Abstract
Patient: Male, 46
Final Diagnosis: Yolk Sac tumor
Symptoms: Shortness of breath
Medication: —
Clinical Procedure: Cardiac MRI • tumor resection
Specialty: Cardiology
Objective:
Rare disease
Background:
Cardiac involvement by a malignant tumor is rare. However, this is a case of right heart failure due to cardiac metastasis from a yolk sac tumor. Although a few case reports of cardiac metastasis from yolk sac tumors have been published, to our knowledge this is the first instance of multiple metastases to the right ventricular of yolk sac tumor in an adult male.
Case Report:
The patient is a 46-year-old male with a history of testicular cancer that presented with dyspnea on exertion. He was found to have two large right sided intracardiac masses on echocardiography. Cardiac magnetic resonance imaging (MRI) was obtained to further investigate these masses. Right ventricular function was decreased and concern for right ventricular outflow tract (RVOT) obstruction was present. The patient was taken to the operating room (OR) for resection of the cardiac masses. Pathology revealed the masses to be yolk sac tumors. Despite urgent resection of the tumors, the patient deteriorated clinically, ultimately succumbing to heart failure.
Conclusions:
This unique presentation of a yolk sac tumor emphasizes the need to keep a broad differential and complete a thorough workup for any cardiac mass. Early diagnosis and treatment of intra-cardiac masses is imperative due to their high rates of mortality. Albeit an uncommon etiology for heart failure, germ cell tumors can potentially metastasize to the heart and present with such a clinical picture.
MeSH Keywords: Endodermal Sinus Tumor, Heart Failure, Heart Neoplasms
Background
Cardiac involvement by a malignant tumor is rare. Malignant melanoma is the most common cancer to metastasize to the heart [1]. Here we present a case of right heart failure due to cardiac metastasis from a yolk sac tumor. Although a few case reports of cardiac metastasis from yolk sac tumors have been published, to our knowledge this is the first instance of multiple metastases to the right ventricular of yolk sac tumor in an adult male.
Case Report
The patient is a 46-year-old male with a three months’ history of progressive dyspnea on exertion. His past medical history was significant testicular cancer involving the right testes 32 years prior with pulmonary and widespread lymph nodal metastasis. He was treated at that time with orchiectomy, and chemotherapy along with radiation to the brachial plexus for distant metastasis. Eight years later, the patient was found to have a pulmonary nodule on chest x-ray that was treated surgically and was a teratoma. Since then the patient had remained asymptomatic.
As a part of a dyspnea workup, the patient underwent a transthoracic echocardiogram (TTE) that showed two large, well circumscribed, partially mobile right ventricular masses (Figure 1A, 1B). One was in the right ventricular outflow tract (RVOT) measuring 2.8×2.0 cm) and the other was toward the RV apical free wall (3.3×2.9 cm). The RV and left ventricle (LV) systolic function was normal though the RV was mildly dilated. There was color flow turbulence across the RVOT indicating some obstruction to flow, though velocity was not elevated. The patient was subsequently admitted to the hospital with Cardiothoracic Surgery and Oncology consults for further workup of his RV masses.
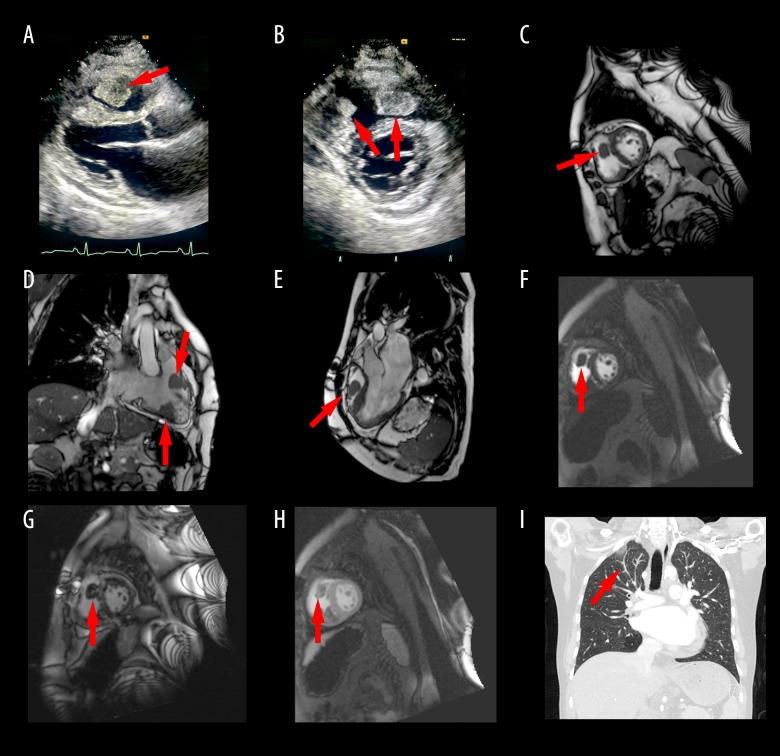
Figure 1.
