph-theory parameter analysis
The Brain Connectivity Toolbox (BCT) (https://sites.google.com/site/bctnet/) was used to analyse the graph characteristics (both global and local aspects) of the networks obtained based on the ICs resulting from the ICA analysis. To ensure the same number of edges in the graphs from the different groups, a sparsity threshold needs to be fixed (Achard and Bullmore, 2007, Stam, 2014). Sparsity value was defined as the number of connections between nodes in a network divided by the total possible connections in that network. We selected sparsity of 0.34 to maximise global and local efficiency (Achard and Bullmore, 2007).
The global parameters (Bullmore and Sporns, 2009, Wang et al., 2011) assessed were: 1) Global efficiency defined as the efficiency of the network to transmit the information through the network; 2) Clustering coefficient of a network defined as the mean of clustering coefficients of each node in the network.
The local parameters assessed (Bullmore and Sporns, 2009, Wang et al., 2011) were: 1) Local efficiency defined as the efficiency of transmission of information from one node to other close nodes; 2) Clustering coefficient defined as the number of existing connections divided by the maximum number of possible connections; 3) Betweenness centrality reflected the relevance of a node in a network, and was defined as the number of shortest connections between two other nodes that should go through that node.
Graph-theoretical parameter analyses were adjusted with Bonferroni correction considering the number of intergroup comparisons.
2.9. Ethics statement
The study protocol was approved by the Ethics Committee at the Health Department of the Basque Mental Health System in Spain and the Ethics Committee of the University of Deusto. All subjects were volunteers and provided written informed consent prior to their participation in the study, in accordance with the Declaration of Helsinki.
2.10. Statistical analyses
The SPSS 22.0 was used to perform statistical analyses. Demographic, clinical and behavioral variables were tested for normality using the Shapiro-Wilk test. Sociodemographic differences between groups were tested with the Analysis of Variance (ANOVA) or Kruskal-Wallis test for 3-group comparisons and 2-tailed t-test or U-Mann Whitney for 2-group comparisons and chi-squared test for qualitative variables.
Regarding neuroimaging analysis, differences between groups were performed including age as covariate, following previous recommendations (Allen et al., 2011). LEDD was also included as covariate when assessing the differences between PD-MCI and PD-NC, due to its influence in fMRI signal (Mattay et al., 2002). Statistical differences between groups were performed using two sample t-tests. Finally, effect size was calculated with Cohen's d, considering 0.2, 0.5 and 0.8, small, medium and large effect sizes respectively (Hojat and Xu, 2004).
3. Results
3.1. Sociodemographic and cognitive characteristics of the sample
Twenty-three PD patients were classified as PD-MCI and 12 PD patients as PD-NC. Sociodemographic, clinical and disease characteristics of the sample are shown in Table 1. In addition, mean values of each cognitive domain and statistical differences between groups are shown in Table 2. Post-hoc analyses revealed significant impairment in all the cognitive domains assessed in the PD-MCI group compared with the PD-NC and HC (see Table 2).
Table 2.
Group differences in cognitive domains.
| HC n = 26 |
PD-NC n = 12 |
PD-MCI n = 23 |
F | p⁎ | |
|---|---|---|---|---|---|
| Attention and working memory | 0.32 (0.71) | 0.27 (0.44) | − 0.56 (0.82) | 4.24 | 0.019 |
| Memory | 0.42 (0.63) | 0.24 (0.33) | − 0.87 (0.49) | 17.59 | < 0.001 |
| Executive functions | 0.34 (0.34) | 0.40 (0.33) | − 0.83 (0.96) | 10.54 | < 0.001 |
| Language | 0.43 (0.84) | 0.24 (0.73) | − 0.76 (0.69) | 4.78 | 0.012 |
| Visuospatial | 0.39 (0.26) | 0.16 (0.34) | − 0.57 (0.83) | 7.94 | 0.001 |
Values are expressed in Z scores, mean (standard deviation).
HC = healthy controls; PD-NC = PD patients with normal cognition; PD-MCI = PD patients with mild cognitive impairment.
Significant differences were found between PD-MCI < HC and PD-MCI < PD-NC.
