Abstract
ZNF804A rs1344706 (A/C) was the first SNP that reached genome-wide significance for schizophrenia. Recent studies have linked rs1344706 to functional connectivity among specific brain regions. However, no study thus far has examined the role of this SNP in the entire functional connectome. In this study, we used degree centrality to test the role of rs1344706 in the whole-brain voxel-wise functional connectome during the resting state. 52 schizophrenia patients and 128 healthy controls were included in the final analysis. In our whole-brain analysis, we found a significant interaction effect of genotype × diagnosis at the precuneus (PCU) (cluster size = 52 voxels, peak voxel MNI coordinates: x = 9, y = − 69, z = 63, F = 32.57, FWE corrected P < 0.001). When we subdivided the degree centrality network according to anatomical distance, the whole-brain analysis also found a significant interaction effect of genotype × diagnosis at the PCU with the same peak in the short-range degree centrality network (cluster size = 72 voxels, F = 37.29, FWE corrected P < 0.001). No significant result was found in the long-range degree centrality network. Our results elucidated the contribution of rs1344706 to functional connectivity within the brain network, and may have important implications for our understanding of this risk gene's role in functional dysconnectivity in schizophrenia.
Keywords: ZNF804A, Schizophrenia, Functional magnetic resonance imaging, Degree centrality, Brain network, PCC/PCU
Highlights
-
•
This study was the first to report the effect of ZNF804A rs1344706 on the property of the whole-brain network.
-
•
We found a significant interaction of rs1344706 genotype × diagnosis on the functional connectivity of the PCU.
1. Introduction
Schizophrenia is a serious and debilitating mental disorder with a lifetime prevalence rate of about 1%. Its heritability has been estimated to be about 81% (Sullivan et al., 2003). Recent whole genome-wide association studies of schizophrenia have suggested many risk genetic polymorphisms. Among them, rs1344706 (A/C) in the gene of zinc finger protein 804A (ZNF804A) was the first SNP that reached genome-wide significance level (O'Donovan et al., 2008). Our recent study also found a significant association between the same SNP and schizophrenia (Zhang et al., 2016). To identify the neural systems linked to this SNP, multiple fMRI studies using working memory tasks (e.g., the N-back task) have been reported (Esslinger et al., 2009, Paulus et al., 2013, Rasetti et al., 2011, Zhang et al., 2016). Although these studies did not find significant genotype effects on regional brain activation, they consistently found significant genotype effects on functional connectivity seeded from the right dorsolateral prefrontal cortex (rDLPFC) to the left hippocampus formation (lHF). Our recent study reconstructed the fiberic and accordant functional subconnections between the rDLPFC and the lHF (with anterior cingulate cortex (ACC) and posterior cingulate cortex/precuneus (PCC/PCU) as the intervening nodes) and found a significant association between rs1344706 and PCC/PCU -lHF connection (Zhang et al., 2016).
One limitation of the previous functional connectivity studies (Esslinger et al., 2009, Paulus et al., 2013, Rasetti et al., 2011, Zhang et al., 2016) is that they relied on a priori selection of seeds. No study thus far has examined the role of this SNP in the entire functional connectome, perhaps because it was only recently when the development of computational neuroimaging made it possible to examine the whole-brain voxel-wise functional connectome during the resting state (Buckner et al., 2009, Dai et al., 2015, Liang et al., 2013, Zuo et al., 2012). Instead of using seeds, the new approach computes degree centrality (a measure of network in graph theory) of a specific grey matter voxel based on the total correlations of its time series with those of the rest of the network. Brain regions with high degree centrality are deemed to be the hubs that play central roles in functional integrity of the whole-brain network (Crossley et al., 2014, Dai et al., 2015, Guo et al., 2015a, Liang et al., 2013, Rubinov and Sporns, 2010).
In the current study, we used the whole-brain connectome approach to identify key brain regions within the brain network that were linked to rs1344706. The final sample included 52 schizophrenia patients and 128 healthy controls who were scanned using fMRI during the resting state. We hypothesized that the PCC/PCU may be one of the key brain regions linked to rs1344706 due to four reasons: (1) The PCC/PCU is an important brain hub in the human brain with high connectivities with other brain areas (Buckner et al., 2009, Cao et al., 2014, van den Heuvel and Sporns, 2013); (2) Perhaps due at least partially to such connectivities, cerebral blood flow and metabolic rate of the PCC/PCU are 40% greater than average (Raichle et al., 2001); (3) Altered degree centrality of the PCC/PCU has been found in schizophrenia (Guo et al., 2015a, van Lutterveld et al., 2014, Wang et al., 2015a, Wang et al., 2014) and has been associated with some symptoms of schizophrenia such as hallucination (van Lutterveld et al., 2014); and (4) Our recent study as mentioned above found a significant role of rs1344706 in functional connection of the PCC/PCU (Zhang et al., 2016).
