Abstract
Background
The Cognitive Avoidance Theory of Worry argues that worry is a cognitive strategy adopted to control the physiological arousal associated with anxiety. According to this theory, pathological worry, as in Generalized Anxiety Disorder (GAD), is verbal in nature, negative and abstract, rather than concrete. Neuroimaging studies link the expression of worry to characteristic modes of brain functional connectivity, especially in relation to the amygdala. However, the distinctive features of worry (verbal, abstract, negative), and their relationship to physiological arousal, have not so far been mapped to brain function.
Methods
We addressed this omission by undertaking a resting-state functional magnetic resonance neuroimaging study of 19 patients with GAD and 21 controls, before and after induction of perseverative cognitions, while measuring emotional bodily arousal from heart rate (HR). Seed-based analyses quantified brain changes in whole brain functional connectivity from the amygdala.
Results
In GAD, the induction increased negative thoughts and their verbal content. In line with predictions, the verbal expression of worry in GAD was associated with higher HR at baseline and attenuated HR increases after induction of perseverative cognitions. Within brain, the increased use of words during worry, and the associated dampening of HR after induction were mediated by the strength of functional connectivity between the amygdala and default mode network ‘hubs’ and the opercular cortex. The negative content of worry was further related to functional communication between amygdala and cingulo-opercular and temporal cortices.
Conclusions
Findings provide a neurobiological basis for the impact of verbal worry on HR in GAD.
Abbreviations: GAD, Generalized Anxiety Disorder; rsfMRI, resting-state functional magnetic resonance neuroimaging; HR, heart rate; NYC-Q, New York Cognition Questionnaire; DMN, default mode network; HC, Healthy Controls; SCID, Structured Clinical Interview for DSM; BDI, Beck Depression Inventory; RRS, Ruminative Response Scale; PSWQ, Penn State Worry Questionnaire; STAI, Spielberger State Trait Anxiety Inventory; EPI, echoplanar imaging; BOLD, blood oxygenation level dependent; PCC, posterior cingulate cortex
Keywords: Generalized anxiety disorder, Amygdala, Functional connectivity, Worry, Heart rate, New York Cognition Questionnaire
Highlights
-
•
More negative worrisome thoughts have more words in GAD and more images in controls.
-
•
Thinking in words is associated with reduced cardiac reactivity during worry.
-
•
Verbal, abstract, and negative features of worry have unique neural correlates.
-
•
Amygdala functional connectivity mediates use of words and HR decrease during worry.
-
•
A neurobiological basis for the impact of verbal worry on HR in GAD is provided.
1. Introduction
Worry describes repetitive thoughts about potentially negative events in the future. Although worry is common to almost everybody's experience, it becomes chronic and uncontrollable in Generalized Anxiety Disorder (GAD; DSM-5, American Psychiatric Association, 2013). One puzzle underlying pathological worry is why individuals engage in this form of cognition in the absence of evidence that it serves an adaptive purpose.
The Cognitive Avoidance Theory of Worry is an influential account for understanding why patients with GAD spend so much time worrying. This theory proposes that worry is implemented by patients as an avoidance strategy, aimed at controlling physiological arousal engendered by anxiety (Borkovec, 1994, Borkovec et al., 2004). Pathological worry is typically verbal: both healthy and psychopathological individuals shift the nature of their cognitions towards negative verbal thoughts when instructed to think about a current concern (Behar et al., 2005, Borkovec and Inz, 1990, Stöber et al., 2000). Moreover, successful therapeutic outcomes for patients with GAD are accompanied by reduction in the ratio between reported words and mental imagery (Borkovec and Inz, 1990). Consistent with the model, verbal articulation of fearful thoughts attenuates cardiovascular reactivity, whereas mental imagery of the same material elicits exaggerated cardiovascular responses (e.g., Borkovec and Hu, 1990, Borkovec et al., 1993, Hazlett-Stevens and Borkovec, 2001, Peasley-Miklus and Vrana, 2000, Vrana et al., 1989). Lastly, in support of the notion that individuals with GAD use their worry to try to control emotional arousal, when asked why they worry, patients answer that ‘worry helps distract me from more emotional topics’ or ‘prepare for the worst’ (Borkovec and Roemer, 1995, Davey et al., 1996, Freeston et al., 1994). Nevertheless, this mechanism can also explain the chronic nature of anxiety in GAD patients: the reduction in emotional processing following ‘successful’ verbal worrying limits exposure to anxiety-provoking material, and thereby prevents normal adaptive habituation (Borkovec et al., 2004). Despite this evidence supporting the Cognitive Avoidance Theory, not all studies show attenuated physiological arousal during worry (see Ottaviani et al., 2016a for a meta-analysis) and alternative theoretical explanations have emerged (e.g., the Contrast Avoidance Model by Newman and Llera, 2011).
Our study combined functional brain imaging with peripheral physiological monitoring to test for a hypothesized association between worry and up-regulation of regions linked to language and cognitive control processes (including lateral prefrontal cortex and inferior frontal gyrus) with concurrent down-regulation of brain regions implicated in affective processing and emotion-related cardiovascular reactivity. This work extends previous observations regarding the neurobiological signatures of pathological worry that are consistent with the Cognitive Avoidance Theory of Worry. A negative association between worry scores and amygdala-prefrontal functional connectivity was observed after a worry induction, suggesting efficient top down suppression of fearful imagery associated with the worry (Meeten et al., 2016). Moreover, stronger engagement of the prefrontal cortex (reflected by increased connectivity with bilateral amygdala) is associated with attenuation of dysregulated autonomic arousal during worry in individuals with GAD, confirming that worry may act as to suppress physiologic arousal in this clinical population (Makovac et al., 2016b).
Building on these results, our study aimed to characterize patterns of resting state functional connectivity in relation to the modality of participants' thoughts during worry in patients with GAD and healthy controls. We employed a validated measure of ongoing cognition, the New York Cognition Questionnaire (NYC-Q; Gorgolewski et al., 2014, see also Sanders et al., 2017). The questionnaire has established utility in neuroimaging studies, where self-generated verbal cognitions, across a large normative sample, were inversely associated with engagement of regions of retrosplenial cortex closely linked to the hippocampus (Gorgolewski et al., 2014). In our study, we specifically tested how the content and form of worry, measured by the NYC-Q, related to the functional connectivity of the amygdala at rest and bodily arousal indexed by heart rate (HR). We focused on the dimensions of the NYC-Q that measure well-recognized characteristics of worrisome thoughts (i.e., verbal, abstract/vague, and negative). To obtain measures of bodily arousal and the form of thoughts during worry, a behavioural induction of perseverative cognition was used. We predicted that the induced worry would be predominantly verbal, negative, and vague particularly in individuals with GAD. Considering that functional connectivity under multiple mental states is essential to disentangle connectivity differences that are transient versus those that represent more stable, trait-like characteristics of an individual (Geerligs et al., 2015), we measured functional connectivity at rest, and also quantified changes in functional connectivity from pre to post-induction of perseverative cognitions. As hypothesized by the Cognitive Avoidance Theory of Worry, we predicted that there are differences in amygdala functional connectivity during verbal, compared to imagery-related, worry and that these differences are linked to attenuation of physiological arousal after worry induction. Borkovec's Theory not only describes worrisome thoughts as verbal, but also as negative and abstract in nature; therefore, we also tested how distinct patterns of amygdala functional connectivity were associated with these specific features of worry. Previous findings linking resting state functional connectivity in healthy individuals to dimensions of the NYC-Q, led us to broadly predict that functional connectivity to the limbic region and to default mode network (DMN) respectively would be associated with more negative and more abstract thoughts.