3.2. Dynamic FC differences
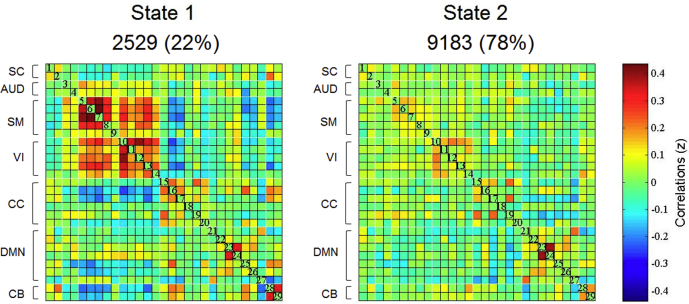
Following the elbow criterion, the whole sample showed 2 different states during the rs-fMRI acquisition (see Fig. 2). State 1 (22% of the windows) was characterized by the presence of stronger connectivity, with positive correlation between SM and VI networks, and anti-correlations between CC, DMN and CB; State 2 (78% of the windows) was characterized by weaker connectivity within and between networks, showing some modularity in SM, VI and DMN (see Fig. 2). PD-MCI patients showed significantly reduced mean dwell time in state 2, characterized by weaker connectivity, compared with the HC (t = 2.14; p = 0.030; Cohen'd = 0.61) (see Fig. 3). As well, PD-MCI patients showed significantly increased state transition compared with the HC (t = 2.82; p = 0.007; Cohen'd = 0.80) (see Fig. 3). PD-NC patients showed no significant differences in mean dwell time or the number of transitions between states compared with the HC or PD-MCI groups (see Fig. 3).
Fig. 2.
Dynamic FC states in the whole sample (k = 2).
Cluster centroids are shown for each state. The total number of occurrences and the percentage of total occurrences are shown for each state. The color bar represents the value of the correlations: Red color represents positive correlations, Blue color represents negative correlations. SC = Subcortical Network; AUD = Auditory Network; SM = Somatomotor Network; VI = Visual Network; CC = Cognitive Control Network; DMN = Default Mode Network; CB = Cerebellar Network. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Dynamic FC differences between groups.
*Significant differences between groups. HC = healthy controls; PD-NC = PD patients with normal cognition; PD-MCI = PD patients with mild cognitive impairment; FC = functional connectivity; SD = Standard deviation.
Additionally, FC differences were calculated between groups in each dynamic FC state. In state 2, significant FC differences between groups were found in PD-MCI patients compared with HC. PD-MCI patients showed reduced inter-network connectivity compared with the HC group (see Table 3, Fig. 4). Connectivity reductions were found mostly between the SM and CC networks, but SM-VI, SM-AUD, CC-VI and SC-DMN reductions were also found in PD-MCI compared with the HC (see Table 3, Fig. 4). We found no significant differences in state 1 between PD-MCI and HC and no significant differences were found between any other groups.
Table 3.
FC reductions in state 2 in PD-MCI compared with HC.
| Inter-network connectivity reduction | t⁎ | Cohen'd | |
|---|---|---|---|
| SM-CC | |||
| BA4R- Precentral gyrus | BA8R- Middle frontal gyrus | 3.91 | 1.11 |
| BA4L- Precentral gyrus | BA8R- Middle frontal gyrus | 3.20 | 0.91 |
| BA47L- Inferior frontal gyrus | 3.29 | 0.94 | |
| BA6L- Paracentral lobe | BA40R- Inferior parietal lobe | 3.51 | 1.00 |
| SM-VI | |||
| BA4R- Precentral gyrus | BA19R- Inferior Occipital Lobe | 3.51 | 1.00 |
| BA6L- Paracentral lobe | BA19R- Cuneus (Occipital Lobe) | 3.78 | 1.08 |
| SM-AUD | |||
| BA6L- Paracentral lobe | BA22R- Superior temporal gyrus | 4.38 | 1.25 |
| CC-VI | |||
| BA8R- Middle frontal gyrus | BA19R- Inferior Occipital Lobe | 5.31 | 1.52 |
| SC-DMN | |||
| Thalamus L | BA36- Uncus (Limbic Lobe) | 3.10 | 0.88 |
FC = Functional Connectivity; PD-MCI = PD patients with mild cognitive impairment; HC = healthy controls; SM = Somatomotor Network; CC = Cognitive Control Network; VI = Visual Network; AUD = Auditory Network; SC = Subcortical Network; DMN = Default Mode Network; BA = Brodmann area. R = Right; L = Left.