2. Materials and methods
2.1. Participants
The initial sample consisted of 55 schizophrenia patients and 137 healthy controls. Due to their excessive head motion (> 3° or 3 mm) and failure of registration (due to low quality of T1 images), 3 patients and 9 healthy controls were excluded. Due to the small number of the minor-allele homozygotes (10 patients and 28 controls), we combined the CC genotype with the CA genotype as the non-risk allele carriers. The final data analysis included 52 schizophrenia patients (34 CA/CC and 18 AA) and 128 healthy controls (96 CA/CC and 32 AA). The patients were recruited from the inpatients of the Beijing Anding Hospital. All patients fulfilled the DSM-IV criteria for schizophrenia according to the diagnostic consensus of two experienced psychiatrists based on structured interview (SCID). The positive and negative syndrome scale (PANSS) was used to assess each patient's positive (SAPS) and negative (SANS) symptoms. The mean scores of the SAPS and SANS are shown in Table 1. All patients were treated with stable doses of atypical antipsychotics for > 2 weeks. Antipsychotic drugs included clozapine (5 patients), olanzapine (13 patients), risperidone (22 patients), aripiprazole (6 patients), and haloperidol (6 patients). No antidepressants were involved. Patients took 2.5–5 mg diazepam occasionally for their insomnia. However, patients were asked not to take diazepam or other benzodiazepines on the night before the scan. If they did, their appointments for fMRI scans were canceled. Exclusion criteria for the patients included a history of other psychiatric disorders and severe brain injury (any closed or open injuries that may be related to current symptoms or cognitive functions), current substance abuse, and currently having acute psychotic episodes. The healthy controls were recruited from the same geographical region as the patients by advertisement and received unstructured clinical interview by experienced psychiatrists to screen for any personal or family history of psychiatric disorders. Most healthy controls were involved in our previous studies (Yu et al., 2016, Zhang et al., 2016). All patients and controls had normal or corrected-to-normal vision and were right-handed as assessed by the Edinburgh handedness inventory. None of the patients and controls reported any history of hard drug use (e.g., cocaine, crack, heroin, methamphetamine, etc.). Additional demographic and clinical information for both patients and controls is shown in Table 1.
Table 1.
Demographics and clinical characteristics of the participants.
| SCZ |
Healthy control |
Total |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD |
F or χ2 | P | Mean ± SD |
F or χ2 | P | Mean ± SD |
F or χ2 | P | ||||
| AA | CA/CC | AA | CA/CC | AA | CA/CC | |||||||
| Age (years) | 27.44 ± 6.81 | 28.24 ± 8.78 | 0.11 | 0.74 | 26.84 ± 4.57 | 27.20 ± 6.11 | 0.91 | 0.76 | 27.06 ± 5.42 | 27.47 ± 6.88 | 0.14 | 0.71 |
| Gender (male/female) | 18 (15/3) | 34 (30/4) | 0.24 | 0.68 | 32 (25/7) | 96 (74/22) | 0.02 | 1.00 | 50 (40/10) | 130 (104/26) | 0.00 | 1.00 |
| Education (years) | 12.44 ± 2.85 | 13.00 ± 3.19 | 0.38 | 0.54 | 13.62 ± 2.64 | 12.59 ± 3.19 | 2.72 | 0.10 | 13.20 ± 2.75 | 12.70 ± 3.18 | 0.96 | 0.33 |
| PANSS positive score | 19.33 ± 11.12 | 17.91 ± 10.28 | 0.21 | 0.65 | NA | NA | NA | NA | NA | NA | NA | NA |
| PANSS negative score | 16.28 ± 10.49 | 17.62 ± 9.96 | 0.21 | 0.65 | NA | NA | NA | NA | NA | NA | NA | NA |
| PANSS total score | 62.89 ± 38.88 | 68.35 ± 33.99 | 0.28 | 0.60 | NA | NA | NA | NA | NA | NA | NA | NA |
| Number of episodes | 2.72 ± 1.56 | 2.85 ± 1.51 | 0.09 | 0.76 | NA | NA | NA | NA | NA | NA | NA | NA |
| Age of onset (years) | 20.88 ± 3.99 | 21.74 ± 4.48 | 0.46 | 0.49 | NA | NA | NA | NA | NA | NA | NA | NA |
| Illness duration (months) | 72.66 ± 59.14 | 70.28 ± 71.29 | 0.02 | 0.90 | NA | NA | NA | NA | NA | NA | NA | NA |
| Medication dosage (mg/day)a | 310.72 ± 273.52 | 224.88 ± 172.53 | 1.92 | 0.17 | NA | NA | NA | NA | NA | NA | NA | NA |
Chlorpromazine equivalents.
This study's protocol was reviewed and approved by the Institutional Review Board of the Institute of Cognitive Neuroscience and Learning at Beijing Normal University. This study was conducted in accordance with the approved protocol. All participants were Han Chinese and gave written informed consent for this study.