2. Methods and materials
2.1. Participants
The present study is based on a secondary analysis of data from a larger longitudinal functional magnetic imaging (fMRI) study (Makovac et al., 2016a, Makovac et al., 2016b, Makovac et al., 2016c, Meeten et al., 2016, Ottaviani et al., 2016b). Nineteen patients (17 women, 2 men; mean age = 29.58 ± 6.93 years) who met diagnostic criteria for GAD and 21 healthy controls (HC; 18 women, 3 men; mean age = 28.67 ± 9.45 years) participated in the study. Only 1 participant was non-Caucasian. Patients and HC were recruited from public advertisement. All participants were right-handed, native English speakers, and had normal or corrected-to-normal vision. Exclusion criteria were: age below 18 years, past head injury or neurological disorders, history of major medical or psychiatric disorder (other than GAD and co-morbid depression in the patients), cognitive impairment, history of substance or alcohol abuse or dependence, heart disease, obesity (body mass index > 30 kg/m2), pregnancy, claustrophobia or other MRI exclusions. None of the participants had a formal diagnosis of comorbid major depressive disorder. Two patients with GAD were included who took long-term medication (1 Citalopram, 1 Pregabalin) at the time of the study. All other participants were medication free. All participants provided written informed consent. The study was approved by the National Research Ethics Service (NRES) with local approval the Brighton and Sussex Medical School Research Governance and Ethics Committee. Participants were compensated for their time.
2.2. Procedure
The Structured Clinical Interview for DSMIV (SCID) was administered to patients and controls to confirm/exclude the diagnosis of GAD. Participants then completed a series of online sociodemographic and dispositional traits questionnaires. Participants were subsequently familiarized with the neuroimaging environment, connected to the physiological recording equipment, and then underwent the MRI protocol.
2.3. Questionnaires
Each participant completed a set of questionnaires accessing socio-demographic and lifestyle information (nicotine, alcohol, and caffeine consumption, physical activity), and dispositional measures of: 1) depressive mood (Beck Depression Inventory, BDI; Beck et al., 1961); 2) anxious worry (Penn State Worry Questionnaire, PSWQ; Meyer et al., 1990); 3) depressive rumination (Ruminative Response Scale, RRS; Nolen-Hoeksema and Morrow, 1991); and 4) state and trait anxiety (Spielberger State Trait Anxiety Inventory, STAI; Spielberger, 1989).
The New York Cognition Questionnaire (NYC-Q; Gorgolewski et al., 2014) was used to assess content and form of worry during the resting state period following the perseverative cognition induction. All participants completed the questionnaire at the end of MRI scanning session, immediately after the last resting state scan. For each question (e.g., “During the task my thoughts were in the form of words”), participants are asked to indicate how well each statement described their thoughts on a scale from 1 - Completely did not describe my thoughts to 9 - Completely did describe my thoughts.
Scoring followed a previously described procedure (Gorgolewski et al., 2014): questions were decomposed using exploratory factor analysis to find interpretable latent components and the number of factors was estimated using parallel analysis (Horn, 1965). Factors were estimated using principal axis factor analysis. Individual-level scores were the calculated (ten Berge et al., 1999). After obtaining a weight matrix, individual-level question scores were transformed into individual-level factor scores. Given our specific hypothesis on the verbal, abstract, and negative form of worry (Borkovec, 1994), other dimensions of the NYC-Q (past/future, positive, and social) were not entered into our analyses.
2.4. Experimental design
In the scanner, each participant underwent a series of four 5-min resting state periods, each followed by a 6-min easy visuomotor tracking task (described elsewhere; Ottaviani et al., 2016b). During resting state periods the participant was instructed to rest with eyes open without thinking of anything and not falling asleep. After the second or third resting block, randomly, the participant underwent a recorded verbal induction procedure designed to engender perseverative cognition:
‘Next I would like you to recall an episode that happened in the past year that made you feel sad, anxious, or stressed or something that may happen in the future that worries you. Then, I would like you to think about this episode in detail, for example about its possible causes, consequences, and your feelings about it. Please keep thinking about this until the end of the next tracking task. Thank you. Please take as much time as you need to recall the episode and press the button whenever you are ready.’
The rationale for collapsing worrisome and ruminative thoughts into a single phenomenal category (i.e. perseverative cognition) is corroborated by studies showing no differences between these two processes on their impact on appraisals and strategies (Segerstrom et al., 2000, Watkins et al., 2005) and by the incremental benefits of using perseverative cognition as a transdiagnostic symptom (McEvoy et al., 2013, Spinhoven et al., 2015).
2.5. Physiological data processing
Heartbeats were monitored using MRI-compatible finger pulse oximetry (8600FO; Nonin Medical), recorded digitally as physiological waveforms (via a CED power 1401, using Spike2 v7 software; Cambridge Electronic, Design CED). Pulse data were manually checked and corrected for artifacts.
To quantify the impact of the induction on cardiac activity, change scores (Δ HR) were computed by subtracting the average HR before the induction from the average HR after the induction.
2.6. MRI acquisition and preprocessing
MRI images were acquired on a 1.5-Tesla Siemens Magnetom Avanto scanner. Structural volumes were obtained using the high-resolution three-dimension magnetization-prepared rapid gradient-echo sequence (HiResMPRAGE). Functional datasets used T2*weighted echoplanar imaging (EPI) sensitive to blood oxygenation level dependent (BOLD) signal (TR = 2.52 s, TE = 43 ms, flip-angle 90°, 34 slices, 3 mm slice thickness, 192 mm FOV, voxel size 3 × 3 × 3 mm).
Data were pre-processed using Statistical Parametric Mapping (Wellcome Department of Imaging Neuroscience; SPM8, http://www.fil.ion.ucl.ac.uk/spm/), and in-house software implemented in Matlab (Mathworks Inc., Natick, Massachussetts, USA). For each participant, the first four volumes of the fMRI series were discarded to allow for T1 equilibration effects. The pre-processing steps included correction for head motion, compensation for slice-dependent time shifts, normalization to the EPI template in standard space (MNI) coordinates provided with SPM8, and smoothing with a 3D Gaussian Kernel with 8 mm3 full-width at half maximum. The global temporal drift was removed using a 3rd order polynomial fit. To remove other potential sources of bias, data was further filtered regressing against the realignment parameters. As global signal removal can potentially change functional connectivity distributions and result in increased negative correlations (Saad et al., 2012), it was avoided in our pre-processing. Translational movement in millimeters (x, y, z) and rotational motion in degrees (pitch, roll, yaw) was calculated based on the SPM8 parameters for motion correction. None of the participants had a maximum absolute shift > 2 mm translation or exceed 1.5° of maximum absolute rotation. Then, all images were filtered by a phase-insensitive band-pass filter (pass band 0.01–0.08 Hz) to reduce the effect of low frequency drift and high frequency physiological noise.
2.7. Statistical analyses
All data are expressed as means (± SD). Differences at p ≤ 0.05 are regarded as significant. Data analysis was performed with SPSS 22.0 for Windows (SPSS Inc., USA). There were no baseline group differences on self-report socio-demographic measures (Makovac et al., 2016a).
Correlation analysis was performed to examine the associations between NYC-Q factors and HR and validated measures of worry, rumination and anxiety. To spot disease-specificity in content and form of perseverative cognition, intercorrelations among NYC-Q factors were performed separately for the GAD and HC groups.
A Spearman correlation was run to assess the associations between NYC-Q factors and HR, and for dispositional measures of anxiety, worry, depressive symptoms, and depressive rumination.
2.8. Seed-based fMRI analysis
Anatomical ROIs for bilateral amygdala were constructed using an anatomical toolbox in SPM (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The average resting state fMRI time-series over the ROIs were extracted for patients with GAD and HC. This time series were then used as a regressor in a 1st level SPM analysis, extracting the voxels in the brain showing a significant correlation with it (Makovac et al., 2016a, Makovac et al., 2016b, Makovac et al., 2016c). Statistical threshold was set at p < 0.05 - FWE-corrected at cluster level (cluster size defined using uncorrected voxel-level threshold p < 0.001).