(A) RV mass noted on para-sternal long axis view on TTE. (B) Masses on RV free wall and RVOT noted on para-sternal short axis on TTE. (C–E) Cardiac MRI showing the two RV masses. (F–G) Late gadolinium enhancement of the RV mass on cardiac MRI. (H) Central necrosis of the RV mass on cardiac MRI. (I) CT chest showing adenopathy and wedge infarct concerning for tumor emboli. RV – right ventricle; TTE – transthoracic echocardiogram; RVOT – right ventricular outflow tract; MRI – magnetic resonance imaging; CT – computerized tomography.
Cardiac magnetic resonance imaging (MRI) also showed these two intra-cardiac masses in the RV (Figure 1C–1E). One was located in the RVOT and the other in the diaphragmatic wall of the RV. Dynamic perfusion MRI demonstrated central delayed hyper-enhancement suggesting necrosis (Figure 1F–1H). These findings were thought to be suggestive of tumor. RV systolic function was low normal. Chest computerized tomography (CT) re-demonstrated the RV masses but also showed scattered areas of nodularity in the peripheral pulmonary arteries with areas of peripheral wedge-shaped consolidation and ground glass opacity likely related to tumor emboli and associated pulmonary infarcts (Figure 1I). Supraclavicular and gastro-hepatic adenopathy was noted that was concerning for metastatic disease (Figure 1I). These findings were new compared to prior CT from two years ago. Testicular ultrasound was negative for recurrence of cancer in the solitary remaining testicle.
Laboratory data demonstrated elevated brain natriuretic peptide at 466 pg/mL (normal 0–100 pg/mL), minimal elevations in α-fetoprotein (AFP) to 32 ng/mL (normal 0–15 ng/mL), and LDH to 300 U/L (normal 100–210 U/L). B-HCG was negative. Mild thrombocytopenia was also noted with a platelet count of 132,000 U/mL. Troponin was negative at <0.02 ng/mL (normal 0.00–0.07 ng/mL).
Given concerns for RVOT obstruction, early RV failure and multiple tumor emboli to the lungs, patient was taken to the operating room by Cardiothoracic Surgery team for resection of the masses. On intra-operative gross visualization, the first mass was noted to be a pedunculated mass with a thin stalk while the second mass was a sessile mass imbedded in the wall of the ventricle. The pedunculated mass was easily resected in total. The sessile mass was debulked so as to not compromise the ventricular wall or damage the tricuspid valve.
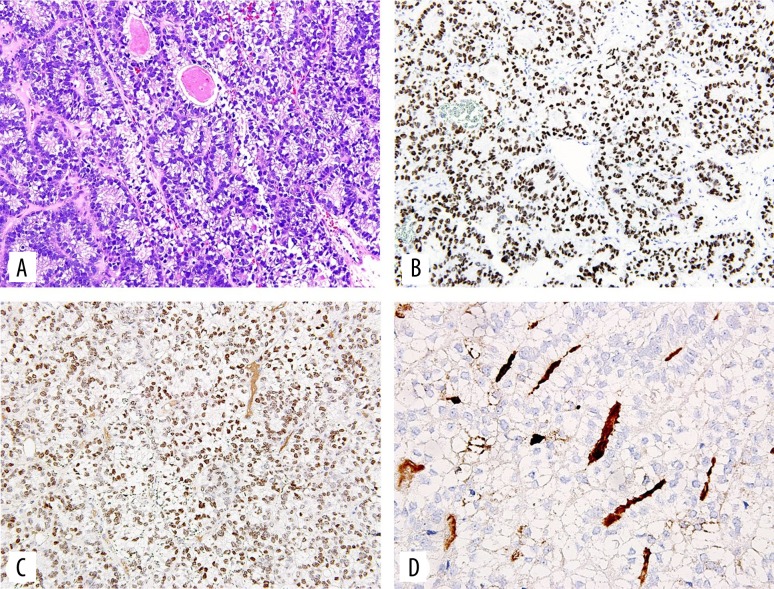
The specimens were sent to pathology. Both tumors were positive for glypican 3, pancytokeratin, antibody to CDX2, and AFP (Figure 2). They were negative for OCT4, TTF-1, PAX-8, vimentin, WT-1, and podoplanin. Ki-67 showed a high proliferative index. Several histological features of these masses were consistent with yolk sac carcinoma with endodermal differentiation. The first finding consistent with yolk sac carcinoma was presence of periodic acid Schiff positive hyaline bodies known as Schiller-Duval bodies [2,3]. Second was the presence of mixed mesenchymal and epithelial cells, tubular and papillary structures and possibly an eosinophilic basement membrane known as Reichert’s membrane [3,4]. Lastly, immunochemical staining for AFP was used to confirm the diagnosis.