2.2. Genotyping
Genomic DNA was extracted using the standard method. The SNP rs1344706 was genotyped using TaqMan allele-specific assays on the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.). The sample success rate was 100%. The reproducibility of the genotyping was 100% based on a duplicate analysis of 40% of the genotypes.
2.3. Imaging data acquisition
All imaging data were acquired at the Brain Imaging Center of Beijing Normal University. All participants were scanned on a Siemens 3 T scanner (Siemens, Erlangen, Germany) with their head snugly fixed with straps and foam pads to restrict head movement. Resting-state images (240 volumes) were acquired first, followed by the T1 scan. During the resting-state fMRI data collection (lasting about 8 min), all subjects were required to keep their eyes closed, to stay still but relaxed, and not to think of anything in particular and not to fall asleep.
The resting-state images were collected axially using echo-planar imaging (EPI) sequence: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; field of view (FOV) = 200 × 200 mm2; matrix size = 64 × 64; axial slices = 31; 4.0 mm slice thickness without gap; voxel size = 3.125 × 3.125 × 4.0 mm3. Structural images were acquired using a T1-weighted sagittal 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence: TR = 2530 ms; TE = 3.45 ms; FA = 7o; FOV = 256 × 256 mm2; matrix size = 256 × 256; slices = 176; thickness = 1.0 mm; voxel size = 1 × 1 × 1 mm3.
2.4. fMRI data preprocessing
Functional imaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK). Preprocessing of resting-state fMRI data included slice timing (to correct the acquisition time differences between slices), realignment (participants with > 3 mm maximum displacement in any of the x, y, z directions or > 3° of angular rotation about any axis were excluded), normalization to the MNI space (each participant's T1-weighted images were first coregistered to the mean functional image after head motion correction and then segmented into white matter, grey matter, and cerebrospinal fluid, and generated segmentation parameters were then used to normalize functional images to the MNI standard space), resampling to voxel size 3 × 3 × 3 mm3, spatial smoothing with a 4-mm full width at half maximum (FWHM) Gaussian kernel, and removal of linear trends and temporal filter (0.01–0.1 Hz, to reduce low-frequency drifts and high-frequency physiological noise). To further reduce the effects of confounding factors, 6 head motion parameters and white matter and cerebrospinal fluid signals were included as covariates. The global mean signal was not included as a covariate (Fox et al., 2009, Guo et al., 2015b, Murphy et al., 2009, Saad et al., 2013, van Lutterveld et al., 2014, Wang et al., 2015a).
2.5. Degree centrality analysis
Degree centrality (whole-range) is a measure in graph theory that estimates the total connectivity between a voxel and the rest of the brain (Buckner et al., 2009, Dai et al., 2015, Zuo et al., 2012). It has often been used to identify the hub regions of the brain network (Dai et al., 2015, Guo et al., 2015a, Liang et al., 2013, Rubinov and Sporns, 2010). Specifically, Pearson's correlation coefficients were computed in all possible pairs of voxels, which resulted in a whole-brain functional connectivity matrix. This step was restricted to a predefined grey matter mask that was the same as in previous studies (Zhou et al., 2014, Zuo et al., 2012). For a given voxel, its degree centrality was calculated using the following equation (Buckner et al., 2009, Zuo et al., 2012):
where rij was Pearson's correlation coefficient between voxel i and voxel j, and r0 was a correlation threshold that was used to eliminate possible spurious correlations arising from noise. In the current study, r0 = 0.2. It is worth noting that different correlation thresholds did not change the connection pattern significantly, which is consistent with previous reports (Dai et al., 2015, Guo et al., 2015a, Liang et al., 2013). A degree centrality map for each individual was produced and then standardized to a z-score map (Buckner et al., 2009, Yan et al., 2013, Zhou et al., 2016, Zuo et al., 2012).
Degree centrality can be affected by connectivity distance between regions. Therefore, for further analysis, we subdivided the connections and associated degree centrality between all possible pairs of voxels into long-range (> 75 mm) and short-range (< 75 mm) according to their anatomical distance (Achard et al., 2006, He et al., 2007, Liang et al., 2013) by using the GRaph thEoreTical Network Analysis (GRETNA) toolbox (Wang et al., 2015b).
2.6. Statistical analysis
We did full factorial two-way ANOVA across the whole brain to test the genotype effect, the diagnosis effect, and their interaction, with genotype (AA vs. CA/CC) and diagnosis (schizophrenia vs. controls) as fixed factors. If significant main effects of genotype or significant interaction effects of genotype × diagnosis were found, genotype effects were further explored using two-sample t-test across the whole brain in the patients and controls separately. To correct for multiple comparisons, significance was determined using a voxel-level threshold of P < 0.001 and cluster-level family-wise error (FWE) corrected P < 0.05.
3. Results
No deviation from Hardy–Weinberg equilibrium was found for rs1344706 (P > 0.05). As shown in Table 1, there were no significant differences between genotypes in their demographic factors.