Results at p < 0.05 FDR-corrected.
Fig. 4.
FC reductions in state 2 in PD-MCI compared with HC.
FC = Functional Connectivity; PD-MCI = PD patients with mild cognitive impairment; HC = healthy controls; BA = Brodmann area. R = Right; L = Left; S = Superior; I = Inferior; P = Posterior.
Results at p < 0.05 FDR-corrected.
3.3. Graph topological parameters
Results revealed that PD-MCI patients showed a reduced clustering coefficient in the right precentral gyrus (BA4; SM) compared with the HC (t = 3.54; p < 0.001; Cohen'd = 1.03). As well, PD-MCI patients also showed reduced betweenness centrality in the left paracentral gyrus (BA6; SM) compared with PD-NC patients (t = 3.57; p < 0.001; Cohen'd = 1.03). We found no significant differences in graph parameters between PD-NC and the HC.
4. Discussion
The aim of this study was to evaluate dynamic FC and local/global connectivity in PD-MCI and PD-NC using the combination of dynamic FC and graph-theoretical approaches during rs-fMRI. Findings suggest dynamic FC alternations in PD-MCI patients during rs-fMRI that were accompanied by graph topological dysfunctions in the SM network and reduced FC between networks, whereas PD-NC patients showed similar patterns of FC compared with HC.
The whole sample presented two different connectivity states, a hyper-connected state and a hypo-connected state. The sparsely connected state was present 78% of the time. Results showed that PD-MCI patients exhibited dynamic FC alterations compared with the HC. In a recent dynamic FC study in PD, the sample also showed two dynamic FC states, and PD patients significantly spent less time in the hypo-connected state compared to HC group (Kim et al., 2017). Similarly, previous dynamic FC studies have also been performed with schizophrenia patients, and also showed alterations in mean dwell time compared with the HC (Damaraju et al., 2014, Du et al., 2016, Lottman et al., 2017). Specifically, the present study analysed the dynamic FC pattern in PD patients with MCI and with normal cognition. Results showed that PD-MCI patients spent significantly less time in the state characterized by hypo-connectivity compared with the HC. These differences were not found in PD-NC patients. Therefore, findings suggest that the dynamic FC pattern could be associated with the presence of MCI in PD patients. Previous static FC studies in PD also showed a relationship between FC pattern during rs-fMRI and MCI (Amboni et al., 2015, Baggio et al., 2015, Gorges et al., 2015), and the present study suggest that temporal properties of FC in PD could also be related to cognitive performance.
Moreover, PD-MCI patients showed increased number of changes between states compared with the HC. Contrary to PD-MCI patients, there were no dynamic FC significant differences between PD-NC and the HC group, neither in mean dwell time nor state transitions. However, despite not finding significant differences, PD-NC patients showed slight increased state transitions compared to the HC. The previous dynamic FC study in PD subjects without MCI diagnosis showed no significant differences in state transitions compared to HC, but the number of transitions was also slightly elevated compared to HC (Kim et al., 2017). Results in both studies might represent a gradual dysfunctional pattern in PD patients that increases with more severe cognitive deterioration. Dynamic FC analysis could add relevant information about the neural substrates of PD-MCI deterioration, and its differences with PD-NC patients.
Furthermore, FC differences between groups in each dynamic FC state were also investigated. PD-MCI patients in this study showed reduced FC in the hypo-connected state compared with the HC, showing reduced inter-network connectivity mostly between the SM and CC networks, but also between the SM-VI, SM-AUD, CC-VI and SC-DMN networks. Previous studies in PD-MCI patients also found reduced FC compared with the HC. Interestingly, reduced FC in PD-MCI has also been found between the precentral gyrus and middle frontal gyrus (in this study: SM-CC) (Baggio et al., 2015), reduced FC between Rolandic operculum and cuneus (in this study: SM-VI) (Göttlich et al., 2013), reduced connectivity between medial frontal lobe and cuneus in PD (in this study: CC-VI) (Göttlich et al., 2013) and reduced connectivity between the medial frontal and occipital lobes in PD-MCI (in this study: CC-VI) (Baggio et al., 2015). The disconnection between networks has only been found in the PD-MCI group, while PD-NC patients showed no significant differences compared with the HC. These results, added to previous results in PD patients with MCI (Amboni et al., 2015, Baggio et al., 2015, Gorges et al., 2015, Lucas-Jiménez et al., 2016), suggest that reduced connectivity is linked to the presence of cognitive deficits in PD patients.