Figure 2.
(A) Light microscopy on hematoxylin and eosin stained slide shows a tumor displaying a glandular, enteric like growth pattern with prominent subnuclear and supranuclear clear vacuoles within the cuboidal or cylindrical cells. (B) Immunohistochemistry with the antibody to SALL4 shows nuclear staining. (C) Immunohistochemistry with the antibody to CDX2 shows nuclear staining. (D) Immunohistochemistry with the antibody to alpha fetoprotein shows focal staining of the intraluminal material in some glands, (160×).
Post procedure the patient continued to deteriorate clinically with worsening right-sided heart failure, requiring multiple vasopressor medications. Initial plan was for PET/CT of the abdomen/pelvis for identification of primary tumor and staging followed by chemotherapy. However, the patient was never hemodynamically stable to undergo further testing and unfortunately passed away 14 days after the surgery. The family opted to forgo an autopsy so complete staging of the cancer was left incomplete.
Discussion
Our report presents an unusual etiology of right-sided heart failure from a rare cardiac tumor. Although primary cardiac tumors are rare (generally between 0.01% and 0.1% on postmortem analysis), the frequency of secondary metastatic tumors to the pericardium, myocardium, great vessels, or coronary arteries is between 0.7% and 3.5% at autopsy in the general population and as high as 9.1% in patients with known malignancies [1]. The risk of cardiac metastasis increases with disease burden. Although nearly all types of tumors can metastasize to the heart, some are more common than others. Primary lung cancer represents 36% to 39% of cardiac metastases, followed by breast cancer (10–12%) and hematologic malignancies (10–21%) [1]. Pleural mesothelioma and melanoma have exceptionally high rates of cardiac involvement with estimates of 28–56% of patients with metastatic melanoma having some cardiac involvement [1]. Other primary cancers with high incidence of cardiac metastasis are ovarian, gastric, renal, and pancreatic carcinomas [1]. To the best of our knowledge, this is the first reported case of multiple metastases of yolk sac tumor metastases to the right ventricle in the absence of recurrent gonadal tumor.
Diagnosis of such an uncommon tumor requires a multi-modal approach combining histological, clinical, and immunohistochemical data. One of the mainstays of yolk sac tumor diagnosis is obtaining serum alpha fetoprotein (AFP) levels. Serum AFP is a relatively sensitive marker in patients with yolk sac tumors and is commonly found to be elevated to 100–1,000 ng/mL [5]. In a case series by Kurman et al., 65 patients with ovarian endodermal sinus tumors were all found to have positive AFP staining on pathology [6]. AFP staining, although sensitive for these endodermal sinus tumors is not specific, as hepatic and gastrointestinal tumors often produce AFP as well [7,8]. Glypican-3 and pancytokeratin are more modern markers that are useful in aiding in the diagnosis of a yolk sac tumor albeit with limited specificity as well [8]. It is often the constellation of these positive markers in combination with histological findings that lead to confirmation of the diagnosis.
There are few reported cases of yolk sac tumor with intracardiac involvement, specifically of the right ventricle. A review of the literature revealed two reports of primary intracardiac yolk sac tumors: a two-year-old male and a three-year-old female [9,10]. In 2013, Nunes et al. published a comprehensive review of germ cell tumors with intracardiac involvement [11], totaling 18 cases including their own cases. Only four of these cases had discrete right ventricular involvement and all were metastatic disease. Two cases were adults with mixed germ cell, thrombus-like tumors extending from the vena cava to the right atrium and into the right ventricle [12,13]. Taghavi et al. reported a non-seminoma, teratoma RV tumor in a 32year-old male; the tumor was not surgically resected but treated with chemotherapy [14]. Nunes et al. reported a 26-year-old female with mixed germ cell tumor with yolk sac predominance found in the right ventricle [11]. This case held similarity to ours in that the metastasis was from a gonadal primary, which was eventually found on autopsy in the right ovary [11].