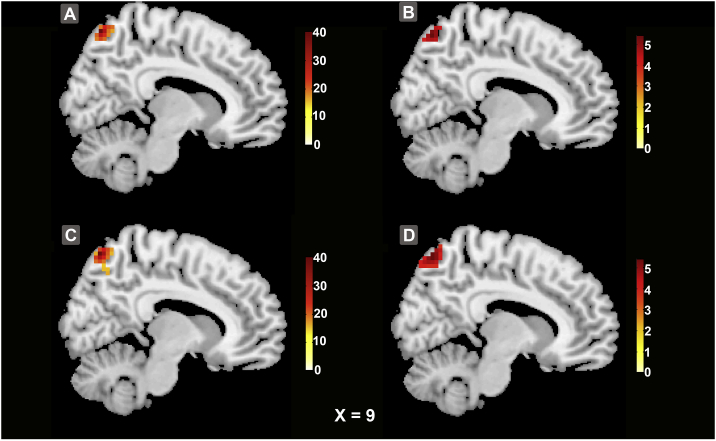
The whole-brain analysis of the whole-range degree centrality network revealed no significant genotype effects, but a significant interaction effect of genotype × diagnosis within the PCU (cluster size = 52 voxels, peak voxel MNI coordinates: x = 9, y = − 69, z = 63, F = 32.57, FWE corrected P < 0.001) (see Fig. 1A). We then did the whole-brain analysis for the genotype effect in patients and healthy controls separately and only found a significant genotype effect in patients, at the PCU with the same peak (cluster size = 27 voxels, T = 5.35, FWE corrected P = 0.042, see Fig. 1B and Supplementary Fig. S2A). The patients who were risk allele homozygotes showed significantly increased degree centrality as compared to risk allele non-carriers. No significant result was found in healthy controls (FWE corrected P > 0.05) although its sample size was three times as large as that of patients. It needs to be mentioned that, the main effect of diagnosis within the PCU was also significant with patients showing higher degree centrality (cluster size = 29 voxels, peak voxel MNI coordinates: x = − 3, y = − 66, z = 33, F = 17.55, FWE corrected P = 0.029) than healthy controls (see Supplementary Fig. S1), which was consistent with previous reports (Guo et al., 2015a, van Lutterveld et al., 2014, Wang et al., 2015a).
Fig. 1.
Full factorial ANOVA across the whole-brain whole-range degree centrality network showed a significant interaction effect of genotype × diagnosis at the PCU (Panel A, the maximal peak voxel MNI coordinates: x, y, z = [9–69 63]). Further analysis found that the interaction effect was limited to short-range connections (Panel C). Separate analyses for patients and controls found that these effects were specific to patients (Panel B for whole-range, Panel D for short-range).
Because distance is one of the major potential confounds when studying functional connectivity, we further subdivided the degree centrality network into long- and short-range connections. The whole-brain analysis found significant results only for short-range connections, with a significant interaction effect of genotype × diagnosis at the PCU with the same peak as above (cluster size = 72 voxels, F = 37.29, FWE corrected P < 0.001) (see Fig. 1C). Separate analyses for patients and controls confirmed that the genotype effect was limited to patients, within the PCU at the same peak (cluster size = 66 voxels, T = 5.68, FWE corrected P < 0.001, see Fig. 1D and Supplementary Fig. S2B). The patients who were risk allele homozygotes showed significantly increased short-range degree centrality. No significant result was found in healthy controls (FWE corrected P > 0.05).
4. Discussion
This study was the first to report the effect of ZNF804A rs1344706 on the property of the whole-brain network. Using the case-control design, we found a significant interaction of rs1344706 genotype × diagnosis on the functional connectivity of the PCU (specifically, short-range degree centrality), indicating a diverging effect of rs1344706 in schizophrenia patients and healthy controls.
The interaction effect of rs1344706 genotype × diagnosis may be one of the most consistent results in previous studies. In addition to this study, there were at least 10 other studies that also used the case-control design to test the effect of rs1344706, 8 of which found significant interaction effects of rs1344706 genotype × diagnosis (Donohoe et al., 2011, Hashimoto et al., 2010, Kuswanto et al., 2012, Nenadic et al., 2015, Schultz et al., 2014, Walters et al., 2010, Wei et al., 2012, Wei et al., 2015). Within the 8 studies, two were behavioral studies and the other six examined structural MRI characteristics, such as grey matter volume (Donohoe et al., 2011, Nenadic et al., 2015), cortical thickness (Schultz et al., 2014, Wei et al., 2015), surface area (Wei et al., 2015), white matter density (Wei et al., 2012), or white matter integrity (Kuswanto et al., 2012). Although the location of the genotype × diagnosis interaction effect was not the same across these studies, the PCC/PCU was one of the most often reported regions (Donohoe et al., 2011, Schultz et al., 2014, Wei et al., 2015). This study, for the first time, suggested an interaction effect of genotype × diagnosis in fMRI data and localized it to the PCU. However, due to lacks of detailed information on diagnosis effect within PCC/PCU in the previous studies (Donohoe et al., 2011, Schultz et al., 2014, Wei et al., 2015) which could indicate the characteristic changes of schizophrenia patients, it is impossible to compare the direction of the interaction effect across different studies directly. It needs to mention that the position of the PCU identified in this study is very close to that of the PCU whose functional connectivity (during the N-back task and resting state) with the hippocampus formation differed significantly by rs1344706 genotype (Zhang et al., 2016).