Dynamic FC alterations in PD-MCI patients were accompanied by impairment in graph topological parameters in two nodes located in the SM network (right BA4 and left BA6). Nodes with high level of betweenness centrality are important in a network, due to its crucial role in transferring the information (Bullmore and Sporns, 2009). The reduced betweenness centrality in the node BA6 (left hemisphere) in PD-MCI group suggests a poorer communication between the adjacent nodes in the network. Moreover, the clustering coefficient of a specific node is related with an efficient communication; therefore, PD-MCI patients might show a loss of efficiency when transferring the information in the node BA4 (right hemisphere). Both nodes were located in the SM network and remarkably, most of the reduced FC found in the PD-MCI group, was located between these two nodes and other brain regions. This may suggest that the reduced efficiency when transferring the information in the SM network could have influenced the poorer FC between SM network and other networks in PD-MCI patient.
Graph theoretical analyses could add information to the FC results in order to better understand the neurobiological process of cognitive deterioration in PD. Previous graph theoretical studies in PD also found reduced clustering coefficient (Tinaz et al., 2016) and reduced betweenness centrality (Koshimori et al., 2016) in different nodes of the SM network in PD patients compared with the HC. In the present study, the dysfunctional graph parameters were found in PD-MCI patients and not in PD-NC. A previous study that analysed graph differences between PD-MCI and PD-NC, found graph alterations in PD-MCI patients compared with the HC but not in PD-NC patients (Baggio et al., 2014). Both studies suggest a relationship between cognitive impairment and graph theoretical dysfunctions in PD that are not present in PD-NC. Contrary to results in this study, previous PD studies also found increased graph parameters compared to the HC (Baggio et al., 2014, Göttlich et al., 2013, Zhang et al., 2016). Specifically Baggio et al. (2014) reported that PD-MCI patients showed increased graph characteristics in nodes that normally have lower network relevance, as a compensatory mechanism for the reduced graph parameters found in more important nodes. PD-MCI patients in the present study were older, showed longer disease duration, higher scores on UPDRS III (motor complications) and lower scores on MMSE compared to the PD-MCI patients from the study of Baggio et al. (2014). We hypothesized that all these characteristic differences could be related to the absence of brain compensatory mechanisms found in the PD-MCI sample from this study.
Some limitations of the study must be considered. First, the length of the rs-fMRI acquisition is 7′39″. A recent study suggested that dynamic FC analysis should be performed with rs-fMRI acquisitions of > 10 mins (Hindriks et al., 2016). Also, the TE of the resting-state fMRI acquisition in this study is quite low. In addition, independent monitoring of wakefulness is a more accurate technique to control if any patients fell asleep during the rs-fMRI acquisition. Moreover, all patients from this study were on medication. It would be interesting to analyse dynamic FC in drug-naïve PD patients because of the relevance of PD medication in fMRI signal (Mattay et al., 2002). Moreover, PD-MCI patients were not distinguished between single domain MCI and multiple domain MCI. Future studies should assess the dynamic FC and graph theoretical characteristic differences between PD-MCI subtypes. Finally, longitudinal follow-up assessment would give us information about the dynamic FC progression of PD-MCI and PD-NC patients.
5. Conclusions
This is the first study to assess the dynamic FC characteristics in PD-MCI and PD-NC. Findings suggest that the temporal connectivity alterations found in PD-MCI such as reduced mean dwell time in the hypo-connected state and reduced state transitions, could be related to the presence of cognitive impairment in PD. Dynamic FC has proven to be a useful approach in the study of PD brain dysfunction, and more research on the disease needs to be done with this technique. Future studies should evaluate the use of dynamic FC to monitor and predict MCI in PD. Moreover, the loss of graph properties in nodes of the SM network could be related to the reduced FC between the SM and other networks in PD-MCI group compared to HC. The combination of neuroimaging approaches such as graph theory and FC analyses could help in the understanding of neurobiological substrates of MCI in PD.