A direct comparison between baseline functional connectivity in the two groups as well as results of the Group by Time interaction have been already described elsewhere and will not be repeated here (Makovac et al., 2016b). Similarly, the effectiveness of induction in eliciting significant increase in worrisome thoughts in both groups has already been reported (Makovac et al., 2016a).
t-Tests were used to test for association between amygdala functional connectivity at baseline (i.e., before the induction) and the content and form of thoughts and cardiac reactivity after the induction, with Group (GAD vs. HC) as the between-subject variable and NYC-Q factors and Δ HR as covariates of interest.
We further calculated the shift in amygdala functional connectivity from pre to post-induction in the two groups (Δ functional connectivity; post minus pre-induction), and correlated this Δ functional connectivity with NYC-Q factors, to investigate whether the impact of a perseverative cognition induction on the brain could be associated with the specific form of thoughts in response to the induction.
Following the specific hypothesis regarding the verbal form of worry dampening cardiovascular reactivity (Borkovec et al., 2004), scores on the Words factor of the NYC-Q (indicating how much participants were thinking in words) and the impact of the induction on HR (Δ HR) were entered into the same model to test for mediation by shared regional neural substrates. To do so, the Words by Δ HR interaction was entered as covariate of interest and Group (GAD vs. HC) was entered as the between-subject variable in two models having respectively amygdala functional connectivity at baseline and Δ functional connectivity as outcome.
3. Results
3.1. Questionnaires
Individuals within the GAD group had significantly higher baseline scores on the PSWQ, the STAI, and the RRS when compared to the HC group. Moreover, patients with GAD had overall higher HR when compared to HC. Perseverative cognition induction significantly increased HR in both groups (Makovac et al., 2016b).
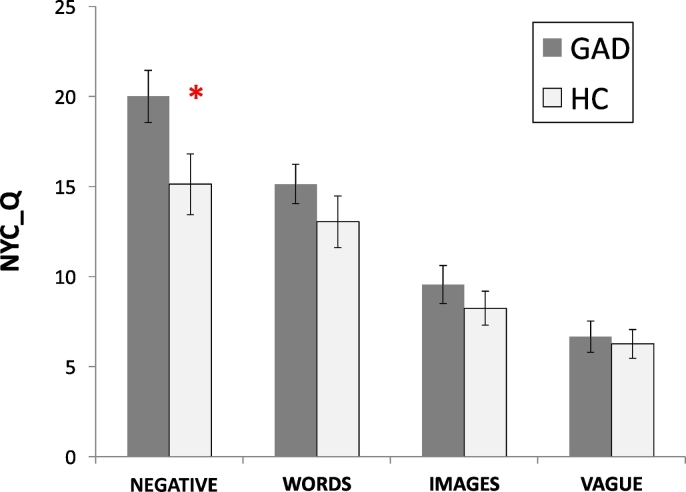
Based on the NYC-Q, patients with GAD reported more negative thoughts than HC (Negative factor, t(38) = 2.73, p < 0.01). No other significant group differences in the form and content of thoughts emerged (Fig. 1).
Fig. 1.
Differences between patients with Generalized Anxiety Disorder (GAD) and Healthy Controls (HC) on the examined New York Cognition Questionnaire (NYC-Q) factors.
3.2. Correlations between NYC-Q factors, anxiety, and heart rate
In GAD patients, an increase in reported negative thoughts was accompanied by an increase in the number of verbal thoughts. Conversely in HC, an increase in negative thoughts was accompanied by an increase in the number of thoughts in the form of images, which were also described as more vague/less specific. Negative thinking was positively correlated with levels of depression (BDI), state and trait anxiety (STAI), worry (PSWQ), and depressive rumination (RRS) (Table 1).
Table 1.
Spearman's intercorrelations among NYC-Q factors in the two groups and Spearman's intercorrelations between NYC-Q factors and a) depression (BDI), b) trait and state measures of anxiety (STAI), c) trait rumination (RRS), d) trait worry (PSWQ); and e) cardiac responses at baseline and in response to the induction.
| Words | Images | Negative | Vague | |||
|---|---|---|---|---|---|---|
| GAD | Words | − 0.20 | 0.56⁎ | − 0.34 | ||
| Images | − 0.20 | 0.40 | 0.14 | |||
| Negative | 0.56⁎ | 0.40 | − 0.14 | |||
| Vague | − 0.34 | 0.14 | − 0.14 | |||
| HC | Words | − 0.12 | 0.40 | 0.24 | ||
| Images | − 0.12 | 0.48⁎ | − 0.46⁎ | |||
| Negative | 0.40 | 0.48⁎ | − 0.23 | |||
| Vague | 0.24 | − 0.46⁎ | − 0.23 | |||
| BDI | PSWQ | RRS | STAIy1 | STAIy2 | ||
| Words | 0.39⁎ | 0.22 | 0.38⁎ | 0.43⁎⁎ | 0.35⁎ | |
| Images | 0.13 | 0.21 | 0.12 | 0.33⁎ | 0.23 | |
| Negative | 0.54⁎⁎ | 0.41⁎⁎ | 0.49⁎⁎ | 0.61⁎⁎ | 0.55⁎⁎ | |
| Vague | − 0.05 | − 0.07 | − 0.02 | 0.04 | − 0.05 | |
| HR | HR | |
|---|---|---|
| Words | 0.38⁎ | − 0.32 |
| Images | − 0.04 | − 0.13 |
| Negative | 0.29 | − 0.17 |
| Vague | − 0.06 | − 0.10 |
p < 0.01.
p < 0.001. GAD = Generalized anxiety disorder; HC = Healthy controls; BDI = Beck Depression Inventory; PSWQ = Penn State Worry Questionnaire; RRS = Ruminative Response Scale; STAI Y1 = state subscale of the State-trait anxiety inventory; STAI Y2 = trait subscale of the State-trait anxiety inventory.
Verbal thoughts also correlated positively with levels of depression (BDI), state and trait anxiety (STAI), baseline HR (pre-induction) and correlated negatively with HR changes from pre to post-induction (Δ HR). Thinking in images correlated positively with state anxiety (STAI). No correlation emerged between having vague thoughts and any of the other examined variables.
3.3. Associations between the use of words vs images during perseverative cognition and baseline amygdala functional connectivity
We tested to see if baseline functional connectivity with amygdala was differently associated with increase in verbal or imagery thoughts after perseverative cognition induction in GAD and controls. No correlations between thinking in words or images and the functional connectivity of either the left or right amygdala seed emerged.
3.4. Associations between the use of words vs images during perseverative cognition and pre to post induction changes in amygdala functional connectivity
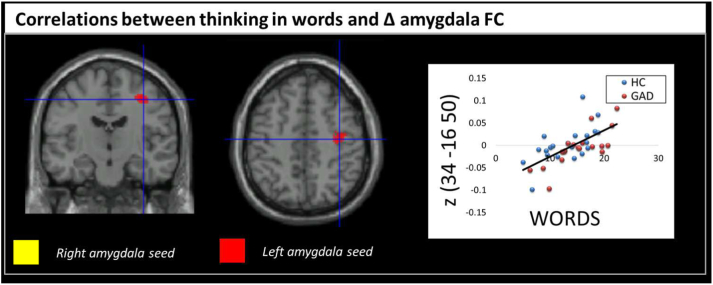
We next tested to see if pre- to post-induction changes in functional connectivity with amygdala was differently associated with changes in verbal or imagery thoughts after perseverative cognition induction in GAD and controls. Across groups, more verbal thoughts were associated with increased functional connectivity between left amygdala and right precentral gyrus following induction (Table 2, Fig. 2).
Table 2.