Because the PCU is one of the most important hubs of the whole-brain network (Buckner et al., 2009, Cao et al., 2014, van den Heuvel and Sporns, 2013), it is likely that ZNF804A rs1344706 could affect the overall organization of functional communication in the brain network, rather than only the activity of specific brain regions. Such a conjecture would explain the fact that most previous fMRI studies using working memory tasks did not find significant effects of rs1344706 on regional brain activation, but instead found significant effects on functional connectivity (Esslinger et al., 2009, Paulus et al., 2013, Rasetti et al., 2011, Zhang et al., 2016). It needs to be mentioned that two fMRI studies using a theory-of-mind (ToM task (Mohnke et al., 2014, Walters et al., 2010) consistently found significant effects of rs1344706 on the deactivation of PCC/PCU. As a key node of the DMN, the PCC/PCU may contribute to cognitive processing through its functional connections rather than its activation level (Elton and Gao, 2015, Leech and Sharp, 2014). In that sense, our finding may help us to understand the above finding from the two fMRI studies using a ToM task (Mohnke et al., 2014, Walters et al., 2010). The energy demand of the PCU is very high due to its central role in optimizing overall brain communication (Bullmore and Sporns, 2012, Liang et al., 2013). Accordingly, the PCU becomes one of the weakest points within the brain network and is vulnerable to any potential insult. Previous studies found that injuries to the hubs led to greater impairments of the brain's overall communication efficiency than did injuries to non-hub regions (van den Heuvel and Sporns, 2011). Taken together, the finding that the risk allele homozygotes had significantly higher degree centrality at the PCU hub suggests that this region would have a higher demand for energy and hence greater susceptibility to dysfunctions including those leading to diseases such as schizophrenia.
In addition to the significant interaction effect of genotype × diagnosis, the PCU also showed a significant diagnosis effect in this study. Briefly, schizophrenia patients showed significantly higher degree centrality at this region than did healthy controls. It seems that schizophrenia risk factors such as the risk allele of rs1344706 could affect the etiology of schizophrenia through the functional connectivity of the PCU. There have been 4 other independent resting-state fMRI studies that found significant differences between patients and controls in degree centrality within PCC/PCU (Guo et al., 2015a, van Lutterveld et al., 2014, Wang et al., 2015a, Wang et al., 2014). Three previous studies and the current one reported increased degree centrality in PCC/PCU for schizophrenia patients (Guo et al., 2015a, van Lutterveld et al., 2014, Wang et al., 2015a), but one found decreased degree centrality in schizophrenia patients (Wang et al., 2014). The discrepancy between these results may have been due to the fact that four studies did not account for the global mean signal (Guo et al., 2015a, van Lutterveld et al., 2014, Wang et al., 2015a), whereas the fifth one did (Wang et al., 2014). Indeed, Wang et al. (2015a) analyzed their data both ways–with and without accounting for global mean signal –and reported that the elevated degree centrality of PCC in schizophrenia patients was more obvious when global signals were not accounted for than when they were (Wang et al., 2015a). Previously, the global mean signals at the resting-state were presumed to reflect physiological noise and were thus commonly accounted for. However, it is now suggested that such signals may also reflect important neuronal components (Hahamy et al., 2014). So it seems more reasonable not to remove the global mean signals when we analyze the fMRI data.
When we subdivided the whole-range degree centrality network according to anatomic distance into long-range and short-range connections, we further found that the significant interaction effect of genotype × diagnosis was limited to short-range connections. The balance between long- and short-range cortical-cortical interactions is important for efficient cortical processing (Mesulam, 1998). The brain network in children is dominated by short-range connections, but long-range connections become dominant as children mature (Fair et al., 2009, Fair et al., 2007, Kelly et al., 2009). Our result seems to suggest that the brain network of schizophrenia patients who are risk allele homozygotes may be underdeveloped.