Acknowledgments
Acknowledgements
The authors would like to thank ASPARBI and all of the patients involved in the study. This study was supported by the Department of Health of the Basque Government [2011111117], the Spanish Ministry of Economy and Competitiveness [PSI2012-32441], and the Department of Education and Science of the Basque Government (Equipo A) [IT946-16].
Conflict of interest
The authors declare no conflict of interest.
The following are the supplementary data related to this article.
Peak coordinates of the ICNs.
Contributor Information
María Díez-Cirarda, Email: maria.dcirarda@deusto.es.
Antonio P. Strafella, Email: Antonio.Strafella@camh.ca.
Jinhee Kim, Email: jin-hee.kim@camhpet.ca.
Javier Peña, Email: javier.pena@deusto.es.
Natalia Ojeda, Email: nojeda@deusto.es.
Alberto Cabrera-Zubizarreta, Email: acabrera@osatek.net.
Naroa Ibarretxe-Bilbao, Email: naroa.ibarretxe@deusto.es.
References
- Aarsland D., Bronnick K., Larsen J.P., Tysnes O.B., Alves G., Norwegian ParkWest Study Group Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. 2007;3 doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R. A baseline for the multivariate comparison of resting-state networks. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb.Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amboni M., Tessitore A., Esposito F., Santangelo G., Picillo M., Vitale C., Giordano A., Erro R., de Micco R., Corbo D. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J. Neurol. 2015;262:425–434. doi: 10.1007/s00415-014-7591-5. [DOI] [PubMed] [Google Scholar]
- Baggio H., Sala-Llonch R., Segura B., Marti M., Valldeoriola F., Compta Y., Tolosa E., Junqué C. Functional brain networks and cognitive deficits in Parkinson's disease. Hum. Brain Mapp. 2014;35:4620–4634. doi: 10.1002/hbm.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio H., Segura B., Sala-Llonch R., Marti M., Valldeoriola F., Compta Y., Tolosa E., Junque C. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum. Brain Mapp. 2015;36:199–212. doi: 10.1002/hbm.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H., Schretlen D., Groninger L., Dobraski M., Shpritz B. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol. Assess. 1996;8:145. [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kotter R., Li S.J., Lin C.P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.J., Veijola J., Villringer A., Walter M., Wang L., Weng X.C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.F., Zhang H.Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin. Neuropsychol. 1991;5:125–142. [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calhoun V., Adali T., Pearlson G., Pekar J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Miller R., Pearlson G., Adalı T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Glover G.H. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.J., Chen Q.F., Liu J., Shi H.B. Aberrant salience network and its functional coupling with default and executive networks in minimal hepatic encephalopathy: a resting-state fMRI study. Sci. Rep. 2016;6:27092. doi: 10.1038/srep27092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher L., Strafella A.P. Neuroimaging of brain changes associated with cognitive impairment in Parkinson's disease. 2013;7:225–240. doi: 10.1111/jnp.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., Wendt G.J., Turski P.A., Moritz C.H., Quigley M.A., Meyerand M.E. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Damaraju E., Allen E., Belger A., Ford J., McEwen S., Mathalon D., Mueller B., Pearlson G., Potkin S., Preda A. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Pearlson G.D., Yu Q., He H., Lin D., Sui J., Wu L., Calhoun V.D. Interaction among subsystems within default mode network diminished in schizophrenia patients: a dynamic connectivity approach. Schizophr. Res. 2016;170:55–65. doi: 10.1016/j.schres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G.W., Firbank M.J., Yarnall A.J., Khoo T.K., Brooks D.J., Barker R.A., Burn D.J., O'Brien J.T. Gray and white matter imaging: a biomarker for cognitive impairment in early Parkinson's disease? 2016;31:103–110. doi: 10.1002/mds.26312. [DOI] [PubMed] [Google Scholar]