Brain areas showing significant associations between baseline or pre- to post-induction changes in amygdala functional connectivity (Δ FC) and the examined New York Cognition Questionnaire (NYC-Q) factors, the shift in HR from pre- to post-induction (Δ HR), and the interaction of these two variables.
| Cluster |
Voxel |
||||||
|---|---|---|---|---|---|---|---|
| Contrast of interest | Brain area | Seed | k | p FWE | Side | T | MNI xyz |
| (1) WORDS | |||||||
| ΔFC (post-pre induction) | |||||||
| Pos. correlation with WORDS | |||||||
| Precentral gyrus | LA | 131 | 0.012 | R | 6.17 | 34–16 50 | |
| (2) WORDS × ΔHR | |||||||
| Baseline | |||||||
| WORDS × △HR | |||||||
| Precuneus | 270 | 0.000 | R | 4.86 | 16–54 48 | ||
| Superior frontal gyrus | RA | 305 | 0.001 | L | 5.09 | − 20 36 54 | |
| Middle frontal gyrus | L | 5.00 | − 38 26 46 | ||||
| Temporal fusiform cortex | 308 | 0.017 | R | 5.08 | 40–18 -30 | ||
| Temporal fusiform cortex | 105 | 0.002 | L | 4.85 | − 36 -14 -34 | ||
| Words/Images factor × Group (GAD, HC) | |||||||
| Parietal opercular cortex | RA | 184 | 0.003 | R | 4.63 | 42–26 16 | |
| ΔFC (post-pre induction) | |||||||
| WORDS × △HR | |||||||
| Frontal pole | RA | 112 | 0.025 | R | 5.28 | 26 48–2 | |
| Frontal pole | 1482 | 0.000 | L | 6.15 | − 28 48 36 | ||
| Cerebelllum | 464 | 0.000 | L | 5.65 | − 2 -40 − 16 | ||
| Inferior temporal gyrus | 135 | 0.000 | R | 5.45 | 50–56 -18 | ||
| Middle temporal gyrus | 207 | 0.001 | R | 5.16 | 56–22 -10 | ||
| Putamen | 124 | 0.000 | R | 5.42 | 32–4 -12 | ||
| Lat occipital cortex | 151 | 0.006 | R | 4.97 | 30–74 -28 | ||
| Precentral gyrus | LA | 144 | 0.006 | R | 6.33 | 38–12 52 | |
| Cerebellum, V | 242 | 0.000 | L | 6.40 | -16 -44 -18 | ||
| Paracingulate gyrus | 158 | 0.022 | R | 3.68 | 10 24 36 | ||
| Superior frontal gyrus | 215 | 0.000 | L | 6.14 | − 8 -38 52 | ||
| Posterior inf temporal gyrus | 198 | 0.000 | R | 6.04 | 46–28 -10 | ||
| Temporooccipital inf temporal gyrus | 192 | 0.004 | R | 5.97 | 56–66 -10 | ||
| Words/Images factor × Group (GAD, HC) | |||||||
| Precentral gyrus | RA/LA | 163 | 0.003 | L | 5.86 | − 44 -18 62 | |
| Postcentral gyrus | L | 5.14 | − 52 -18 52 | ||||
| Occipital cortex | 181 | 0.002 | R | 5.24 | 52–72 -8 | ||
| 124 | 0.016 | R | 5.34 | 28 38 30 | |||
| Sup frontal gyrus | 167 | 0.002 | R | 4.97 | 12 34 50 | ||
| (3) NEGATIVE | |||||||
| Baseline | |||||||
| Group × NEGATIVE interaction | |||||||
| Temporal pole | LA | 233 | 0.001 | R | 6.14 | 26 10–30 | |
| Middle temporal gyrus | 162 | 0.007 | R | 4.90 | 50 4–26 | ||
| Central opercular cortex | 185 | 0.003 | L | 5.97 | − 50 6–4 | ||
| 121 | 0.029 | R | 4.32 | 58 2 4 | |||
| ΔFC (post-pre induction) | |||||||
| Neg. correlation with NEGATIVE | |||||||
| Anterior cingulate cortex | RA | 104 | 0.041 | L | 6.16 | − 12 38 18 | |
| (4) VAGUE | |||||||
| Baseline | |||||||
| Neg. correlation with VAGUE | |||||||
| LA | 349 | 0.000 | R | 5.37 | 0 50–10 | ||
| ΔFC (post-pre induction) | |||||||
| Precuneus | 74 | 0.014 | R | 4,59 | 6–46 14 | ||
Fig. 2.
Upper panel: brain regions showing significant correlations between the ‘Words’ factor of the New York Cognition Questionnaire (NYC-Q) and the shift in amygdala FC from pre- to post-induction (Δ FC) in HC and patients with GAD.
3.5. Associations between increased use of words and reduced cardiac reactivity during perseverative cognition and baseline amygdala functional connectivity
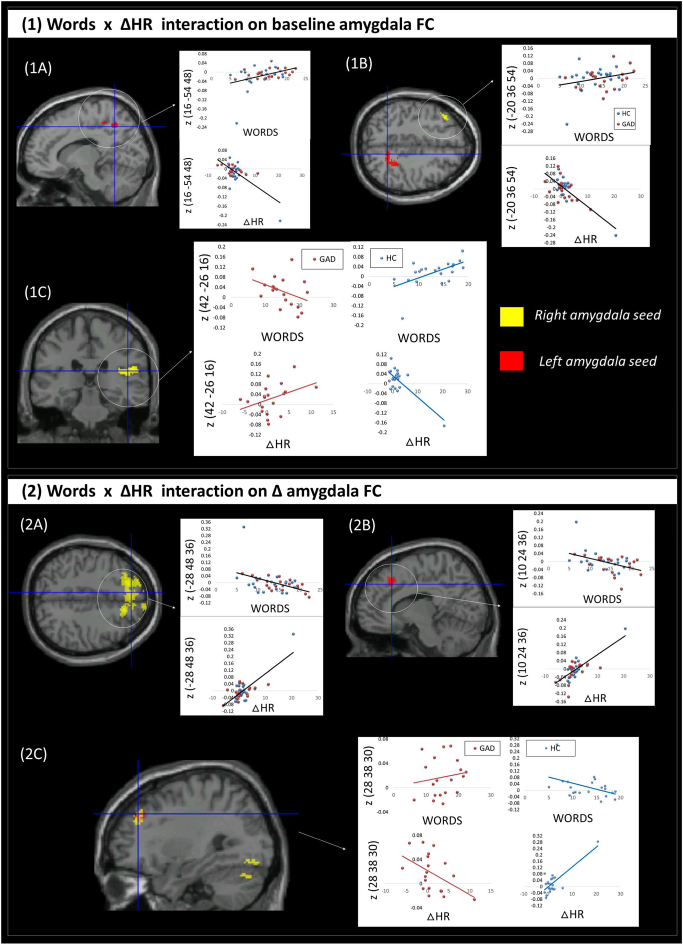
We tested whether baseline functional connectivity with amygdala mediates both increase in verbal thoughts and reduced cardiac reactivity during perseverative cognition. Changes in functional connectivity between left amygdala and precuneus expressed a significant Words × Δ HR interaction. Across both groups of participants, this effect was driven principally by the co-expression of a positive correlation with verbal thinking with a negative correlation with increased HR. Thus stronger left amygdala connectivity with this brain region was associated on one hand with more thoughts in the form of words and on the other hand with decreases in HR following perseverative cognition induction (Table 2, Fig. 3, Fig. 1A).
Fig. 3.
Upper panel: brain regions showing a significant interaction between the ‘Words’ factor of the New York Cognition Questionnaire (NYC-Q) and the shift in HR from pre to post-induction (Δ HR) on left and right amygdala functional connectivity (FC) at baseline (i.e., pre-induction) in Healthy Controls (HC) and patients with Generalized Anxiety Disorder (GAD). Lower panel: brain regions showing a significant interaction between the ‘Words’ factor of the New York Cognition Questionnaire (NYC-Q) and the shift in amygdala FC from pre- to post-induction (Δ FC) in HC and patients with GAD.
A Words × Δ HR interaction was also observed across groups for right amygdala functional connectivity with frontotemporal cortices, namely left superior frontal gyrus, left middle frontal gyrus, and bilateral temporal fusiform cortex. This effect was driven principally by a negative correlation with Δ HR, meaning that a stronger connectivity between these brain regions was associated with decreases in HR following perseverative cognition induction (Table 2, Fig. 3, Fig. 1B).