The presence of cardiac metastases in the absence of recurrent testicular tumor or other recurrent primary tumor was a unique condition in our case. In our case, it was likely that the metastases were secondary to the patient’s original testicular tumor. Germ cell tumors are known to recur after many years after both surgical and chemotherapeutic treatment. However, it is important to point out that metastatic disease with regression of the testicular primary is a possibility. In cases where no primary tumor is ever identified, it may be that the original testicular tumor has regressed. Balzer et al. published a study of 42 cases of regression of testicular germ cell tumor, originally confirmed on pathology, of which two yolk sac tumors developed widespread metastasis [15].
Conclusions
This unique presentation of a yolk sac tumor emphasizes the need to keep a broad differential and complete a thorough workup for any cardiac mass. Early diagnosis and treatment of intracardiac masses is imperative due to their high rate of mortality. Albeit an uncommon etiology for heart failure, germ cell tumors can potentially metastasize to the heart and present with such a clinical picture.
Footnotes
Conflicts of interest
None.
References:
- 1.Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation. 2013;128(16):1790–94. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 2.Alkatan HM, Al-Kofide A, Al-Hussain H. Yolk sac tumor: Histopathologic report of 2 cases. Can J Ophthalmol. 2008;43(1):125–26. doi: 10.3129/i07-198. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, Green JD. Yolk sac carcinoma. South Med J. 1976;69(6):728–31. doi: 10.1097/00007611-197606000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Norris HJ. Endodermal sinus tumor of the ovary: A clinical and pathologic analysis of 71 cases. Cancer. 1976;38(6):2404–19. doi: 10.1002/1097-0142(197612)38:6<2404::aid-cncr2820380629>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Talerman A, Haije WG, Baggerman L. Serum alphafetoprotein (AFP) in patients with germ cell tumors of the gonads and extragonadal sites: Correlation between endodermal sinus (yolk sac) tumor and raised serum AFP. Cancer. 1980;46(2):380–85. doi: 10.1002/1097-0142(19800715)46:2<380::aid-cncr2820460228>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Norris HJ. Endodermal sinus tumor of the ovary: A clinical and pathologic analysis of 71 cases. Cancer. 1976;38(6):2404–19. doi: 10.1002/1097-0142(197612)38:6<2404::aid-cncr2820380629>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Margo CE, Folberg R, Zimmerman LE, Sesterhenn IA. Endodermal sinus tumor (yolk sac tumor) of the orbit. Ophthalmology. 1983;90(12):1426–32. doi: 10.1016/s0161-6420(83)34364-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Liu A, Peng Y, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. Am J Surg Pathol. 2009;33(10):1529–39. doi: 10.1097/PAS.0b013e3181ad25d5. [DOI] [PubMed] [Google Scholar]
- 9.Morin MJ, Hopkins RA, Ferguson WS, Ziegler JW. Intracardiac yolk sac tumor and dysrhythmia as an etiology of pediatric syncope. Pediatrics. 2004;113(4):374–76. doi: 10.1542/peds.113.4.e374. [DOI] [PubMed] [Google Scholar]
- 10.Parvathy U, Balakrishnan KR, Ranjit MS, et al. Primary intracardiac yolk sac tumor. Pediatr Cardiol. 1998;19(6):495–97. doi: 10.1007/s002469900368. [DOI] [PubMed] [Google Scholar]
- 11.Nunes MC, Moreira DR, Ferrari TC. Cardiac metastasis from yolk sac tumor: Case report and review. Exp Hematol Oncol. 2013;2:13. doi: 10.1186/2162-3619-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JX, Eftimie B, Mortimer J. Intracardiac metastasis of germ cell tumor complicated by pulmonary hypertension and thrombocytopenia. J Clin Oncol. 2007;25(23):3547–49. doi: 10.1200/JCO.2007.12.3562. [DOI] [PubMed] [Google Scholar]
- 13.Savarese DM, Rohrer MJ, Pezzella AT, et al. Successful management of intracardiac extension of tumor thrombus in a patient with advanced nonseminomatous germ cell testicular cancer. Urology. 1995;46(6):883–87. doi: 10.1016/s0090-4295(99)80366-5. [DOI] [PubMed] [Google Scholar]
- 14.Taghavi F, Markar S, Williams M, Large S. Intra-cardiac metastasis from testicular non-seminoma germ cell tumour; to resect or not to resect. Interact Cardiovasc Thorac Surg. 2010;11(6):843–45. doi: 10.1510/icvts.2010.242198. [DOI] [PubMed] [Google Scholar]
- 15.Balzer BL, Ulbright TM. Spontaneous regression of testicular germ cell tumors: An analysis of 42 cases. Am J Surg Pathol. 2006;30(7):858–65. doi: 10.1097/01.pas.0000209831.24230.56. [DOI] [PubMed] [Google Scholar]