- Elgh E., Domellöf M., Linder J., Edström M., Stenlund H., Forsgren L. Cognitive function in early Parkinson's disease: a population-based study. 2009;16:1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- Gao L., Wu T. The study of brain functional connectivity in Parkinson's disease. 2016;5:18. doi: 10.1186/s40035-016-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden C.J. TEA ediciones. 1994. STROOP: Test de colores y palabras: Manual. [Google Scholar]
- Gorges M., Müller H., Lulé D., Pinkhardt E.H., Ludolph A.C., Kassubek J., Consortium LANDSCAPE. To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol. Aging. 2015;36:1727–1735. doi: 10.1016/j.neurobiolaging.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Göttlich M., Münte T.F., Heldmann M., Kasten M., Hagenah J., Krämer U.M. Altered resting state brain networks in Parkinson's disease. 2013;8 doi: 10.1371/journal.pone.0077336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker D.A., Roopchansingh V., Gonzalez-Castillo J., Bandettini P.A. Periodic changes in fMRI connectivity. NeuroImage. 2012;63:1712–1719. doi: 10.1016/j.neuroimage.2012.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely M.A., Reid W.G., Adena M.A., Halliday G.M., Morris J.G. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Himberg J., Hyvärinen A., Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hindriks R., Adhikari M.H., Murayama Y., Ganzetti M., Mantini D., Logothetis N.K., Deco G. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage. 2016;127:242–256. doi: 10.1016/j.neuroimage.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998;50:318–334. doi: 10.1212/wnl.50.2.318. [DOI] [PubMed] [Google Scholar]
- Hojat M., Xu G. A visitor's guide to effect sizes–statistical significance versus practical (clinical) importance of research findings. 2004;9:241–249. doi: 10.1023/B:AHSE.0000038173.00909.f6. [DOI] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M., Della Penna S., Duyn J.H., Glover G.H., Gonzalez-Castillo J. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D.I., Cummings J.L., Ketchel P., Smith V., MacMillan A., Shelley T., Lopez O.L., DeKosky S.T. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J. Neuropsychiatr. Clin. Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kim J., Criaud M., Cho S., Díez-Cirarda M., Mihaescu A., Valli M., Ghadery C., Coakeley S., Jacobs M., Houle S., Strafella A.P. Abnormal intrinsic brain functional network dynamics in Parkinson's disease. 2017;140:2955–2967. doi: 10.1093/brain/awx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimori Y., Cho S., Criaud M., Jacobs M., Ghadery C., Coakeley S., Harris M., Mizrahi R., Hamani C., Lang A.E., Houle S., Strafella A.P. Disrupted nodal and hub organization account for brain network abnormalities in Parkinson's disease. 2016;8:259. doi: 10.3389/fnagi.2016.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi I., McDonald K., Pantula H., Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J. Geriatr. Psychiatry Neurol. 2012;25:208–214. doi: 10.1177/0891988712464823. [DOI] [PubMed] [Google Scholar]
- Litvan I., Goldman J.G., Tröster A.I., Schmand B.A., Weintraub D., Petersen R.C., Mollenhauer B., Adler C.H., Marder K., Williams-Gray C.H. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo A., Saz P., Marcos G., Día J.L., de la Cámara C., Ventura T., Morales Asín F., Fernando Pascual L., Montañés J.Á., Aznar S. 2001. Revalidación y normalización del Mini-Examen Cognoscitivo (primera versión en castellano del Mini-Mental Status Examination) en la población general geriátrica. [PubMed] [Google Scholar]
- Lottman K.K., Kraguljac N.V., White D.M., Morgan C.J., Calhoun V.D., Butt A., Lahti A.C. Vol. 8. 2017. Risperidone Effects on Brain Dynamic Connectivity–a Prospective Resting State fMRI Study in Schizophrenia; p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Jiménez O., Ojeda N., Peña J., Díez-Cirarda M., Cabrera-Zubizarreta A., Gómez-Esteban J.C., Gómez-Beldarrain M.Á., Ibarretxe-Bilbao N. Altered functional connectivity in the default mode network is associated with cognitive impairment and brain anatomical changes in Parkinson's disease. Parkinsonism Relat. Disord. 2016 doi: 10.1016/j.parkreldis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Luo C.Y., Guo X.Y., Song W., Chen Q., Cao B., Yang J., Gong Q.Y., Shang H. Functional connectome assessed using graph theory in drug-naive Parkinson's disease. J. Neurol. 2015;262:1557–1567. doi: 10.1007/s00415-015-7750-3. [DOI] [PubMed] [Google Scholar]