There were group differences in neural interactions between the form of thoughts and physiological arousal: i.e. significant Words × Δ HR × Group interaction was evident for right amygdala functional connectivity with right parietal opercular cortex. This effect was driven by a negative correlation with Words and a positive correlation with HR change in patients with GAD compared to controls. Participants with GAD who responded to the induction with more verbal thought than imagery and who also experienced a decrease in HR, manifest weaker functional connectivity between right amygdala and parietal operculum. The opposite pattern emerged in HC (Table 2, Fig. 3, Fig. 1C).
3.6. Associations between increased use of words and reduced cardiac reactivity during perseverative cognition and pre to post induction changes in amygdala functional connectivity
We then tested for associations between pre- to post-induction changes in amygdala functional connectivity and more negative thoughts and reduced cardiac reactivity during perseverative cognition in GAD and controls. Significant interactions emerged for the pre- to post-induction changes in functional connectivity between right amygdala and right frontal pole (driven by a positive correlation with Words), and between right amygdala and a cluster comprising left frontal pole, brainstem/cerebellum, right inferior temporal gyrus, right middle temporal gyrus, putamen, and right lateral occipital cortex (driven by a negative correlation with Words and positive with Δ HR). Thus, participants who responded to the induction with thoughts characterized by more words than images and had a decrease in HR, also increased functional coupling between right amygdala and right frontal pole and decreased functional coupling between right amygdala and the other brain regions (see Table 2 for the complete list of brain areas; Fig. 3, Fig. 2A).
A Words x Δ HR interaction was also found for the Δ left amygdala functional connectivity between and cerebellum, right paracingulate gyrus, left superior frontal gyrus and temporal areas. Participants who responded to the induction with thoughts characterized by more words than images and a decrease in HR, showed a decrease in functional connectivity between left amygdala and cerebellum, right paracingulate gyrus, left superior frontal gyrus, and temporal areas (Table 2; Fig. 3, Fig. 2A) and in functional connectivity between left amygdala and precentral gyrus from pre to post-induction (Table 2, Fig. 3, Fig. 2B).
A Words x Δ HR x Group interaction was found for functional connectivity between bilateral amygdala with precentral and postcentral gyri, occipital cortex, and frontal areas (see Table 2 for a detailed list of brain areas), driven by a positive correlation with Words and a negative correlation with the change in HR in patients with GAD. Thus, patients who responded to the induction with a relative increase in verbal thoughts accompanied by a decrease in HR, showed an increased functional coupling between amygdala (bilaterally) and these brain regions (Fig. 3, Fig. 2C).
3.7. Associations between negative quality to thoughts during perseverative cognition and baseline amygdala functional connectivity
We next tested for associations between baseline functional connectivity with amygdala and an increase in negative thoughts after perseverative cognition induction in GAD and controls. No significant associations emerged.
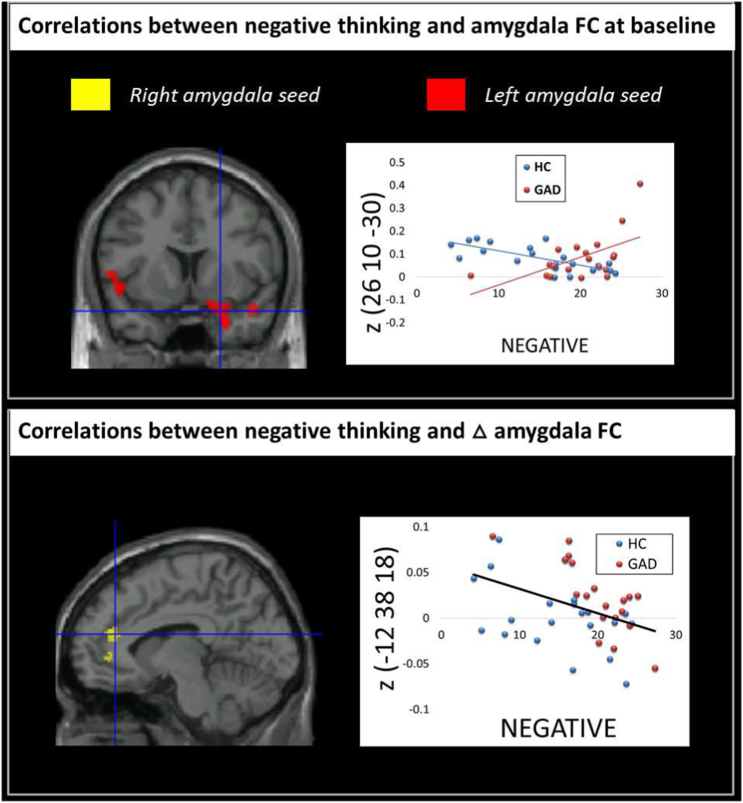
A Negative × Group interaction was observed for the functional connectivity between left amygdala and right temporal pole, right middle temporal gyrus and bilateral central opercular cortex, driven by a positive correlation in GAD and a negative correlation in HC. Whereas patients with GAD who had more negative thoughts had a stronger functional connectivity between these regions, the opposite pattern emerged in HC (Fig. 4, upper panel).
Fig. 4.
Upper panel: brain regions showing significant correlations between the ‘Negative’ factor of the New York Cognition Questionnaire (NYC-Q) and left and right amygdala functional connectivity (FC) at baseline (i.e., pre-induction) in Healthy Controls (HC) and patients with Generalized Anxiety Disorder (GAD). Lower panel: brain regions showing significant correlations between the ‘Negative’ factor of the New York Cognition Questionnaire (NYC-Q) and the shift in amygdala FC from pre- to post-induction (Δ FC) in HC and patients with GAD.
3.8. Associations between negative quality to thoughts during perseverative cognition and pre to post-induction changes in amygdala functional connectivity
We tested to see if pre- to post-induction changes in amygdala functional connectivity were associated with increases in negative worrisome thoughts in GAD and controls. Negative thoughts were associated with diminished pre to post-induction functional connectivity between right amygdala and anterior cingulate cortex (Fig. 4, lower panel).
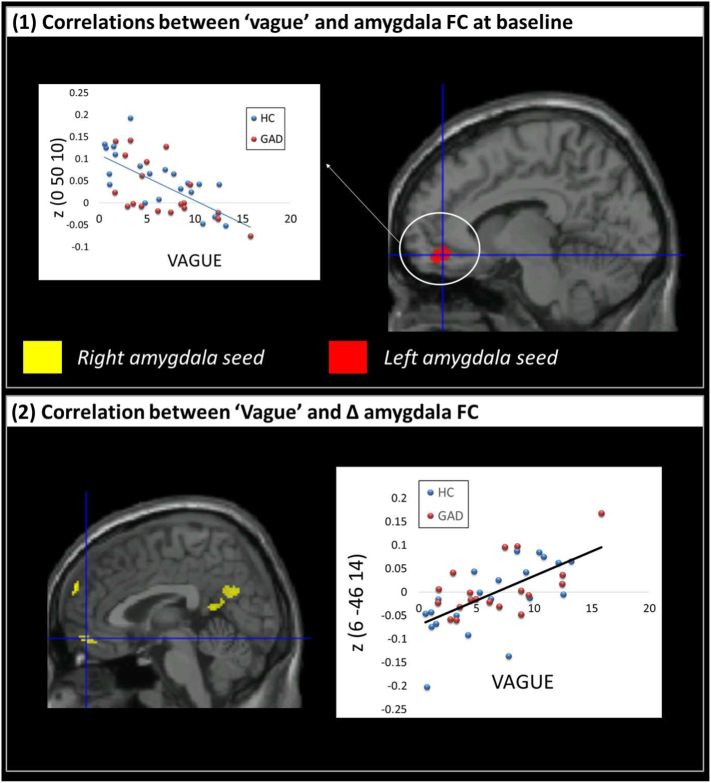
3.9. Associations between vague/specific thoughts during worry and baseline amygdala functional connectivity
We tested for the association between baseline functional connectivity with amygdala and the presence of more vague thoughts after worry induction in GAD and controls.