The PCU is an important component of the default mode network (DMN). Although the exact reason for the role of the DMN in symptoms of schizophrenia is still unknown, two properties of the DMN give us some hints. First, the DMN is very active when the mind is in a passive state or when the mind is involved in tasks that direct attention away from external stimuli (Andreasen et al., 1995). Some researchers have proposed the internal mentation hypothesis that the DMN directly supports internal mentation that is detached from the external world (Andrews-Hanna, 2012, Buckner et al., 2008). It is possible that overactive DMN may weaken or even cut off the link between internal mentation and the external world in schizophrenia patients. Second, the DMN is closely connected to brain regions that are active during episodic memory such as the hippocampus (Kim et al., 2010). When the link between internal mentation and the external world was weakened, patients' thinking and judgement may rely more heavily on their own memory. As a result, they may produce many self-referential thoughts (Buckner et al., 2008). As reported in one previous study (van Lutterveld et al., 2014), increased short-range functional connectivity at the PCU was associated with increased self-referential thoughts, which could further result in hallucinations. It needs to mention that the DMN is deactivated when performing tasks with high cognitive demands such as the ToM task. Some fMRI studies using a ToM task (to measure the ability to understand other people's mental states) have suggested that the significant rs1344706 genotype effect on the deactivation of the PCC/PCU may be due to delusional ideation in schizophrenia patients (Mohnke et al., 2014, Walters et al., 2010). In sum, rs1344706 may be linked to symptoms of schizophrenia due to its role in the PCC/PCU-related brain connectivity.
Three major limitations need to be mentioned. First, the size of our patient sample was relatively small. Second, we did not analyze the task fMRI data due to a lack of a consensus on the network analysis methods for task-fMRI data. Finally, the threshold (voxel-level threshold of P < 0.001 and cluster-level family-wise error (FWE) corrected P < 0.05) used in this study may still be too liberal according to Eklund et al.'s study (2016). Therefore, results of this study should be interpreted with caution.
5. Conclusion
To conclude, the current study found significant effects of ZNF804A rs1344706 on the degree centrality of an important hub, the PCU. Our results suggest a possible mechanism underlying this genetic polymorphism's association with schizophrenia.
Author contributions
Li J, Dong Q and Chen CS designed the study and wrote the protocol. Zhang QM, Zhai JG, Chen M, Du BQ, Deng XX, Ji F, Wang CY, Xiang YT and Wu HJ selected and evaluated the sample. Chen XY, Zhang ZF, and Zhao W conducted the literature searches. Chen XY undertook the statistical analysis and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Financial disclosures
Authors declare no competing financial interests in relation with this manuscript.
Acknowledgements
The authors would like to thank all the volunteers and this work was supported by grants from the National Key Basic Research Program of China (2014CB846103) and the Natural Science Foundation of China (81571045).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.12.017.
Appendix A. Supplementary data
Fig. S1. The main effect of diagnosis across the whole brain in the whole-range degree centrality network using a full factorial ANOVA. Patients showed significantly higher degree centrality than healthy controls in multiple regions, including the PCU.
Fig. S2. Mean functional degree centrality maps for each genotype/diagnosis group. The left panels show the mean degree centrality maps within the whole-range network and the right panels show the mean degree centrality maps within the short-range network. Panels A and B are for schizophrenia patients. Panels C and D are for healthy controls.
References
- Achard S., Salvador R., Whitcher B., Suckling J., Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., O'Leary D.S., Cizadlo T., Arndt S., Rezai K., Watkins G.L., Ponto L.L., Hichwa R.D. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Cao M., Wang J.H., Dai Z.J., Cao X.Y., Jiang L.L., Fan F.M., Song X.W., Xia M.R., Shu N., Dong Q., Milham M.P., Castellanos F.X., Zuo X.N., He Y. Topological organization of the human brain functional connectome across the lifespan. Dev. Cogn. Neurosci. 2014;7:76–93. doi: 10.1016/j.dcn.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley N.A., Mechelli A., Scott J., Carletti F., Fox P.T., McGuire P., Bullmore E.T. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Yan C., Li K., Wang Z., Wang J., Cao M., Lin Q., Shu N., Xia M., Bi Y., He Y. Identifying and mapping connectivity patterns of brain network hubs in Alzheimer's disease. Cereb. Cortex. 2015;25:3723–3742. doi: 10.1093/cercor/bhu246. [DOI] [PubMed] [Google Scholar]