- Mainland B.J., Shulman K.I. Anonymous Cognitive Screening Instruments. Springer; 2013. Clock drawing test; pp. 79–109. [Google Scholar]
- Martinez-Martin P., Gil-Nagel A., Gracia L.M., Gómez J.B., Martínez-Sarriés J., Bermejo F. Unified Parkinson's disease rating scale characteristics and structure. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- Mattay V.S., Tessitore A., Callicott J.H., Bertolino A., Goldberg T.E., Chase T.N., Hyde T.M., Weinberger D.R. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann. Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Pena-Casanova J., Quinones-Ubeda S., Quintana-Aparicio M., Aguilar M., Badenes D., Molinuevo J.L., Torner L., Robles A., Barquero M.S., Villanueva C., Antunez C., Martinez-Parra C., Frank-Garcia A., Sanz A., Fernandez M., Alfonso V., Sol J.M., Blesa R., Study Team NEURONORMA. Spanish multicenter normative studies (NEURONORMA project): norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch. Clin. Neuropsychol. 2009;24:321–341. doi: 10.1093/arclin/acp038. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport L.J., Millis S.R., Bonello P.J. Validation of the Warrington theory of visual processing and the Visual Object and Space Perception Battery. 1998;20:211–220. doi: 10.1076/jcen.20.2.211.1169. [DOI] [PubMed] [Google Scholar]
- Rosenthal E., Brennan L., Xie S., Hurtig H., Milber J., Weintraub D., Karlawish J., Siderowf A. Association between cognition and function in patients with Parkinson disease with and without dementia. 2010;25:1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roweis S.T. 1998. EM Algorithms for PCA and SPCA; pp. 626–632. [Google Scholar]
- Sakoğlu Ü., Pearlson G.D., Kiehl K.A., Wang Y.M., Michael A.M., Calhoun V.D. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. 2010;23:351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D. 1989. Brief Test of Attention. [Google Scholar]
- Shirer W., Ryali S., Rykhlevskaia E., Menon V., Greicius M. Decoding subject-driven cognitive states with whole-brain connectivity patterns. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore F., Korenkevych D., Liu Y., He G., Bullmore E., Pardalos P.M. Connectivity brain networks based on wavelet correlation analysis in Parkinson fMRI data. Neurosci. Lett. 2011;499:47–51. doi: 10.1016/j.neulet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Stam C.J. Modern network science of neurological disorders. 2014;15:683–695. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- Tinaz S., Lauro P., Hallett M., Horovitz S.G. Deficits in task-set maintenance and execution networks in Parkinson's disease. 2016;221:1413–1425. doi: 10.1007/s00429-014-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel, Martijn P., Pol H.E.H. Exploring the brain network: a review on resting-state fMRI functional connectivity. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Varoquaux G., Gramfort A., Poline J., Thirion B. 2010. Brain covariance selection: better individual functional connectivity models using population prior; pp. 2334–2342. [Google Scholar]
- Wang J., Zuo X., Gohel S., Milham M.P., Biswal B.B., He Y. Graph theoretical analysis of functional brain networks: test-retest evaluation on short-and long-term resting-state functional MRI data. 2011;6 doi: 10.1371/journal.pone.0021976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yesavage J.A., Sheikh J.I. 9/Geriatric depression scale (GDS) recent evidence and development of a shorter violence. Clin. Gerontol. 1986;5:165–173. [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zhang D., Liu X., Chen J., Liu B., Wang J. Widespread increase of functional connectivity in Parkinson's disease with tremor: a resting-state FMRI study. 2015;7:6. doi: 10.3389/fnagi.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu I., Tosun D., Foster E., Schuff N. Vol. 11. 2016. Progression of Regional Microstructural Degeneration in Parkinson's Disease: A Multicenter Diffusion Tensor Imaging Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peak coordinates of the ICNs.