A significant negative correlation suggested that having more vague, and less specific, thoughts was associated with reduced functional connectivity between left amygdala and right frontal medial cortex (Fig. 5, upper panel).
Fig. 5.
Upper panel: brain regions showing significant correlations between the ‘Vague’ factor of the New York Cognition Questionnaire (NYC-Q) and left and right amygdala functional connectivity (FC) at baseline (i.e., pre-induction) in Healthy Controls (HC) and patients with Generalized Anxiety Disorder (GAD). Lower panel: brain regions showing significant correlations between the ‘Vague’ factor of the New York Cognition Questionnaire (NYC-Q) and the shift in amygdala FC from pre- to post-induction (Δ FC) in HC and patients with GAD.
3.10. Associations between vague/specific thoughts during perseverative cognition and pre to post-induction changes in amygdala functional connectivity
Lastly, we tested whether pre to post-induction changes in functional connectivity with amygdala were associated with the occurrence of more vague thoughts after perseverative cognition induction in GAD and controls. We found a different pattern of connectivity supporting the level of detail in thought content following the induction. We observed positive correlations between Vague and the Δ functional connectivity (post-pre induction) between right amygdala and precuneus. This pattern means that participants who described their thoughts as more vague rather than specific responded to the induction by increasing the functional connectivity between these areas (Fig. 5, lower panel).
4. Discussion
This study tested hypotheses and mechanisms arising out of the Cognitive Avoidance Theory of Worry, by linking the content and the form of thoughts and cardiac responses evoked by a perseverative cognition induction to measures of neural connectivity associated with the amygdala in patients with GAD and controls. As predicted, worrisome thoughts were more negative in individuals with a diagnosis of GAD compared to non-pathological participants. In patients with GAD, more negative thoughts were characterized by the use of more words. This pattern is consistent with previous reports that people with clinical levels of worry experience more verbal negative thoughts than healthy volunteers (Borkovec and Inz, 1990, Hirsch et al., 2012). Across groups, thinking in words was associated with higher baseline heart rate and reduced cardiac reactivity after the perseverative cognition induction.
Contrary to what emerged in the pathological GAD group, healthy participants who reported more negative thoughts described the latter more in the form of images and as being vague. No association between the verbal nature of worry and negative valence was evident in the control group, suggesting that non-pathological worry can take the form of imagery (Bergman and Craske, 2000, Stokes and Hirsch, 2010). For example, individuals with dispositional tendencies to worry report increases in negative thought intrusions when instructed to worry verbally and a decrease in negative intrusions when trained to worry in images (Stokes and Hirsch, 2010).
In line with the hypothesis that verbal worrying reflects attempts to control emotional aspects of anxiety, more negative verbal thoughts after the induction was correlated with higher levels of dispositional rumination, depressive symptoms, and state and trait anxiety. This association did not emerge for trait worry, which was instead significantly associated with more negative thoughts. The lack of statistical significance is likely due to the fact that negative thoughts were more verbal in nature only in patients with GAD and not in controls, thus obscuring the result on a dimensional level. According to the notion that worrying in images is more adaptive as it elicits the physiological anxiety needed for habituation, thinking in images after the induction was only associated with higher levels of state -but not trait- anxiety. Together, our behavioural and self-reported data are consistent with Borkovec's Cognitive Avoidance Theory of Worry, which states that worrisome thinking in GAD is verbal in nature with the aim to avoid fear-inducing mental imagery and concomitant physiological arousal.
The Cognitive Avoidance Theory of Worry was further supported by our neuroimaging and physiological findings. Strong engagement of the prefrontal cortex (reflected by increased connectivity with bilateral amygdala) is associated with the attenuation of dysregulated autonomic arousal during worry in GAD (Makovac et al., 2016b). Here, we extend these observations by characterizing how the form (words, images, vague) and valence of worrisome thoughts exclusively influence the communication between amygdala and other brain areas both at baseline (before induction) and from pre to post-induction of perseverative cognitions. We demonstrated that the use of words during perseverative cognition significantly correlated with pre- to post-induction changes in amygdala functional connectivity, whereas no significant associations emerged for the use of images. The induction engendered higher verbal content of worry, which was associated with enhanced communication between left amygdala and right precentral gyrus, an area that has been lately implicated in language and speech processing, and in the integration of auditory and motor functions (Glasser and Rilling, 2008). Notably, increased functional connectivity between amygdala and precentral gyrus has been found in bipolar disorder (Vizueta et al., 2012) and has been linked to dissociative states in borderline personality disorder (Krause-Utz et al., 2014).
Given the hypothesized link between verbal worrying and reduced physiological arousal, we investigated which brain areas mediate both the use of words and cardiac reactivity in response to a perseverative cognition induction. As depicted in Fig. 3 (2C), combination of a more pronounced use of words and a corresponding decrease in heart rate following the induction were associated with increased functional connectivity between bilateral amygdala and superior and medial frontal areas, likely reflecting attempts of an effective top-down control of the amygdala on emotional arousal as also indicated by other studies (Etkin et al., 2009). The same pattern of a use of more words, and reduced heart rate during worry, was associated with a decrease in functional connectivity between bilateral amygdala and areas of the cerebellum. This is not surprising since the cerebellum has direct anatomical connections to the amygdala (Heath and Harper, 1974) and growing evidence suggest its role in higher cognitive functions (Schmahmann and Caplan, 2006), verbal working memory (Ng et al., 2016), emotion (Turner et al., 2007), and avoidant behaviors (Laricchiuta and Petrosini, 2014). Moreover, significant alterations in cerebellum functional connectivity have been described in social anxiety disorder (Warwick et al., 2008), post-traumatic stress disorder (Bonne et al., 2003), obsessive-compulsive disorder (Menzies et al., 2007), and GAD (Blair et al., 2008). It is noteworthy that the cerebellum has also direct reciprocal connections to the hypothalamus (for a review see Zhu et al., 2006), which can influence heart rate reactivity. Lastly, we observed group differences in some areas that appeared to mediate the inhibitory control of worry over physiological arousal (heart rate). For example, only individuals with GAD showed increased functional connectivity after perseverative cognition induction between right amygdala and right orbitofrontal cortex, an area that has been associated with attenuation of negative affect (Banks et al., 2007) again suggesting inhibitory control exertion by this psychopathological group.
We also found patterns of functional coupling with the amygdala that were associated with the negative valence of worrisome thoughts. In participants with GAD, enhanced baseline functional connectivity between left amygdala and temporal and central opercular cortex was also associated with more negative thoughts. Neural activity in the opercular cortex (with anterior insula) is linked to cardiac interoception (Critchley et al., 2004). Moreover, biofeedback-guided control of heart rate is associated with activation of the frontal pole (Jones et al., 2015). These previous observations suggest our finding may recruitment of these regions for down-regulation of cardiac arousal by negative worrisome thoughts in patients with GAD.
In both pathological and non-pathological participants, more negative thoughts after the induction of worry were associated with a decrease in functional connectivity between right amygdala and anterior cingulate cortex from pre to post-induction. The cingulo-opercular network includes portions of the dorsal anterior cingulate cortex and operculum/insula and anterior thalamus (Dosenbach et al., 2008) and is implicated in anxiety (Sylvester et al., 2012) in part through its contribution to maintenance of alertness (Sadaghiani et al., 2009).