- Donohoe G., Rose E., Frodl T., Morris D., Spoletini I., Adriano F., Bernardini S., Caltagirone C., Bossu P., Gill M., Corvin A.P., Spalletta G. ZNF804A risk allele is associated with relatively intact gray matter volume in patients with schizophrenia. NeuroImage. 2011;54:2132–2137. doi: 10.1016/j.neuroimage.2010.09.089. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl Acad. Sci. USA. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A., Gao W. Task-positive functional connectivity of the default mode network transcends task domain. J. Cogn. Neurosci. 2015;27:2369–2381. doi: 10.1162/jocn_a_00859. [DOI] [PubMed] [Google Scholar]
- Esslinger C., Walter H., Kirsch P., Erk S., Schnell K., Arnold C., Haddad L., Mier D., Opitz von Boberfeld C., Raab K., Witt S.H., Rietschel M., Cichon S., Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. 2007. Development of Distinct Control Networks Through Segregation and Integration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5 doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Liu F., Xiao C., Liu J., Yu M., Zhang Z., Zhang J., Zhao J. Increased short-range and long-range functional connectivity in first-episode, medication-naive schizophrenia at rest. Schizophr. Res. 2015;166:144–150. doi: 10.1016/j.schres.2015.04.034. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Zhang Z., Liu G., Liu J., Yu L., Xiao C., Zhao J. Increased cerebellar functional connectivity with the default-mode network in unaffected siblings of schizophrenia patients at rest. Schizophr. Bull. 2015;41:1317–1325. doi: 10.1093/schbul/sbv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A., Calhoun V., Pearlson G., Harel M., Stern N., Attar F., Malach R., Salomon R. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 2014;4:395–403. doi: 10.1089/brain.2014.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R., Ohi K., Yasuda Y., Fukumoto M., Iwase M., Iike N., Azechi M., Ikezawa K., Takaya M., Takahashi H., Yamamori H., Okochi T., Tanimukai H., Tagami S., Morihara T., Okochi M., Tanaka T., Kudo T., Kazui H., Iwata N., Takeda M. The impact of a genome-wide supported psychosis variant in the ZNF804A gene on memory function in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B:1459–1464. doi: 10.1002/ajmg.b.31123. [DOI] [PubMed] [Google Scholar]
- He Y., Chen Z.J., Evans A.C. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Rich-club organization of the human connectome. J. Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Network hubs in the human brain. Trends Cogn. Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T., Margulies D.S., Castellanos F.X., Milham M.P. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kim H., Daselaar S.M., Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: roles of the task-positive and task-negative networks. NeuroImage. 2010;49:1045–1054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto C.N., Woon P.S., Zheng X.B., Qiu A., Sitoh Y.Y., Chan Y.H., Liu J., Williams H., Ong W.Y., Sim K. Genome-wide supported psychosis risk variant in ZNF804A gene and impact on cortico-limbic WM integrity in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B:255–262. doi: 10.1002/ajmg.b.32032. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zou Q., He Y., Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lutterveld R., Diederen K.M., Otte W.M., Sommer I.E. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum. Brain Mapp. 2014;35:1436–1445. doi: 10.1002/hbm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.-M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mohnke S., Erk S., Schnell K., Schutz C., Romanczuk-Seiferth N., Grimm O., Haddad L., Pohland L., Garbusow M., Schmitgen M.M., Kirsch P., Esslinger C., Rietschel M., Witt S.H., Nothen M.M., Cichon S., Mattheisen M., Muhleisen T., Jensen J., Schott B.H., Maier W., Heinz A., Meyer-Lindenberg A., Walter H. Further evidence for the impact of a genome-wide-supported psychosis risk variant in ZNF804A on the Theory of Mind Network. Neuropsychopharmacology. 2014;39:1196–1205. doi: 10.1038/npp.2013.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I., Maitra R., Basmanav F.B., Schultz C.C., Lorenz C., Schachtzabel C., Smesny S., Nothen M.M., Cichon S., Reichenbach J.R., Sauer H., Schlosser R.G., Gaser C. ZNF804A genetic variation (rs1344706) affects brain grey but not white matter in schizophrenia and healthy subjects. Psychol. Med. 2015;45:143–152. doi: 10.1017/S0033291714001159. [DOI] [PubMed] [Google Scholar]
- O'Donovan M.C., Craddock N., Norton N., Williams H., Peirce T., Moskvina V., Nikolov I., Hamshere M., Carroll L., Georgieva L., Dwyer S., Holmans P., Marchini J.L., Spencer C.C., Howie B., Leung H.T., Hartmann A.M., Moller H.J., Morris D.W., Shi Y., Feng G., Hoffmann P., Propping P., Vasilescu C., Maier W., Rietschel M., Zammit S., Schumacher J., Quinn E.M., Schulze T.G., Williams N.M., Giegling I., Iwata N., Ikeda M., Darvasi A., Shifman S., He L., Duan J., Sanders A.R., Levinson D.F., Gejman P.V., Cichon S., Nothen M.M., Gill M., Corvin A., Rujescu D., Kirov G., Owen M.J., Buccola N.G., Mowry B.J., Freedman R., Amin F., Black D.W., Silverman J.M., Byerley W.F., Cloninger C.R., Molecular Genetics of Schizophrenia, C Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat. Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Paulus F.M., Krach S., Bedenbender J., Pyka M., Sommer J., Krug A., Knake S., Nothen M.M., Witt S.H., Rietschel M., Kircher T., Jansen A. Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Hum. Brain Mapp. 2013;34:304–313. doi: 10.1002/hbm.21434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R., Sambataro F., Chen Q., Callicott J.H., Mattay V.S., Weinberger D.R. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch. Gen. Psychiatry. 2011;68:1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Saad Z.S., Reynolds R.C., Jo H.J., Gotts S.J., Chen G., Martin A., Cox R.W. Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect. 2013;3:339–352. doi: 10.1089/brain.2013.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C.C., Nenadic I., Riley B., Vladimirov V.I., Wagner G., Koch K., Schachtzabel C., Muhleisen T.W., Basmanav B., Nothen M.M., Deufel T., Kiehntopf M., Rietschel M., Reichenbach J.R., Cichon S., Schlosser R.G., Sauer H. ZNF804A and cortical structure in schizophrenia: in vivo and postmortem studies. Schizophr. Bull. 2014;40:532–541. doi: 10.1093/schbul/sbt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.F., Kendler K.S., Neale M.C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Walters J.T., Corvin A., Owen M.J., Williams H., Dragovic M., Quinn E.M., Judge R., Smith D.J., Norton N., Giegling I., Hartmann A.M., Moller H.J., Muglia P., Moskvina V., Dwyer S., O'Donoghue T., Morar B., Cooper M., Chandler D., Jablensky A., Gill M., Kaladjieva L., Morris D.W., O'Donovan M.C., Rujescu D., Donohoe G. Psychosis susceptibility gene ZNF804A and cognitive performance in schizophrenia. Arch. Gen. Psychiatry. 2010;67:692–700. doi: 10.1001/archgenpsychiatry.2010.81. [DOI] [PubMed] [Google Scholar]
- Wang X., Xia M., Lai Y., Dai Z., Cao Q., Cheng Z., Han X., Yang L., Yuan Y., Zhang Y., Li K., Ma H., Shi C., Hong N., Szeszko P., Yu X., He Y. Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophr. Res. 2014;156:150–156. doi: 10.1016/j.schres.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhou Y., Zhuo C., Qin W., Zhu J., Liu H., Xu L., Yu C. Altered functional connectivity of the cingulate subregions in schizophrenia. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., Xia M., Liao X., Evans A., He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015;9:386. doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Kang Z., Diao F., Shan B., Li L., Zheng L., Guo X., Liu C., Zhang J., Zhao J. Association of the ZNF804A gene polymorphism rs1344706 with white matter density changes in Chinese schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;36:122–127. doi: 10.1016/j.pnpbp.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Wei Q., Li M., Kang Z., Li L., Diao F., Zhang R., Wang J., Zheng L., Wen X., Zhang J., Zhao J., Huang R. ZNF804A rs1344706 is associated with cortical thickness, surface area, and cortical volume of the unmedicated first episode schizophrenia and healthy controls. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015;168B:265–273. doi: 10.1002/ajmg.b.32308. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Craddock R.C., Zuo X.N., Zang Y.F., Milham M.P. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. NeuroImage. 2013;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Chen X., Zhao W., Zhang Z., Zhang Q., Han B., Zhai J., Chen M., Du B., Deng X., Ji F., Wang C., Xiang Y.T., Li D., Wu H., Li J., Dong Q., Chen C. Effect of rs1063843 in the CAMKK2 gene on the dorsolateral prefrontal cortex. Hum. Brain Mapp. 2016;37:2398–2406. doi: 10.1002/hbm.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Chen X., Yu P., Zhang Q., Sun X., Gu H., Zhang H., Zhai J., Chen M., Du B., Deng X., Ji F., Wang C., Xiang Y., Li D., Wu H., Li J., Dong Q., Chen C. Effect of rs1344706 in the ZNF804A gene on the connectivity between the hippocampal formation and posterior cingulate cortex. Schizophr. Res. 2016;170:48–54. doi: 10.1016/j.schres.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang Y., Rao L.L., Liang Z.Y., Chen X.P., Zheng D., Tan C., Tian Z.Q., Wang C.H., Bai Y.Q., Chen S.G., Li S. Disrutpted resting-state functional architecture of the brain after 45-day simulated microgravity. Front. Behav. Neurosci. 2014;8:200. doi: 10.3389/fnbeh.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Hu X., Hu J., Liang M., Yin X., Chen L., Zhang J., Wang J. Altered brain network in amyotrophic lateral sclerosis: a resting graph theory-based network study at voxel-wise level. Front. Neurosci. 2016;10:204. doi: 10.3389/fnins.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Ehmke R., Mennes M., Imperati D., Castellanos F.X., Sporns O., Milham M.P. Network centrality in the human functional connectome. Cereb. Cortex. 2012;22:1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The main effect of diagnosis across the whole brain in the whole-range degree centrality network using a full factorial ANOVA. Patients showed significantly higher degree centrality than healthy controls in multiple regions, including the PCU.
Fig. S2. Mean functional degree centrality maps for each genotype/diagnosis group. The left panels show the mean degree centrality maps within the whole-range network and the right panels show the mean degree centrality maps within the short-range network. Panels A and B are for schizophrenia patients. Panels C and D are for healthy controls.