The engagement of temporal lobe regions by more negative and wordy worrisome thoughts provides anatomical insight into theoretical models of emotional reappraisal, which postulate that frontoparietal ‘control’ regions modulate semantic representations in lateral temporal cortex to influence emotion-related responses via the amygdala (Buhle et al., 2014, Ochsner and Gross, 2005, Ochsner and Gross, 2007, Ochsner et al., 2012) implying a crucial contribution of temporal cortices in emotion regulation (Messina et al., 2015). Moreover, the temporal lobe has a well-established role in the semantic processing that is important for language related processing (Lambon-Ralph et al., 2017, Patterson et al., 2007). Our data extend this view suggesting that the temporal cortex has effects at both behavioural and psychophysiological levels that can impact emotional reappraisal.
The abstract (vague) nature of worry in GAD is widely recognized (e.g., Stöber and Borkovec, 2002), and underpins notions of ‘reduced concreteness’ of worry (Stöber et al., 2000). Since abstract sentences evoke less vivid images when compared to concrete sentences (Paivio, 1991), the reduced concreteness of worrisome thoughts might serve as a strategy to avoid negative imagery. Contrary to our expectations, however, we did not observe a correlation between the abstract nature of worry and state and trait measures of anxiety or states of cardiac arousal. One possible explanation for this lack of association is perhaps the constraints of using the NYC-Q questionnaire to study the content and form of pathological worry. This is first time that the NYC-Q questionnaire has been applied to a clinical group (patients with GAD) exhibiting pathological worry. Future studies will help validate it as a tool in clinical populations. Nevertheless, our approach elicited meaningful results when investigating the brain correlates of the abstract nature of induced worry. Individuals with more vague worrisome thoughts exhibited lower right amygdala functional connectivity with frontal medial cortex. Prior studies suggest that regions of ventromedial prefrontal cortex are important for generating concrete details from schematic knowledge (Bonnici et al., 2012, Kumaran et al., 2009, van Kesteren et al., 2013, Zeithamova et al., 2012). For example, our prior study found connectivity from the hippocampus is linked to the generation of more concrete descriptions of personally relevant information (Medea et al., 2016). This pattern of connectivity is broadly consistent with a large-scale individual difference investigation, which found that connectivity from the hippocampus to the posterior cingulate cortex was linked to more detailed thoughts (Smallwood et al., 2016).
Our study findings reinforce the Cognitive Avoidance Theory of Worry (Borkovec, 1994, Borkovec et al., 2004) by testing and extending its validity through the study of its brain correlates in GAD and controls. Importantly, our participants were not directed to worry verbally or in an imagery form, contrary to approaches adopted by most other studies investigating the impact of the form of worry on the behaviour. Instead, the form and content of worry was assessed a posteriori, allowing participants to engage in their habitual form of worry without being constrained by instruction. This adds ecological validity to our results.
One limitation of this study is the relatively small sample size, due to difficulties in asking GAD patients to receive an MRI scan. The prevalence of women in the sample reflects the unequal gender distribution in the GAD and, despite our best attempts at matching patients and controls, it may have biased the results. Another important limitation is that our measures of functional connectivity do not allow determining the direction of causal relationships between brain regions. Such limitations notwithstanding, our observations provide deeper insight into differential pathophysiological processes associated with component features of worrisome thinking. Moreover we demonstrate a plausible neurobiological basis for the impact of verbal worry on the strategic down-regulation of cardiac arousal in patients with GAD.
Funding
This work was supported by Italian Ministry of Health Young Researcher Grants (GR2010-2312442; GR2011-02348232). JS was supported by the ERC (WANDERINGMINDS - 646927).
Financial disclosure
No financial interests to disclose.
References
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed. (DSM-5) [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala–frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4 doi: 10.1001/archpsyc.1961.01710120031004. (561–471) [DOI] [PubMed] [Google Scholar]
- Behar E., Zuellig A.R., Borkovec T.D. Thought and imaginal activity during worry and trauma recall. Behav. Ther. 2005;36:157–168. [Google Scholar]
- Bergman R.L., Craske M.G. Verbalization and imagery during worry activity. Depress. Anxiety. 2000;11:169–174. doi: 10.1002/1520-6394(2000)11:4<169::AID-DA4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Blair K., Shaywitz J., Smith B.W., Rhodes R., Geraci M., Jones M., McCaffrey D., Vythilingam M., Finger E., Mondillo K., Jacobs M., Charney D.S., Blair R.J., Drevets W.C., Pine D.S. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am. J. Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O., Gilboa A., Louzoun Y., Brandes D., Yona I., Lester H., Barkai G., Freedman N., Chisin R., Shalev A.Y. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol. Psychiatry. 2003;54:1077–1086. doi: 10.1016/s0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- Bonnici H.M., Chadwick M.J., Lutti A., Hassabis D., Weiskopf N., Maguire E.A. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J. Neurosci. 2012;32:16982–16991. doi: 10.1523/JNEUROSCI.2475-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec T.D. The nature, functions, and origins of worry. In: Davey G.C.L., Tallis F., editors. Worrying: Perspectives on Theory, Assessment and Treatment. Wiley; Oxford, England: 1994. pp. 5–33. [Google Scholar]
- Borkovec T.D., Hu S. The effect of worry on cardiovascular response to phobic imagery. Behav. Res. Ther. 1990;28:69–73. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Borkovec T.D., Inz J. The nature of worry in generalized anxiety disorder: a pre- dominance of thought activity. Behav. Res. Ther. 1990;28:69–73. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Borkovec T.D., Roemer L. Perceived functions of worry among generalized anxiety disorder subjects: distraction from more emotionally distressing topics? J. Behav. Ther. Exp. Psychiatry. 1995;26:25–30. doi: 10.1016/0005-7916(94)00064-s. [DOI] [PubMed] [Google Scholar]
- Borkovec T.D., Lyonfields J.D., Wiser S.L., Deihl L. The role of worrisome thinking in the suppression of cardiovascular response to phobic imagery. Behav. Res. Ther. 1993;31:321–324. doi: 10.1016/0005-7967(93)90031-o. [DOI] [PubMed] [Google Scholar]
- Borkovec T.D., Alcaine O., Behar E.S. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg R., Mennin D., Turk C., editors. Generalized Anxiety Disorder: Advances in Research and Practice. Guilford; New York: 2004. pp. 77–108. [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davey G.C.L., Tallis F., Capuzzo N. Beliefs about the consequences of worrying. Cog. Ther. Res. 1996;20:499–520. [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top- down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Freeston M.H., Rheaume J., Letarte H., Dugas M.J., Ladouceur R. Why do people worry? Personal. Individ. Differ. 1994;17:791–802. [Google Scholar]
- Geerligs L., Rubinov M., Cam-Can Henson, R.N. State and trait components of functional connectivity: individual differences vary with mental state. J. Neurosci. 2015;35:13949–13961. doi: 10.1523/JNEUROSCI.1324-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain's language pathways. Cereb. Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K.J., Lurie D., Urchs S., Kipping J.A., Craddock R.C., Milham M.P., Margulies D.S., Smallwood J. A correspondence between individual differences in the brain's intrinsic functional architecture and the content and form of self-generated thoughts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett-Stevens H., Borkovec T.D. Effects of worry and progressive relaxation on the reduction of fear in speech phobia: an investigation of situational exposure. Behav. Ther. 2001;32:503–517. [Google Scholar]
- Heath R.G., Harper J.W. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp. Neurol. 1974;45:268–287. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- Hirsch C.R., Hayes S., Mathews A., Perman G., Borkovec T. The extent and nature of imagery during worry and positive thinking in generalized anxiety disorder. J. Abnorm. Psychol. 2012;121:238–243. doi: 10.1037/a0024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Jones C.L., Minati L., Nagai Y., Medford N., Harrison N.A., Gray M., Ward J., Critchley H.D. Neuroanatomical substrates for the volitional regulation of heart rate. Front. Psychol. 2015;6:300. doi: 10.3389/fpsyg.2015.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A., Elzinga B.M., Oei N.Y.L., Paret C., Niedtfeld I., Spinhoven P., Bohus M., Schmahl C. Amygdala and dorsal anterior cingulate connectivity during an emotional working memory task in borderline personality disorder patients with interpersonal trauma history. Front. Hum. Neurosci. 2014;8:848. doi: 10.3389/fnhum.2014.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Summerfield J.J., Hassabis D., Maguire E.A. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laricchiuta D., Petrosini L. Individual differences in response to positive and negative stimuli: endocannabinoid-based insight on approach and avoidance behaviors. Front. Syst. Neurosci. 2014;8:238. doi: 10.3389/fnsys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovac E., Meeten F., Watson D.R., Garfinkel S.N., Critchley H.D., Ottaviani C. Neurostructural abnormalities associated with axes of emotion dysregulation in generalized anxiety. Neuroimage Clin. 2016;10:172–181. doi: 10.1016/j.nicl.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovac E., Meeten F., Watson D.R., Herman A., Garfinkel S.N., Critchley H.D., Ottaviani C. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol. Psychiatry. 2016;80:786–795. doi: 10.1016/j.biopsych.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Makovac E., Watson D.R., Meeten F., Garfinkel S.N., Cercignani M., Critchley H.D., Ottaviani C. Amygdala functional connectivity as a longitudinal biomarker of symptom changes in generalized anxiety. Soc. Cogn. Affect. Neurosci. 2016;11:1719–1728. doi: 10.1093/scan/nsw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy P.M., Watson H., Watkins E.R., Nathan P. The relationship between worry, rumination, and comorbidity: evidence for repetitive negative thinking as a transdiagnostic construct. J. Affect. Disord. 2013;151:313–320. doi: 10.1016/j.jad.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Medea B., Karapanagiotidis T., Konishi M., Ottaviani C., Margulies D., Bernasconi A., Bernasconi N., Bernhardt B., Jefferies E., Smallwood J. How do we decide what to do? Resting-state connectivity patterns and components of self-generated thought linked to the development of more concrete personal goals. Exp. Brain Res. 2016 doi: 10.1007/s00221-016-4729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeten F., Davey G.C.L., Makovac E., Watson D.R., Garfinkel S.N., Critchley H.D., Ottaviani C. Goal directed worry rules are associated with distinct patterns of amygdala functional connectivity and vagal modulation during perseverative cognition. Front. Hum. Neurosci. 2016;10:553. doi: 10.3389/fnhum.2016.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L., Achard S., Chamberlain S.R., Fineberg N., Chen C.H., del Campo N., Sahakian B.J., Robbins T.W., Bullmore E. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Messina I., Bianco S., Sambin M., Viviani R. Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Front. Psychol. 2015;6:956. doi: 10.3389/fpsyg.2015.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. Development and validation of the Penn State Worry Questionnaire. Behav. Res. Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Newman M.G., Llera S.J. A novel theory of experiential avoidance in generalized anxiety disorder: a review and synthesis of research supporting a contrast avoidance model of worry. Clin. Psychol. Rev. 2011;31:371e382. doi: 10.1016/j.cpr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H.B., Kao K.L., Chan Y.C., Chew E., Chuang K.H., Chen S.H. Modality specificity in the cerebro-cerebellar neurocircuitry during working memory. Behav. Brain Res. 2016;305:164–173. doi: 10.1016/j.bbr.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. A prospective study of depression and posttraumatic stresssymptoms after a natural disaster: the 1989 Loma Prieta earthquake. J. Pers. Soc. Psychol. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The neural architecture of emotion regulation. In: Gross J.J., Thompson R.H., editors. The Handbook of Emotion Regulation. Guilford Press; New York: 2007. pp. 87–109. [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C., Thayer J.F., Verkuil B., Lonigro A., Medea B., Couyoumdjian A., Brosschot J.F. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Watson D.R., Meeten F., Makovac E., Garfinkel S.N., Critchley H.D. Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biol. Psychology. 2016;119:31–34. doi: 10.1016/j.biopsycho.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Paivio A. Dual coding theory: retrospect and current status. Can. J. Psychol. 1991;45:255–287. [Google Scholar]
- Patterson K., Nestor P.J., Rogers T.T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peasley-Miklus C., Vrana S.R. Effect of worrisome and relaxing thinking on fearful emotional processing. Behav. Res. Ther. 2000;38:129e144. doi: 10.1016/s0005-7967(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Ralph M.A., Jefferies E., Patterson K., Rogers T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017;18:42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., Chen G., Jo H.J., Martin A., Cox R.W. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Hesselmann G., Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J. Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.G., Wang H.T., Schooler J., Smallwood J. Can I get me out of my head? Exploring strategies for controlling the self-referential aspects of the mind-wandering state during reading. Q. J. Exp. Psychol. 2017;70:1053–1062. doi: 10.1080/17470218.2016.1216573. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Segerstrom S.C., Tsao J.C.I., Alden L.E., Craske M.G. Worry and rumination: repetitive thought as a concomitant and predictor of negative mood. Cogn. Ther. Res. 2000;24:671–688. [Google Scholar]
- Smallwood J., Karapanagiotidis T., Ruby F., Medea B., de Caso I., Konishi M., Wang H.T., Hallam G., Margulies D.S., Jefferies E. Representing representation: integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. 2nd ed. Consulting Psychologists Press; Palo Alto, CA: 1989. State-trait Anxiety Inventory: Bibliography. [Google Scholar]
- Spinhoven P., Drost J., van Hemert B., Penninx B.W. Common rather than unique aspects of repetitive negative thinking are related to depressive and anxiety disorders and symptoms. J. Anxiety Disord. 2015;33:45–52. doi: 10.1016/j.janxdis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Stöber J., Borkovec T.D. Reduced concreteness of worry in generalized anxiety disorder: findings from a therapy study. Cogn. Ther. Res. 2002;26:89–96. [Google Scholar]
- Stöber J., Tepperwien S., Staak M. Worrying leads to reduced concreteness of problem elaborations: evidence for the avoidance theory of worry. Anxiety Stress Coping. 2000;13:217–227. [Google Scholar]
- Stokes C., Hirsch C.R. Engaging in imagery versus verbal processing of worry: impact on negative intrusions in high worriers. Behav. Res. Ther. 2010;48:418–423. doi: 10.1016/j.brat.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Corbetta M., Raichle M.E., Rodebaugh T., Schlaggar B.L., Sheline Y.I., Zorumski C.F., Lenze E.J. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge J.M.F., Krijnen W.P., Wansbeek T., Shapiro A. Some new results on correlation-preserving factor scores prediction methods. Linear Algebra Appl. 1999;289:311–318. [Google Scholar]
- Turner B.M., Paradiso S., Marvel C.L., Pierson R., Boles Ponto L.L., Hichwa R.D., Robinson R.G. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M.T., Beul S.F., Takashima A., Henson R.N., Ruiter D.J., Fernández G. Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: from congruent to incongruent. Neuropsychologia. 2013;51:2352–2359. doi: 10.1016/j.neuropsychologia.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Vizueta N., Rudie J.D., Townsend J.D., Torrisi S., Moody T.D., Bookheimer S.Y., Altshuler L.L. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am. J. Psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana S.R., Cuthbert B.N., Lang P.J. Processing fearful and neutral sentences: memory and heart rate change. Cognit. Emot. 1989;3:179–195. [Google Scholar]
- Warwick J.M., Carey P., Jordaan G.P., Dupont P., Stein D.J. Resting brain perfusion in social anxiety disorder: a voxel-wise whole brain comparison with healthy control subjects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1251–1256. doi: 10.1016/j.pnpbp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Watkins E.D., Moulds M., Mackintosh B. Comparisons between rumination and worry in a non-clinical population. Behav. Res. Ther. 2005;43:1577–1585. doi: 10.1016/j.brat.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Zeithamova D., Dominick A.L., Preston A.R. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.N., Yung W.H., Kwok-Chong Chow B., Chan Y.S., Wang J.J. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res. Rev. 2006;52:93–106. doi: 10.1016/j.brainresrev.2006.01.003. [DOI] [PubMed] [Google Scholar]